?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Kampo medicines are widely used in Japan; however, their potential to cause drug interactions still remains unclear and needs to be further investigated. The effects of goreisan on the P-glycoprotein (P-gp) and the cytochrome P-450 (CYP), which are associated with drug interactions, were investigated.
The inhibitory effect of goreisan extract on P-gp was evaluated using a Caco-2 cell permeability assay. The results indicated that it inhibited P-gp function in a concentration-dependent manner.
The inhibitory effect of three goreisan ingredients (alisol A, tumulosic acid, and (E)-cinnamic acid) on seven CYP isoforms was evaluated using human liver microsomes (HLM). Of these, tumulosic acid and (E)-cinnamic acid exhibited less than 16% inhibition at concentrations of 10 µmol/L against any of the CYP isoforms tested. Alisol A inhibited only CYP3A but showed no inhibitory effect with pre-incubation.
These results indicate that goreisan extract has inhibitory activity against P-gp and that alisol A, a goreisan ingredient, exhibits an inhibitory effect on CYP3A. However, these are thought to be minor or negligible in vivo. Overall, these findings will be useful to evaluate possible drug interactions and provide support for the interpretation of future clinical drug–drug interaction studies involving goreisan.
Introduction
Clinically, multiple drugs are often used in combination to achieve therapeutic goals. Age-related changes in pharmacokinetics/pharmacodynamics and combination drug use are both major factors attributed to the increase in adverse drug reactions, with a 2016 survey reporting that 40% of elderly people (age ≥ 75) were prescribed five or more drugs at a time in Japan (Ministry of Health, Labour and Welfare, MHLW Citation2018a). Prescription drugs are required to be evaluated for their potential for drug–drug interactions (DDIs) to ensure safety, as DDIs may cause serious side effects or reduce therapeutic efficacy of the individual drugs administered when taken together. The pharmacokinetic DDIs for new drugs are generally evaluated by measuring the potential inhibition or induction of drug candidates on drug transporters and metabolic enzymes in vitro and/or in clinical studies.
Traditional Japanese herbal medicine (Kampo) consists of mixtures of extracts from several crude drugs; extract granules are manufactured by spray-drying decoctions and are mainly used in clinical practice. Kampo medicines are frequently used in combination with various drugs, so understanding the DDI liabilities associated with Kampo medicines will help inform practitioners and patients on their concomitant use with other drugs and will help ensure that these combination treatments are safe and efficacious.
To date, several studies have reported on the DDIs of crude drugs in combination with Kampo medicines. Naringin, a crude drug ingredient, was shown to alter the plasma concentration of co-administered drugs by inhibiting a major drug-metabolizing enzyme, cytochrome P-450 (CYP) 3A4, and organic anion-transporting polypeptide (OATP) 1A2 (Bailey et al. Citation2007; Choi and Kang Citation2008). Furanocoumarins extracted from herbal drugs of the Rutaceae and Umbelliferon families have been reported to inhibit CYP3A4 and P-gp (Iwanaga et al. Citation2010). In addition, studies have shown that yokukansan, rikkunshito, goshajinkigan, and three other Kampo medicines used for gynecological treatments show low potential for serious DDI via CYPs (Ito et al. Citation2008; Ni et al. Citation2018; Nakayama et al. Citation2022) or P-gp (Ito et al. Citation2008; Matsumoto et al. Citation2018). On the other hand, studies have suggested the possibility of DDIs for 50 Kampo medicines containing liquorice root that were reported to inhibit P-gp, which was correlated to the content of the liquorice root extract (Satoh et al. Citation2009), or that P-gp inhibition was observed in daiokanzoto and is correlated to its extraction from rhubarb (Watanabe et al. Citation2012).
Goreisan is a MHLW-approved traditional Japanese Kampo medicine that is used to treat oedema, diarrhoea, vomiting, and dizziness (see the Materials and Methods section for the composition of goreisan). Its effects on the homoeostatic regulation of body fluids have been well documented (Ohnishi et al. Citation2000; Ahn et al. Citation2012; Yano et al. Citation2017; Nakano et al. Citation2018) and it has been successful in treating a wide range of maladies, including chronic subdural haematoma (Katayama et al. Citation2018), lymphedema (Komiyama et al. Citation2015), traumatic cerebral oedema (Yasunaga Citation2015), and oedema resulting from heart failure (Yaku et al. Citation2021). Therefore, it has been suggested for use in combination with various Western medicines. A previous study reported on the effect of goreisan on P-gp and used paclitaxel-resistant HuH-7 (HuH-7/PTX) cells and reported that goreisan and its ingredients, alisols, overcame paclitaxel drug resistance by inhibiting P-gp and its ability to export drugs (Hyuga et al. Citation2012). Although this paper suggests that goreisan may be a P-gp inhibitor, the potential of goreisan to cause DDIs with P-gp substrate drugs in various therapeutic areas of clinical practice remains unclear. The effect of goreisan on the metabolism of the various combination drugs has also not been determined.
