SUMMARY
Amaranth is a pseudocereal that has a potentially greater role to play in reducing malnutrition and promoting animal feed security because of its high quality and ability to adapt to environmental stresses. We review the nutrient content and its value for poultry feeding, as well as uses in industry and for human food that might impact on its availability for poultry. Wit effective processing to detoxify antinutritive components by extrusion or autoclaving, it can be incorporated at up to 40% in the diet of broiler chickens without loss of productivity. Its nutritional value is high, in terms of protein, oil, mineral and antioxidant content, as well as its excellent amino acid balance. Amaranth is likely to be most beneficial as feed grain for poultry in extreme climatic regions, such as those that are drought prone, as long as effective processing can be incorporated into the feed production system.
Introduction
Globally about two million square kilometres of agricultural land are currently used to grow feed for poultry (FAO Citation2018). However, poultry meat has the fastest growth in consumption of any meat worldwide (Whitton et al. Citation2021) and demand for high-quality poultry feeds is expected to increase. At the same time, the growing human population, climate change and wartime export restrictions are increasingly causing concerns about feed security. The predicted effect of an estimated 2°C increase in global temperatures by the end of the century is approximately a 20% reduction in cereal production, dependent on cereal cultivar and species (Fatima et al. Citation2020). Some of this reduction may be avoided by choosing plant species that use C4 photosynthesis and are tolerant to the predicted extremes of soil moisture and temperature (Janmohammadi et al. Citation2022, Citation2023; Kianfar et al. Citation2023; Sarker, Oba, Ercisli et al. Citation2022). Amaranth is one such plant species, and it has been successfully grown in Mexico, Guatemala, Nepal, India, Indonesia, China and Nepal (Acar et al. Citation1988).
There is no global data collated for production of amaranth (Aderibigbe et al. Citation2022). A commercial business analyst estimated the global amaranth market to have had a value of USD 1.8 billion in 2020, increasing to USD 3.5 billion by 2030 (Fortune Business Insights Citation2021). However, the same analyst estimated the US market at $1.48 billion in 2017 (Fortune Business Insights Citation2018). In the US, amaranth seeds retail at about US$0.88/kg (Iowa State University Citationn.d.), which, if reflected globally, suggests that about 1.5 million tonnes are grown worldwide. China, one of the world’s largest producers, was reported in the media as producing 0.8 million tonnes in 2017, but with plans for expansion (Mochan Citation2017). Amaranth grain has a high nutrient value, and it contains many bioactive compounds, as well as being tolerant of droughts and high temperatures (Baraniak and Kania-Dobrowolska Citation2022; Oteri et al. Citation2021; Park et al. Citation2020). According to the report of the World Health Organization (WHO Citation2018; Geneva, Switzerland), amaranth is one of the most neglected and underutilised plant species, in relation to improving feed security, enabling poultry production to be less reliant on dwindling cereal supplies. As such, it is potentially an important tool in combating the effects of climate change (Hosseintabar-Ghasemabad et al. Citation2022; Kianfar et al. Citation2023).
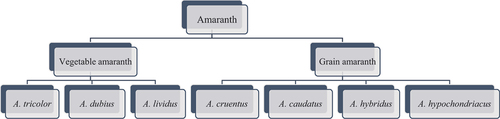
Botanically, amaranth is in the class Dicotyledoneae, subclass Caryophylliid, order Caryphyllales, family Amaranthaceae and genus Amaranthus (Breus Citation1997). It is therefore considered a pseudocereal. It is a dicotyledonous plant with an efficient C4 photosynthetic system due to the presence of phosphoenolpyruvate carboxylase (PEPA) and rubisco enzymes, which are resistant to hot weather and environmental stress. It uses carbon with high efficiency and has low water consumption, as well as having rapid growth and ecological benefits (Jamalluddin et al. Citation2021; Manyelo, Sebola, Hassan, et al. Citation2022; Sarker, Oba, Alsanie et al. Citation2022). There are more than 70 species of Amaranthus, many of which are potentially valuable for animal production. Some of the most important are listed in , as well as those that are ornamental or weeds (Cai et al. Citation2004; Hosseintabar-Ghasemabad et al. Citation2022). Although outside the scope of this review, it should be noted that amaranth leaf meal can be included in the diet of meat chickens at up to 15% of the diet without loss of productivity or adverse effects on meat quality (Manyelo, Sebola, and Mabelebele Citation2022).
In poultry diets, amaranth grain can be of similar value to conventional cereals, in terms of energy content, and it can also be used as a protein supplement and a rich source of digestible fibre, antioxidants (vitamins A, C, E, β-carotene, and calcium), iron, manganese and bioactive compounds (carotenoids, flavonoids, polyphenols and phytosterols) (Karamać et al. Citation2019; Nazeer and Firincioglu Citation2022; Procopet and Oroian Citation2022). The purpose of this review is to examine the nutritive value of amaranth grain for feeding to poultry.
Methodological approach
The current research was conducted with a focus on the introduction of amaranth nutrients and bioactive substances in poultry nutrition and the effects of these compounds on productivity and health. Since most of the costs in the poultry industry are related to nutrition, and the performance, health and quality of poultry products depend on the composition of the diet, the focus of our searches in Google, Google Scholar, ScienceDirect, Scopus and Web of Science databases used the keywords ‘amaranth’, ‘amaranth grain’, ‘chemical composition and bioactive compounds in amaranth grain’, ‘amaranth feeding in broiler chickens’, ‘amaranth feeding in laying hens’, ‘amaranth feeding in other poultry’. The methodological recommendations of Park et al. (Citation2020), Manyelo et al. (Citation2020) and Jimoh et al. (Citation2022) were employed in this study.
Carbohydrates in amaranth grain
Whole grain has 7.1–16.4% crude fibre and 7.6% non-starch polysaccharides (NSP) (Cai et al. Citation2004). Mustafa et al. (Citation2011) reported total fibre (soluble and insoluble) in the grain of three amaranth species, A. caudatus, A. cruentus and A. hypochondriacus, as between 9.8% and 14.5%. Pedersen et al. (Citation1990) found 8–13% crude fibre in amaranth grain, most of which was cellulose and lignin. It is possible to fractionate amaranth grain, which generates a high fibre byproduct, with insoluble and soluble fibre concentrations of 63.9% and 6.86%, respectively (Tosi et al. Citation2001). Neutral detergent insoluble fibre (NDF) and acid detergent insoluble fibre (ADF) concentrations in processed and unprocessed amaranth grains are compared to that in wheat and corn grains in .
Table 1. NDF and ADF concentrations in amaranth grain, with wheat and corn grains for comparison.
Starch is the main component of amaranth grain, and it ranges from 48% to 69% of its dry matter by weight (Cai et al. Citation2004). The starch granules are small, 0.8–2.5 µm, smaller than those in rice (3–8 µm) or potato (15–100 µm) (Cai et al. Citation2004; Corke et al. Citation1997). Light-coloured amaranth grains typically contain more starch than dark-coloured grains (Cai et al. Citation2004; Corke et al. Citation1997). About 86% of the starch is resistant to digestion (Capriles et al. Citation2008). The starch’s stable kneading, high viscosity and gelatinous properties, especially after the application of heat (Cai et al. Citation2004; Corke et al. Citation1997, Citation2016), provide industrial applications in food coating, cosmetic powder, and in making biological and degradable plastic (Karaeva et al. Citation2023; Sayed-Ahmad et al. Citation2022). It is used as a thickener in salad dressings, canned foods, drinks and frozen foods (Cai et al. Citation2004; Dabija et al. Citation2022) and its generally low amylose content and the absence of gluten make it useful in bread and cakes (Balakrishnan and Schneider Citation2022; Cai et al. Citation2004; Tareh et al. Citation2022). Amylose concentrations vary considerably from 7.8% in A. hypochondriacus cultivars to 34.3% in A. retroflexus cultivars, with an average in 124 genotypes of 19.2% (Cai et al. Citation2004; Corke et al. Citation1997). The main sugar in amaranth grain is sucrose, which is slightly more than in wheat, triticale and millet (Becker et al. Citation1981; Schmidt et al. Citation2023); however, the amount of monosaccharide and disaccharide in amaranth is low, just 3–5% or less (between 1.8% and 2.2% in A. cruentus and A. caudatus (Saunders and Becker Citation1983).
Protein and amino acids in amaranth grain
Amaranth has excellent protein biological value, largely due to its good amino acid balance. Lysine and sulphur-containing amino acids make up 4.4% of the total amino acids of amaranth (Schmidt et al. Citation2023). For human nutrition it is considered second only to eggs, and better than cow’s milk (Cai et al. Citation2004; Janmohammadi et al. Citation2022). Since its protein quality is similar to animal sources (Balakrishnan and Schneider Citation2022; Janmohammadi et al. Citation2023), amaranth grains can be considered an alternative to meat in the human diet (Segura-Nieto et al. Citation1994), not only because of the favourable amino acid composition but also because protein is the second most abundant nutrient (Balakrishnan and Schneider Citation2022; Cai et al. Citation2004; Segura-Nieto et al. Citation1994). This near perfect balance and quality of amino acids in amaranth grain is due to the existence of simple spherical proteins (Yánez et al. Citation1994). As a proportion of total proteins, albumin is present at 48.9–65%, glutelin 22.4–42.3%, globulin 13.7–18% and prolamin 1–2.3% (Cai et al. Citation2004; Segura-Nieto et al. Citation1994). The first limiting amino acid in amaranth grain has been mainly reported as threonine (Bressani Citation2003), but also leucine and valine (Bejosano and Corke Citation1998; Pedersen et al. Citation1987, Citation1990). The discrepancy may be due to the fact that different genotypes of amaranth have different amino acid profiles. The amino acid profile of amaranth grains is compared to eggs, wheat and corn in .
Table 2. Amaranth grain amino acid profile percentage compared to eggs (FAO Citation1990), wheat and corn (NRC Citation1994).
Oils and fatty acids in amaranth grain
The oil in amaranth grains is at higher concentrations (5–8%) than cereal grains (). More than 5% of amaranth oil contains a compound called squalene (2, 6, 10, 15, 19, 23-hexamethyl-2, 6, 10, 14, 18, 22-tetracozahexane), which is widely used in pharmaceutical industries (such as in anticancer, steroid and cholesterol-lowering drugs), cosmetics and health (such as skin and hair softeners), as well as in the aviation and electronic industries (such as airplane fuel, disk cleaners and lubricants in computers) and is currently considered one of the most expensive oils. In addition to amaranth, squalene can be obtained from the oil in the liver of sharks and olive leaves at a high cost. Currently, due to wide range of applications and consequently high price of squalene, many countries cultivate amaranth to extract this oil (phytosqualene) (Cai et al. Citation2004; Sayed-Ahmad et al. Citation2022). The range of fatty acids in amaranth oil in Cai et al.‘s report (Citation2004) includes palmitic acid (12–25%), oleic acid (35–19%), linoleic acid (25–62%), stearic acid (2–8.6%) and linolenic acid (0.3–2.2%) ().
