ABSTRACT
Millipedes are slow-moving soil organisms, which do not easily disperse to new habitats. However, species can be transported by humans across large distances and be found in anthropogenic habitats like botanical gardens and glasshouses. Thus, several millipede species have been introduced from around the world to Europe. Here we describe three new species of pinhead millipedes (Polyzoniida): Siphonethus dudleycookeorum sp. nov. from Great Britain with close affinities to specimens from New Zealand, and Siphonethus coxaespinosus sp. nov. and Siphonethus obtusus sp. nov. from Tawhiti Rahi (Poor Knights Islands) from New Zealand. Siphonethus dudleycookeorum sp. nov. was initially discovered in Lamorran House Gardens (Cornwall) in Great Britain and could be traced back to New Zealand with DNA-barcoding and comparison to specimens from the New Zealand Arthropod Collection (NZAC). As generic as well as species characters of the Siphonotidae Cook, 1895 are only poorly worked out, the placement of the new species in the only New Zealand genus Siphonethus Chamberlin, 1920 is provisional. Based on the available material and photographs of the holotypes of S. bellus Chamberlin, 1920 and S. enotatus Chamberlin, 1920, we suggest that the species currently classified as Siphonethus belong to at least two separate genera. It can be expected that New Zealand harbours a great diversity of undescribed polyzoniidan millipedes, as a lot of unsorted specimens are present in various collections and already material from the relatively small island Tawhiti Rahi has yielded two species new to science.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:6D3FF76F-16A2-43ED-B9CC-F34B4EBE7525
http://www.zoobank.org/urn:lsid:zoobank.org:act:F59D9647-6F25-448F-AD36-DE9F86F187D6
http://www.zoobank.org/urn:lsid:zoobank.org:act:A0718515-C5AE-4BE1-B32A-312FB66E9B5C
http://www.zoobank.org/urn:lsid:zoobank.org:act:3D4D8C0C-F30E-42B9-8CBD-E394C635FD87
Introduction
Millipedes (Diplopoda) are relatively small and slow-moving animals, which are often burrowing, and restricted to the leaf litter or upper soil layers (Golovatch & Kime Citation2009). Therefore, they do not easily disperse to new localities. However, some species are known to be transported by human activity across the world and can be found in anthropogenic habitats. Several introduced tropical species are reported from European botanical gardens (Read Citation2007; Stoev et al. Citation2010; Decker et al. Citation2014; Gregory & Lugg Citation2020), such as the tramp species Rhinotus purpureus (Pocock, Citation1894), a representative of the taxonomically rather small millipede taxon Polyzoniida, which probably originated from the Neotropics (Shelley Citation2000) and is now distributed throughout the world (Sierwald & Bond Citation2007; Wesener Citation2014). The wide distribution of Rhinotus purpureus has led to the description of at least four genus- and several species-level synonyms (Millibase). Besides R. purpureus, the only other polyzoniidan millipede of the well-studied British millipede fauna is the native Polyzonium germanicum Brandt, Citation1837 (Blower Citation1985).
Polyzoniida (and Siphonocryptida) are distinct from other millipedes by their minute, conical heads, which bear only a few ommatidia, large antennae and reduced mouthparts (Moritz et al. Citation2022). As in all Colobognatha the 9th and 10th legs are modified in males for sperm transfer (gonopods). With less than 80 described species the Polyzoniida is one of the least diverse taxon within millipedes (Diplopoda), which comprise more than 12,000 described species (Enghoff et al. Citation2015). While the Polyzoniida is species poor in Europe (12 species, Kime & Enghoff Citation2011), the group seems to be more diverse but largely understudied in other parts of the world, such as Oceania, which holds various undescribed species. An unpublished dissertation lists for example 42 potential species in 8 genera for Australia alone (Marek et al. Citation2021). New Zealand is home to several endemic and introduced millipede species as summarized by Johns (Citation1962) with 39 described species (Johns Citation1962; Korsós & Johns Citation2009). However, only two polyzoniidan millipedes of the family Siphonotidae Cook, 1895, Siphonethus bellus Chamberlin, Citation1920 and Siphonethus enotatus Chamberlin, Citation1920, are described from the North Island of New Zealand, but unsorted material from museum collections suggest that the group is quite diverse in New Zealand.
The family Siphonotidae comprises ca. 30 described species in 12 genera, which are mainly distributed in the tropics and southern hemisphere (South America, South Africa, Madagascar, SE Asia, and Oceania). The only millipede species with more than 1,000 legs belongs to the family (Marek et al. Citation2021). Siphonotidae are not well defined (see Shear Citation2016 for a discussion) and diagnostics for the family might be a combination of characters: the position of the male gonopore on the coxa of the 2nd leg, the presence of a long paronychium under the tarsal claw, posterior tergal margins which are not upturned, and a completely fused narrow preanal ring (Hoffman Citation1977; Enghoff et al. Citation2015); a character combination which supposedly differentiates them from the two other polyzoniidan families Polyzoniidae Newport, Citation1844 and Hirudisomatidae Silvestri Citation1896. The taxonomy and genus-specific characters in this group are only poorly worked out with many rather superficial descriptions (Hoffman Citation1977) and only few authors give detailed accounts on taxonomically important characters (e.g. Mauriès & Silva Citation1971; Golovatch Citation2014; Shear Citation2016; Marek et al. Citation2021). This is also true for the only New Zealand genus, Siphonethus Chamberlin, Citation1920, which is seen as problematic by Hoffman (Citation1977) and is in urgent need of revisionary work. As the original description of the genus, based on two female specimens, no other Siphonethus species have been proposed, but several specimens assigned to the genus have been reported from New Zealand (Watt Citation1982; Derraik et al. Citation2001; Sinclair et al Citation2005; Affeld et al. Citation2009). The original descriptions of S. enotatus and S. bellus by Chamberlin (Citation1920) lack illustrations and mainly include somatic characters like colouration, setation and the shape of the head, antennae and body-rings. However, the gonopods (legs modified for copulation in males), which are often highly specific and a main character to distinguish millipede species and higher taxa (e.g. Sierwald & Bond Citation2007), are not described (as both types are female). Therefore, a redescription of material from the type locality is urgently needed, including SEM images of the gonopods and molecular barcoding.