The aim of this study is to evaluate the P-gp- and CYPs-mediated DDI potential of goreisan in clinical use according to the regulatory guidelines for DDI evaluation (MHLW Citation2018b; US Food Drug Administration Citation2020, FDA). Following the oral administration of Kampo medicines, some extract ingredients may act on transporters expressed on the luminal side of the gastrointestinal tract, regardless of whether they are absorbed, and may cause P-gp-mediated DDIs with the concomitant drugs. In contrast, some of the ingredients transferred into systemic circulation have the potential to cause DDIs by inhibiting hepatic enzymes. In this study, we investigated the P-gp inhibitory effect of goreisan in Caco-2 cell monolayers using a bi-directional permeability assay. In addition, the three ingredients that have been detected in plasma after oral administration of goreisan to rats (Hida et al. Citation2021) were evaluated in this study for their CYP inhibitory effects on CYP isoforms (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A) using HLMs.
Materials and methods
Test substances and reagents
To assess the inhibitory effect of goreisan extract on drug transporters, extract powder (lot no. 2200017010), the base powder of goreisan without excipients, was obtained from Tsumura & Co. (Tokyo, Japan). It is prepared by spray-drying a hot water extract of a mixture of the following five crude drugs: Alismatis Tuber (the tuber of Alisma orientale Juzepczuk, 27.6%), Atractylodis Lanceae Rhizoma (the roots of Atractylodes lancea De Candolle, Atractylodes chinensis Koidzumi, 20.7%), Polyporus (the sclerotium of Polyporus umbellatus Fries, 20.7%), Poria (the sclerotium of Wolfiporia cocos Ryvarden et Gilbertson, 20.7%), and Cinnamomi Cortex (the bark or periderm of Cinnamomum cassia Blume, 10.3%). The drug was manufactured according to Good Manufacturing Practices as defined by the MHLW. [3H]-Digoxin (969.4 GBq/mmol) and [3H]-mannitol (588 GBq/mmol) were obtained from Perkin-Elmer, Inc. (Waltham, MA, USA). Digoxin was purchased from Alfa Aesar (Ward Hill, MA, USA). Mannitol was purchased from Fujifilm Wako Pure Chemical Industries (Osaka, Japan). Verapamil hydrochloride was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Foetal bovine serum, nonessential amino acids, and a mixed solution of penicillin, streptomycin, and glutamine used for Caco-2 cell culture were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
To assess the inhibitory effect of goreisan ingredients on CYP isoforms, (E)-cinnamic acid and alisol A were obtained from Fujifilm Wako Pure Chemical Industries and tumulosic acid was purchased from Biosynth Carbosynth (Compton, UK). Standard probe substrates for each CYP isoform and each substrate-derived metabolite were obtained from various companies: acetaminophen, diclofenac sodium salt, 4′-hydroxydiclofenac, 1′-hydroxybufuralol maleate, testosterone, and 6β-hydroxytestosterone were obtained from Sigma-Aldrich (St. Louis, MO, USA); bupropion hydrochloride, hydroxybupropion, (S)-mephenytoin, and 4′-hydroxymephenytoin from Toronto Research Chemicals (Toronto, ON, Canada); paclitaxel and midazolam from Fujifilm Wako Pure Chemical Industries; and 6α-hydroxypaclitaxel and 1′-hydroxymidazolam from Cayman Chemical (Ann Arbour, MI, USA). Phenacetin and bufuralol hydrochloride were obtained from Tokyo Chemical Industry (Tokyo, Japan) and Sumika Chemical Analysis Service (Tokyo, Japan), respectively. Pooled HLMs (pool of 50 human donors; lot no. PL050E-C) and β-nicotinamide adenine dinucleotide phosphate (reduced form) tetrasodium salt (β-NADPH) were obtained from Thermo Fisher Scientific and Oriental Yeast (Tokyo, Japan), respectively. All other chemicals were purchased at chemical grade and used as is.
Studies of the inhibitory effect of goreisan extract on P-gp
Caco-2 cell culture
Caco-2 cells obtained from the American Type Culture Collection (Manassas, VA, USA) were routinely cultured in Dulbecco’s Modified Eagle Medium, high glucose (Fujifilm Wako Pure Chemical Industries), containing 10% foetal bovine serum, 0.1 mol/L nonessential amino acids, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 g/mL streptomycin, and incubated at 37 °C in an atmosphere of 5% CO2. The cells used in this study were less than 60 passages.
Effect of goreisan on digoxin transport across a caco-2 cell monolayer
Caco-2 cells (2.7 × 105 cells/insert) were seeded into a transwell insert (0.4 μm pore size; polyethylene terephthalate; Corning, NY, USA) in a 24-well tissue culture plate. After culturing for 20 days, PBS in the apical chamber and the medium in the basolateral chamber were replaced with HEPES-buffered HBSS (HBSS-HEPES, pH 7.4).
