SUMMARY
Feather pecking (FP) by laying hens is a significant welfare issue in the poultry industry. Pecking at and pulling out feathers of conspecifics can seriously reduce the well-being of birds and causes economic losses for the farmer. Records of FP in laying hen flocks from the last 20 years show a prevalence of between 24% and 94%. Several research groups worldwide have hypothesised about the causes of FP. From a nutritional point of view, re-directed behaviour and feather eating seem to be the most plausible causes. The gut microbiome seems to be involved in FP due to its influence on hormonal pathways and as it is influenced by the diet, which might include feathers ingested by the hens. Bird experiences during the rearing period are related to FP in later life by possible effects on the physiological development of the pullets. Most likely, pullets experience a sensitive period within the first few weeks post-hatch during which FP can develop due to various factors such as hormonal influences, nutrition and (the lack of) environmental enrichment. Nutrition could influence FP in two ways. Imbalances in certain nutrients, such as amino acids may have a direct effect on physiological mechanisms that trigger FP. Furthermore, ingredients such as roughages, fibres and non-nutritive ingredients may have an effect on exploratory and foraging or feeding behaviour. Literature (mainly in adult layers) shows that nutritional interventions increased eating time by 23–45% and/or the mean retention time of feed in the gut by 2.9–6.0 min/g fibre, and reduced or delayed FP. Using nutritional strategies (i.e. provision of specific AA profiles and/or high fibrous ingredients) during the sensitive period during rearing could prevent ultimately the development of FP, by altering the pullets’ (gut) physiology and/or her time allocation. Research focussing on critical periods during rearing should be initiated.
Introduction
Feather pecking (FP) is a serious problem in the laying hen sector. It is an abnormal behaviour that has severe impact on welfare, as well as on economics. FP can result in plumage damage (PD), skin damage, increased disease susceptibility (Green et al., Citation2000), productivity decrease, increase of food consumption and increased mortality (Rodenburg et al., Citation2013). FP occurs in all housing systems (Bilcik and Keeling Citation1999).
In practice, to prevent FP and reduce the degree of damage, the beaks of the laying hens are trimmed (Nicol Citation2018). This occurs within conventional systems, since beak trimming is not permitted in organic egg production (EU Council Regulation No. Citation1804/1999). Beak trimming itself does not influence pecking preferences or frequencies (Blokhuis and van der Haar Citation1989), but simply decreases the impact of the pecking, as hens with intact beaks show substantial more FP damages than de-beaked hens (Lambton et al., Citation2010). On the other hand, the interference of trimming itself is quite painful, which can lead to at least short-term pain or trauma. Beak trimming has an impact on the effectiveness of preening behaviour (Nicol Citation2018) and can affect oral sensing (Cheng Citation2007). When part of the beak is removed, the laying hen may lose these functions.
Ethical and societal concerns about the beak trimming procedure are increasing in conventional egg production (Vanhonacker et al., Citation2010; Heng et al., Citation2013; Heleski et al., Citation2015). As of January 2019, Dutch legislation banned beak trimming in all egg producing systems, since law forbids any mutilation of an animal. In Denmark and Germany, pursuing the organic egg producing sector, the poultry industry has voluntary agreed to stop beak trimming. In several European countries, legislation prohibited beak trimming (Switzerland, Sweden, Norway, Finland, Germany and Austria; Jung and Knierim Citation2017). In loose housing systems, FP has more impact since the large numbers of potential victims (Keeling Citation1994). The aim of this review is to give an overview of factors affecting FP, including the extent, ontogeny and potential nutritional solutions of the problem. Most reported experiments in this paper reflect studies that have been performed in in-house rearing and egg production systems.
Pecking behaviour in poultry
Five types of pecking are distinguished: (1) aggressive pecking (AP), (2) gentle feather pecking (GFP), (3) severe feather pecking (SFP), (4) tissue pecking (TP) and (5) vent pecking (VP) (Savory Citation1995). SFP, TP and VP are considered injurious pecking and can cause cannibalism. AP is a natural behaviour and has a clear purpose, which is not comparable to the other types. Directed at the conspecifics head and neck region, AP is used by hens to establish a stable dominance hierarchy (Savory Citation1995), which, hence, is known as the ‘pecking order’. GFP and SFP are non-AP. Both are abnormal and destructive behaviours, which are only seen in birds in captivity (Bestman Citation2002).
Extent of FP
Most studies claim that FP is a big problem, without giving proper support. The prevalence of FP has not been recorded extensively in the last decade. Nevertheless, the laying hen sector still claims to have problems with FP outbreaks. provides an overview of studies in the last two decades that focussed on the extent of FP. The use of PD as an indicator for FP must be interpreted with caution. Whenever a FP outbreak occurs, the plumage is damaged and denuded areas arise rapidly. However, it is possible that a further increase of FP is not observed, since the plumage of the victims is already impaired. In practical settings with large groups of laying hens, it is difficult to observe the same individual twice, whereby increases in size or severity of denuded areas might be overlooked. Epidemiological studies to determine the prevalence of FP usually use questionnaires or interviews with farmers or by direct observations by researchers. When comparing studies, it is suggested by Nicol et al. (Citation2013) that farmers may (i.e. because of lack of recognition or lack of systematic recording) underestimate the prevalence of FP in their barns.
Table 1. Prevalence of feather pecking (FP), severe feather pecking (SFP), gentle feather pecking (GFP) and plumage damage (PD) since 1999 of beak trimmed (Y), intact (N) and trimming unknown (U) flocks.
Although FP is a common problem, not all hens show FP behaviour. Previous studies calculated that 9% (Keeling Citation1994) or 12% (Wechsler et al., Citation1998) in a flock are severe peckers. Dutch poultry farmers estimate that between 5% and 20% of the hens within a group perform SFP (Bestman Citation2002). According to Daigle et al. (Citation2015), once the behaviour has developed, around 5% of the FP hens remain consistent feather peckers, whereas around 30% are consistent victims throughout their life.
Theories regarding FP
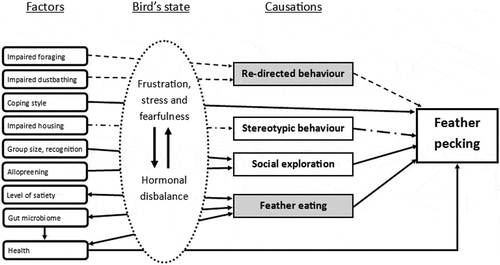
In the past 35–40 years, the causation and the factors affecting FP have been extensively researched. However, no clear cause or one defining factor has been identified, while FP is a multifactorial problem. Several research groups postulated hypotheses about the ontogeny of FP, and the most plausible causations and the corresponding factors are shown in . Some of the causes have influences or a relation with other causes. In the next two sections, redirected behaviour and feather eating as potential causes will be discussed extensively. These causes seem to be most related to nutrition and could potentially be counterbalanced by nutritional interventions.
Re-directed behaviour
The most commonly accepted causation hypotheses derive from redirected ground pecking behaviour (Savory Citation1995), with the lack of (i) foraging or (ii) dustbathing behaviour, as the possible onset. These theories showed the same issues, i.e. sufficient foraging or dustbathing substrate is missing in the environment, which results in an obstruction, or the bird has a strong motivation to express ground pecking behaviour, which cannot be fulfilled. The hens get frustrated, since motivated behaviour is blocked. Therefore, the hens use other possibilities to direct the pecking behaviour towards (i.e. feathers and/or conspecifics). Frustration can directly lead to FP, or, indirectly, by increasing stress in the hens.