Here we describe three new species, which are provisionally placed in the genus Siphonethus for the reasons above: One species from Great Britain with close affinities to siphonotid millipedes from the island Tawhiti Rahi (New Zealand), and two species from the same island.
Material & methods:
Museum acronyms and abbreviations
MCZ - Museum of Comparative Zoology, Harvard, USA
NZAC - New Zealand Arthropod Collections, Manaaki Whenua, Landcare Research, Auckland, New Zealand
SEM - Scanning electron microscopy
ZFMK - Zoological Research Museum A. Koenig, LIB, Bonn, Germany
Material examined
Specimens of Siphonethus dudleycookeorum sp. nov. were collected by Steve Gregory in Lamorran House Gardens (Great Britain, Cornwall, St. Mawes, Lamorran House Gardens, SW 843331 / VC2) on 9th September 2020, and on 28th November 2021, fixed in 95% ethanol and are stored in the collection of the Zoological Research Museum A. Koenig (ZFMK, Bonn, Germany). Images of the holotypes of Siphonethus bellus Chamberlin, Citation1920 (MCZ4878) and Siphonethus enotatus Chamberlin, Citation1920 (MCZ4885), which are stored at the Museum of Comparative Zoology (MCZ, Harvard, USA) were provided by Laura Leibensperger; a loan was not possible due to restriction on international loans during the pandemic (COVID-19). Furthermore, we investigated specimens stored in the New Zealand Arthropod Collection (NZAC) from Tawhiti Rahi (Poor Knight Islands, New Zealand; Siphonethus aff. dudleycookeorum, S. coxaespinosus sp. nov. and S. obtusus sp. nov.) from a previous study by Watt (Citation1982) and specimens from Little Barrier Island (New Zealand; Siphonethus sp.) from a study by Drummond et al. (Citation2015).
Morphological investigation
Specimens were investigated using a Zeiss Discovery V12 stereo microscope.
For scanning electron microscopy specimens fixed in 95% or 75% ethanol were dehydrated in an ascending ethanol series and critical point dried using a Leica EM CPD 300. The specimens were mounted to SEM-stubs using conductive tape and sputtered with gold using the Cressington Sputter Coater 108auto. SEM images were obtained with a Zeiss Sigma 300 VP scanning electron microscope at the ZFMK.
Genetic analysis
A DNA barcode (Hebert et al. Citation2003) of the cytochrome c oxidase subunit 1 (CO1) gene was obtained for one female specimen of Siphonethus dudleycookeorum sp. nov. (ZFMK MYR10095; GenBank: ON023662) from Great Britain and two specimens of Rhinotus purpureus (the only member of the family known from Europe) from the Botanical Garden Bonn, Germany, using the COI JJ primer (Astrin & Stüben Citation2008). For DNA extraction muscle tissue from legs and body-rings was used. DNA extraction, amplification, and sequencing were done as outlined in previous studies (e.g. Wesener Citation2015). A BLAST search (Altschul et al. Citation1997) was performed for the DNA COI fragment. The BLAST search led to the discovery of two closely related specimens (<15% p-distance) from New Zealand, labeled as ‘Pancrustacea sp.’ and ‘Mandibulata sp.’ (Genbank accession numbers KP421754.1 and KP421302.1). All additional 18 Polyzoniida COI sequences from Genbank (12.2021) were downloaded and added to our dataset. These additional sequences only contained three species determined to genus- or species-level, Eumillipes persephone, Polyzonium germanicum and Angarozonium aduncum, as well as several sequences determined to the family-level as Hirudisomatidae and Polyzoniidae.
Our sequences and those of the Polyzoniida from GenBank () were aligned by hand in Bioedit (Hall Citation1999). Our total dataset contained 23 Polyzoniida sequences () with 657 base pairs. The dataset was translated into amino-acids using the ‘invertebrate’ code in MEGA6 (Tamura et al. Citation2013) to rule out pseudogenes. All three new sequences were deposited in GenBank (see ).
Table 1. CO1 sequences used in this study.
The number of base differences per site between sequences was calculated. The analysis involved 23 nucleotide sequences. Codon positions included were 1st+2nd+3rd. All ambiguous positions were removed for each sequence pair. There were a total of 657 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 (Tamura et al. Citation2013).
The best fitting substitution model for a maximum likelihood analysis was calculated with Modeltest (Tamura & Nei Citation1993) as implemented in MEGA6. Models with the lowest BIC scores (Bayesian Information Criterion) are considered to describe the substitution pattern the best (Nei & Kumar Citation2000). The best fitting model was the Hasegawa, Kinshino, Yano Model (Hasegawa et al. Citation1985) with gamma distribution and invariant sites (lnL = −3682.469, Invariant = 0.37586, Gamma = 0.82789, R = 3.158; Freq A: 0.28, T: 0.317, C: 0.24, G: 0.16). Phylogenetic analyses were performed in MEGA6 based on the Hasegawa, Kinshino, Yano Model (HKY + G+I). A species tree was constructed using maximum likelihood method with gamma distribution of five categories. The tree with the highest log likelihood (−3682.4690) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbour-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.8279)). The rate variation model allowed for some sites to be evolutionarily invariable ([ + I], 37.5869% sites). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 23 nucleotide sequences. Codon positions included were 1st+2nd+3rd. All positions with less than 5% site coverage were eliminated. That is, fewer than 95% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 657 positions in the final dataset. The bootstrap consensus tree was calculated from 1000 replicates (Felsenstein Citation1985) in MEGA6 (Tamura et al. Citation2013). The obtained tree was edited in Adobe Illustrator CS2.