Transepithelial electrical resistance (TEER) was used to evaluate the integrity of the cell monolayer, which was measured using a Millicell ER-2 (Millipore, Billerica, MA, USA). For further verification of the tight junctions in the monolayer, warm HBSS-HEPES buffer containing mannitol (10 μmol/L, 0.037 GBq/L) was loaded into the apical chambers, and the monolayer permeability of mannitol was measured using the same procedure as that of the test substances. The integrity of the cell monolayer was confirmed by measuring the TEER, and mannitol permeability was used for evaluating the test substance.
The effect of goreisan extract on the permeability of digoxin through the cell monolayer was performed as previously described with minor modifications (Matsumoto et al. Citation2018). Briefly, 37 °C HBSS-HEPES buffer containing digoxin (10 µmol/L, 0.030 GBq/L) was added to either the apical or basolateral chamber in the absence or presence of various concentrations of goreisan extract with or without the P-gp inhibitor verapamil (50 µmol/L). Goreisan extract was prepared in HBSS-HEPES containing DMSO (final concentration of DMSO was ≤1 vol.%). The solution in the apical or basolateral chamber was collected after incubation at 37 °C for 120 min, mixed with scintillation cocktail, and analysed in a liquid scintillation counter (AccuFLEX LSC-7200, Hitachi Aloka Medical, Tokyo, Japan).
Liquid chromatography–tandem mass spectrometry (LC–MS/MS) system to measure CYP metabolites
To quantitate CYP metabolites using LC–MS/MS, 50 μL of methanol and the same volume of internal standard (dissolved in methanol) solution was mixed with the reaction mixture after stopping the reaction with acetonitrile, followed by centrifugation at 1600 × g at 4 °C for 10 min. To prepare a calibration and the quality control (QC) samples, 50 μL of working solution at various concentrations prepared in methanol were mixed with reaction mixture instead of 50 μL of methanol. The supernatant was collected after centrifugation and mixed with water or water plus methanol solution, and then, a 5 μL aliquot was injected into an LC–MS/MS system. The LC–MS/MS system consisted of a triple quadruple MS (TripleQuad5500; SCIEX, Framingham, MA, USA) and a prominence HPLC system (Shimadzu, Kyoto, Japan). The CYP metabolites were separated on a reverse-phase column (InertSustain C18, 3 µm, 2.1 × 50 mm, GL Sciences, Tokyo, Japan) maintained at 40 °C. The mobile phase consisted of solvent A (5 mmol/L ammonium formate) and solvent B (acetonitrile) at a flow rate of 0.25 mL/min. The gradient programs and MS/MS conditions are provided in Supplementary Table 1.
LC–MS/MS method validation
Bioanalytical method validation was done to assess the validity of selectivity, the lower limit of quantification (LLOQ), calibration curve, and accuracy. The selectivity for detection of each CYP metabolite was determined by comparing the chromatograms of the LLOQ samples and the reaction sample containing each goreisan ingredient. As a result, the chromatogram for the reaction sample containing each goreisan ingredient showed no significant interfering peak near the retention time of any analyte. The accuracy of each calibration point for each CYP metabolite was within 100.0 ± 15.0% of the nominal value for all measurements. Furthermore, QC samples were also evaluated to determine whether they met the acceptance criteria, of which the accuracy was within 100.0 ± 15.0% of at least one sample at each concentration (n = 2/each concentration) and four out of six QC samples and consequently satisfied the acceptance criteria for all measurements.
Studies of the inhibitory effect of goreisan ingredients on CYP isoforms
To evaluate the inhibitory effects of the individual goreisan ingredients (alisol A, (E)-cinnamic acid, and tumulosic acid) on the seven CYP isoforms (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A), the production of the metabolite from each probe substrate produced by the reaction in HLMs was measured. Representative inhibitors for each CYP were examined with the goreisan ingredients to verify the reliability of the method. Information on the probe substrates, inhibitors, and CYP metabolites used is summarised in . Goreisan ingredients were dissolved in either acetonitrile/water (1:1, v/v), methanol/water (1:1, v/v), or methanol for alisol A, (E)-cinnamic acid, or tumulosic acid, respectively, at the final concentrations of 0.001, 0.01, 0.1, 1, or 10 µmol/L.
Table 1. The metabolic reaction for each CYP isoform.
Representativeinhibitor
CYP inhibition occurs either as reversible direct inhibition or as irreversible time-dependent inhibition (Pelkonen et al. Citation2008). In the time-dependent inhibition assay, the active intermediate metabolite of the test compound forms an irreversible covalent bond with the enzyme, which hinders the enzyme activity, and the activity is not immediately recovered. Pre-incubation of the enzyme in the presence of NADPH prior to addition of the compound is known to increase the inhibitory effect of the test compound in a time-dependent manner (Perloff et al. Citation2009).