From a nutritional point of view, the foraging theory is more interesting and more likely to play a role. As described by Hartcher et al. (Citation2016), foraging consists of food-searching and consumption. In modern laying hen husbandry, high nutrient content diets are provided that lead to early satiation and therefore the motivation to consume feed is quickly fulfilled. The motivation to search for feed, however, remains unfulfilled, especially when appropriate litter material is not provided. The motivation to forage is very high, hens will even use excreta or dirty litter material (von Waldburg-zeil et al., Citation2019). In the absence of an appropriate foraging material, feathers of conspecifics can be a good replacement. Numerous studies have shown that the absence of litter material affects pecking behaviour. The redirected behaviour theory cannot explain the occurrence of FP in both experimental and practical settings, in which foraging material is adequately provided. Newberry et al. (Citation2007) performed a longitudinal study on the development of SFP in individual domestic fowl. The study found no evidence that SFP substituted foraging behaviour. Using structural equation models, Bessei et al. (Citation2018) could not confirm the hypothesis that foraging in young pullets is a cause for FP. Furthermore, a study by Cronin et al. (Citation2018) reported more injurious pecking and higher mortality in groups which received foraging material.
Feather eating
Another well-defined theory about the cause of FP includes feather eating. Lutz et al. (Citation2016) showed, with structural equation models, that an increase of feather eating led to an increase in FP. A study performed by Ramadan and von Borell (Citation2008) showed that SFP in the laying period was higher in hens which were reared in litter material containing loose feathers vs. litter where loose feathers were removed. During SFP, the feathers are usually ingested by the peckers. Although non-peckers can ingest feathers, feather peckers consume more than non-peckers (Harlander-Matauschek and Häusler Citation2009). These feathers clearly have an effect, i.e. food pellets that contained feathers decreased SFP and improved the plumage scores (Kriegseis et al., Citation2012). The absence of loose feathers on the floor is, in practice, the first sign of a possible FP outbreak. Previous findings showed that the number of short feathers on the floor was negatively correlated with pecking and the number of droppings containing feathers was positively correlated with AP. These findings showed that damaging pecking at a young age was mostly directed at the tail and preen gland, where feathers are easily accessible and have the favoured eating size of 2–6 cm (Mckeegan and Savory Citation1999). Feathers of that particular area are coated with preen oil, which might attract the peckers (Mckeegan and Savory Citation2001).
It has been suggested that the motivation for feather eating is regulated and enhanced by positive reinforcement. Harlander-Matauschek et al. (Citation2008) showed that feather eating could be increased when the hens had a positive experience, i.e. when ingesting feathers covered with a palatable substance. Furthermore, birds acquired an aversion to feathers when they were covered with an unpalatable substance. Hence, the ingestion of tasty feathers could be experienced as a reward, which can motivate the bird to eat more of them.
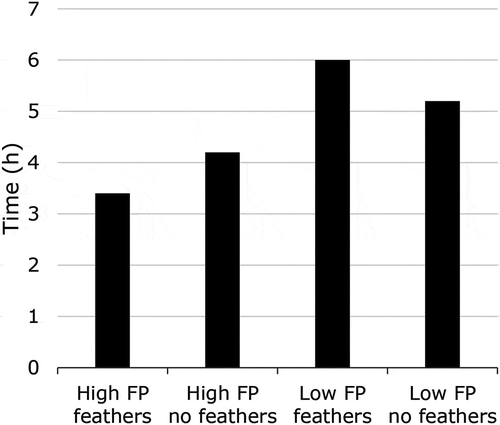
Chicken feathers consist over 90% of protein, mainly beta-keratin (Saravanan and Dhurai Citation2012). Since laying hens cannot break down keratin in the digestive tract, feathers do not have any nutritional value for the chicken (Mckeegan and Savory Citation1999). However, some studies suggested that the ingestion of feathers might aid digestion, as they act as insoluble fibre. Harlander-Matauschek et al. (Citation2006) showed that feathers provided to hens selected for high FP (HFP) increased the passage rate and could have similar effects as insoluble fibre, although passage rate decreased in hens selected for low FP (LFP) (). The lower excretion rate in LFP hens might be due to the low number of feathers that were ingested by these hens. Nonetheless, it remains unclear which is the actual factor related to feather-eating that makes the hens start FP. It could be that the lack of those components causes stress or frustration, and therefore induces redirected behaviour, or maybe the hens are not satiated, and are looking to increase gut fill.
Figure 2. The time when 50% of the plateau level of TiO2 excretion was reached (p < 0.05): FP = feather pecking, feathers = access to feathers, no feathers = no access to feathers. Adapted from Harlander-Matauschek et al. (Citation2006).
Additionally, it is possible that something is missing in the diet (e.g. fibre or amino acids) which accelerates a physiological response, in terms of a hormonal disequilibrium or the production of metabolites in the gut by bacteria that influence gut health. Behaviour is, in that situation, the ‘visible’ response to an intrinsic physiological imbalance that is not yet understood.
The gut microbiome
Apart from influences on digestion, the ingestion of feathers can influence the gut microbiota composition of the chicken due to bacterial feather degradation (Meyer et al. Citation2012). The influence of microbiota and its metabolites on the gut–brain axis is a rather unexplored field of study, although they are known to have direct effects on behaviour. Studies in rats and mice show that the gut microbiota communicates with the central nervous system (CNS) through neural, endocrine and immune pathways (Cryan and Dinan Citation2012).
Some studies in laying hens have focussed on possible differences in the microbiome between feather peckers and non-feather peckers. Meyer et al. (Citation2013) found differences in the microbial metabolites between high and low FP hens, which were most pronounced in the caeca. Total short-chain fatty acids (SCFA), propionate and n-butyrate were higher in laying hens from the HFP lines that were involved in more FP. These authors suggested that SCFA and propionate are important because of their direct and indirect effects on behaviour and the brain.
Recently, van der Eijk et al. (Citation2019) showed higher amounts of Clostridiales and lower amounts of Lactobacillus and Staphylococcus spp. in luminal microbiota (ileum, caeca and colon combined) of HFP compared to LFP hens, especially in non-pecking hens. They reported a higher diversity in both caeca mucosa-associated and luminal microbiota in the HFP line. In line with these results, Birkl et al. (Citation2018) indicated lower amounts of Lactobacillus spp. in the caeca of HFP hens. The authors suggested that Lactobacillus spp. could have direct effects on neurotransmission within the CNS, potentially reducing birds’ sensitivity to stress-related behaviour. The exact pathways and roles of i.e. propionate and Lactobacillus spp. in hen's gut-brain axis still need to be determined. In mice, supplementation with L. rhamnosus altered neurotransmission in the brain trough the vagus nerve, moreover, plasma corticosterone and depression- and anxiety-related behaviours were reduced (Bravo et al., Citation2011).
In this rather new field regarding behaviour, it is unclear if the microbiome is altered because of FP and/or feather eating behaviour, or whether a certain gut microbial composition provokes FP behaviour.
Other factors affecting FP
Many factors or combinations of factors may affect FP behaviour, mainly because they influence the hens’ welfare (i.e. stress). An elevation in GFP and/or SFP has been observed in chickens fed pelleted diets, via ‘skip-a-day’ feeding management, housed in large groups, without environmental enrichment or litter material, reared in suboptimal conditions, kept in high-density flocks, high light intensity or excessive lights, with a different genetic background and being mixed with conspecifics from other groups (Lambton et al., Citation2010; van Krimpen Citation2008; Bestman and Wagenaar Citation2003). Some factors have shown to decrease GFP and SFP, such as the quality and quantity of environmental enrichment, access to elevated perches, the use of warm-white or UV lights. All these factors however, contribute to the underlying motivational FP system, which is connected to the possible causations. Despite an abundance of research reporting several factors influencing FP, the egg industry is still struggling with this issue. Furthermore, the sheer abundance of potential factors influencing FP is the reason why the control of the behaviour fails under practical conditions.
How important is the rearing period?