Table 3. Concentrations of oil and squalene in amaranth grain and other crops.
Table 4. Fatty acid profile in amaranth grain (%).
In amaranth grain, the ratio of saturated fatty acid (S) to unsaturated fatty acid (U) is 0.1–0.5 (S/U) (Cai et al. Citation2004; León-Camacho et al. Citation2001). The values of C18:1 and C18:2 in amaranth are similar to cottonseed oil and sesame oil (Gamel et al. Citation2007). Phospholipid concentrations in amaranth oil have been measured at 5% (Becker et al. Citation1981) and 3.6% (Opute Citation1979), with the latter containing 13.3% cephalin, 16.3% lectin, and 2.8% phosphoinositol. Different measurement techniques probably produced these quantitative differences between the various reports (Wirkowska-Wojdyła et al. Citation2022).
Minerals and vitamins in amaranth grain
Minerals in the amaranth plant family vary with species, climatic conditions and soil type, but have been reported to be twice as much as other grains (Gamel et al. Citation2006). Amaranth is rich in calcium and phosphorus, with a Ca:P ratio of 1.2–1.9. In humans, amaranth consumption has been recommended for avoiding osteoporosis and heart disease (Duke Citation1999). Amaranth is also a rich source of phosphorus, magnesium, potassium, and vitamin C, and has good concentrations of other vitamins. Its concentrations of riboflavin, vitamins C and E, and folic acid compare favourably with wheat (Gamel et al. Citation2006). The amounts of total folate (TF), folic acid (FA), 5-methyltetrahydrofolate (MTHF-5) and 10-formyl tetrahydrofolate (10-CHOTHF) in amaranth grain has been measured at 228 μg/100 g DM, and boiling, steaming and malting reduced TF by 58, 22 and 21%, respectively (Motta et al. Citation2017). According to the European Food Safety Authority (EFSA), a small portion (35 g) of raw amaranth grain provides 25% of the Dietary Reference Intake (DRI) (EFSA Citation2014) for folate in humans (Motta et al. Citation2017), which suggests that its folate content would be beneficial for poultry diets.
Bioactive compounds in amaranth grain
Amaranth grain has many bioactive compounds with high antioxidant activity, which may be used to improve the health of poultry (Smolentsev et al. Citation2023). They are also beneficial for humans living in semi-arid and arid regions prone to drought who are experiencing malnutrition and metabolic diseases (Sarker, Oba, Alsanie et al. Citation2022). These include phenolic compounds, which exist in grain at a concentration of 168–329 mg/kg, although the extractable compounds are only 70–140 mg/kg (Tang and Tsao Citation2017). Feeds containing phenolic compounds are valuable for their potential antioxidant properties and strong antimicrobial properties (Ahmed et al. Citation2013). Vitamin E analogs are potent antioxidants with multiple physiological functions, such as regulating metabolic processes, improving anti-inflammatory and anti-cancer properties (Joana Gil‐Chávez et al. Citation2013; Tang and Tsao Citation2017).
There are many other bioactive compounds in amaranth grain, some of which have beneficial effects, such as antioxidants and E group vitamins, and some have anti-nutritional effects, such as saponins and phytates. The bioactive compounds of gallic, protocatechuic and p-hydroxy-benzoic acid are present at 11–440, 4.7–136 and 5.8–20.9 mg/kg, respectively (Klimczak et al. Citation2002; Woodhead and Swain Citation1974). The main betacyanin active compounds in amaranth are amaranthine and isoamaranthine, with estimated concentrations of approximately 19 mg/kg (Cai et al. Citation2004; Repo-Carrasco-Valencia et al. Citation2010). Vitamin E homologs include tocopherol and tocotrienols and all four isomeric forms (α, β, γ and δ) have been detected in amaranth grain tocopherols. The highest values have been recorded for γ-tocopherol at 47–53 mg/kg, followed by α-tocopherol at 17–26 mg/kg (Tang and Tsao Citation2017). β and δ-tocopherol were at trace concentrations. The concentrations (mg/kg) of tocotrienols in amaranth grain have been reported as: α (1.4–31.6), β (0.53–43.86), γ (8.69–0.06) and δ (0.01–48.79) (Bruni et al. Citation2002; Lehmann et al. Citation1994; Tang and Tsao Citation2017). In the study of Hosseintabar-Ghasemabad et al. (Citation2022), amaranth grain had the highest concentration of β+γ tocopherol (293 units), followed by Δ-tocopherol (219 units), with least α-tocopherol (18.60 units). The total content of carotenoids in amaranth has been reported as 17.61–17.69 mg/kg. According to Janmohammadi et al. (Citation2023), the most common phytosterol compounds in amaranth grain are β-sitosterol (37%), Δ-5-avena sterol (25%) and stigma sterol (19%). The carotenoids in this category of plants include lutein and zeaxanthin, which have many benefits in improving the health of consumers (Tang et al. Citation2016). An active compound in amaranth, 20-hydroxyacedizone (20HE), present at 148–484 mg/kg), can affect muscle protein synthesis and reduce blood glucose, thus helping to control obesity and diabetes, at least in mice (Foucault et al. Citation2012).
Saponins in amaranth grain have been measured at 0.9–4.91 mg/kg and 0.9–1.0 mg/kg DM, with concentration increasing linearly 24–240 hours after germination of amaranth seeds (Oleszek et al. Citation1999). Several types of saponins have been identified in amaranth grain, and they can be divided into subgroups oleanolic acid, hederagenin or phytolaccagenic acid as aglycones (Junkuszew et al. Citation1998). 3-O-β-di-glucopyranosyl oleanolic acid is a sapogenin with anti-inflammatory activity (Kuljanabhagavad and Wink Citation2009).
Phytic acid (myinositol hexaphosphoric acid) in amaranth has been reported to be between 2 and 8 g/kg (Breene Citation1991; Escudero et al. Citation2004; Gamel et al. Citation2006; Kozioł Citation1992; Tang and Tsao Citation2017), reduced by 15–20% with thermal processing (Gamel et al. Citation2007). Germination is mainly a catabolic process to deliver nutrients to the growing plant through the hydrolysis of stored nutrients, and phytic acid plays an important role as a source of phosphorus and cations during this process. There is more phytic acid in amaranth than in rice (1–1.4 g/kg), but less than in corn (9 g/kg) or in wheat (9.8–14.3 g/kg) (Cai et al. Citation2004; Lorenz and Wright Citation1984). In addition to phytic acid and saponin, amaranth has other anti-nutritional compounds such as tannins, oxalate and various inhibitors of trypsin, chymotrypsin and amylase (Chemeda and Bussa Citation2018). Lorenz and Wright (Citation1984) found the phytate to be between 5.2 and 6.1 g/kg in eight varieties of amaranth grain, higher than rice and millet but less than corn and wheat. A. cruentus and A. hybridus cultivars have the most tannins (Lorenz and Wright Citation1984). Dehulling the grain significantly reduces tannin and improves protein digestibility because the amount of tannin in the shell is more than six times that of dehulled grain (Lorenz and Wright Citation1984).
Amaranth grain feeding to the major poultry groups
The effects of incorporating amaranth grain into the diets of the major poultry groups is summarised in and detailed below.
Table 5. The effect of using different amounts and forms of amaranth grain on bird performance, health, meat and egg quality, listed in chronological order of publication.
Amaranth grain feeding to broiler chickens
It is almost half a century since the first studies of the use of amaranth (A. edulis) grain in poultry nutrition (Connor et al. Citation1980), which found that it was rich in the essential amino acids, lysine (0.94%), methionine and cysteine (0.66%), arginine (1.54%), and threonine (0.59%). Thus, it had a better amino acid balance than many cereals. The apparent metabolisable energy contents, corrected to zero nitrogen balance (AMEn), were 3145 and 3475 kcal/kg, for raw and processed grain, respectively. Thermal processing allowed up to 70% of conventional high protein concentrates to be replaced by amaranth grain, without loss of performance.
In the 1980s, many studies were conducted on amaranth grain in poultry diets. Laovoravit et al. (Citation1986) found that A. cruentus grain, with 13.5% protein and 0.96% lysine (Laovoravit Citation1982), could be included at 30% of the diet of broiler chickens if autoclaved. Supplementing amaranth-based diets with thiamine sources (diet containing wheat) was recommended, particularly because thiamine is important in regulating chicken growth.
Later, it was confirmed that heat processing can increase protein digestibility (Písaříková et al. Citation2005); however, the amount and duration of heating should be carefully programmed, because protein quality depends not only on amino acid composition, but also on bioavailability and digestibility of protein. The digestibility of protein, available lysine, net protein utilisation (NPU), protein efficiency ratio (PER) or biological value (BV) are very high in amaranth compared to cheese in human nutrition. The average protein digestibility of amaranth is variously reported to be 74% (Bejosano and Corke Citation1998) and 80–86% (Gamel et al. Citation2006). Roasting at 170–190°C gives the best increase in digestibility (Písaříková et al. Citation2005). However, in the popping process, trypsin inhibitors and other anti-nutritional agents are deactivated, but it also causes the loss of several essential amino acids, especially tyrosine, phenylalanine, methionine, and lysine, and subsequently, a noticeable decrease in protein quality (Gamel et al. Citation2006). This suggests a decrease in the biological value of protein at very high temperatures, and the possibility of denaturation and Maillard reaction, and in parallel, a decrease in the nutritional value of amaranth grain (Bressani et al. Citation1993). Steam dehulling of amaranth grain can also lead to improved performance in broilers (P. G. Peiretti et al. Citation2017).
Tillman and Waldroup (Citation1986) studied the usefulness of amaranth grain (A. cruentus), processed either by autoclaving or extrusion, to replace corn/soyabean diets for broilers at 20% or 40%. This study and that of Waldroup et al. (Citation1985) indicated that inclusion of amaranth grains at 40% depresses growth rate unless the grain is suitably processed by autoclaving or extrusion. It did not depress growth, even at 40% inclusion rate, if it was properly processed by dry extrusion or autoclaving for 30–45 min. Later work by Tillman and Waldroup (Citation1988c) confirmed that at 50% replacement growth reductions occurred in the finisher period.