In the CYP direct inhibition assay, each goreisan ingredient at various concentrations or the representative inhibitors and each CYP probe substrate are added to the reaction mixture containing 0.1 mg protein/mL HLMs, 0.05 mmol/L EDTA, and 100 mmol/L Na-K phosphate buffer (pH 7.4). The mixture was pre-warmed at 37 °C for 5 min and then incubated with 50 μL of 5 mmol/L NADPH solution at 37 °C. The incubation time varied between 5–20 minutes for each CYP isoform as shown in . The final solvent concertation of the mixture was ≤1% vol. The reactions were finished by adding 250 μL acetonitrile. In the time-dependent inhibition assay, each goreisan ingredient at various concentrations or representative CYP inhibitors were added to the reaction mixture and were pre-warmed at 37 °C for 5 min, followed by a pre-incubation with NADPH at 37 °C for 30 min. The incubation was then initiated with the addition of the test probe substrates. The final solvent concertation of the mixture was ≤1% vol. Reaction incubation and stopping procedures were then performed under the same conditions as used for the direct inhibition assay.
Data analysis
The apparent permeability coefficient (Papp) was calculated as follows:
where
(nmol/s) was the flux rate, A (cm2) was the effective surface area of the cell monolayer, and C0 (nmol/mL) was the initial drug concentration used in the donor chamber. The efflux ratio was calculated by dividing Papp, BA (mean of n = 3) to Papp, AB (mean of n = 3). The inhibition ratio of P-gp from the goreisan extract was determined as follows:
The IC50 of P-gp inhibition was calculated with a nonlinear least squares method using Phoenix WinNonlin 8.3 software (Certara USA, Inc.) as follows:
where E0 was the inhibitory effect at C = 0.
The metabolic activity of each sample (individual value) was calculated as follows:
where C is the metabolite concentration (nmol/L), P (mg protein/mL) is microsomal protein concentration, and t (min) is incubation time. The remaining activity was calculated as follows:
where As is the metabolic activity of the test substance or representative CYP inhibitor (mean value from n = 2) and Ac is the metabolic activity of the vehicle control (mean value from n = 2). In analyses where the remaining activity in the presence of 10 µmol/L goreisan ingredients was 50.0% or less, the IC50 values were calculated using a nonlinear least squares method using pharmacokinetics analysis software (Phoenix WinNonlin 8.1) as follows:
where E0 was the inhibitory effect at C = 0. In the case of competitive inhibition of the CYP isoforms, the apparent Michaelis constant was calculated as follows:
where [I] is the predicted maximum concentration in the human gastrointestinal tract.
Statistical analysis
The statistical significance of Papp versus control was evaluated with Dunnett’s multiple comparison test using SAS 9.2 software (SAS Institute, Inc.). A value of p < 0.05 was considered significant.
Results
Effect of goreisan extract on digoxin transport across the caco-2 cell monolayer
To confirm the expression and function of P-gp in the cell culture monolayer, the transport of digoxin, a well-established P-gp probe substrate, through the cell monolayer was tested (). The efflux ratio, an index for permeability, was inhibited from 3.09 to 1.07 after co-incubating with verapamil (50 µmol/L), a typical P-gp inhibitor, which indicated that P-gp was expressed in the cell monolayer and was sufficient for evaluating potential P-gp-mediated DDIs.
Table 2. Effect of goreisan extract on apparent permeability (Papp) and efflux ratio of bi-directional [3H]-digoxin (0.030 μmol/L) transport across polarised Caco-2 cell monolayers.
Goreisan extract significantly inhibited the digoxin transport in a basolateral-to-apical (BA) direction in a dose-dependent manner, and the percent of control decreased by 16.8% and 75.8% at 250 and 4000 μg/mL, respectively. The IC50 value of the goreisan extract was calculated using the efflux ratio nonlinear regression analysis and was found to be 2.09 mg/mL ().
Effect of goreisan ingredients on metabolism by CYPs in HLMs
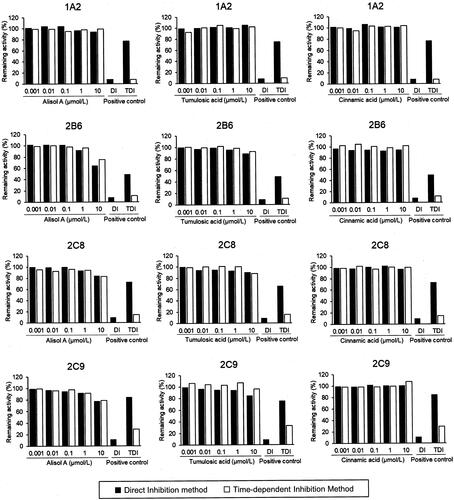
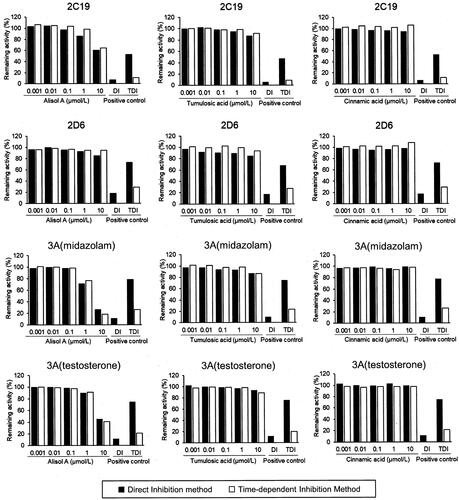
The inhibitory effects by simultaneous treatment with three goreisan ingredients (alisol A, tumulosic acid, and (E)-cinnamic acid) at 0.001–10 µmol/L on seven CYP isoforms, including CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A, were tested (). However, no effect was observed for tumulosic acid and (E)-cinnamic acid, even at the maximum tested concentration of 10 μmol/L. Only alisol A at high concentrations inhibited the metabolic reaction of midazolam and testosterone by CYP3A but did not inhibit the other CYP isoforms (). The IC50 values of alisol A for CYP3A are listed in . Pre-incubation with NADPH had no additional impact on the CYP inhibitory effect of alisol A, tumulosic acid and (E)-cinnamic acid in HLM, indicating the absence of time-dependent inhibition.