Multiple studies that focus on FP during the laying period have suggested that experiences during early life are important in the development of FP. For example, rearing pullets on a wire floor resulted in more FP, less dust bathing behaviour and a higher mortality rate (Johnsen et al., Citation1998; de Jong et al., Citation2013). Young rats subjected to early life stress showed an altered stress response in adult life, compared to a control group. Furthermore, stress in early life has been shown to affect both the gut–brain axis and the gut microbiome (O’Mahony et al., Citation2009). Animals that have been raised in a germ-free environment have an underdeveloped gut microbiome. When exposed to stress, they show exaggerated responses of the hypothalamus-pituitary-adrenal axis (HPA axis), which is the pathway associated with stress responses.
As reviewed by Campbell et al., (Citation2018), laying hens should be enriched with physical, sensory and stimulatory aspects to aid the pullets potential of behavioural and (neuro)biological development. From a physiological point of view, the rearing period is an important stage in the development of the pullet and experiences can have large influences on FP development (Gilani et al., Citation2013). A study comparing rearing farms showed that the disruption and limitation of litter supply at early age increased SFP already at five weeks of age (de Haas Citation2014). Van Niekerk et al. (Citation2013) studied the influence of litter material in early rearing on FP in both rearing and laying. They found an increase of SFP in laying hens which were reared on plastic mats compared to rearing on litter material. Furthermore, Liebers et al. (Citation2019) performed a study in which environmental enrichment during the rearing period increased plumage quality at 17 weeks of age. In line with these results, Tahamtani et al. (Citation2017) found that the provision of environmental enrichment in the rearing period resulted in less feather damage during the production phase. Janczak and Riber (Citation2015) showed that perches, mashed feed, similar housing conditions and appropriate litter substrates throughout the rearing and production phases could be effective in reducing FP.
A study in rats showed a delay of brain maturation when the environment during the rearing period was poor (Narducci et al., Citation2018). Post-hatching, the pullet’s brain continues to grow and develop until week 10 (Atkinson et al., Citation2008). Synapse formation takes place during the first three weeks (de Haas Citation2014), which sets the potential for the function of neurotransmitters. It is possible that within the first weeks of the rearing period, pullets go through a sensitive period in which they develop FP due to various factors, such as imbalances in hormones, nutrition and environmental enrichment. As reviewed by Hartcher et al. (Citation2016), the first 10-day post-hatch seem to be critical for pullets to learn to interact with environmental enrichment. FP has already been observed within the first days post-hatch (Riedstra and Groothuis Citation2002). However, since this involves mainly GFP and the hens will still moult, the onset of FP can be easily overlooked. Most FP expression is observed during the production period. Furthermore, it is important to note that the study by Newberry et al. (Citation2007) did not find any evidence that individual behaviour variables recorded in pullets (such as foraging, pecking, resting and dust bathing), was identified with the development of SFP when adult. However, negative experiences at a young age do affect behavioural development in later life. Pinpointing particular ‘sensitive periods’ where birds can develop FP during rearing, and the physiological processes that coincide with the onset, have not yet been researched.
Role of serotonin in the development of FP
Even though there are many hypotheses, speculations and studies on the causes and factors associated with FP, underlying physiological mechanisms still remain unclear. Fundamental research has shown the involvement of the serotonergic system in FP (van Hierden Citation2003; Kops Citation2014). Serotonin is a central brain neurotransmitter and peripheral signalling molecule, and the serotonergic system can be influenced by gut microbiota (O’Mahony et al., Citation2015). Central serotonin has an influence on processes such as behaviour, mood, metabolic processes, sleep and growth (de Haas and van der Eijk Citation2018). Mostly deficiencies in central serotonin during the rearing period have been associated with the onset of FP (Kops et al., Citation2017). Amongst other hormones, peripheral serotonin can be found in the gut, where it has numerous physiological functions (Spohn and Mawe Citation2017). Lower peripheral serotonin levels have been found in hens that were phenotyped as feather peckers, both in late rear and during lay (de Haas and van der Eijk Citation2018). There are no data available regarding possible differences in peripheral serotonin around the onset of FP during early lay. To prevent the development of FP, one beneficial strategy may be to set serotonin to adequate levels in the rearing period.
Potential nutritional strategies during rearing to prevent or reduce FP later in life
Nutrition can be considered as a promising way to influence animal behaviour. Effects can be observed quickly, and fast adjustments are feasible as well. Nutrition may influence FP in two ways. The first is by the effect of specific nutrients directly on physiological mechanisms. This route is mostly driven by deficiencies and imbalances, which have been proven to enhance FP development (Kjaer and Bessei Citation2013). Thus, adequate nutrition administrated at the right life stage of the bird could be a method to reduce FP or prevent a FP outbreak. Individual layers may have a specific appetite for certain nutrients (Roura and Cho, Citation2017). Such individual differences might be explained by a genetic variation in expression and/or sensitivity of nutrient sensors in these hens, and this may induce behavioural problems, such as FP (Roura et al., Citation2013).
The second route is the effect that nutrition could have on feeding behaviour. This focusses mostly on prolonging eating time, as shown by increasing exploratory or foraging behaviour by occupation or increasing the level of satiety, which reduces the drive to peck. Within this strategy, pullets are distracted from FP and simply have less time to perform this behaviour. As mentioned above, the rearing period is important, since many trials suggest that FP begins during the developmental stages of a young hen.
Nutrient specific effects on FP
Dietary protein and amino acids
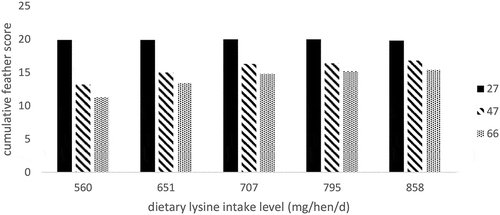
Several studies have shown that low protein diets can increase FP (Kjaer and Bessei Citation2013). Deficiencies in dietary lysine alone have been associated with FP. Kumar et al. (Citation2018) fed several levels of dietary lysine to laying hens and measured their plumage over time (). In total, five areas (neck, back, vent, wings and breast) were scored with each measurement a score from 1 (completely featherless) to 4 (fully feathered) at 27, 47 and 66 weeks of age. Over time, feather score decreased for all dietary treatments. However, the mean cumulative scores of all areas clearly showed the biggest decrease in feather scores in birds the lowest amount of dietary lysine. The authors found a positive correlation between dietary lysine level and feather score.
Figure 3. Effect of lysine intake level (mg/hen/day) on mean cumulative feather score from 1 (completely featherless) to 4 (fully feathered) at five body areas: neck, back, vent, wings and breast, of laying hens fed different dietary lysine levels at 27 (black), 47 (striped) and 66 (dotted) weeks of age. Adapted from Kumar et al. (Citation2018).
Additionally, the levels of Arg (Conson and Petersen Citation1986; Bozakova and Yotova Citation2009), Met and Cys (Elwinger et al., Citation2002; Kjaer and Bessei Citation2013) seem to influence FP. Unfortunately, these studies did not investigate physiological processes that might be altered because of imbalances of these amino acids (AA). It seems that laying hens are sensitive to changes in the diet and use their behaviour to cope with whatever is missing. Arg, Cys, Met and Val are important for the development of feathers (van Emous and van Krimpen Citation2019), which could be a reason why pullets are trying to counteract deficiencies of these AA by eating of or pecking at feathers.
Tryptophan (Trp) is a pre-cursor for serotonin, which means that the AA is involved in serotonin synthesis. Because Trp is an essential AA, serotonin production is limited by its availability in the diet. Research in adult laying hens showed that higher supplementation of dietary Trp reduces FP (Savory et al., Citation1999; van Hierden et al., Citation2004). Most likely, additional Trp increases serotonin synthesis, as these compounds are clearly connected, and depletion or supplementation of Trp is widely used to study serotonergic functions. However, the exact physiological mechanisms between Trp, peripheral and central serotonin and FP in laying hens remain unclear. A study by (Birkl et al., Citation2017b) showed that an acute Trp depletion (ATD) in the diet could seriously alter blood AA levels. A 50% decrease of Trp plasma baseline levels, a 70% decrease of the Trp ratio to large neutral AA or a 60% decrease of the Trp ratio to aromatic AA, can have effects on all kind of physiological functions. Another recent study by Birkl et al. (Citation2019) showed that ATD increases GFP in both high and low FP lines, suggesting that ATD influences hens’ social behaviour. The effects of depletion or supplementation of Trp on the central and peripheral serotonin of laying hens has not been studied yet.