Several different forms of raw, autoclaved and processed amaranth grain were evaluated for feeding to broiler chickens by Acar et al. (Citation1988). Their control diet of primarily corn and soybean meal contained 3060 kcal/kg of metabolisable energy and 23.6% protein, and all the experimental diets were formulated with similar metabolisable energy and protein contents. The various treatments tested compared whole grain flour amaranth (61.46% of diet), fat-free amaranth flour (62.10% of diet), perisperm (49.50% of diet), amaranth grain bran (35.30% of diet) and popped amaranth flour (61.10% of the diet). Crude protein (CP) contents varied between 9.6% and 29.6% for the different forms of amaranth studied. Ether extract, crude protein, crude fibre and ash in bran were lower than whole grain flour and the lowest values were for perisperm. Autoclaving whole grain flour increased ADF, NDF, cellulose and lignin contents, with new complexes formed after thermal processing. There was no effect on metabolisable energy concentrations, except in the perisperm in which it was reduced. Bran had the highest protein, alanine, arginine, glutamic acid, proline and tyrosine concentrations. Heat-treatment reduced lysine concentrations, but autoclaving improved broiler performance, probably due to reduced levels of antinutritive compounds and improved bioavailability of some nutrients.
In a similar study, Ravindran et al. (Citation1996) evaluated the nutritional value of A. hypochondriacus, in raw and autoclaved form for feeding to broiler chickens. The raw grain contained 168, 58, 60, 26, 2.2 and 5.6 grams per kilogram crude protein, crude fat, crude fibre, total ash, calcium and total phosphorus, respectively. Replacement of the basal maize/soyabean meal/meat and bonemeal diet with raw amaranth grain depressed growth and increased FCR. However, they were able to overcome these negative effects by autoclaving the grain at 130°C for one hour, suggesting that there were heat-labile antinutritive elements within the grain. The authors suggest that phenolics, in particular tannins, were most likely responsible, though lectins, trypsin inhibitors and troponins may also have been implicated. The authors conclude that processing increased AME from 11.9 to 13.1 MJ/kg DM and confirmed that processed amaranth grain can be included at up to 40% of the diet without loss of performance.
Yaghobfar (Citation2001) determined the chemical composition of amaranth grain as, in g/kg, crude protein 166, crude fat 73, linoleic acid 50.8% of total fat, ash 35, cellulose 250, hemicellulose 45, cell wall excluding hemicellulose 205, lignin 86, protein digestibility 140, lysine 10.3, methionine 3.4, cysteine 2.5, alanine 5.7, arginine 11.0, asparagine 14, leucine 10.4, proline 10.5, phenylalanine 6.4, threonine 5.6, histidine 3.8, glycine 12.0, glutamine 29.5, tyrosine 5.7, valine 6.0, isoleucine 5.5 and glucose 75. The metabolisable energy concentration for raw amaranth was 1805 and extruded amaranth Citation2275 kcal/kg.
Several studies have found no change in growth of broiler chickens fed unheated amaranth, compared with a control diet Waldroup et al. (Citation1985), Serratos (Citation1996), Roučková et al. (Citation2004) and Písaříková et al. (Citation2005), but this is only when it was included at low rates in the diet. Waldroup et al. (Citation1985) found that there was no effect on growth of inclusion of A. hypochondriacus or A. cruentus grains at 20% of the diet for broiler chickens in the raw and autoclaved forms, but at 40% there was an adverse effect on performance, particularly for A. hypochondriacus. Autoclaving reduced these negative effects on the growth of this species. Extrusion, rather than autoclaving, is probably the best method of processing, based on practical considerations and potential benefits (Tillman and Waldroup Citation1986).
Amaranth grains were able to replace animal origin protein sources, such as meat and bone meal, which are increasingly hard to source, for feeding to broilers (Roučková et al. Citation2004). Amaranth grain is also a suitable substitute for fishmeal (Pisarikova et al. Citation2006), in the raw, heat-processed and dried grains. Pelleting amaranth-containing diets leads to improvement of body weight, feed consumption, feed efficiency and reduction of carcass fat in broiler chickens (Alizadeh-Ghamsari et al. Citation2021). The popping process leads to a change in the content of chemical compounds and protein digestibility, and the differences in nutrient contents between raw and popped amaranth grain were, in g/kg: crude protein 158.1 for raw amaranth, which after popping became 168.5, fat 71.5 for raw amaranth, which after popping became 69.4, NDF 99.2 for raw amaranth, which after popping became 111.8, cellulose 86.6 for raw amaranth, which after popping became 60.0, cysteine 4.2 for raw amaranth, which after popping became 4.1, threonine 6.0 for raw amaranth, which after popping became 6.5, alanine 8.8 for raw amaranth, which after popping became 9.2, valine 6.8 for raw amaranth, which after popping became 7.4, isoleucine 5.2 for raw amaranth, which after popping became 5.6, lysine 9.2 for raw amaranth, which after popping became 8.8, arginine 12.8 for raw amaranth, which after popping became 14.2, in vitro protein digestibility 68.1 for raw amaranth, which after popping became 50.6%, respectively (Písaříková et al. Citation2005). Similar improvement was reported by Bressani et al. (Citation1993) and Andrasofszky et al. (Citation1998). Inclusion at up to 10% was deemed appropriate. Inclusion at 5% and 10% led to some decrease in performance in the work of Longato et al. (Citation2017), but the antioxidant in serum increased from 177 (μEq/l) to 219 and 250 units, and lipid peroxidation in the serum of chickens decreased from 700 micromol/ml to 419 and 262 units, respectively. Alizadeh-Ghamsari and Hosseini (Citation2020, Citation2021) considered that an inclusion at 2% or less gave the maximum performance. Incorporating amaranth grain at 8% of the diet of meat chickens reduced both feed intake and growth rate, but did not affect FCR, in the study of Orczewska-Dudek et al. (Citation2018).
Extruded amaranth (A. cruentus) grain is reported to contain AME and AMEn values of 3556 and 3415 kcal/kg DM, respectively (Tillman and Waldroup Citation1988a). The AMEn content of raw and processed amaranth has been determined in Ross 308 broiler chickens (Janmohammadi et al. Citation2022). The main constituents of the basal diet were replaced by between 0% and 60% amaranth, in the untreated or heat-treated form, and with or without an enzyme additive. Total excreta collection was used to create regression models to determine AMEn content, which was estimated at 3260 and 3900 kcal/kg for untreated and heat-treated grain, respectively. Enzyme addition produced a small benefit in AMEn content. These energy concentrations were 230 and 470 kcal/kg more than corn and wheat grains, respectively. In addition, the amaranth grain had about twice the crude protein content of corn.
In the digestibility studies of feeding poultry with amaranth, Tillman and Waldroup (Citation1988b) found that amaranth could be included at up to 40% in the diet, but above this level growth rate was reduced. However, abdominal fat pad, liver and gizzard weight was increased with the proportion of amaranth in the diet.
Amaranth grain incorporation into the diet of meat chickens increased the n-3 fatty acid in breast muscle and, amaranth being rich in antioxidants, protects against excessive oxidation of lipids, leading to better sensory properties (Orczewska-Dudek et al. Citation2018). As well, it reduced triglycerides in blood and abdominal and subcutaneous fat content.
Being rich in antioxidants, in particular quercetins such as rutin, amaranth vegetative growth could potentially be used as a phytobiotic replacement for antibiotics in the diet of broiler chickens (Drannikov et al. Citation2021). Quercetin contents vary with amaranth species, being greatest for A. caudatus (16 mg/kg DW) and least for A. tricolor (<2 mg/kg DW).
Amaranth grain feeding to laying hens
The use of amaranth in the nutrition of laying hens is of special importance due to the risks posed by cholesterol in egg yolks and its challenges to the health of consumers in the form of non-communicable diseases. Some cholesterol is necessary in the human diet – the American Heart Association reported that the consumption of 300 mg of cholesterol per day is sufficient to maintain health, hence a large egg containing 210 mg of cholesterol can be consumed daily provided that other foods consumed do not increase cholesterol intake to dangerous levels (Baghban-Kanani et al. Citation2019, Citation2020; Rodríguez-Ríos et al. Citation2020). Several methods can be used to reduce the cholesterol content of eggs: genetic modification (although the cholesterol content is not well inherited), hypocholesterolemic drugs, and flock management (Elkin Citation2007), but incorporating amaranth into the diet is likely to be one of the most effective.
The ratio of polyunsaturated fatty acids (PUFA) to saturated fatty acids (SFA) in eggs is about 0.5 (Mine Citation2008). Increasing this ratio can decrease serum cholesterol of egg consumers (Mine Citation2008). Amaranth reduces cholesterol in eggs through multiple mechanisms, which generates a reduction in cholesterol in consumers’ blood and liver (Mine Citation2008). In particular, compounds such as crude fibre, squalene, phytosterols, tocopherols and tocotrienol found in amaranth can reduce the biosynthesis of cholesterol in poultry (P. Peiretti Citation2018). Some unique compounds in amaranth grain can limit the production rate of HMG-CoA reductase enzyme in the liver, which will lead to cholesterol reduction. Crude fibre in amaranth reduces cholesterol by binding to it and removing it from the animal’s body. Abundant linoleic acid in amaranth grain can play an important role in eliminating bile acids and reducing cholesterol. Also, an exclusive component in amaranth grain is squalene, which, if consumed, is sequestered by the liver with the help of chylomicrons and used to make steroids and bile acids. Bile acids (cholic and deoxycholic) combine with glycine and taurine and produce bile salts, and by increasing the recycling of bile secretions, reduce yolk cholesterol (Kianfar et al. Citation2023). Nutritional modification of cholesterol in chickens’eggs is therefore feasible. Other feeds to reduce egg cholesterol in the diet of laying hens include black cumin (Nigella sativa) (Aydin et al. Citation2008), zucchini (Cucurbita maxima duchesne) (Orozco-Martínez et al. Citation2012), rapeseed (Brassica napus) (Caston and Leeson Citation1990), chia (Salvia hispanica) (Salazar-Vega et al. Citation2009), sunflower meal (Helianthus annuus) (Baghban-Kanani et al. Citation2018), seaweed (Sargassum spp) (Carrillo et al. Citation2012), Artemisia (Artemisia annua) (Baghban-Kanani et al. Citation2019), spirulina algae (Spirulina platensis) (Tufarelli et al. Citation2021), and flaxseed (Linum usitatissimum) (Tufarelli et al. Citation2022).