Figure 2. Inhibitory effect of alisol A, tumulosic acid, and (E)-cinnamic acid on CYP450 enzyme activity. The remaining activity was calculated from the mean activity of duplicate samples. Positive controls to evaluate direct inhibition included CYP1A2, α-naphthoflavone (0.05 μmol/L); CYP2B6, ticlopidine (3 μmol/L); CYP2C8, montelukast (1 μmol/L); CYP2C9, sulfaphenazole (5 μmol/L); CYP2C19, tranylcypromine (50 μmol/L); CYP2D6, quinidine (5 μmol/L); and CYP3A, ketoconazole (0.2 μmol/L). Positive control to evaluation for time-dependent inhibition: CYP1A2, furafylline (1 μmol/L); CYP2B6, ticlopidine (0.1 μmol/L); CYP2C8, gemfibrozil 1-O-β-glucuronide (10 μmol/L); CYP2C9, tienilic acid (0.3 μmol/L); CYP2C19, S-fluoxetine (10 μmol/L); CYP2D6, paroxetine (0.3 μmol/L); and CYP3A, verapamil (3 μmol/L).
Table 3. IC50 values of alisol A for CYP3A activity in human liver microsomes.
Discussion
Since Caco-2 cells express various cell surface transporters that are commonly expressed in intestinal epithelial cells including P-gp, breast cancer-resistant protein (BCRP), and multidrug resistance-associated protein 2 (MRP2), they are useful in preclinical drug development studies to help evaluate the inhibitory effects of test compounds on transporters and to confirm whether a test compound is a substrate or an inhibitor of P-gp (Anderle et al. Citation1998; Hirohashi et al. Citation2000; Taipalensuu et al. Citation2001). In this study, we examined the inhibitory effects of goreisan extract and its ingredients on and their ability to inhibit the functionality of P-gp and CYP isoforms. Caco-2 cells, a human colon cancer cell line, exhibit a high morphological and functional similarity to human intestinal epithelial cells and are therefore widely used in experiments to predict drug absorption in humans (Yee Citation1997; van Breemen and Li Citation2005). Digoxin, a typical P-gp substrate, exhibited an efflux ratio of 3.09 in Caco-2 cells, which sufficiently indicated the transporter function. In addition, the transport of digoxin was inhibited by verapamil (50 μmol/L), a typical inhibitor of P-gp, indicating that this experimental setup was a valid system for evaluating P-gp-mediated DDIs.
DDI guidelines from the MHLW and FDA mention that the maximum concentration of an inhibitor in the gastrointestinal tract (Igut) is most appropriately reflected by using its concentration in the gastrointestinal tract (dose/250 mL) rather than its concentration in the blood. Since the maximum single oral dose of goreisan extract is 1 g, the Igut value is estimated to be 4 mg/mL, which is 1.9 times higher than its inhibitory IC50 against P-gp (2.09 mg/mL). The guidelines state that the detailed DDI study in humans is recommended if the gastrointestinal concentration of a test drug exceed 10 times the IC50. Therefore, based on this guideline criteria, the highest single dose of goreisan was unlikely to cause P-gp-mediated inhibition. Furthermore, we evaluated the DDI potential of goreisan with fexofenadine, a P-gp substrate drug frequently used as an antiallergic agent, as one example. The Igut of fexofenadine at a single dose of 60 mg was 479 µmol/L, which was 18 times higher than its reported Km value for P-gp (Takano et al. Citation2016). The apparent Michaelis constant Km, app with goreisan was calculated to be 2.9 Km, app. Here, the digoxin concentration was set low enough in our study so that the Km, app value of goreisan was able to be calculated using Ki as the IC50. Based on the Michaelis-Menten formula: the ratio of (Km, app + S) in combination with (Km + S) in fexofenadine with/without goreisan was about 0.91, indicating that fexofenadine had little effect on the v. If the Km of the substrate drugs were lower, the effect of goreisan on v would have been even lower.