Results
Order Polyzoniida Newport, Citation1844
Family Siphonotidae Cook, 1895
Remarks: All investigated specimens can be placed in the family Siphonotidae based on the following character combination: Tarsus with paronychium, preanal ring narrow and forming a complete ring around paraprocts, male gonopore positioned on coxa of leg two, tergal margins not upturned.
Genus Siphonethus Chamberlin, Citation1920
Chamberlin, Citation1920: 99 (first description); Jeekel Citation1971: 44 (list).
Type species: Siphonethus enotatus Chamberlin, Citation1920
Other species included:
Siphonethus bellus Chamberlin, Citation1920
Siphonethus dudleycookeorum sp. nov.
Siphonethus coxaespinosus sp. nov.
Siphonethus obtusus sp. nov.
Remarks: The placement of the three new species in the genus Siphonethus is provisional. A redescription of S. enotatus () and S. bellus (), based on fresh material from the type localities is urgently needed, including detailed descriptions of the gonopods and molecular analyses.
Figure 1. Holotype of Siphonethus enotatus Chamberlin, Citation1920 (MCZ4885), photographs by Laura Leibensperger (MCZ). (a) Habitus, lateral view. (b) Head and anterior body-rings, ventral view. (c) Preanal ring and posterior body-rings, lateral view.

Figure 2. Holotype of Siphonethus bellus Chamberlin, Citation1920 (MCZ4878), photographs by Laura Leibensperger (MCZ). (a) Habitus, lateral view. (b) Head and anterior body-rings, lateral view. (c) Preanal ring and posterior body-rings, lateral view.

We place the newly described species in the genus Siphonethus because this is the only Polyzoniida genus known from New Zealand, and based on the blast results of the specimens from Great Britain (with close morphological affinities to specimens from New Zealand), showing the best match for the specimens with unidentified ‘Mandibulata sp.’ (Sequence ID: KP421302.1; Voucher: NZAC 03011232) with 13.9% p-distances and ‘Pancrustacea sp.’ (Sequence ID: KP421754.1; Voucher: NZAC 03012785) with 14.3% p-distance. The latter two specimens are from Little Barrier Island (New Zealand) from a study by Drummond et al. (Citation2015) and show a p-distance of 0.2% to one another. The short description of the genus by Chamberlin (Citation1920) does not allow an in-depth comparison of morphological characters. Chamberlin (Citation1920: 99) states as diagnosis for the genus: ‘Distinguished from Siphonotus by having two ocelli on each side instead of one, and by having the head excavated on each side above for the reception of the antennae.’. We suggest to provisionally include all species from New Zealand in the genus Siphonethus until a detailed examination of the type material is possible.
Siphonethus dudleycookeorum sp. nov. shows distinct morphological differences to S. coxaespinosus sp. nov. and S. obtusus sp. nov., which might justify placing the species in two different genera (), but at the moment we hesitate to create a new genus, without revision of the holotypes and topotypic material of S. enotatus and S. bellus (which might not be congeneric either), to avoid creating further chaos in the group. As outlined below S. dudleycookeorum sp. nov. shows closer morphological similarities to S. enotatus, while S. coxaespinosus sp. nov. and S. obtusus sp. nov. show closer affinities to S. bellus.
Table 2. Comparison of Siphonethus species. Information for S. enotatus and S. bellus taken from the original description (Chamberlin Citation1920) and inferred from photographs of types. *might be an artefacts due to preservation. Inverted commas (‘ … ’) indicate text directly quoted from Chamberlin (Citation1920: 99). Question marks (?) indicate unknown character states.
Siphonethus enotatus Chamberlin, Citation1920
Chamberlin, Citation1920: 99 (first description). Jeekel Citation1971: 44 (list);
Original description (Chamberlin, Citation1920: 99): ‘Color uniform fulvoferruginous, or rather more ferruginous at anterior end, with collum, head and antennae somewhat dusky. Face below level of eyes broadly triangular, sides nearly straight, inferior end acute. Ocelli on mesodorsal side of antennal socket, two in number on each side of which the upper one is the smaller. Head rather deeply excavated on each side for insertion or reception of antennae. Antennae cylindrical, enlarging distad, the lower end of face reaching to near middle of fourth article. Face transversely depressed or furrowed at lower level of antennal notches.
Collum three times as wide as the head. Body hemicylindrical. Each segment transversely furrowed or constricted. Hairs of body moderately long, sparsely and nearly uniformly distributed. Number of segments, forty-nine. Width, 0.93 mm.’
Material examined (based on photographs, ): Holotype: Female (MCZ4885), NEW ZEALAND, Taumarunui (‘Taumarunni’ in Chamberlin (Citation1920: 99)), leg. W.M. Wheeler.
Remarks: The type species of the genus Siphonethus, Siphonethus enotatus is of uniform orange-brown colouration (a) and has a rather pointed head and longer cylindrical antennae (b) similar to S. dudleycookeorum sp. nov., and is densely covered by long setae (). Gonopod characters are unknown as the type is a female specimen.
Siphonethus bellus Chamberlin, Citation1920
Chamberlin, Citation1920: 99 (first description).
Original description (Chamberlin, Citation1920: 99): ‘Distinguished readily by its colour-pattern. The general color yellow with a median dorsal longitudinal black line and a broader but still narrow submarginal black stripe on each side, a small yellow dot enclosed in the black of the latter on each segment. Collum, face, and antennae dusky. Legs fulvous. Antennae stout, moderately clavate; reaching to caudal edge of second tergite. Face below antennae triangular, the sides convex, the lower end acutely rounded. Each segment constricted across middle, the furrow with longitudinal striae. Body of type nearly glabrous, the hairs few. Number of segments, thirty-six. Width, .92 mm.’
Material examined (based on photographs, ): Holotype: Female (MCZ4878), NEW ZEALAND, Day's Bay, near Wellington, leg. W.M. Wheeler.