Qureshi et al. (Citation1996) reported that supplementing the diet of chickens with A. hypochondriacus and A. cruentus led to a 10–30% reduction in blood cholesterol of birds. LDL decreased by 7–70%. In the control group, a 10–18% decrease in the enzyme activity of cholesterol 7-alpha hydroxylase, 3-hydroxy-3-methylglutaryl coenzyme A reductase was observed in the liver compared to other experimental groups, which presumably caused the decrease in blood cholesterol in experimental birds. Activation of 7-alpha hydroxylase enzyme leads to the formation of bile acids from cholesterol and increases the catabolism and excretion of cholesterol. It has been shown in hamsters that the cholesterol-lowering effects of amaranth grain were probably due to peptides reducing HMG-CoA reductase enzyme (Mendonça et al. Citation2009; Soares et al. Citation2015). Additional studies showed that the presence of a special phytochemical compound in amaranth grain called 20-hydroxyacedizone (20HE) can lead to increased anti-obesity effects (reduction of triglyceride and cholesterol) and LDL) and even antidiabetic in mice (Foucault et al. Citation2012; Pond et al. Citation1989; Tang and Tsao Citation2017). Also, the tocopherols in amaranth are more than in cereals, which can reduce the synthesis of cholesterol, low-density lipoprotein and the enzyme lipoprotein lipase, and ultimately regulate and reduce cholesterol in the blood (León-Camacho et al. Citation2001; Qureshi et al. Citation1996; Santiago et al. Citation2014; Schnetzler and Breene Citation1994; Tang and Tsao Citation2017). Hood (Citation1998) reported that eight isomers of vitamin E present in amaranth grain oil cause a 30% and 70% reduction of cholesterol and LDL, respectively, in chickens. β-phytosterol in amaranth grain has also a major effect in reducing LDL cholesterol and increasing HDL blood without side effects (Awad et al. Citation2000; Marcone et al. Citation2003; Tang et al. Citation2016).
These theoretical benefits of including amaranth have been repeatedly demonstrated in practice: Króliczewska et al. (Citation2008) incorporated 2–10% A. cruentus grain into the diet of layers and observed a decrease in blood low-density lipoprotein cholesterol and triglyceride, and an increase in aspartate aminotransferase and alanine aminotransferase activity, compared to the control group. Popiela et al. (Citation2013) investigated the performance, blood characteristics, egg quality traits and the composition of egg yolk fatty acids in sixty brown Lohmann laying hens offered diets containing 0%, 5% and 10% extruded amaranth grain. Birds fed with a diet containing 5% amaranth had the lowest FCR and the highest egg production, and birds on this diet had decreased and increased linoleic acid (omega-6) and linolenic acid (omega-3), respectively. Blood glucose, aspartate aminotransferase and alanine aminotransferase decreased with increasing levels of extruded amaranth grain. Tillman and Waldroup (Citation1987) incorporated extruded A. cruentus grain in the diet of 20, 65-week-old White Leghorn laying hens at 0–30% of the diet. Inclusion of amaranth tended to increase the rate of lay, but reduced egg weight, whilst improving feed conversion efficiency. Yolk colour declined as the proportion of amaranth in the diet increased. Punita and Chaturvedi (Citation2000) incorporated A. paniculatus grain in diet of laying hens and found that the amount of linoleic acid increased by 100% and cholesterol in the eggs decreased by 14%. The highest cholesterol reduction and highest amounts of linoleic acid was observed in a treatment containing mixed red palm oil + popped amaranth grain. Reklewska et al. (Citation1995) similarly reported a reduction of triacyl-glycerides and saturated fatty acids and cholesterol in egg yolk. Kianfar et al. (Citation2023) including untreated and autoclaved A. hybridus amaranth grain in the diet of experimental birds separately, with 0%, 5%, 10% and 15% levels, for six weeks, found that the 5% and 10% levels decreased blood glucose, cholesterol and triglycerides and produced eggs with low cholesterol and triglyceride content. However, the content of omega-6 in eggs and subsequently the ratio of omega-6 to omega-3 in the yolk increased.
No reduction in egg yolk cholesterol was observed when amaranth was included at 15–45% of the diet (Rodríguez-Ríos et al. Citation2020). However, feeding amaranth grain (Amaranthus hybridus chlorostachys) that had been thermally processed and included at up to 40% in the diet did reduce yolk cholesterol (Hosseintabar-Ghasemabad et al. Citation2022), by 10% at a 30–40% inclusion rate. In this study feeding amaranth at 30–40% of the diet reduced egg mass and increased feed conversion ratio; however, inclusion of a multienzyme preparation, including cellulase, xylanase, pectinase, β glucanase, α-amylase, protease and phytase in the diet negated these adverse effects. Amaranth inclusion caused a decrease in triglyceride, cholesterol, LDL, atherogenic index and a parallel increase in HDL and total antioxidant capacity. Janmohammadi et al. (Citation2023) confirmed this and attributed egg cholesterol reduction to the abundance of phytosterol and tocopherol bioactive compounds in raw amaranth grain, along with the presence of squalene. More broadly, amaranth inclusion in the diet of poultry can help combat climate stress, because of the drought-resistant properties of the plant, thereby helping to prevent malnutrition and hunger in humans and livestock (Nwadinigwe et al. Citation2019).
Amaranth grain feeding to other poultry
Breeder chickens
Including 3–7% of amaranth grain in the feeding of breeder hens accelerated sexual maturity, increased the number and weight of eggs and the rate of hatchability and chick production (Vishtakalyuk et al. Citation2001). The existence of the desired profile and types of unsaturated fatty acids in amaranth grain, along with significant amounts of squalene, produces a positive effect on sperm motility and can reduce the risk of defects, because omega-3 and omega-6 fatty acids can significantly protect chromosomes against abnormalities, and in parallel, the presence of squalene in amaranth grain sources can neutralise mitochondrial function disorders and assist in sperm development (Singhal and Kulkarni Citation1988). Also, Vishtakalyuk et al. (Citation2001) observed an increase in egg white and yolk %. Khiroug et al. (Citation2001) investigated low-cholesterol nutrition of birds, and after adding amaranth grain polysaccharides to the feed of breeder hens, they increased the weight of eggs and white %. Thus, amaranth grain in the feeding of breeder chickens can affect the morphological composition of the egg (Szczerbinska et al. Citation2015).
Cockerels
The AMEn values of amaranth have been determined by feeding amaranth to adult Leghorn cockerels (Hosseintabar-Ghasemabad et al. Citation2020). AMEn values for untreated amaranth with enzyme were 3433 kcal/kg and untreated amaranth without enzyme 3250 kcal/kg. Processed amaranth had similar values, and overall enzyme addition led to a 5–6% improvement in ME value.
Quail
Jakubowska et al. (Citation2013) investigated the effects of adding amaranth grain (at 0%, 4% and 7%) in standard isocaloric and isoprotein quail diets, in a study using thirty-six adult female quails (20 weeks). The low level of amaranth led to the improvement of breast muscle tenderness and the high-level reduced breast meat palatability. Szczerbinska et al. (Citation2015) investigated A. cruentus grain incorporation at 0%, 4% and 7% in the diet of quail and found that feeding birds with rations containing amaranth had improved egg hatchability. The percentage of yolk in eggs in the group fed with 4% amaranth was more than the other experimental groups, and at 7% there was an increase in the concentration of cholesterol, triglyceride, LDL and albumin, and a decrease in liver enzymes.
Turkeys
Organically grown amaranth has been compared with buckwheat (Fagopyrum esculentum), white and yellow corn and wheat for feeding to adult turkeys (Jacob et al. Citation2008). However, in this study the amaranth seeds were not ground because of their small size, whereas the cereal grains were. Although the GE and CP contents of the amaranth were highest, the nitrogen-corrected ME values were less than all the cereals except buckwheat. It was less well utilised by turkeys than chickens.
Conclusions
This review showed that amaranth grain has a favourable content of nutrients, including carbohydrates, protein, fat, minerals and vitamins compared to cereals. In addition, the profile of amino acids, fatty acids and bioactive compounds in the grain indicate potential benefits of including amaranth in feed programmes, provided that it is effectively processed and not included at more than 40% of the diet. Heat processing the grain reduces the effects of anti-nutritive compounds and can improve bioavailability of nutrients. A particular benefit of including amaranth in the diet of layers is the reduction in egg cholesterol content. The antioxidant compounds and squalene, as well as the ideal amino acid balance in amaranth, can be effective in improving the health of the flock, as well as increasing the quality and quantity of poultry products, without affecting the performance negatively.
Author contributions
A.S. co-ordinated the project. C.J.C.P. wrote the main part of the principle draft, to which B.H.G., A.R.D.R., H.J., M.I.S., I.F.G., A.A.M., and A.S. had contributed. All authors approved the final draft. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
Financial support by Rasht Branch, Islamic Azad University, grant number 17.16.1.575 is gratefully acknowledged. The work was done in accordance with the State task of the State Scientific Institution NIIMMP.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Acar, N., P. Vohra, R. Becker, G. Hanners, and R. Saunders. 1988. “Nutritional Evaluation of Grain Amaranth for Growing Chickens.” Poultry Science 67 (8): 1166–1173. https://doi.org/10.3382/ps.0671166.
- Aderibigbe, O. R., O. O. Ezekiel, S. O. Owolade, J. K. Korese, B. Sturm, and O. Hensel. 2022. “Exploring the Potentials of Underutilized Grain Amaranth (Amaranthus Spp.) Along the Value Chain for Food and Nutrition Security: A Review.” Critical Reviews in Food Science and Nutrition 62 (3): 656–669. https://doi.org/10.1080/10408398.2020.1825323.
- Ahmed, S. A., S. Hanif, and T. Iftkhar. 2013. “Phytochemical Profiling with Antioxidant and Antimicrobial Screening of Amaranthus Viridis L. Leaf and Seed Extracts.” Open Journal of Medical Microbiology. https://doi.org/10.4236/ojmm.2013.33025.
- Alizadeh-Ghamsari, A. H., and S. A. Hosseini. 2020. “Determination the Optimum Level of Amaranth Grain in Broilers Pelleted Diet Based on Multiple Attribute Decision Making Method (TOPSIS Model) and Production Index.” Research on Animal Production 11 (27): 1–8. https://doi.org/10.5398/tasj.2021.44.1.71.
- Alizadeh-Ghamsari, A. H., S. Hosseini, M. Soleymani, and R. Nahavandi. 2021. “Performance, Intestinal Histomorphology, and Blood Variables of Broilers Fed Amaranth Grain in Pellet Diet.” Tropical Animal Science Journal 44 (1): 71–78. https://doi.org/10.29252/rap.11.27.1.