A previous study reported that goreisan and its ingredients, alisols, inhibited drug export by P-gp and increased cell sensitivity to paclitaxel, making these goreisan ingredients potentially useful P-gp inhibitors in anticancer therapy (Hyuga et al. Citation2012). Although the P-gp inhibitory effects of tumulosic acid, (E)-cinnamic acid, and other goreisan ingredients have not been elucidated, it is likely that alisols play a role in the overall P-gp inhibitory effect of goreisan. Our results suggest that goreisan is a weak P-gp inhibitor, but that its low DDI potential shows promise in its potential usefulness as shown by Hyuga et al. In addition to P-gp, other efflux transporters including MRP2 and BCRP are involved in promoting multidrug resistance (MDR). Various natural products, including flavonoids, alkaloids, terpenoids, and coumarins, are being explored as potential safe MDR modulators (Kumar and Jaitak Citation2019). Investigations on the effects of goreisan on these other transporters will deepen our understanding of the relative efficacy and safety of goreisan.
Some ingredients contained in Kampo medicines are absorbed into the body without being metabolised, whereas others are absorbed after being metabolised by enterobacterium in the gastrointestinal tract. For example, glycyrrhizic acid from Glycyrrhiza is hardly absorbed in the intestines but after it is hydrolysed by microbiota in the intestines into glycyrrhetinic acid, it is readily absorbed into the blood (Takeda et al. Citation1996). Furthermore, the evaluation of goreisan extracts may not provide appropriate information on their action against hepatic drug-metabolizing enzymes since not all the ingredients contained in Kampo medicine are necessarily absorbed. Alisol A, tumulosic acid, and (E)-cinnamic acid were detected in plasma following a pharmacokinetic study of goreisan in rats after their oral administration (Hida et al. Citation2021) and show the potential to cause DDIs due to their inhibition of hepatic enzymes. We selected these three ingredients and tested their inhibitory effect on CYP isoforms with HLMs.
Among the three ingredients evaluated, tumulosic acid and (E)-cinnamic acid showed no inhibition against the CYP isoforms tested, as their IC50 values around 10 μmol/L or above. There was no effect observed after pre-incubating the HLMs and each of the ingredients. Tumulosic acid and (E)-cinnamic acid were also not time-dependent inhibitors of the CYP isoforms tested. On the other hand, alisol A inhibited CYP3A with an IC50 value of 2.99–8.39 μmol/L but did not exhibit time-dependent inhibition. Thus, alisol A seems to have competitively inhibited the CYP3A-mediated metabolism of midazolam or testosterone.
At present, there is an absence of data on the DDI potential of alisol A in hepatic metabolism since there are no reported studies on the blood concentrations of alisol A after administration of goreisan. Further research of the absorbed ingredients, their unbound concentrations, and the CYP inhibitory effecof goreisan after administration will help elucidate of the CYP-mediated DDI potential of goreisan in clinical use.
Given that some drugs have a significant first-pass effect by CYP3A in the small intestine, this may increase their bioavailability when used in combination with CYP3A inhibitors. Furanocoumarins contained in grapefruit juice are well known to strongly inhibit CYP3A4 in the gastrointestinal tract in a time-dependent manner (Chan et al. Citation1998). A previous study reported that Kampo medicines including herbal drugs from the Umbelliferae and Rutaceae families that contain furanocoumarins inhibited CYP3A4 in the gastrointestinal tract (Iwanaga et al. Citation2010).
The effect of goreisan on CYP3A in the gastrointestinal tract was evaluated based on the criteria defined in DDI guidelines. The DDI guidelines state that if the value calculated from < 11, the DDI potential due to inhibition of CYPs in the gastrointestinal tract is minimal. The concentration of alisol A in the goreisan extract used in this study were around 380 μg/g (0.77 μmol/g). The content analysis of the ingredients in this extract was performed based on a previously reported method (Hida et al. Citation2021). The Igut value of alisol A after a maximum single dose of goreisan was estimated to be 3.08 μmol/L. This concentration is comparable to the Ki value for the assumed competitive inhibition of CYP3A by alisol A. Therefore, the
value was lower than 11, suggesting that the amount of alisol A present in the highest dose of goreisan would be unlikely to cause DDI solely due to CYP3A inhibition in the gastrointestinal tract. The same results were obtained from the two other goreisan ingredients and an approximate IC50 for CYP3A was calculated to be 10 μmol/L.
In this study, the CYP inhibitory activity of alisol A, an absorbed ingredient from goreisan was examined and various ingredients from the extract showed possible activity on CYP3A in the gastrointestinal tract. Further studies will be needed to identify the specific ingredients in goreisan that inhibit CYP3A activity and to elucidate their unbound concentrations, specifically in intestinal epithelial cells where CYP3A are commonly localised, for a more accurate evaluation of DDI potential.