If Trp can directly influence the synthesis of central serotonin, supplementation during rearing could be a method to prevent FP, since central serotonin levels during rearing are low (Kops et al., Citation2017). In a study focussing on the availability of Trp in plasma, low levels in young laying hens (aged 24 weeks) were preceded by the onset of aggressive injurious pecking the week before (Birkl et al., Citation2017a).
However, Trp has to compete with other large AA in order to pass the blood-brain barrier (de Haas and van der Eijk Citation2018) and is not exclusively used for serotonin synthesis. Therefore, only providing extra Trp might not work, and a more complete range of AA may need to be provided in the diet. Prescilla et al. (Citation2018) studied the combination of Trp, methionine and glycine on plumage condition, and modelled the optimal inclusion rate for plumage score. Their model predicted that if these three AA were provided well above recommendations (relative increase of 23%, 10% and 49% of TRP, methionine and glycine respectively), the maximal likelihood of observing perfect plumage conditions was 61%. Furthermore, providing laying hens with a higher Trp:Lys ratio decreased FP (Helmbrecht et al., Citation2015). Since there is not an abundance of studies focussing on combining Trp and the other AA (Met, Gly, Lys), or their combinations that potentially influence FP and Trp, the area of AA profile is worthwhile to research in more detail.
Satiety and foraging behaviour with dietary fibres
High fibre contents in diets have shown to induce a consistent FP reducing effect (Nicol Citation2019). Furthermore, including high non-starch polysaccharide (NSP) concentrations in the diet may increase weights of the gizzard and its content, and aid digestion (increase in retention time by 2.9–6.0 min/g fibre) (Hetland et al., Citation2003; van Krimpen Citation2008). By providing high coarse fibre content in the diet, hens might be more satiated, especially when the feed remains longer in the crop and gizzard. It has been suggested that insoluble NSP sources accumulate in the gizzard and increase the mean retention time (MRT) in the foregut (van Krimpen Citation2008). The MRT is often used as a measure for satiety. However, the exact physiological mechanisms involved in the regulation of satiety and feed intake in chickens are not yet completely understood.
Satiety in humans is regulated by gastrointestinal mechanisms, in which gastric expansion is most important to stop eating and the presence of nutrients in the small intestine is required to feel satiated (Read Citation1992; Schwartz et al., Citation2000). It is possible that expansion in the crop and gizzard of chicken might have a similar effect.
Conversely, low fibrous diets have been related to increased foraging behaviour. Most likely, low fibre content decreases satiety, which motivates the hens to perform more foraging behaviour, which might decrease FP (Nicol Citation2019). The increased foraging behaviour due to providing a low fibrous diet could have a negative effect as well. As mentioned before, when the hens are missing fibre in their diets, they might use feathers as a replacement, especially if litter material is absent or inadequate. Hens do not have a specific preference for foraging material (de Jong et al., Citation2007). Since excreta has proven to be sufficient as foraging material, hens may start to forage on loose feathers on the floor as well, which could result in the onset of FP. These observations result in a bit of a dilemma. Reduction of FP and increasing foraging behaviour are both favourable as an aim for nutritional strategies: providing a low fibrous diet can be beneficial to prevent the development of FP but could be the onset of FP as well.
The gut microbiome
The diet consumed by an animal has an influence on its gut microbiome. The ingredients used in the diet are therefore important. For example, providing feathers in a diet are proven to have an effect on the composition, e.g. an increase of bacterial species that hydrolyse keratin in the ileum and caecum (Enterococcus facium and different strains of Lactobacillus spp.) and lower bacterial diversity in the caecum (Meyer et al., Citation2012). Other nutrients might have an influence as well; for example providing probiotics (Bravo et al., Citation2011), prebiotics, SCFA and oxidised fatty acids (Zhang and Davies Citation2016). Novel ingredients that act as or enhance pre-, pro- or antibiotic function could be promising as well.
The gut microbiota affects the synthesis of serotonin, both centrally and peripherally. It may utilise or produce TRP, produce SCFA involved in the synthesis and release of neurotransmitters or directly influence serotonin by the vagus nerve (de Haas and van der Eijk Citation2018). Thus, changes in or influences on the gut microbiome might influence FP via several routes. However, causal relations between the gut microbiome and FP have yet to be published.
Feeding-related behavioural effects on FP
Occupation
Instead of prolonging feeding time, FP can also be prevented by occupying the hens with other pursuits. This could potentially increase foraging behaviour by providing environmental enrichment or special ingredients such as roughages. For example, providing laying hens with grass haylage as an enrichment material has improved hens welfare by lowering SFP and cannibalism (Albiker and Zweifel, Citation2017). A study done by Steenfeldt et al. (Citation2007) tested high fibrous diet ingredients, i.e. maize silage, barley-pea silage and carrots, as a potential foraging enrichment. At 53 weeks of age, all three enrichments resulted in lower total, severe and gentle pecking bouts compared to the control group. Mortality was low in the groups receiving enrichment (0.5–2.5%) as compared to the control group (15%). In the control group, half of the mortality was due to cannibalism. Furthermore, total plumage condition of the control group was worse than the groups that received maize silage or barley-pea silage. Although eating time was not observed, the authors suggested that the hens spent more time on feeding, which leaves less time to spend on FP.
Unfortunately, both studies did not investigate the effect of the enrichment on the length of other behaviours, such as foraging or eating. It could be expected that providing extra nutrients next to the normal diet increases foraging behaviour. Additionally, non-nutritive materials could occupy and distract the hens from pecking. Zepp et al. (Citation2018) found a relationship between FP and enrichment pecking (defined as direct pecking at enrichment) when testing enrichment in the form of a pecking block, pecking stone and lucerne bale. If enrichment pecking occurred, GFP, SFP and AP was lower.
Prolonging eating time
Another strategy to prevent or reduce FP is to aim for prolonging the time hens are eating. Studies have been performed on the effect of energy and nutrient dilution on consumption time. Energy content has been demonstrated to influence FP, where a high energy content resulted in FP and a low energy content decreased FP. When feeding a low energy diet, hens increased feed intake to maintain their energy intake, and, as a consequence, spent more time eating (+23–45%). The increase in eating time can be achieved by nutrient dilution, i.e. providing sand or grit or high NSP raw materials. Even though studies have found effects of NSP on FP, not all results agree. In a study providing high and normal NSP levels and normal and energy diluted diets in a FP prone flock, only a delay in feather damage has been observed (van Krimpen Citation2008).
One of the layer studies included the rearing period, and found that hens showed increased feed intake during rearing on a low NSP + energy dilution by sand diet and on a high NSP + energy dilution by oat hulls diet, whereby a similar energy intake was ensured (van Krimpen Citation2008). In the same study, energy dilution of the low NSP diets did not affect eating time, while eating time was prolonged in the diluted high NSP diets, indicating that, in particular, the NSP-rich ingredients were responsible for prolonging eating time. In line with these findings, another study found that dietary dilution with insoluble NSP from common feed ingredients, like barley and sunflower seed extract, affected the time-budget of pullets during rearing, resulting in an increased eating time and decreased FP (Qaisrani et al., Citation2013). Furthermore, when pullets received NSP-diluted diets during rearing and laying, FP was reduced in the laying period (van Krimpen et al., Citation2010). Based on these results, it can be concluded that dietary dilution with insoluble NSP indeed affects the time-budget of pullets, allowing training during rearing towards more time spend on eating, which can affect FP in later life.