Remarks: The second species currently placed in Siphonethus, S. bellus shows longitudinal stripes (a) similar to Burinia Attems, 1926 from South America and South Africa (Golovatch Citation2014) and several undescribed species from New Zealand. S. bellus has an apically rounded and stouter head (b) than S. enotatus, with stouter clavate antennae similar to S. coxaespinosus sp. nov. and S. obtusus sp. nov., and its body is covered by few shorter setae similar to S. coxaespinosus sp. nov. (). Based on these characters, we suspect that S. bellus is not a Siphonethus but might represent a second native genus of Siphonotidae present in New Zealand, to which also S. coxaespinosus sp. nov. and S. obtusus sp. nov. may belong. Gonopod characters are unknown as the type is a female specimen.
Siphonethus dudleycookeorum sp. nov.
Figure 3. Habitat of Siphonethus dudleycookeorum sp. nov. in Lamorran HouseGardens (Great Britain, Cornwall), photographs by S. J. Gregory. (a) Path lined by planted tree ferns from New Zealand. (b) Siphonethus dudleycookeorum sp. nov.

Figure 4. Siphonethus dudleycookeorum sp. nov. from Lamorran House Gardens (Great Britain, Cornwall), female. (a) Habitus, lateral view. (b) Anterior body rings, dorsal view. (c) Midbody-rings dorsal view.

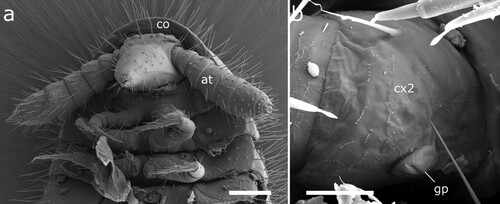
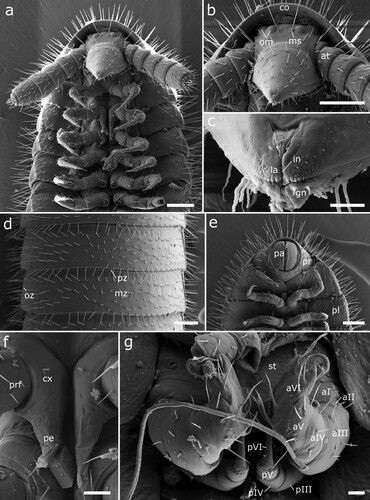
Figure 5. Siphonethus dudleycookeorum sp. nov., male holotype (ZFMK-MYR11376) from Great Britain, head, SEM images. (a) Head, frontal view. (b) Head, lateral view. (c) Detail of labrum. (d) Gnathochilarium. (e) Antennae, overview. (f) Antennae apical antennomeres, apical view. (g) Antennae apical antennomeres, lateral view. Scale: a, b, d, e = 100 µm, c, f = 10 µm, g = 20 µm. Abbreviations: I-VII = antennomere 1-7, ac = apical cones, at = antennae; co = collum, gn = gnathochilarium, in = incision of labrum, la = labrum, ms = macrosetae, om = ommatidia, sb = sensilla basiconica.
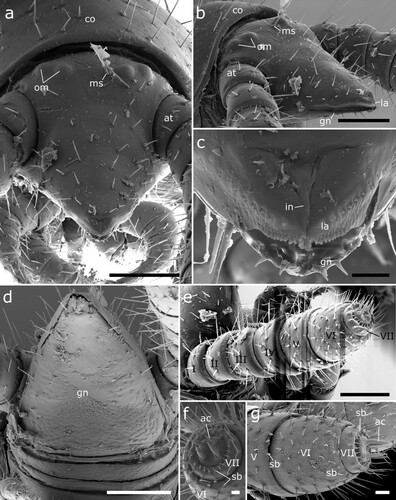
Figure 6. Siphonethus dudleycookeorum sp. nov., female paratype (ZFMK-MYR10094; a-e) and male holotype (ZFMK-MYR11376; f) from Great Britain, body-rings and legs, SEM images. (a) Body-ring, dorsal view. (b) Details (mesal rectangular structure) of (a). (c) Posterior body-rings and preanal ring, lateral view. (d) Posterior body-rings and preanalring, ventral view. (e) Ultimate walking leg. (f) Detail of claw with paronychium. Scale: a, c-e = 100 µm, b = 10 µm, f = 20 µm. Abbreviations: cl = claw, cx = coxa, fe = femur, mz = metauonite, oz = ozopore, pa = paraproct, pof = postfemur, pl = pleurite, pn = paronychium, pr = preanal ring, prf = prefemur, ta = tarsus, ti = tibia, tr = trochanter.