Based on the results of this study, goreisan extract and three bioavailable ingredients from it (alisol A, tumulosic acid, and (E)-cinnamic acid) are predicted to exhibit minor or negligible effects on P-gp and CYPs in vivo, although further studies are needed. These findings will be useful in considering the drug interactions between goreisan and substrates of P-gp and CYP3A and will provide support for interpretating future clinical DDI studies of goreisan.
Conclusion
This study revealed that goreisan extract exhibits inhibitory activity on the drug efflux transporter, P-gp, and that alisol A, an ingredient contained in goreisan, has an inhibitory effect on CYP3A. Its inhibition was low enough that alisol A would be insufficient at causing clinically significant drug interactions. Nevertheless, understanding the pharmacokinetics of alisol A in humans and the inhibitory effects of other goreisan ingredients are important. The findings obtained in this study will be useful for predicting putative drug interactions.
Supplemental Material
Download PDF (106.3 KB)Acknowledgments
The authors thank Dr. Chiharu Sadakane and Ms. Mari Hida of Tsumura Advanced Technology Research Laboratories for their assistance in editing and proofreading this manuscript and sample analysis. The authors also thank Mikako Torii (LSI Medience Corporation, Tokyo, Japan) for evaluating the effect of goreisan ingredients on the CYP isoforms.
Disclosure statement
MT, TM, NK, YM, KO, and KM are employees of Tsumura & Co. The authors declare that, except for income received from the employer, no financial support or compensation has been received from any individual or corporate entity and there is no conflict of interest. EW received a research grant from Tsumura & Co.
References
- Ahn YM, Cho KW, Kang DG, Lee HS. 2012. Oryeongsan (Wulingsan), a traditional Chinese herbal medicine, induces natriuresis and diuresis along with an inhibition of the renin-angiotensin-aldosterone system in rats. J Ethnopharmacol. 141(3):780–785.
- Anderle P, Niederer E, Rubas W, Hilgendorf C, Spahn-Langguth H, Wunderli-Allenspach H, Merkle HP, Langguth P. 1998. P-Glycoprotein (P-gp) mediated efflux in Caco-2 cell monolayers: the influence of culturing conditions and drug exposure on P-gp expression levels. J Pharm Sci. 87(6):757–762.
- Bailey DG, Dresser GK, Leake BF, Kim RB. 2007. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 81(4):495–502.
- Chan WK, Nguyen LT, Miller VP, Harris RZ. 1998. Mechanism-based inactivation of human cytochrome P450 3A4 by grapefruit juice and red wine. Life Sci. 62(10):PL135–142.
- Choi JS, Kang KW. 2008. Enhanced tamoxifen bioavailability after oral administration of tamoxifen in rats pretreated with naringin. Arch Pharm Res. 31(12):1631–1636.
- Hida M, Sadakane C, Mizuhara Y, Watanabe J, Isohama Y. 2021. Pharmacokinetics of ingredients after a single oral administration of Goreisan extraxt in rats. Jpn Pharmacol Ther. 49(6):849–860.
- Hirohashi T, Suzuki H, Chu XY, Tamai I, Tsuji A, Sugiyama Y. 2000. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2). J Pharmacol Exp Ther. 292(1):265–270.
- Hyuga S, Shiraishi M, Hori A, Hyuga M, Hanawa T. 2012. Effects of Kampo medicines on MDR-1-mediated multidrug resistance in human hepatocellular carcinoma HuH-7/PTX cells. Biol Pharm Bull. 35(10):1729–1739.
- Ito K, Satoh T, Watanabe Y, Ikarashi N, Asano T, Morita T, Sugiyama K. 2008. Effects of Kampo medicines on CYP and P-gp activity in vitro. Biol Pharm Bull. 31(5):893–896. eng.
- Iwanaga K, Hayashi M, Hamahata Y, Miyazaki M, Shibano M, Taniguchi M, Baba K, Kakemi M. 2010. Furanocoumarin derivatives in Kampo extract medicines inhibit cytochrome P450 3A4 and P-glycoprotein. Drug Metab Dispos. 38(8):1286–1294.
- Katayama K, Matsuda N, Kakuta K, Naraoka M, Takemura A, Hasegawa S, Akasaka K, Shimamura N, Itoh K, Asano K, et al. 2018. The effect of goreisan on the prevention of chronic subdural hematoma recurrence: multi-center randomized controlled study. J Neurotrauma. 35(13):1537–1542.
- Komiyama S, Takeya C, Takahashi R, Yamamoto Y, Kubushiro K. 2015. Feasibility study on the effectiveness of Goreisan-based Kampo therapy for lower abdominal lymphedema after retroperitoneal lymphadenectomy via extraperitoneal approach. J Obstet Gynaecol Res. 41(9):1449–1456.
- Kumar A, Jaitak V. 2019. Natural products as multidrug resistance modulators in cancer. Eur J Med Chem. 176:268–291.