- Andrasofszky, E., Z. Szöcs, S. Fekete, and K. Jelenits. 1998. “Evaluation of the Nutritional Value of the Amaranth Plant. I. Raw and Heat-Treated Grain Tested in Experiments on Growing Rats.” Acta Veterinaria Hungarica 46 (1): 47–59. https://pubmed.ncbi.nlm.nih.gov/9704510/.
- Awad, A. B., K. C. Chan, A. C. Downie, and C. S. Fink. 2000. “Peanuts as a Source of β-Sitosterol, a Sterol with Anticancer Properties.” Nutrition and Cancer 36 (2): 238–241. https://doi.org/10.1207/S15327914NC3602_14.
- Aydin, R., M. Karaman, T. Cicek, and H. Yardibi. 2008. “Black Cumin (Nigella sativa L.) Supplementation into the Diet of the Laying Hen Positively Influences Egg Yield Parameters, Shell Quality, and Decreases Egg Cholesterol.” Poultry Science 87 (12): 2590–2595. https://doi.org/10.3382/ps.2008-00097.
- Baghban-Kanani, P., B. Hosseintabar-Ghasemabad, S. Azimi-Youvalari, A. Seidavi, T. Ayaşan, V. Laudadio, and V. Tufarelli. 2018. “Effect of Different Levels of Sunflower Meal and Multi-Enzyme Complex on Performance, Biochemical Parameters and Antioxidant Status of Laying Hens.” South African Journal of Animal Science 48 (2): 390–399. https://doi.org/10.4314/sajas.v48i2.20.
- Baghban-Kanani, P., B. Hosseintabar-Ghasemabad, S. Azimi-Youvalari, A. Seidavi, V. Laudadio, D. Mazzei, and V. Tufarelli. 2020. “Effect of Dietary Sesame (Sesame Indicum L) Seed Meal Level Supplemented with Lysine and Phytase on Performance Traits and Antioxidant Status of Late-Phase Laying Hens.” Asian-Australasian Journal of Animal Sciences 33 (2): 277. https://doi.org/10.5713/ajas.19.0107.
- Baghban-Kanani, P., B. Hosseintabar-Ghasemabad, S. Azimi-Youvalari, A. Seidavi, M. Ragni, V. Laudadio, and V. Tufarelli. 2019. “Effects of Using Artemisia Annua Leaves, Probiotic Blend, and Organic Acids on Performance, Egg Quality, Blood Biochemistry, and Antioxidant Status of Laying Hens.” The Journal of Poultry Science 56:120–127. https://doi.org/10.2141/jpsa.0180050.
- Balakrishnan, G., and R. G. Schneider. 2022. “The Role of Amaranth, Quinoa, and Millets for the Development of Healthy, Sustainable Food Products—A Concise Review.” Foods 11 (16): 2442. https://doi.org/10.3390/foods11162442.
- Baraniak, J., and M. Kania-Dobrowolska. 2022. “The Dual Nature of Amaranth—Functional Food and Potential Medicine.” Foods 11 (4): 618. https://doi.org/10.3390/foods11040618.
- Becker, R., E. Wheeler, K. Lorenz, A. Stafford, O. Grosjean, A. Betschart, and R. Saunders. 1981. “A Compositional Study of Amaranth Grain.” Journal of Food Science 46 (4): 1175–1180. https://doi.org/10.1111/j.1365-2621.1981.tb03018.x.
- Bejosano, F. P., and H. Corke. 1998. “Protein Quality Evaluation of Amaranthus Whole Meal Flours and Protein Concentrates.” Journal of the Science of Food and Agriculture 76 (1): 100–106. https://doi.org/10.1002/(SICI)1097-0010(199801)76:1<100:AID-JSFA931>3.0.CO;2-B.
- Breene, W. 1991. “Food Uses of Grain Amaranth.” Cereal Foods World 36 (5): 426–430.
- Bressani, R. 2003. “Amaranth.” In Encyclopedia of Food Science and Nutrition, edited by L. Trugo, and P. M. Finglas, 166–173. Oxford: Academic Press.
- Bressani, R., E. De Martell, and C. De Godinez. 1993. “Protein Quality Evaluation of Amaranth in Adult Humans.” Plant Foods for Human Nutrition 43 (2): 123–143. https://doi.org/10.1007/BF01087917.
- Breus, I. 1997. “Productivity, Chemical Composition and Fertilizing of Amaranth Growing for Green Matter Yield.” Agricultural Chemistry 10:52–74.
- Bruni, R., A. Guerrini, S. Scalia, C. Romagnoli, and G. Sacchetti. 2002. “Rapid Techniques for the Extraction of Vitamin E Isomers from Amaranthus Caudatus Seeds: Ultrasonic and Supercritical Fluid Extraction.” Phytochemical Analysis: An International Journal of Plant Chemical and Biochemical Techniques 13 (5): 257–261. https://doi.org/10.1002/pca.651.
- Cai, Y., H. Corke, and H. Wu. 2004. “Amaranth.” In Encyclopedia of Grain Science, edited by C. Wrigley, H. Corke, and C. Walker, 10. Amsterdam: Elsevier.
- Capriles, V., K. Coelho, A. Guerra‐Matias, and J. Arêas. 2008. “Effects of Processing Methods on Amaranth Starch Digestibility and Predicted Glycemic Index.” Journal of Food Science 73 (7): H160–H164. https://doi.org/10.1111/j.1750-3841.2008.00869.x.
- Carrillo, D., A. Bahena, V. Casas, J. Carranco, C. Calvo, and G. Ávila. 2012. “The Algae Sargassum Spp. As an Alternative for Reducing the Cholesterol Content of Eggs.” Cuban Journal of Agriculture Science 46 (2): 1–6. https://cjascience.com/index.php/CJAS/article/view/67.
- Caston, L., and S. Leeson. 1990. “Research Note: Dietary Flax and Egg Composition.” Poultry Science 69 (9): 1617–1620. https://doi.org/10.3382/ps.0691617.
- Chemeda, A. S., and N. F. Bussa. 2018. “Effect of Processing Methods on Nutritional and Anti-Nutritional Value of Amaranth Grain; and Potential Future Application of Amaranth Grain in Injera Making.” International Journal of Fermented Foods 7 (1): 11–20. https://doi.org/10.30954/2321-712X.01.2018.2.
- Chung, O. K., J.-B. Ohm, M. Ram, S. H. Park, and C. A. Howitt. 2009. “Wheat Lipids.” In Wheat: Chemistry and Technology, edited by K. Khan, and P. Shewry, 363–399. Amsterdam: Elsevier.
- Connor, J., R. Gartner, B. M. Runge, and R. Amos. 1980. “Amaranthus edulis: An Ancient Food Source Re-Examined.” Australian Journal of Experimental Agriculture 20 (103): 156–161. https://doi.org/10.1071/EA9800156.
- Corke, H., Y. Cai, and H. Wu. 2016. “Amaranth: Overview.” https://www.cabdirect.org/cabdirect/abstract/20093327182.
- Corke, H., H. Wu, S. Yue, and H. Sun. 1997. Developing Specialty Starches from New Crops: A Case Study Using Grain Amaranth. In Cereals: Novel Uses and Processes, edited by G. M. Campbell, C. Webb, and S. L. McKee, 91–102. New York: Plenum Press. https://doi.org/10.1007/978-1-4757-2675-6_12.
- Dabija, A., M. E. Ciocan, A. Chetrariu, and G. G. Codină. 2022. “Buckwheat and Amaranth as Raw Materials for Brewing, a Review.” Plants 11 (6): 756. https://doi.org/10.3390/plants11060756.
- Drannikov, A., A. Derkanosova, A. Korotaeva, A. Orinicheva, A. Y. Iskusnykh, and E. Litvinov. 2021. “Phytobiotics as an Alternative to Antibiotics in Feeding Farm Birds.” In International Conference on Production and Processing of Agricultural Raw Materials (P2ARM 2021), Voronezh, Russia, September 21–24, 2021. Vol. 1052. IOP Conference Series, Earth and Environment.
- Duke, J. A. 1999. The Green Pharmacy: New Discoveries in Herbal Remedies for Common Diseases and Conditions from the World’s Foremost Authority on Healing Herbs. Ammaeus, PA: Rodale Books.
- EFSA (European Food Safety Authority). Panel on Dietetic Products, Nutrition and Allergies. 2014. “Scientific Opinion on Dietary Reference Values for Folate.” The EFSA Journal 12 (11): 3893. https://doi.org/10.2903/j.efsa.2014.3893.
- Elkin, R. G. 2007. “Reducing Shell Egg Cholesterol Content. II. Review of Approaches Utilizing Non-Nutritive Dietary Factors or Pharmacological Agents and an Examination of Emerging Strategies.” World’s Poultry Science Journal 63:5–32. https://doi.org/10.1017/S0043933907001249.
- Escudero, N., M. De Arellano, J. Luco, M. Giménez, and S. Mucciarelli. 2004. “Comparison of the Chemical Composition and Nutritional Value of Amaranthus Cruentus Flour and Its Protein Concentrate.” Plant Foods for Human Nutrition 59 (1): 15–21. https://doi.org/10.1007/s11130-004-0033-3.
- FAO (Food and Agriculture Organisation) 1990. “Protein Quality Evaluation: Report of a Joint FAO/WHO Expert Consultation.” Organizacion de las Naciones Unidas para la Agricultura y la Alimentacion.
- FAO (Food and Agriculture Organisation). 2018. Food Outlook: Biannual Report on Global Food Markets. FAO Rome. https://doi.org/10.4060/cc2864en.
- Fatima, Z., M. Ahmed, M. Hussain, G. Abbas, S. Ul-Allah, S. Ahmad, N. Ahmed, M. A. Ali, G. Sarwar, and E. U. Haque. 2020. “The Fingerprints of Climate Warming on Cereal Crops Phenology and Adaptation Options.” Scientific Reports 10 (1): 18013. https://doi.org/10.1038/s41598-020-74740-3.
- Fortune Business Insights. 2018. “Amaranth Market Size, Industry Share.” Accessed December 8, 2023. www.fortunebusinessinsights.com/amaranth-market-103213.
- Fortune Business Insights. 2021. “Amaranth Market Size, Industry Share Forecast 2030.” Accessed December 8, 2023. www.fortunebusinessinsights.com/amaranth-market-103213.
- Foucault, A. S., V. Mathé, R. Lafont, P. Even, W. Dioh, S. Veillet, D. Tomé, J. F. Huneau, D. Hermier, and A. Quignard‐Boulangé. 2012. “Quinoa Extract Enriched in 20‐Hydroxyecdysone Protects Mice from Diet‐Induced Obesity and Modulates Adipokines Expression.” Obesity 20 (2): 270–277. https://doi.org/10.1038/oby.2011.257.