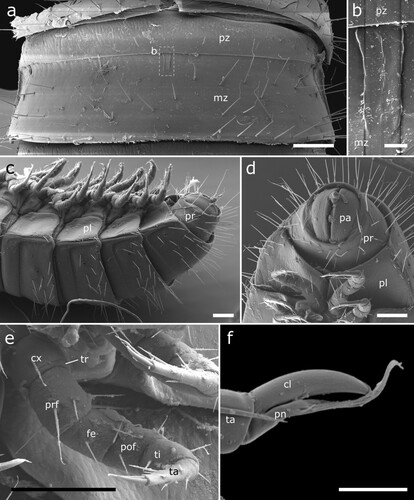
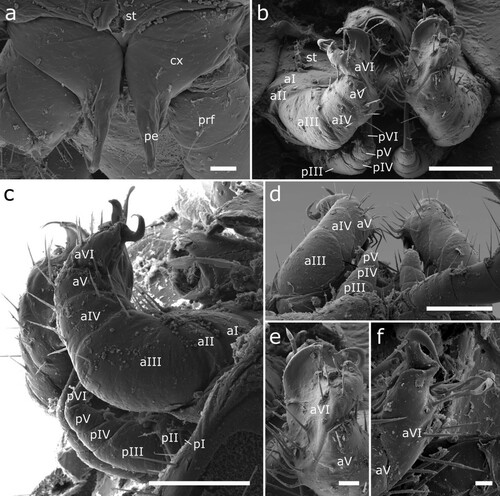
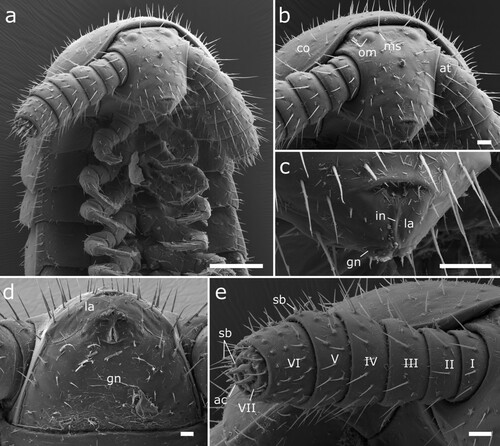
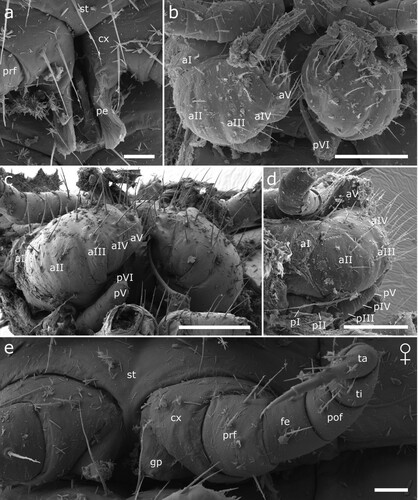
Figure 7. Siphonethus dudleycookeorum sp. nov., male holotype (ZFMK-MYR11376) from Great Britain, male sexual characters, SEM images. (a) Leg pair 2 with penes. (b) Gonopods, ventral view. (c) Gonopods, lateral view. (d) Gonopods posterior view. (e & f) detail of anterior gonopod apical podomeres. Scale: a = 20 µm, b-d = 100 µm, e = 20, f = 10 µm. Abbreviations: aI-aVI = podomeres of anterior gonopod, cx = coxa, pe = penis, pI-pVI = podomeres of posterior gonopod, prf = prefemur, st = sternite.
Material examined: Holotype: Male (ZFMK-MYR11376), GREAT BRITAIN, Cornwall, St. Mawes, Lamorran House Gardens, SW 843331/ VC2, Lat 50.158535 Lon −5.0199108, 09.ix.2020, leg. S.J. Gregory; In soil under tree ferns and beneath cut stems lying on the ground of tree ferns from New Zealand (NZ) and Australia (AU) (Cyatheas dealbata (NZ), Australis smithii (AU, introduced to NZ), Sphaeropteris medullaris (NZ), Dicksonia antarctica (AU), D. squarrosa (NZ), D. fibrosa (NZ)) (a). Paratypes: 3 females (ZFMK-MYR10094, ZFMK-MYR10095, ZFMK-MYR11377) same data as holotype. Paratypes: Male (ZFMK-MYR11433) 14 Females (ZFMK-MYR11419 – ZFMK-MYR11432)), same location as holotype, 28.xi.2021.
Etymology: The species name dudleycookeorum honours Robert and Maria-Antoinette Dudley-Cooke, who own and manage Lamorran House Gardens, the type locality of the species, and who allowed Steve J. Gregory to collect there. Noun in apposition.
Diagnosis: Two pairs of ommatidia and mesal rectangular structure on anterior margin of metazonite are present as in S. coxaespinosus sp. nov. and S. obtusus sp. nov.. Differs from S. coxaespinosus sp. nov. and S. obtusus sp. nov. by a more pointed head, which tapers strongly anterior of the antennae (as in S. enotatus), with a transverse depression, a unique sculptured labrum, and the positon of the macrosetae anterior of the ommatidia (opposed to between ommatidia in S. coxaespinosus sp. nov. and S. obtusus sp. nov.). Differs from S. coxaespinosus sp. nov. and S. obtusus sp. nov. by the shape of the apical podomere of the gonopod, which is divided into two lobes of which the outer lobe carries a conspicuous curved spine. Differs from S. coxaespinosus sp. nov. by longer setation and the absence of coxal projections.
Description
Colouration of specimens freshly fixed in ethanol: Brownish-orange overall colour (b, 4a). Collum with a single median white field and a pair of large lateral white fields (b). Tergites anteriorly and posterior margin brownish-orange, purple transverse band in between. Medially the margin of the purple band is incised (v-shape). Tergites laterally with pair of white fields in purple area (b, c).
Measurements: Ca. 10 times as long as wide. Length: 9 mm, Width: 0.8-0.9 mm. up to 43 tergites + preanal ring and 78 leg pairs.
Head: Head small, long, conical. The head capsule is slightly excavated and tapers anterior of the antennal base (a, b). Labrum split longitudinally, posteriorly delimited by transverse fold resulting in a t-shaped or triangular field. Margin of labrum sculptured with tubercles, which are prominent anteriorly and flatten laterally, mesally two pairs of longer tubercles. On each side dorsally of the labral sculptures lies a single row of up to 12 cones, which are sunken into pits (c). Two pairs of ommatidia, one lateral posterior pair and one pair positioned mesal and slightly anterior of the other. One pair of macrosetae mesally of the anterior pair of ommatidia. Head covered by few setae (ca. 30) anterior of ommatidia. Mandibles not visible externally (b), except for posterior margin of mandibular cardo in ventral view (d).
Gnathochilarium: A pointed triangular plate, with anterior incision, creating two apical areas (remnants of stipites?), which are not fully divided from remaining gnathochilarium. Apically with two pairs of small setae (rudimental palps?). The gnathochilarium is covered by few setae, especially well developed at its lateral margins (d).