Physiological processes that may interact with the influences of diluted diets on prolonged eating time, however, have not yet been researched. Satiety and its regulatory processes might play a role in this matter. Satiety could be explained by influences of energy dilution on physical thresholds for gut fill or by influencing the metabolic state of the bird (i.e. blood glucose levels). By diluting the diet, hens might feel less satiated and therefore increase their eating time. Conversely, the diluted diet could have a higher MRT in various part of the gastral-intestinal tract (van Krimpen Citation2008), meaning the hens would feel more satiated. Future research should investigate physiological parameters, such as hormones involved in satiety. When these mechanisms are fully understood, specialised diets could be formulated to influence satiety and prevent FP.
Conclusions
FP remains a welfare issue in the laying hen sector. The prevalence of SFP in laying hen flocks measured over the last 20 years remains above 50%. Changing legislation within Europe after the ban on beak trimming might increase the severity of the problem. From a nutritional point of view, re-directed foraging behaviour and feather eating theories seem the most plausible causes of FP. Furthermore, experiences during the rearing period seem to be linked to FP in later life. Nutrition can be used to reduce FP by direct effects on physiological mechanisms such as the serotoninergic system, satiety and the gut microbiome. Moreover, feed can be used to prolong eating time, to occupy the hens and to increase foraging behaviour, which indirectly decreases FP behaviour. Applying such nutritional strategies in the rearing period might alter the pullets’ physiology or train them in their time budget allocation, which both could result in the prevention of FP.
To prevent the development of FP, the ontogeny of FP needs to be studied more closely. The sensitive period in which pullets are most susceptible, as well as the role of the gut–brain axis needs to be unravelled. Future research should focus on the impact of nutritional strategies during early rearing on the development of FP and the gut microbiome. Thereby, involved physiological and neuro-endocrine mechanisms of known and new nutritional strategies should be resolved for developing more effective strategies to prevent FP. It is important to study the role of peripheral serotonin, the gut microbiome, energy metabolism and satiety, and predisposing and increasing normal behaviours, such as foraging and eating.
Acknowledgments
This research is co-financed by Lohmann Tierzucht GmbH, Ter Heerdt N.V., Agromix Broederij en Opfokintegratie B.V., De Heus Voeders B.V., Fonds voor Pluimveebelangen, Wageningen Livestock Research and Wageningen University, within the framework of the public private partnership ‘Feed4Foodure’, partially funded by the Dutch Ministry of Economic Affairs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
A.J.W. Mens
Annemarie Mens MSc works as a researcher poultry nutrition at Wageningen Livestock Research. In her research, she focusses on the birds’ physiology, understanding the mechanisms related to welfare, behaviour, reproduction and health of poultry, mainly in laying hens and broiler breeders. Furthermore, in her work the main emphasis is to use nutrition as a tool to influence and improve these mechanisms. As part of her employment, in 2018, she started her PhD about the ontogeny of feather pecking during rearing of laying hens and developing feeding strategies to prevent feather pecking.
M.M. van Krimpen
Dr Marinus van Krimpen finished his PhD-project regarding the effect of nutritional factors on reducing feather pecking behaviour in laying hens. Since 2010, he was appointed as senior researcher in animal nutrition at the same institute. The focus of his projects regarded nutritional requirements of laying hens and broilers, development of alternative protein sources, improving the phosphorus and nitrogen efficacy of pigs and poultry, improving bird welfare, and stimulating immune competence by nutrition.
R.P. Kwakkel
Dr Rene Kwakkel is Associate Professor, working mainly in the field of Poultry Nutrition, with interests in feed intake behaviour, novel feeding strategies and Ca/P metabolism. He has published over 70 papers in peer-reviewed journals, up to 13 PhD-students graduated successfully under his supervision and he acted as external examiner in 14 international PhD examinations. He currently supervises another 10 PhD students. Next to this job he serves as Programme Director of the BSc & MSc in Animal Sciences at WUR.
References
- Albiker, D., and R. Zweifel. 2017. “Performance, behaviour und plumage of brown layers with intact beaks.” 21st European Symposium on Poultry Nutrition, Salou/Vila-seca, Spain.
- Atkinson, R., P. V. Migues, M. Cammarota, J. M. Kavanagh, M. Hunter, and J. A. Rostas. 2008. “Biochemical, Behavioural and Electrophysiological Investigations of Brain Maturation in Chickens.” Brain Research Bulletin 76: 217–223.
- Au, E. H., and M. Singh. 2018. “Prevalence of plumage damage and investigation of associated nutritional factors for free range laying hens in Australia.” 29th Annual Australian poultry science symposium, Sydney.
- Bessei, W., V. Lutz, J. Kjaer, M. Grashorn, and J. Bennewitz. 2018. “Relationships between Foraging and Open-field Activity in Young Chicks and Feather Pecking in Adult Birds: Results of Analyses Using Quantitative Genetics and Structural Equation Models.” European Poultry Science 82: 282.
- Bestman, M. 2002. Kippen Houden Zonder Verenpikken. De Biologische Legpluimveehouderij Als Uitgangspunt. In Dutch: Louis Bolk Instituut Report.
- Bestman, M. W. P., and J. P. Wagenaar. 2003. “Farm Level Factors Associated with Feather Pecking in Organic Laying Hens.” Livestock Production Science 80: 133–140. doi:10.1016/S0301-6226(02)00314-7.
- Bilcik, B., and L. J. Keeling. 1999. “Changes in Feather Condition in Relation to Feather Pecking and Aggressive Behaviour in Laying Hens.” British Poultry Science 40: 444–451. doi:10.1080/00071669987188.
- Birkl, P., A. Bharwani, J. B. Kjaer, W. Kunze, P. Mcbride, P. Forsythe, and A. Harlander-Matauschek. 2018. “Differences in Cecal Microbiome of Selected High and Low Feather-pecking Laying Hens.” Poultry Science 97: 3009–3014. doi:10.3382/ps/pey167.
- Birkl, P., J. Chow, P. Mcbride, J. B. Kjaer, W. Kunze, P. Forsythe, and A. Harlander-Matauschek. 2019. “Effects of Acute Tryptophan Depletion on Repetitive Behavior in Laying Hens.” Frontiers in Veterinary Science 6: 230. doi:10.3389/fvets.2019.00230.
- Birkl, P., J. B. Kjaer, W. Szkotnicki, P. Forsythe, and A. Harlander-Matauschek. 2017b. “Acute Tryptophan Depletion: The First Method Validation in an Avian Species (Gallus Gallus Domesticus).” Poultry Science 96: 3021–3025. doi:10.3382/ps/pex142.
- Birkl, P., L. Franke, T. Bas Rodenburg, E. Ellen, and A. Harlander-Matauschek. 2017a. “A Role for Plasma Aromatic Amino Acids in Injurious Pecking Behavior in Laying Hens.” Physiology & Behavior 175: 88–96. doi:10.1016/j.physbeh.2017.03.041.
- Blokhuis, H. J., and J. W. van der Haar. 1989. “Effects of Floor Type during Rearing and of Beak Trimming on Ground Pecking and Feather Pecking in Laying Hens.” Applied Animal Behaviour Science 22: 359–369. doi:10.1016/0168-1591(89)90030-0.
- Bozakova, N., and I. Yotova. 2009. “Ethological Aspects of Improving the Welfare of Turkey Breeders in the Hot Summer Period by Dietary L-arginine Supplementation.” Bulgarian Journal of Veterinary Medicine 12: 185–191.
- Bravo, J. A., P. Forsythe, M. V. Chew, E. Escaravage, H. M. Savignac, T. G. Dinan, J. Bienenstock, and J. F. Cryan. 2011. “Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve.” Proceedings of the National Academy of Sciences 108: 16050–16055. doi:10.1073/pnas.1102999108.
- Campbell, D. L. M., E. N. de Haas, and C. Lee. 2018. “A Review of Environmental Enrichment for Laying Hens during Rearing in Relation to Their Behavioral and Physiological Development.” Poultry Science 98: 9–28. doi:10.3382/ps/pey319.