- Gamel, T. H., J. P. Linssen, A. S. Mesallam, A. A. Damir, and L. A. Shekib. 2006. “Effect of Seed Treatments on the Chemical Composition of Two Amaranth Species: Oil, Sugars, Fibres, Minerals and Vitamins.” Journal of the Science of Food and Agriculture 86 (1): 82–89. https://doi.org/10.1002/jsfa.2318.
- Gamel, T. H., A. S. Mesallam, A. A. Damir, L. A. Shekib, and J. P. Linssen. 2007. “Characterization of Amaranth Seed Oils.” Journal of Food Lipids 14 (3): 323–334. https://doi.org/10.1111/j.1745-4522.2007.00089.x.
- Hood, R. L. 1998. “Tocotrienols in Metabolism.” In Phytochemicals: A New Paradigm, edited by W. R. Bidlack, S. T. Omaye, M. S. Meskin, and D. K. W. Topham, 33–47. Boca Raton, FL: CRC Press.
- Hosseintabar-Ghasemabad, B., H. Janmohammadi, A. Hosseinkhani, S. Alijani, and M. Oliyai. 2020. “Determination of Chemical Composition and Apparent Metabolizable Energy Corrected for Nitrogen (AMEn) Content of Amaranth Grain with and without Enzyme in Adult Leghorn Roosters by Regression Method.” Iranian Journal of Applied Animal Science 10 (4): 705–716.
- Hosseintabar-Ghasemabad, B., H. Janmohammadi, A. Hosseinkhani, S. Amirdahri, P. Baghban-Kanani, I. F. Gorlov, M. I. Slozhenkina, A. A. Mosolov, L. S. Ramirez, and A. Seidavi. 2022. “Effects of Using Processed Amaranth Grain with and without Enzyme on Performance, Egg Quality, Antioxidant Status and Lipid Profile of Blood and Yolk Cholesterol in Laying Hens.” Animals 12 (22): 3123. https://doi.org/10.3390/ani12223123.
- “Iowa State University, Extension and Outreach, Alternatives, Amaranth.” n.d. Accessed December 8, 2023. www.extension.iastate.edu/alternativeag/amaranth.
- Jacob, J., S. Noll, and J. Brannon. 2008. “Comparison of Metabolic Energy Content of Organic Cereal Grains for Chickens and Turkeys.” Journal of Applied Poultry Research 17 (4): 540–544. https://doi.org/10.3382/japr.2008-00026.
- Jakubowska, M., J. Gardzielewska, Z. Tarasewicz, D. Szczerbinska, T. Karamucki, K. Rybak, E. Polawska, and J. Garczewska. 2013. “The Effect of Amaranth Seed Added to the Standard Diet Upon Selected Meat Quality Traits in the Quail.” Animal Science Papers and Reports 31 (4): 355–362.
- Jamalluddin, N., F. J. Massawe, S. Mayes, W. K. Ho, A. Singh, and R. C. Symonds. 2021. “Physiological Screening for Drought Tolerance Traits in Vegetable Amaranth (Amaranthus tricolor) Germplasm.” Agriculture 11 (10): 994. https://doi.org/10.3390/agriculture11100994.
- Janmohammadi, H., B. Hosseintabar-Ghasemabad, S. Amirdahri, I. F. Gorlov, K. E. Vladimirovna, M. I. Slozhenkina, R. M. Bilal, A. Seidavi, and C. J. C. Phillips. 2022. “The Energy Value for Broiler Chickens of Heat-Treated and Untreated Amaranth Grain, with and without Enzyme Addition.” Agriculture 12 (11): 1810. https://doi.org/10.3390/agriculture12111810.
- Janmohammadi, H., B. Hosseintabar-Ghasemabad, M. Oliyai, S. Alijani, I. F. Gorlov, M. I. Slozhenkina, A. A. Mosolov, L. Suarez Ramirez, A. Seidavi, and V. Laudadio. 2023. “Effect of Dietary Amaranth (Amaranthus hybridus chlorostachys) Supplemented with Enzyme Blend on Egg Quality, Serum Biochemistry and Antioxidant Status in Laying Hens.” Antioxidants 12 (2): 456. https://doi.org/10.3390/antiox12020456.
- Jimoh, M. O., K. Okaiyeto, O. O. Oguntibeju, and C. P. Laubscher. 2022. “A Systematic Review on Amaranthus-Related Research.” Horticulturae 8 (3): 239. https://doi.org/10.3390/horticulturae8030239.
- Joana Gil‐Chávez, G., J. A. Villa, J. Fernando Ayala‐Zavala, J. Basilio Heredia, D. Sepulveda, E. M. Yahia, and G. A. González‐Aguilar. 2013. “Technologies for Extraction and Production of Bioactive Compounds to Be Used as Nutraceuticals and Food Ingredients: An Overview.” Comprehensive Reviews in Food Science and Food Safety 12 (1): 5–23. https://doi.org/10.1111/1541-4337.12005.
- Junkuszew, M., W. Oleszek, M. Jurzysta, S. Piancente, and C. Pizza. 1998. “Triterpenoid Saponins from the Seeds of Amaranthus Cruentus.” Phytochemistry 49 (1): 195–198. https://doi.org/10.1016/S0031-9422(97)00904-7.
- Kabuage, L., P. Mbugua, B. Mitaru, T. Ngatia, and K. Schafer. 2002. “Effect of Fortifying Amaranth Diets with Amino Acids, Casein and Ethylene Diamine Tetra Acetate on Broiler Performance, Amino Acid Availability and Mineral Utilisation.” South African Journal of Animal Science 32 (2): 144–153. https://doi.org/10.4314/sajas.v32i2.3757.
- Karaeva, J., S. Timofeeva, S. Islamova, K. Bulygina, F. Aliev, V. Panchenko, and V. Bolshev. 2023. “Pyrolysis of Amaranth Inflorescence Wastes: Bioenergy Potential, Biochar and Hydrocarbon Rich Bio-Oil Production.” Agriculture 13 (2): 260. https://doi.org/10.3390/agriculture13020260.
- Karamać, M., F. Gai, E. Longato, G. Meineri, M. A. Janiak, R. Amarowicz, and P. G. Peiretti. 2019. “Antioxidant Activity and Phenolic Composition of Amaranth (Amaranthus caudatus) During Plant Growth.” Antioxidants 8 (6): 173. https://doi.org/10.3390/antiox8060173.
- Khiroug, S., A. Vishtakaliuk, A. Lapin, N. Sosnina, V. Koksin, A. Konovalov, and U. Barbeau. 2001. “Availability and Prospective Usage of Amaranth in the Production of Low Cholesterol Foodstuff for Aviculture.” Chemistry and Computational Simulation Butlerov Communications 2:17–20.
- Kianfar, R., A. R. Di Rosa, N. Divari, H. Janmohammadi, B. Hosseintabar-Ghasemabad, M. Oteri, I. F. Gorlov, M. I. Slozhenkina, A. A. Mosolov, and A. Seidavi. 2023. “A Comparison of the Effects of Raw and Processed Amaranth Grain on Laying Hens’ Performance, Egg Physicochemical Properties, Blood Biochemistry and Egg Fatty Acids.” Animals 13 (8): 1394. https://doi.org/10.3390/ani13081394.
- Klimczak, I., M. Małecka, and B. Pachołek. 2002. “Antioxidant Activity of Ethanolic Extracts of Amaranth Seeds.” Food/nahrung 46 (3): 184–186. https://doi.org/10.1002/1521-3803(20020501)46:3<184:AID-FOOD184>3.0.CO;2-H.
- Kozioł, M. J. 1992. “Chemical Composition and Nutritional Evaluation of Quinoa (Chenopodium quinoa Willd.).” Journal of Food Composition and Analysis 5 (1): 35–68. https://doi.org/10.1016/0889-1575(92)90006-6.
- Króliczewska, B., W. Zawadzki, A. Bartkowiak, and T. Skiba. 2008. “The Level of Selected Blood Indicators of Laying Hens Fed with Addition of Amaranth Grain.” Electronic Journal of Polish Agricultural Universities 11 (2): #18. http://www.ejpau.media.pl/volume11/issue2/art-18.html.
- Kuljanabhagavad, T., and M. Wink. 2009. “Biological Activities and Chemistry of Saponins from Chenopodium Quinoa Willd.” Phytochemistry Reviews 8 (2): 473–490. https://doi.org/10.1007/s11101-009-9121-0.
- Laovoravit, N. 1982. “The Nutritional Value of Amaranth for Feeding Chickens.” M.S. Thesis,University of California, Davis. https://doi.org/10.3382/ps.0651365.
- Laovoravit, N., F. Kratzer, and R. Becker. 1986. “The Nutritional Value of Amaranth for Feeding Chickens.” Poultry Science 65 (7): 1365–1370. https://doi.org/10.3382/ps.0651365.
- Lehmann, J. W., D. H. Putnam, and A. A. Qureshi. 1994. “Vitamin E Isomers in Grain Amaranths (Amaranthus Spp.).” Lipids 29 (3): 177–181. https://doi.org/10.1007/BF02536726.
- León-Camacho, M., D. L. García-González, and R. Aparicio. 2001. “A Detailed and Comprehensive Study of Amaranth (Amaranthus Cruentus L.) Oil Fatty Profile.” European Food Research and Technology 213 (4–5): 349–355. https://doi.org/10.1007/s002170100340.
- Longato, E., M. Georgia, and P. G. Peiretti. 2017. “Effect of Amaranthus Caudatus Supplementation to Diets Containing Linseed Oil on Oxidative Status, Blood Serum Metabolites, Growth Performance and Meat Quality Characteristics in Broilers.” Animal Science Papers and Reports 35:71–86. https://doi.org/10.1007/s11947-017-1978-0.
- Lorenz, K., and B. Wright. 1984. “Phytate and Tannin Content of Amaranth.” Food Chemistry 14 (1): 27–34. https://doi.org/10.1016/0308-8146(84)90015-3.
- Manyelo, T. G., N. A. Sebola, Z. M. Hassan, J. W. Ng’ambi, W. J. Weeks, and M. Mabelebele. 2022. “Chemical Composition and Metabolomic Analysis of Amaranthus Cruentus Grains Harvested at Different Stages.” Molecules 27 (3): 623. https://doi.org/10.3390/molecules27030623.