Antennae: Antennae stout ca. twice as long as head, with 7 antennomeres, covered by setae arranged in rows. Antennomeres 1–5 and 7 wider than long, antennomere 6 longest, ca. as wide as long (e). Apical margin of antennomere 5 and 6 with a row of sensilla basiconica, 3 or 4 on 5th, 5 on 6th. Apical (7th) antennomere with a marginal row of 12 sensilla, which are swollen in their middle. Apical disc with 4 sensory cones (f, g).
Collum: Collum large, concealing posterior part of head and posterior pair of ommatidia from above, ca. twice as long as following body-ring. Posterior margin nearly straight in dorsal view, only slightly rounded. Covered by setae (b, a).
Body-rings: Up to 43 tergites + preanal ring. Body-rings semi-circular in cross section, tergites well separated from pleurites and sternites. Tergites with distinct transverse division into pro- and metazonite, few longitudinal short striae on metazonite along division. Prozonite length ca. 1/3 of body-ring, anterior margin with net-like ornamentation. Metazonite with three more or less regular transverse rows of setae (a). Metazonite mesally at anterior margin with small demarcated rectangular area, which flattens out posteriorly (b). Ozopores starting from body-ring 5 on metazonite in some distance from lateral margin of tergite, surrounded by an inconspicuous rim (a, c). Sternites carrying tracheal opening lateral of legs from leg-pair 3 onwards, slightly elevated (c), tracheal opening also present lateral of the gonopods. Last body-ring anterior of preanal ring apodous, pleurites overlapping (c, d).
Preanal ring: Preanal ring narrow cylindrical, forming completely fused ring around paraprocts (anal valves). Covered by 3 rows of particularly long setae. Paraprocts free of setae (c, d).
Legs: Up to 78 leg pairs (female). Leg 1 and 2 with 6 podomeres, from leg 3 onwards 7 podomeres (with short trochanter) (e). 1st leg pair slightly smaller than following legs. Coxa carrying eversible sacs (coxal pouches) from leg pair 3 onwards, except for ultimate leg pairs. Tarsus long narrow with apical claw and cylindrical paronychium, paronychium longer than claw (f).
Male sexual characters: Male gonopore positioned on coxa of leg pair 2, on an elongated lobe (gonapophysis or pseudopenis) (a). Leg pairs 9 and 10 modified to well-developed leg-like gonopods (b–f).
Anterior gonopod consisting of 6 podomeres (segmentation not visible in all views). Podomere 3 largest, ca. as long as podomere 4 + 5. Podomeres 1–5 with few setae (b–d). Apical podomere (6) strongly modified, apically divided into two lobes, an outer lobe and an inner lobe. The outer lobe is triangular in shape and carries laterally a long pointed spine, which bends inwards towards the inner lobe. At base of outer lobe a group of 4 setae. Inner lobe apically and mesally rounded, lateral margin straight (e, f). Inner side of apical podomere incised by groove/fold, extending along its whole length and along inner margin of inner lobe. Within fold lies apical portion of posterior gonopod’s apical podomere, engulfed by anterior gonopod (d). Inner side of apical podomere base with a group of prominent setae, posterior of mesal groove/fold. Sternite carrying anterior gonopod with a pair of swellings medially of gonopods, each carrying a group of setae.
Posterior gonopod leg-like, consisting of 6 podomeres. Podomeres 1 and 2 minute, ca. half as long as podomere 4. Podomere 3 as long as podomere 4 + 5. Podomere 6 longest, ca. 1.5 times as long as remaining posterior gonopod. Apical podomere (6) elongated and narrows anteriorly, tip hollow. Apical podomere lies within fold formed by anterior gonopod, surpassing anterior gonopod. Posterior gonopod devoid of setae (c, d).
Female sexual character: Coxae of second leg pair free, not touching each other. Female gonopores on coxae of second leg pair, small circular, no division visible.
Remarks: Very similar to NZAC03038958 () from Poor Knights Islands. Shares with Siphonethus enotatus Chamberlin, Citation1920 a pointed acuminate head, cylindrical long antennae, moderately long and dense setae on the body.
Figure 8. Siphonethus aff. dudleycookeorum sp. nov. (NZAC03038958), male, from New Zealand, SEM images. (a) Anterior body, ventral view. (b) Head, frontal view. (c) Detail of labrum. (d) Mid body-ring, dorsal view. (e) Posterior body-rings and preanal ring, ventral view (f) Leg pair 2 with penes, ventral view. (g) Gonopods, ventral view. Scale: a, b, d, e = 100 µm, c = 10 µm f, g = 20 µm. Abbreviations: aI-aVI = podomeres of anterior gonopod, at = antennae, co = collum, cx = coxa, gn = gnathochilarium, in = incision of labrum, la = labrum, ms = macrosetae, mz = metazonite, om = ommatidia, oz = ozopore, pa = paraproct, pe = penis, pI-pVI = podomeres of posterior gonopod, pl = pleurite, pr = preanal ring, pz = prozonite.
Siphonethus aff. dudleycookeorum sp. nov.
&
Material examined: 7 males, 2 females, 5 juveniles (NZAC03038958), NEW ZEALAND, Poor Knights Island, Tawhiti Rahi, 5.xii.1980, leg. G. Kuschel. Sifted decayed wood & litter on plateau 80/136.
Remarks: These specimens resemble Siphonethus dudleycookeorum sp. nov. from Great Britain in their shape of the head (a, b, 9a), the sculpture of the labrum (c), in setation (d, e, 9a), and in the structure and shape of the gonopods ( g). The specimens differ slightly from S. dudleycookeorum sp. nov. in the proportions of the anterior gonopod’s apical lobes, although little is known about intraspecific variation within Colobognatha. Furthermore, some of the specimens from New Zealand show darker longitudinal lateral stripes differing from the colour pattern of S. dudleycookeorum sp. nov., although this might be the results of colour fading in ethanol. However, no genetic barcodes could be obtained and taxonomic characters as well as inter- and intraspecific variations are only poorly understood for the Siphonotidae. Therefore, we currently hesitate to view these specimens as identical to S. dudleycookeorum sp. nov..