- Cheng, H. 2007. “Morphopathological Changes and Pain in Beak Trimmed Laying Hens.” World’s Poultry Science Journal 62: 41–52. doi:10.1079/WPS200583.
- Conson, M., and J. Petersen. 1986. “Feathering Score of Laying Hybrids Reared under Different Conditions.” Archiv Fur Geflugelkunde 50: 159–168.
- Coton, J., M. Guinebretière, V. Guesdon, G. Chiron, C. Mindus, A. Laravoire, G. Dennery, et al. 2017. “Epidemiological Study about Feather Pecking in Laying Hens Housed in Free-range and Furnished Cage Farms in France.” Xth European Symposium on Poultry Welfare, 109, Ploufragan, France.
- Cronin, G. M., R. L. Hopcroft, P. J. Groves, E. J. S. Hall, D. N. Phalen, and P. H. Hemsworth. 2018. “Why did severe feather pecking and cannibalism outbreaks occur? An unintended case study while investigating the effects of forage and stress on pullets during rearing.” Poultry Science. doi:10.3382/ps/pey022.
- Cryan, J. F., and T. G. Dinan. 2012. “Mind-altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour.” Nature Reviews. Neuroscience 13: 701. doi:10.1038/nrn3346.
- Daigle, C. L., T. B. Rodenburg, J. E. Bolhuis, J. C. Swanson, and J. M. Siegford. 2015. “Individual Consistency of Feather Pecking Behavior in Laying Hens: Once a Feather Pecker Always a Feather Pecker?” Frontiers in Veterinary Science 2: 6. doi:10.3389/fvets.2015.00006.
- de Haas, E. N. 2014. “The Fearful Feather Pecker.” Ph. D. Thesis, Wageningen University.
- de Haas, E. N., and J. A. J. van der Eijk. 2018. “Where in the Serotonergic System Does It Go Wrong? Unravelling the Route by Which the Serotonergic System Affects Feather Pecking in Chickens.” Neuroscience and Biobehavioral Reviews 95: 170–188. doi:10.1016/j.neubiorev.2018.07.007.
- de Jong, I. C., B. F. J. Reuvekamp, and H. Gunnink. 2013. “Can Substrate in Early Rearing Prevent Feather Pecking in Adult Laying Hens?” Animal Welfare 22: 305–314. doi:10.7120/09627286.22.3.305.
- de Jong, I. C., M. Wolthuis-Fillerup, and C. G. van Reenen. 2007. “Strength of Preference for Dustbathing and Foraging Substrates in Laying Hens.” Applied Animal Behaviour Science 104: 24–36. doi:10.1016/j.applanim.2006.04.027.
- Decina, C., O. Berke, N. van Staaveren, C. F. Baes, T. M. Widowski, and A. Harlander-Matauschek. 2019. “An Investigation of Associations between Management and Feather Damage in Canadian Laying Hens Housed in Furnished Cages.” Animals (Basel) 9: 135. doi:10.3390/ani9040135.
- EC_1804/1999. 1999. “Council Regulation (EC) No 1804/1999, Supplementing Regulation (EEC) No 2092/91.”
- Elwinger, K., R. Tauson, M. Tufvesson, and C. Hartmann 2002. “Feedingof Layers Kept in an Organic Feed Environment.” 11th. European Poultry Conference, Bremen.
- Gilani, A.-M., T. G. Knowles, and C. J. Nicol. 2013. “The Effect of Rearing Environment on Feather Pecking in Young and Adult Laying Hens.” Applied Animal Behaviour Science 148: 54–63. doi:10.1016/j.applanim.2013.07.014.
- Green, L. E., K. Lewis, A. Kimpton, and C. J. Nicol. 2000. “Cross-sectional Study of the Prevalence of Feather Pecking in Laying Hens in Alternative Systems and Its Associations with Management and Disease.” Veterinary Record 147: 233–238. doi:10.1136/vr.147.9.233.
- Gunnarsson, S., L. J. Keeling, and J. Svedberg. 1999. “Effect of Rearing Factors on the Prevalence of Floor Eggs, Cloacal Cannibalism and Feather Pecking in Commercial Flocks of Loose Housed Laying Hens.” British Poultry Science 40: 12–18. doi:10.1080/00071669987773.
- Häne, M., B. Huber-Eicher, and E. Fröhlich. 2007. “Survey of Laying Hen Husbandry in Switzerland.” World’s Poultry Science Journal 56: 21–31. doi:10.1079/WPS20000003.
- Harlander-Matauschek, A., F. Wassermann, J. Zentek, and W. Bessei. 2008. “Laying Hens Learn to Avoid Feathers.” Poultry Science 87: 1720–1724. doi:10.3382/ps.2007-00510.
- Harlander-Matauschek, A., H. P. Piepho, and W. Bessei. 2006. “The Effect of Feather Eating on Feed Passage in Laying Hens.” Poultry Science 85: 21–25. doi:10.1093/ps/85.1.21.
- Harlander-Matauschek, A., and K. Häusler. 2009. “Understanding Feather Eating Behaviour in Laying Hens.” Applied Animal Behaviour Science 117: 35–41. doi:10.1016/j.applanim.2008.11.003.
- Hartcher, K. M., S. J. Wilkinson, P. H. Hemsworth, and G. M. Cronin. 2016. “Severe Feather-pecking in Non-cage Laying Hens and Some Associated and Predisposing Factors: A Review.” World’s Poultry Science Journal 72: 103–114. doi:10.1017/S0043933915002469.
- Heerkens, J. L. T., E. Delezie, I. Kempen, J. Zoons, B. Ampe, T. B. Rodenburg, and F. A. M. Tuyttens. 2015. “Specific Characteristics of the Aviary Housing System Affect Plumage Condition, Mortality and Production in Laying Hens.” Poultry Science 94: 2008–2017. doi:10.3382/ps/pev187.
- Heleski, C. R., A. G. Mertig, and A. J. Zanella. 2015. “Stakeholder Attitudes toward Farm Animal Welfare.” Anthrozoös 19: 290–307. doi:10.2752/089279306785415439.
- Helmbrecht, A., C. Elwert, and M. Lepoudere. 2015. “Effect of different tryptophan to lysine ratios on laying performance and phenotypical stress parameters in laying hens under two stocking densities in floor pens.” Actes des 11èmes Journées de la Recherche Avicole et Palmipèdes à Foie Gras, Tours, France.
- Heng, Y., H. H. Peterson, and X. H. Li. 2013. “Consumer Attitudes toward Farm-Animal Welfare: The Case of Laying Hens.” Journal of Agricultural and Resource Economics 38: 418–434.
- Hetland, H., B. Svihus, S. Lervik, and R. Moe. 2003. “Effect of Feed Structure on Performance and Welfare in Laying Hens Housed in Conventional and Furnished Cages.” Acta Agriculturae Scandinavica, Section A - Animal Science 53: 92–100. doi:10.1080/09064700310002387.
- Huber Eicher, B. 1999. “A Survey of Layer-type Pullet Rearing in Switzerland.” World’s Poultry Science Journal 55: 83–91. doi:10.1079/WPS19990007.
- Huber Eicher, B., and F. Sebo. 2001. “The Prevalence of Feather Pecking and Development in Commercial Flocks of Laying Hens.” Applied Animal Behaviour Science 74: 223–231. doi:10.1016/S0168-1591(01)00173-3.
- Janczak, A. M., and A. B. Riber. 2015. “Review of Rearing-related Factors Affecting the Welfare of Laying Hens.” Poultry Science 94: 1454–1469. doi:10.3382/ps/pev123.
- Johnsen, P. F., K. S. Vestergaard, and G. Norgaard-Nielsen. 1998. “Influence of Early Rearing Conditions on the Development of Feather Pecking and Cannibalism in Domestic Fowl.” Applied Animal Behaviour Science 60: 25–41. doi:10.1016/S0168-1591(98)00149-X.