- Manyelo, T. G., N. A. Sebola, and M. Mabelebele. 2022. “Effect of Amaranth Leaf Meal on Performance, Meat, and Bone Characteristics of Ross 308 Broiler Chickens.” PLoS One 17 (8): e0271903. https://doi.org/10.1371/journal.pone.0271903.
- Manyelo, T. G., N. A. Sebola, E. J. van Rensburg, and M. Mabelebele. 2020. “The Probable Use of Genus Amaranthus as Feed Material for Monogastric Animals.” Animals 10 (9): 1504. https://doi.org/10.3390/ani10091504.
- Marcone, M. F., Y. Kakuda, and R. Y. Yada. 2003. “Amaranth as a Rich Dietary Source of β-Sitosterol and Other Phytosterols.” Plant Foods for Human Nutrition 58 (3): 207–211. https://doi.org/10.1023/B:QUAL.0000040334.99070.3e.
- Mendonça, S., P. H. Saldiva, R. J. Cruz, and J. A. Arêas. 2009. “Amaranth Protein Presents Cholesterol-Lowering Effect.” Food Chemistry 116 (3): 738–742. https://doi.org/10.1016/j.foodchem.2009.03.021.
- Mine, Y. 2008. Egg Bioscience and Biotechnology. New York, USA: John Wiley and Sons.
- Mochan, K. 2017. “Amaranth Trials in Australia to Replace Traditional Stockfeed of Wheat, Oats and Corn.” ABC Country Hour. https://www.abc.net.au/news/rural/2017-10-10/amaranth-trials-in-australia-to-replace-stockfeed/9023124?utm_campaign=abc_news_web&utm_content=link&utm_medium=content_shared&utm_source=abc_news_web.
- Motta, C., I. Delgado, A. S. Matos, G. B. Gonzales, D. Torres, M. Santos, M. V. Chandra-Hioe, J. Arcot, and I. Castanheira. 2017. “Folates in Quinoa (Chenopodium quinoa), Amaranth (Amaranthus sp) and Buckwheat (Fagopyrum esculentum): Influence of Cooking and Malting.” Journal of Food Composition & Analysis 64:181–187. https://doi.org/10.1016/j.jfca.2017.09.003.
- Mustafa, A. F., P. Seguin, and B. Gélinas. 2011. “Chemical Composition, Dietary Fibre, Tannins and Minerals of Grain Amaranth Genotypes.” International Journal of Food Sciences and Nutrition 62 (7): 750–754. https://doi.org/10.3109/09637486.2011.575770.
- Nazeer, S., and S. Y. Firincioglu. 2022. “Amaranth in Animal Nutrition.” Journal of Agriculture, Food, Environment and Animal Sciences 3 (2): 195–211.
- NRC (National Research Council). 1994. Nutrient Requirements of Poultry: 1994. 9th Revised Edition. Washington, DC: National Academies Press.
- Nwadinigwe, A., C. Amadi, A. Amujiri, C. Nwadinigwe, and C. Ozokolie. 2019. “Effects of Pharmaceutical Effluents on Germination, Growth and Development of Amaranthus hybridus L.” Nordic Journal of Botany 32 (2). www.researchgate.net/publication/362388500_EFFECTS_OF_PHARMACEUTICAL_EFFLUENTS_ON_GERMINATION_GROWTH_AND_DEVELOPMENT_OF_AMARANTHUS_HYBRIDUS_L_Nwadinigwe#fullTextFileContent.
- Oleszek, W., M. Junkuszew, and A. Stochmal. 1999. “Determination and Toxicity of Saponins from Amaranthus Cruentus Seeds.” Journal of Agricultural and Food Chemistry 47 (9): 3685–3687. https://doi.org/10.1021/jf990182k.
- Opute, F. I. 1979. “Seed Lipids of the Grain Amaranths.” Journal of Experimental Botany 30 (3): 601–606. https://doi.org/10.1093/jxb/30.3.601.
- Orczewska-Dudek, S., M. Pietras, and J. Nowak. 2018. “The Effect of Amaranth Seeds, Sea Buckthorn Pomace and Black Chokeberry Pomace in Feed Mixtures for Broiler Chickens on Productive Performance, Carcass Characteristics and Selected Indicators of Meat Quality.” Annals of Animal Science 18 (2): 501. https://doi.org/10.2478/aoas-2018-0002.
- Orozco-Martínez, R., E. Del-Val, R. Lindig-Cisneros, H. Paz, M. Quesada, and E. de la Barrera. 2012. “Evaluation of Three Organic Fertilizers for Growing the Widely Cultivated Crop Cucurbita pepo L.” African Journal of Agricultural Research 7 (7): 1087–1097. https://doi.org/10.5897/AJAR11.1166.
- Oteri, M., F. Gresta, A. Costale, V. Lo Presti, G. Meineri, and B. Chiofalo. 2021. “Amaranthus Hypochondriacus L. as a Sustainable Source of Nutrients and Bioactive Compounds for Animal Feeding.” Antioxidants 10 (6): 876. https://doi.org/10.3390/antiox10060876.
- Palombini, S. V., T. Claus, S. A. Maruyama, A. K. Gohara, A. H. P. Souza, N. E. D. Souza, J. V. Visentainer, S. T. M. Gomes, and M. Matsushita. 2013. “Evaluation of Nutritional Compounds in New Amaranth and Quinoa Cultivars.” Food Science & Technology 33 (2): 339–344. https://doi.org/10.1590/S0101-20612013005000051.
- Park, S.-J., A. Sharma, and H.-J. Lee. 2020. “A Review of Recent Studies on the Antioxidant Activities of a Third-Millennium Food: Amaranthus Spp.” Antioxidants 9 (12): 1236. https://doi.org/10.3390/antiox9121236.
- Pedersen, B., L. Kalinowski, and B. Eggum. 1987. “The Nutritive Value of Amaranth Grain (Amaranthus caudatus) 1. Protein and Minerals of Raw and Processed Grain.” Plant Foods for Human Nutrition 36 (4): 309–324. https://doi.org/10.1007/BF01892352.
- Pedersen, B., K. B. Knudsen, and B. Eggum. 1990. “The Nutritive Value of Amaranth Grain (Amaranthus caudatus) 3. Energy and Fibre of Raw and Processed Grain.” Plant Foods for Human Nutrition 40 (1): 61–71. https://doi.org/10.1007/BF02193780.
- Peiretti, P. 2018. “Amaranth in Animal Nutrition: A Review.” Livestock Research for Rural Development 30 (5).
- Peiretti, P. G., G. Meineri, F. Gai, E. Longato, and R. Amarowicz. 2017. “Antioxidative Activities and Phenolic Compounds of Pumpkin (Cucurbita Pepo) Seeds and Amaranth (Amaranthus caudatus) Grain Extracts.” Natural Product Research 31 (18): 2178–2182. https://doi.org/10.1080/14786419.2017.1278597.
- Písaříková, B., S. Kráčmar, and I. Herzig. 2005. “Amino Acid Contents and Biological Value of Protein in Various Amaranth Species.” Czech Journal of Animal Science 50 (4): 169–174. https://doi.org/10.17221/4011-CJAS.
- Pisarikova, B., Z. Zraly, S. Kracmar, M. Trckova, and I. Herzig. 2006. “The Use of Amaranth (Genus Amaranthus L.) in the Diets for Broiler Chickens.” Veterinární Medicína 51 (7): 399. https://doi.org/10.17221/5560-VETMED.
- Pond, W., J. Lehmann, and R. Clark. 1989. “Utilization of Four Cultivars of Grain Amaranth for Growth in Rats.” Nutrition Reports International 39:1081–1089.
- Popiela, E., B. Króliczewska, W. Zawadzki, S. Opaliński, and T. Skiba. 2013. “Effect of Extruded Amaranth Grains on Performance, Egg Traits, Fatty Acids Composition, and Selected Blood Characteristics of Laying Hens.” Livestock Science 155:308–315. https://doi.org/10.1016/j.livsci.2013.05.001.
- Procopet, O., and M. Oroian. 2022. “Amaranth Seed Polyphenol, Fatty Acid and Amino Acid Profile.” Applied Sciences 12 (4): 2181. https://doi.org/10.3390/app12042181.
- Punita, A., and A. Chaturvedi. 2000. “Effect of Feeding Crude Red Palm Oil (Elaeis Guineensis) and Grain Amaranth (Amaranthus paniculatus) to Hens on Total Lipids, Cholesterol, PUFA Levels and Acceptability of Eggs.” Plant Foods for Human Nutrition 55 (2): 147–157. https://doi.org/10.1023/A:1008197931085.
- Qureshi, A. A., J. W. Lehmann, and D. M. Peterson. 1996. “Amaranth and Its Oil Inhibit Cholesterol Biosynthesis in 6-Week-Old Female Chickens.” The Journal of Nutrition 126 (8): 1972–1978. https://doi.org/10.1093/jn/126.8.1972.
- Ravindran, V., R. Hood, R. Gill, C. Kneale, and W. Bryden. 1996. “Nutritional Evaluation of Grain Amaranth (Amaranthus Hypochondriacus) in Broiler Diets.” Animal Feed Science and Technology 63 (1–4): 323–331. https://doi.org/10.1016/S0377-8401(96)00997-2.
- Raw, P. 2012. “Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference, Release 25.” United States Department of Agriculture (USDA).
- Reklewska, B., T. Nalecz-Tarwacka, J. Niemiec, and A. Karaszewska. 1995. “Yolk Triacylglycerol Composition and Egg Quality Following the Diet Containing Amaranth Meal.” Animal Science Papers and Reports 13 (2–3): 73–80.
- Repo-Carrasco-Valencia, R., J. K. Hellström, J.-M. Pihlava, and P. H. Mattila. 2010. “Flavonoids and Other Phenolic Compounds in Andean Indigenous Grains: Quinoa (Chenopodium quinoa), Kañiwa (Chenopodium pallidicaule) and Kiwicha (Amaranthus caudatus).” Food Chemistry 120 (1): 128–133. https://doi.org/10.1016/j.foodchem.2009.09.087.
- Rodríguez-Ríos, H., J. Campos-Parra, R. Astudillo-Neira, J. Grande-Cano, S. Carrillo-Domínguez, and F. Pérez Gil-Romo. 2020. “Amaranthus Cruentus L. Como Alternativa Alimentaria en Gallinas Ponedoras Para Disminuir el Colesterol en Huevos.” Chilean Journal of Agricultural & Animal Sciences 36 (1): 78–85. https://doi.org/10.29393/chjaas36-5d20005.
- Rostagno, H., L. Albino, J. Donzele, P. Gomes, R. Oliveira, D. Lopes, A. Ferreira, S. T. Barreto, and R. Euclides. 2011. Brazilian Tables for Poultry and Swine: Composition of Feedstuffs and Nutritional Requirements. Viçosa, MG, Brazil: Animal Science Department UFV.