Siphonethus coxaespinosus sp. nov.
Material examined: Holotype: Male (NZAC03038955), NEW ZEALAND, Poor Knights Is., Tawhiti Rahi, Shag Bay, 30 m, 12.ix.1980, leg. J.C. Watt, Litter 80/72. Paratypes: 22 females, 16 males, 18 juveniles, same data as holotype.
Paratypes: 5 males, 7 immature males, 1 female, 12 juveniles (NZAC03038954), NEW ZEALAND, Poor Knights Island, Tawhiti Rahi, Shag Bay, 40 m, 12.ix.1980, leg. J.C. Watt, Litter 80/73.
Etymology: The species name coxaespinosus is derived from the latin coxae = hips and spinose = thorny, and refers to the characteristic spine on the coxae of the male specimens. Noun in apposition.
Diagnoses: In S. coxaespinosus sp. nov. two pairs of ommatidia and mesal rectangular structure on anterior margin of metazonite are present similar to S. dudleycookeorum sp. nov. and S. obtusus sp. nov.. Differs from Siphonethus dudleycookeorum sp. nov. and S. obtusus sp. nov. by the shorter setation on the body-rings and head. Shares with S. obtusus sp. nov. a single marginal row of setae on the pleurites, a head, which is stouter than in S. dudleycookeorum sp. nov., a labrum lacking sculpture, and the position of the macrosetae between the ommatidia (opposed to anterior of ommatidia). The apical podomere of the anterior gonopod differs from S. dudleycookeorum sp. nov. in its shape, it is not divided into two lobes, and by the absence of the lateral curved spine. S. coxaespinosus sp. nov. differs from S. dudleycookeorum sp. nov. and S. obtusus sp. nov. by the presence of setae which carry apically a brush of spines on the anterior gonopods, the presence of well-developed paired protuberances on the anterior gonopod’s sternite, and by the presence of coxal projections in males, as well as flattened paronychia on the anterior legs. In females coxae of leg-pair 2 fused and sternite fused to syncoxosternite, vulvae opening behind syncoxosternite.
Description
Colouration in ethanol: Specimens are pale, whitish and no colour pattern could be observed ().
Measurements: Length: 8 mm, Width: 0.5 mm. Up to 41 tergites + preanal ring and 75 leg-pairs (18 juv (10–16 T), 22f (18–26 T), 16 m (18–28 T); Up to 41 tergites and 75 leg pairs (5M (24, 31, 36, 41, 41 T), 1F (25 T). Several juveniles and immature males with10–18 tergites (a).
Head: Head conical, stout (a, b). Labrum rounded, split longitudinally with triangular field. Margin of labrum not sculptured, lined by a row of setae (c). Two pairs of ommatidia. One pair of macrosetae mesally of ommatidia. Head covered by few setae anterior of ommatidia. Mandibles not visible externally (b).
Figure 11. Siphonethus coxaespinosus sp. nov. (NZAC03038955) from New Zealand, male, SEM images. (a) Anterior body, ventral view. (b) Head, frontal view. (c) Detail of labrum. (d) Gnathochilarium, ventral view. (e) Antennae. Scale: a = 100 µm, b, c, e = 20 µm, d = 10 µm. Abbreviations: I-VII = antennomere 1-7, ac = apical cones, at = antennae; co = collum, gn = gnathochilarium, in = incision of labrum, la = labrum, ms = macrosetae, om = ommatidia sb = sensilla basiconica.
Gnathochilarium: Stout triangular plate, which is longitudinally incised anteriorly. Apically with two pairs of setae (rudimentary palps?). Covered by few setae, longest along margin (d).
Antennae: Antennae consisting of 7 antennomeres, covered by setae arranged in row. Antennomeres wider than long, antennomere 6 longest, antennomere 4 and 5 widest. Sensilla basiconica in row on antennomere 5-7. Apical disc with 4 sensory cones (e).
Collum: Collum large, saddle shaped, concealing posterior part of head, reaching to posterior ommatidia. Ca. twice as long and as wide as following tergites. Covered by setae (a).
Body-rings: Up to 41 tergites + preanal ring. Body-rings semicircular, tergites well separated from pleurites and sternites. Tergites divided into prozonite and metazonite with distinct margin. Metazonite with faint longitudinal lines on anterior margin (a) and mesal rectangular structure (b). Metazonite with short setae arranged in 3 more or less regular rows. Ozopores starting on body-ring 5, laterally on metazonite with inconspicuous rim, not elevated (a). Pleurites almost rectangular, with lateral marginal row of setae. Sternites carrying tracheal openings lateral of legs from leg 3 onwards, slightly elevated, tracheal opening also present lateral of the gonopods. Last body-ring anterior of preanal ring apodous, pleurites overlapping (c).
Figure 12. Body-rings and legs of Siphonethus coxaespinosus sp. nov. (NZAC03038955) from New Zealand, male, SEM images. (a) Body-ring, dorsal view. (b) Details (mesal rectangular structure) of (a). (c) Posterior body-rings and preanal ring, ventral view. (d) Anterior leg. (e) Posterior leg. Scale: a, c = 100 µm, b = 2 µm, d, e = 20 µm. Abbreviations: cl = claw, cs = coxal sacs, cx = coxa, fe = femur, mz = metauonite, oz = ozopore, pa = paraproct, pof = postfemur, pl = pleurite, pn = paronychium, pr = preanal ring, prf = prefemur, ta = tarsus, ti = tibia, tr = trochanter.
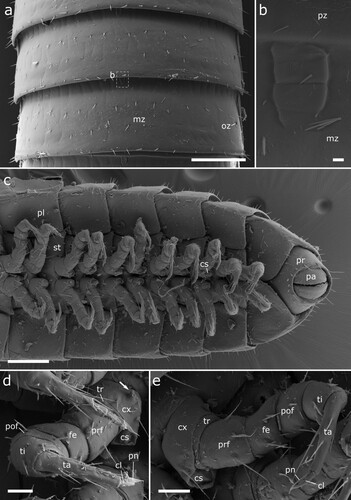
Preanal ring: Preanal ring narrow cylindrical, forming completely fused ring around paraprocts. Paraprocts surrounded by row of setae, longer setae dorsally, shorter setae ventrally. Paraprocts free of setae (c).