- Jung, L., and U. Knierim. 2017. “Are Practice Recommendations for the Prevention of Feather Pecking in Laying Hens in Non-cage Systems in Line with the Results of Experimental and Epidemiological Studies?” Applied Animal Behaviour Science 200: 1–12. doi:10.1016/j.applanim.2017.10.005.
- Keeling, L. J. 1994. “Feather Pecking - Who in the Group Does It, How Often and under What Circumstances?” Proceedings of the 9th European Poultry Conference, 288–289, Glasgow.
- Keeling, L. J. 1994. “Feather Pecking and Cannibalism in Layers.” Poultry International 46–50.
- Kjaer, J. B., and W. Bessei. 2013. “The Interrelationships of Nutrition and Feather Pecking in the Domestic Fowl - A Review.” Archiv Fur Geflugelkunde 77: 1–9.
- Kops, M. S. 2014. “Feather pecking and monoamines-a behavioral and neurobiological approach.” PhD diss., Utrecht University.
- Kops, M. S., J. B. Kjaer, O. Gunturkun, K. G. C. Westphal, G. A. H. Korte-Bouws, B. Olivier, S. M. Korte, and J. E. Bolhuis. 2017. “Brain Monoamine Levels and Behaviour of Young and Adult Chickens Genetically Selected on Feather Pecking.” Behavioural Brain Research 327: 11–20. doi:10.1016/j.bbr.2017.03.024.
- Kriegseis, I., W. Bessei, B. Meyer, J. Zentek, H. Wurbel, and A. Harlander-MATAUSCHEK. 2012. “Feather-pecking Response of Laying Hens to Feather and Cellulose-based Rations Fed during Rearing.” Poultry Science 91: 1514–1521. doi:10.3382/ps.2011-01865.
- Kumar, D., C. Raginski, K. Schwean-Lardner, H. L. Classen, and J. Plaizier. 2018. “Assessing the Response of Hen Weight, Body Composition, Feather Score, Egg Quality, and Level of Excreta Nitrogen Content to Digestible Balanced Protein Intake of Laying Hens.” Canadian Journal of Animal Science 98: 619–630. doi:10.1139/cjas-2017-0134.
- Lambton, S. L., T. G. Knowles, C. Yorke, and C. J. Nicol. 2010. “The Risk Factors Affecting the Development of Gentle and Severe Feather Pecking in Loose Housed Laying Hens.” Applied Animal Behaviour Science 123: 32–42. doi:10.1016/j.applanim.2009.12.010.
- Liebers, C. J., A. Schwarzer, M. Erhard, P. Schmidt, and H. Louton. 2019. “The Influence of Environmental Enrichment and Stocking Density on the Plumage and Health Conditions of Laying Hen Pullets.” Poultry Science 98: 2474–2488. doi:10.3382/ps/pez024.
- Lutz, V., J. B. Kjaer, H. Iffland, M. Rodehutscord, W. Bessei, and J. Bennewitz. 2016. “Quantitative Genetic Analysis of Causal Relationships among Feather Pecking, Feather Eating, and General Locomotor Activity in Laying Hens Using Structural Equation Models.” Poultry Science 95: 1757–1763. doi:10.3382/ps/pew146.
- Mckeegan, D. E. F., and C. J. Savory. 1999. “Feather Eating in Layer Pullets and Its Possible Role in the Aetiology of Feather Pecking Damage.” Applied Animal Behaviour Science 65: 73–85. doi:10.1016/S0168-1591(99)00051-9.
- Mckeegan, D. E. F., and C. J. Savory. 2001. “Feather Eating in Individually Cagedhens Which Differ in Their Propensity to Feather Peck.” Applied Animal Behaviour Science 73: 131–140. doi:10.1016/S0168-1591(01)00124-1.
- Meyer, B., J. Zentek, and A. Harlander-Matauschek. 2013. “Differences in Intestinal Microbial Metabolites in Laying Hens with High and Low Levels of Repetitive Feather-pecking Behavior.” Physiology & Behaviour 110–111: 96–101. doi:10.1016/j.physbeh.2012.12.017.
- Meyer, B., W. Bessei, W. Vahjen, J. Zentek, and A. Harlander-Matauschek. 2012. “Dietary Inclusion of Feathers Affects Intestinal Microbiota and Microbial Metabolites in Growing Leghorn-type Chickens.” Poultry Science 91: 1506–1513. doi:10.3382/ps.2011-01786.
- Narducci, R., L. Baroncelli, G. Sansevero, T. Begenisic, C. Prontera, A. Sale, M. C. Cenni, N. Berardi, and L. Maffei. 2018. “Early Impoverished Environment Delays the Maturation of Cerebral Cortex.” Scientific Reports 8: 1187. doi:10.1038/s41598-018-19459-y.
- Newberry, R. C., L. J. Keeling, I. Estevez, and B. Bilčík. 2007. “Behaviour When Young as a Predictor of Severe Feather Pecking in Adult Laying Hens: The Redirected Foraging Hypothesis Revisited.” Applied Animal Behaviour Science 107: 262–274. doi:10.1016/j.applanim.2006.10.010.
- Nicol, C. 2018. “Feather Pecking and Cannibalism: Can We Really Stop Beak Trimming?” In Advances in Poultry Welfare, 175–197. Duxford: Woodhead Publishing.
- Nicol, C. J. 2019. “Feather Pecking in Laying Hens: Why They Do It, and Welfare Implications.” In Poultry Feathers and Skin: The Poultry Integument in Healt and Welfare, edited by Oluyinka A. Oluski, 31–46. Oxfordshire: CABI.
- Nicol, C. J., M. Bestman, A. M. Gilani, E. N. de Haas, I. C. de Jong, S. Lambton, J. P. Wagenaar, C. A. Weeks, and T. B. RODENBURG. 2013. “The Prevention and Control of Feather Pecking: Application to Commercial Systems.” World’s Poultry Science Journal 69: 775–788. doi:10.1017/S0043933913000809.
- O’Mahony, S. M., G. Clarke, Y. E. Borre, T. G. Dinan, and J. F. Cryan. 2015. “Serotonin, Tryptophan Metabolism and the Brain-gut-microbiome Axis.” Behavioural Brain Research 277: 32–48. doi:10.1016/j.bbr.2014.07.027.
- O’Mahony, S. M., J. R. Marchesi, P. Scully, C. Codling, A. M. Ceolho, E. M. Quigley, J. F. Cryan, and T. G. Dinan. 2009. “Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses.” Biological Psychiatry 65: 263–267. doi:10.1016/j.biopsych.2008.06.026.
- Potzsch, C. J., K. Lewis, C. J. Nicol, and L. E. Green. 2001. “A Cross-sectional Study of the Prevalence of Vent Pecking in Laying Hens in Alternative Systems and Its Associations with Feather Pecking, Management and Disease.” Applied Animal Behaviour Science 74: 259–272. doi:10.1016/S0168-1591(01)00167-8.
- Prescilla, K. M., G. M. Cronin, S. Y. Liu, and M. Singh. 2018. “Estimation of optimal methionine, glycine and tryptophan levels to improve plumage condition in Isa Brown laying hens.” 29th Annual Australian Poultry Science Symposium, Sydney.
- Qaisrani, S. N., M. M. van Krimpen, and R. P. Kwakkel. 2013. “Effects of Dietary Dilution Source and Dilution Level on Feather Damage, Performance, Behavior, and Litter Condition in Pullets.” Poultry Science 92: 591–602. doi:10.3382/ps.2012-02378.
- Ramadan, S. G., and E. von Borell. 2008. “Role of Loose Feathers on the Development of Feather Pecking in Laying Hens.” British Poultry Science 49: 250–256. doi:10.1080/00071660802094180.