- Roučková, J., M. Trčková, and I. Herzig. 2004. “The Use of Amaranth Grain in Diets for Broiler Chickens and Its Effect on Performance and Selected Biochemical Indicators.” Czech Journal of Animal Science 49 (12): 532–541. https://doi.org/10.17221/4341-CJAS.
- Salazar-Vega, M. I., J. G. Rosado-Rubio, L. A. Chel-Guerrero, D. A. Betancur-Ancona, and A. F. Castellanos-Ruelas. 2009. “Composición en Acido Graso Alfa Linolénico (w3) en Huevo y Carne de Aves Empleando Chia (Salvia hispánica L.) en el Alimento.” Interciencia 34:209–213.
- Santiago, P., K. Tenbergen, E. Vélez-Jiménez, and M. Cardador-Martínez. 2014. “Role of Cellular Magnesium in Human Diseases.” Austin Journal of Nutrition and Food Sciences 2 (10): 1010.
- Sarker, U., S. Oba, W. F. Alsanie, and A. Gaber. 2022. “Characterization of Phytochemicals, Nutrients, and Antiradical Potential in Slim Amaranth.” Antioxidants 11 (6): 1089. https://doi.org/10.3390/antiox11061089.
- Sarker, U., S. Oba, S. Ercisli, A. Assouguem, A. Alotaibi, and R. Ullah. 2022. “Bioactive Phytochemicals and Quenching Activity of Radicals in Selected Drought-Resistant Amaranthus Tricolor Vegetable Amaranth.” Antioxidants 11 (3): 578. https://doi.org/10.3390/antiox11030578.
- Saunders, R., and R. Becker. 1983. “Amaranthus: A Potential Food and Feed Resource.” In Advances in Cereal Science and Technology, edited by Y. Pomeranz, 357–396. Vol. VI.
- Sayed-Ahmad, B., M. Urrutigoïty, A. Hijazi, Z. Saad, M. Cerny, P. Evon, T. Talou, and O. Merah. 2022. “Amaranth Oilseed Composition and Cosmetic Applications.” Separations 9 (7): 181. https://doi.org/10.3390/separations9070181.
- Schmidt, D., M. R. Verruma-Bernardi, V. A. Forti, and M. T. M. R. Borges. 2023. “Quinoa and Amaranth as Functional Foods: A Review.” Food Reviews International 39 (4): 2277–2296. https://doi.org/10.1080/87559129.2021.1950175.
- Schnetzler, K. A., and W. Breene. 1994. “Food Uses and Amaranth Product Research: A Comprehensive Review.” In Amaranth Biology, Chemistry, and Technology, edited by K. Schnetzler, 30. Boca Raton, FL: CRC Press. https://doi.org/10.1201/9781351069601-9
- Segura-Nieto, M., A. Barba De La Rosa, and O. Paredes-López. 1994. “Biochemistry of Amaranth Proteins.” In Amaranth Biology, Chemistry, and Technology, edited by K. Schnetzler. Boca Raton, FL: CRC Press.
- Serratos, A. 1996. “Amaranth (Amaranthus hypochondriacus) Grain in Broiler Feeding.” Advances En Investigacion Agropecuaria 5 (3): 46–50.
- Singhal, R., and P. Kulkarni. 1988. “Amaranth Oils: A New Source of Squalene.” Amaranth Newsletter 3:7–8.
- Smolentsev, S., I. Strelnikova, N. Kislitsyna, N. Burova, M. Gugkaeva, A. Kornaeva, N. Shkaeva, and T. Abduramanova 2023. “The Effect of Amaranth on the Meat Productivity of Broiler Chickens.” In Fundamental and Applied Scientific Research in the Development of Agriculture in the Far East (AFE-2022), edited by K. S. Zokirjon Ugli, A. Muratov, and S. Ignateva. AFE 2023. Lecture Notes in Networks and Systems. Cham: Springer. https://doi.org/10.1007/978-3-031-36960-5_9.
- Soares, R. A. M., S. Mendonça, L. Í. A. De Castro, A. C. C. C. C. Menezes, and J. A. G. Arêas. 2015. “Major Peptides from Amaranth (Amaranthus Cruentus) Protein Inhibit HMG-CoA Reductase Activity.” International Journal of Molecular Sciences 16 (2): 4150–4160. https://doi.org/10.3390/ijms16024150.
- Szczerbinska, D., B. Pyka, E. Szabelska, M. Ligocki, D. Majewska, K. Romaniszyn, and M. Sulik. 2015. “The Effect of Diet with Amaranth (Amaranthus Cruentus) Seeds on Japanese Quail (Coturnix coturnix japonica) Performance, Somatic Development, Hatching Results and Selected Blood Biochemical Parameters.” Veterinary Medicine Zootechnics 70:67–72.
- Tang, Y., and R. Tsao. 2017. “Phytochemicals in Quinoa and Amaranth Grains and Their Antioxidant, Anti‐Inflammatory, and Potential Health Beneficial Effects: A Review.” Molecular Nutrition and Food Research 61 (7): 1600767. https://doi.org/10.1002/mnfr.201600767.
- Tang, Y., B. Zhang, X. Li, P. X. Chen, H. Zhang, R. Liu, and R. Tsao. 2016. “Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and Α-Glucosidase and Pancreatic Lipase Inhibitory Effects.” Journal of Agricultural and Food Chemistry 64 (8): 1712–1719. https://doi.org/10.1021/acs.jafc.5b05761.
- Tareh, V., S. Zarringhalami, and A. Ganjloo. 2022. “Optimization of Amaranth Flour, Whey Protein Powder, and Xanthan Gum Levels in the Development of Gluten‐Free Barbari Bread Using Response Surface Methodology.” Journal of Food Processing and Preservation 46 (8): e16787. https://doi.org/10.1111/jfpp.16787.
- Tillman, P., and P. Waldroup. 1986. “Processing Grain Amaranth for Use in Broiler Diets.” Poultry Science 65 (10): 1960–1964. https://doi.org/10.3382/ps.0651960.
- Tillman, P., and P. Waldroup. 1987. “Effects of Feeding Extruded Grain Amaranth to Laying Hens.” Poultry Science 66 (10): 1697–1701. https://doi.org/10.3382/ps.0661697.
- Tillman, P., and P. Waldroup. 1988a. “Assessment of Extruded Grain Amaranth as a Feed Ingredient for Broilers.: 1. Apparent Metabolizable Energy Values.” Poultry Science 67 (4): 641–646. https://doi.org/10.3382/ps.0670641.
- Tillman, P., and P. Waldroup. 1988b. “Assessment of Extruded Grain Amaranth as a Feed Ingredient for Broilers.: 2. Apparent Amino Acid Availability Values.” Poultry Science 67 (4): 647–651. https://doi.org/10.3382/ps.0670647.
- Tillman, P., and P. Waldroup. 1988c. “Performance and Yields of Broilers Fed Extruded Grain Amaranth and Grown to Market Weight.” Poultry Science 67 (5): 743–749. https://doi.org/10.3382/ps.0670743.
- Tosi, E., E. Ré, H. Lucero, and R. Masciarelli. 2001. “Dietary Fiber Obtained from Amaranth (Amaranthus Cruentus) Grain by Differential Milling.” Food Chemistry 73 (4): 441–443. https://doi.org/10.1016/S0308-8146(00)00326-5.
- Tufarelli, V., K. Baghban, Y. Azimi, G. Hosseintabar, M. Slozhenkina, and I. Gorlov. 2021. “Effects of Horsetail (Equisetum arvense) and Spirulina (Sspirulina platensis) Dietary Supplementation on Laying Hens Productivity and Oxidative Status.” Animals 11 (2): 335. https://doi.org/10.3390/ani11020335.
- Tufarelli, V., P. Baghban-Kanani, S. Azimi-Youvalari, B. Hosseintabar-Ghasemabad, M. Slozhenkina, I. Gorlov, F. M. Viktoronova, A. Seidavi, and V. Laudadio. 2022. “Effect of Dietary Flaxseed Meal Supplemented with Dried Tomato and Grape Pomace on Performance Traits and Antioxidant Status of Laying Hens.” Animal Biotechnology 33 (7): 1525–1532. https://doi.org/10.1080/10495398.2021.1914070.
- Vishtakalyuk, A., S. Khirug, M. Ezhkova, A. Lapin, and V. Lisov. 2001. “Amaranth Vitamin-Herbal Flour Ration in Feeding Hens: Perspectives of Their Increased Egg-Laying Productivity.” Chemistry and Computational Simulation Butlerov Communications 2:25–27.
- Waldroup, P., H. Hellwig, D. Longer, and C. Endres. 1985. “The Utilization of Grain Amaranth by Broiler Chickens.” Poultry Science 64 (4): 759–762. https://doi.org/10.3382/ps.0640759.
- Whitton, C., D. Bogueva, D. Marinova, and C. J. Phillips. 2021. “Are We Approaching Peak Meat Consumption? Analysis of Meat Consumption from 2000 to 2019 in 35 Countries and Its Relationship to Gross Domestic Product.” Animals 11 (12): 3466. https://doi.org/10.3390/ani11123466.
- WHO (World Health Organization). 2018. “The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition.” Page:99, Box:14: Enhancing the Contribution of Neglected and Underutilized Species (NUS) to Food Security and Income. Food and Agriculture Org.
- Wirkowska-Wojdyła, M., E. Ostrowska-Ligęza, A. Górska, and J. Bryś. 2022. “Application of Chromatographic and Thermal Methods to Study Fatty Acids Composition and Positional Distribution, Oxidation Kinetic Parameters and Melting Profile as Important Factors Characterizing Amaranth and Quinoa Oils.” Applied Sciences 12 (4): 2166. https://doi.org/10.3390/app12042166.
- Woodhead, S., and T. Swain. 1974. “Effect of Light on Betalain and Cinnamic Acid Biosynthesis in Amaranthus Caudatus.” Phytochemistry 13 (6): 953–956. https://doi.org/10.1016/S0031-9422(00)91428-6.
- Yaghobfar, A. 2001. “Feeding Value of Extruded and Raw Amaranth Grain in Laying Quail Rations.” British Poultry Science 42:S110.
- Yánez, E., I. Zacarías, D. Granger, M. Vásquez, and A. M. Estevez. 1994. “Chemical and Nutritional Characterization of Amaranthus (Amaranthus Cruentus).” Archivos Latinoamericanos de Nutricion 44 (1): 57–62.