- Matsumoto T, Kaifuchi N, Mizuhara Y, Warabi E, Watanabe J. 2018. Use of a Caco-2 permeability assay to evaluate the effects of several Kampo medicines on the drug transporter P-glycoprotein. J Nat Med. 72(4):897–904.
- Ministry of Health, Labour and Welfare 2018b. Guideline on Drug Interaction for Drug Development and Appropriate Provision of Information. https://www.pmda.go.jp/files/000228122.pdf.
- Ministry of Health, Labour and Welfare 2018a. Guidance on Appropriate Medication for Elderly Patients (general). https://www.pmda.go.jp/files/000232249.pdf.
- Nakano T, Nishigami C, Irie K, Shigemori Y, Sano K, Yamashita Y, Myose T, Tominaga K, Matsuo K, Nakamura Y, et al. 2018. Goreisan prevents brain edema after cerebral ischemic stroke by inhibiting aquaporin 4 upregulation in mice. J Stroke Cerebrovasc Dis. 27(3):758–763.
- Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. 2022. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 76(1):59–67.
- Ni H, Matsumoto T, Watanabe J, Makino T. 2018. Inhibitory effect of japanese traditional kampo formula frequently prescribed in gynecological clinics on CYP3A4. Evid Based Complement Alternat Med. 2018:4259603.
- Ohnishi N, Nagasawa K, Yokoyama T. 2000. The verification of regulatory effects of Kampo formulations on body fluid using model mice. J Tradit Med. 17:131–136.
- Pelkonen O, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H. 2008. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol. 82(10):667–715.
- Perloff ES, Mason AK, Dehal SS, Blanchard AP, Morgan L, Ho T, Dandeneau A, Crocker RM, Chandler CM, Boily N, et al. 2009. Validation of cytochrome P450 time-dependent inhibition assays: a two-time point IC50 shift approach facilitates kinact assay design. Xenobiotica. 39(2):99–112.
- Satoh T, Watanabe Y, Ikarashi N, Ito K, Sugiyama K. 2009. Effects of Kampo medicines on P-glycoprotein. Biol Pharm Bull. 32(12):2018–2021.
- Taipalensuu J, Tornblom H, Lindberg G, Einarsson C, Sjoqvist F, Melhus H, Garberg P, Sjostrom B, Lundgren B, Artursson P. 2001. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 299(1):164–170.
- Takano J, Maeda K, Bolger MB, Sugiyama Y. 2016. The prediction of the relative importance of CYP3A/P-glycoprotein to the nonlinear intestinal absorption of drugs by advanced compartmental absorption and transit model. Drug Metab Dispos. 44(11):1808–1818. doi:10.1124/dmd.116.070011.
- Takeda S, Ishthara K, Wakui Y, Amagaya S, Maruno M, Akao T, Kobashi K. 1996. Bioavailability study of glycyrrhetic acid after oral administration of glycyrrhizin in rats; relevance to the intestinal bacterial hydrolysis. J Pharm Pharmacol. 48(9):902–905.
- US Food Drug Administration 2020. Guidance for industry; In vitro drug interaction studies-cytochrome P450 enzyme-and transporter-mediated drug interactions. https://www.fda.gov/media/134582/download.
- van Breemen RB, Li Y. 2005. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol. 1(2):175–185.
- Watanabe Y, Ikarashi N, Satoh T, Ito K, Ochiai W, Sugiyama K. 2012. Inhibitory effects of daiokanzoto (da-huang-gan-cao-tang) on p-glycoprotein. Evid Based Complement Alternat Med. 2012:361516.
- Yaku H, Kaneda K, Kitamura J, Kato T, Kimura T. 2021. Kampo medicine for the holistic approach to older adults with heart failure. J Cardiol. doi:10.1016/j.jjcc.2021.12.011.
- Yano Y, Yano H, Takahashi H, Yoshimoto K, Tsuda S, Fujiyama K, Izumo-Shimizu Y, Motoie R, Ito M, Tanaka J, et al. 2017. Goreisan inhibits upregulation of aquaporin 4 and formation of cerebral edema in the rat model of juvenile hypoxic-ischemic encephalopathy. Evid Based Complement Alternat Med. 2017:3209219.
- Yasunaga H. 2015. Effect of Japanese herbal Kampo medicine goreisan on reoperation rates after Burr-Hole surgery for chronic subdural hematoma: analysis of a national inpatient database. Evid Based Complement Alternat Med. 2015:817616.
- Yee S. 1997. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man–fact or myth. Pharm Res. 14(6):763–766.

![Figure 1. Inhibitory effect of goreisan extract on the efflux ratio across the Caco-2 cell monolayer for the P-gp substrate, [3H]-digoxin. The open circle represents the observations, a regression curve is shown, and a nonlinear regression model was used to determine the IC50 values.](/cms/asset/c5f38caa-a312-414b-9ce9-7545f9d59dfb/ixen_a_2078750_f0001_b.jpg)