- Read, N. W. 1992. “Role of Gastrointestinal Factors in Hunger and Satiety in Man.” Proceedings of the Nutrition Society 51: 7–11. doi:10.1079/PNS19920004.
- Riber, A. B., and L. K. Hinrichsen. 2016. “Feather Eating and Its Associations with Plumage Damage and Feathers on the Floor in Commercial Farms of Laying Hens.” Animal 10: 1218–1224. doi:10.1017/S1751731116000057.
- Riber, A. B., and L. K. Hinrichsen. 2017. “Effects of Omitting Beak-trimming on Plumage, Skin and Keel Bone Conditions in Barn Layers.” Xth European Symposium on Poultry Welfare, 49, Ploufragan, France.
- Riedstra, B., and T. G. G. Groothuis. 2002. “Early Feather Pecking as a Form of Social Exploration: The Effect of Group Stability on Feather Pecking and Tonic Immobility in Domestic Chicks.” Applied Animal Behaviour Science 77: 127–138. doi:10.1016/S0168-1591(02)00031-X.
- Rodenburg, T. B., M. M. van Krimpen, I. C. de Jong, E. N. de Haas, M. S. Kops, B. J. Riedstra, R. E. Nordquist, J. P. Wagenaar, M. Bestman, and C. J. Nicol. 2013. “The Prevention and Control of Feather Pecking in Laying Hens: Identifying the Underlying Principles.” World’s Poultry Science Journal 69: 361–374. doi:10.1017/S0043933913000354.
- Rorvang, M. V., L. K. Hinrichsen, and A. B. Riber. 2019. “Welfare of Layers Housed in Small Furnished Cages on Danish Commercial Farms: The Condition of Keel Bone, Feet, Plumage and Skin.” British Poultry Science 60: 1–7. doi:10.1080/00071668.2018.1533632.
- Roura, E., M. W. Baldwin, and K. C. Klasing. 2013. “The Avian Taste System: Potential Implications in Poultry Nutrition.” Animal Feed Science and Technology 180: 1–9. doi:10.1016/j.anifeedsci.2012.11.001.
- Roura, E., and S. Cho. 2017. “Physiology of feed intake regulation in chickens: a chemosensory perspective.” 21st European Symposium on Poultry Nutrition, Salou/Vila-seca, Spain, 8–11th May.
- Saravanan, K., and B. Dhurai. 2012. “Exploration on Amino Acid Content and Morphological Structure in Chicken Feather Fiber.” Journal of Textile and Apparel, Technology and Management 7: 1–6.
- Savory, C. J. 1995. “Feather Pecking and Cannibalism.” World’s Poultry Science Journal 51: 215–219. doi:10.1079/WPS19950016.
- Savory, C. J., J. S. Mann, and M. G. Macleod. 1999. “Incidence of Pecking Damage in Growing Bantams in Relation to Food Form, Group Size, Stocking Density, Dietary Tryptophan Concentration and Dietary Protein Source.” British Poultry Science 40: 579–584. doi:10.1080/00071669986936.
- Schwartz, M. W., S. C. Woods, P. Y. Porte, R. J. Seeley, and D. G. Bas Kin. 2000. “Central Nervous System Control of Food Intake.” Nature 404: 661–671. doi:10.1038/35007534.
- Sherwin, C. M., G. J. Richards, and C. J. Nicol. 2010. “Comparison of the welfare of layer hens in 4 housing systems in the UK„. British Poultry Science 51 (4): 488–99. doi:10.1080/00071668.2010.502518.
- Spohn, S. N., and G. M. Mawe. 2017. “Non-conventional Features of Peripheral Serotonin Signalling - the Gut and Beyond.” Nature Reviews. Gastroenterology & Hepatology 14: 412–420. doi:10.1038/nrgastro.2017.51.
- Steenfeldt, S., J. B. Kjaer, and R. M. Engberg. 2007. “Effect of Feeding Silages or Carrots as Supplements to Laying Hens on Production Performance, Nutrient Digestibility, Gut Structure, Gut Microflora and Feather Pecking Behaviour.” British Poultry Science 48: 454–468. doi:10.1080/00071660701473857.
- Tahamtani, F. M., M. Brantsæter, J. Nordgreen, E. Sandberg, T. B. Hansen, A. Nødtvedt, T. B. Rodenburg, R. O. Moe, and A. M. Janczak 2017. “Effects of Litter Provision during Early Rearing and Environmental Enrichment during the Production Phase on Feather Pecking and Feather Damage in Laying Hens.” Xth European Symposium on Poultry Welfare, 51, Ploufragan, France.
- van der Eijk, J. A. J., H. de Vries, J. B. Kjaer, M. Naguib, B. Kemp, H. Smidt, T. B. Rodenburg, and A. Lammers. 2019. “Differences in Gut Microbiota Composition of Laying Hen Lines Divergently Selected on Feather Pecking.” Poultry Science 98: 7009–7021. doi:10.3382/ps/pez336.
- van Emous, R. A., and M. M. van Krimpen. 2019. “Effects of nutritional interventions on feathering of poultry - a review.” In Poultry Feathers and Skin: The Poultry Integument in Health and Welfare, edited by Oluyinka A. Olukosi, 133–150. Oxfordshire: CABI.
- van Hierden, Y. M. 2003. “Behavioural Neurobiology on Feather Pecking.” Ph. D. thesis, University of Groningen.
- van Hierden, Y. M., J. M. Koolhaas, and S. M. Korte. 2004. “Chronic Increaseof Dietary L-tryptophan Decreases Gentle Feather Pecking Behaviour.” Applied Animal Behaviour Science 89: 71–84. doi:10.1016/j.applanim.2004.05.004.
- van Krimpen, M. M. 2008. “Impact of Nutritional Factors on Eating Behaviour and Feather Damage of Laying Hens.” Ph. D. thesis, Wageningen University.
- van Krimpen, M. M., W. W. de Bruin, R. Veer, and G. P. Binnendijk 2010. “Effect of Diluted NSP-high Diets under Feather Pecking Prone Conditions on the Development of Feather Damage and Performance of Rearing and Laying Hens.” Wageningen Livestock Research Report 420. pp. 1–46.
- van Niekerk, T. G. C. M., I. C. D. Jong, M. M. V. Krimpen, B. F. J. Reuvekamp, and E. N. de Haas. 2013. Effect of UV-light, High Fiber Feed or Litter Provision in Early Rearing on Feather Pecking in Rearing and Laying Period. Lelystad: Wageningen Livestock Research.
- Vanhonacker, F., E. van Poucke, F. Tuyttens, and W. Verbeke. 2010. “Citizens’ Views on Farm Animal Welfare and Related Information Provision: Exploratory Insights from Flanders, Belgium.” Journal of Agricultural & Environmental Ethics 23: 551–569. doi:10.1007/s10806-010-9235-9.
- von Waldburg-zeil, C. G., N. van Staaveren, and A. Harlander-Matauschek. 2019. “Do Laying Hens Eat and Forage in Excreta from Other Hens?” Animal 13: 367–373. doi:10.1017/S1751731118001143.
- Wechsler, B., B. Huber Eicher, and D. R. Nash. 1998. “Feather Pecking in Growers: A Study with Individually Marked Birds.” British Poultry Science 39: 178–185. doi:10.1080/00071669889097.
- Zepp, M., H. Louton, M. Erhard, P. Schmidt, F. Helmer, and A. Schwarzer. 2018. “The Influence of Stocking Density and Enrichment on the Occurrence of Feather Pecking and Aggressive Pecking Behavior in Laying Hen Chicks.” Journal of Veterinary Behavior 24: 9–18. doi:10.1016/j.jveb.2017.12.005.
- Zhang, L. S., and S. S. Davies. 2016. “Microbial Metabolism of Dietary Components to Bioactive Metabolites: Opportunities for New Therapeutic Interventions.” Genome Medicine 8: 46. doi:10.1186/s13073-016-0296-x.