Legs: Up to 75 leg pairs. Leg 1 and 2 with 6 podomeres, from leg 3 onwards 7 podomeres (with short trochanter). 1st leg slightly smaller than following. Coxa carrying eversible sacs (coxal pouches) from leg pair 3 onwards, except for ultimate leg pairs (c–e). Coxa with anteriad projection, most conspicuous on coxa of 2nd leg in males (), not visible on posterior leg-pairs (c). Tarsus long narrow with apical claw and paronychium, which is longer than the claw. Paronychium of anterior legs flattened (spatulate?) (d), paronychium of posterior legs cylindrical (e).
Male sexual characters: Male gonopore positioned on coxa of leg pair 2, on an elongated laterally flattened lobe (gonapophysis or pseudopenis). Coxa 2 carries a conspicuous projection anterior of penis (a). Leg pairs 9 and 10 modified into well-developed gonopods (b–e).
Figure 13. Male (a-e) and female (f) sexual characters of Siphonethus coxaespinosus sp. nov. from New Zealand, male, SEM images. (a) Anterior coxae of male (NZAC03038954), ventral view. (b-f) Gonopods of NAZAC03038955. (b) Ventral view. (c) Posterior view. (d) Lateral view. (e & f) Detail of apical podomere of anterior gonopod. (e) Ventral view. (f) Gonopore and 2nd leg pair of female, ventral view. Scale: a, c = 100 µm, b, d-f = 20 µm. Abbreviations: aI-aV = podomeres of anterior gonopod, bs = brush-like setae, cx = coxa, go = gonopore, pe = penis, pI-pVI = podomeres of posterior gonopod, prf = prefemur, st = sternite, stp = sternal projection, ve = external valve of vulva, vi = internal valve of vulva.
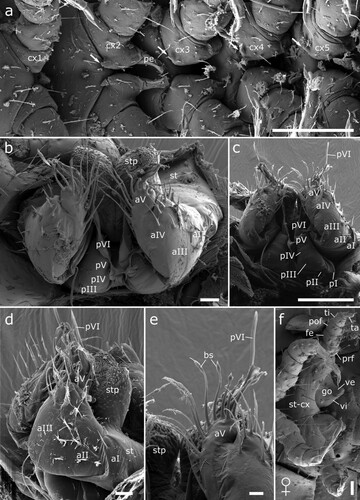
Anterior gonopod consisting of 5 podomeres (segmentation barely visible even in SEM images), covered by setae. Podomere 3 and 4 with apical row of setae (b–d). Podomere 5 extends in apical lobe, in which apical podomere of posterior gonopod rest. Base of lobe with 13–15 long setae, carrying spines, which form a brush- or cone-like structure terminally on outer setae, in apical third on inner setae (e).
Posterior gonopod leg-like, consisting of 6 podomeres, apical podomere (6) extending into long, flattened and twisted flagellum, which rests in lobe of anterior gonopod and extends beyond it (b, c).
Sternites anterior of anterior gonopod carry paired conspicuous projections, covered by spines and almost as large as anterior gonopod (d).
Female sexual characters: Second coxae and sternite fused to syncoxosternite. Female gonopore (vulva) positioned behind syncoxosternite behind leg 2, cone-shaped, divided by furrow. Second leg consisting of 5 podomeres. Apical podomere of leg-pair 2 a third as long as tarsi on remaining legs, lacking a claw (f).
Siphonethus obtusus sp. nov.
–
Figure 14. Siphonethus obtusus sp. nov. (NZAC03038954) from New Zealand. (a) Three male specimens). (b) Male, habitus, lateral.

Figure 15. Head of Siphonethus obtusus sp. nov. (NZAC03038954) from New Zealand, male, SEM images. (a) Anterior body, ventral view. (b) Head, frontal view. (c) Detail of labrum. (d) Gnathochilarium, ventral view. (e) Antennae. Scale: a = 200 µm, b, c, e = 100 µm, d = 20 µm. Abbreviations: I-VII = antennomere 1-7, ac = apical cones, at = antennae; co = collum, gn = gnathochilarium, in = incision of labrum, la = labrum, ms = macrosetae, om = ommatidia, sb = sensilla basiconica.
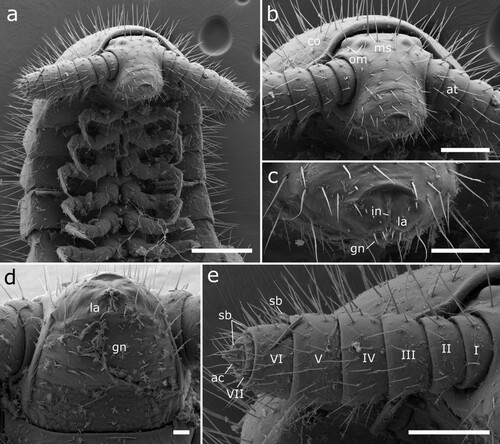
Figure 16. Body-rings and legs of Siphonethus obtusus sp. nov. (NZAC03038954) from New Zealand, male, SEM images. (a) Body-ring, dorsal view. (b) Details (mesal rectangular structure) of (a). (c) Posterior body-rings and preanal ring, ventral view. (d) Posterior leg. Scale: a, c = 100 µm, b = 10 µm, d = 20 µm. Abbreviations: cl = claw, cs = coxal sacs, cx = coxa, fe = femur, mz = metauonite, oz = ozopore, pa = paraproct, pof = postfemur, pl = pleurite, pn = paronychium, pr = preanal ring, prf = prefemur, ta = tarsus, ti = tibia, tr = trochanter.
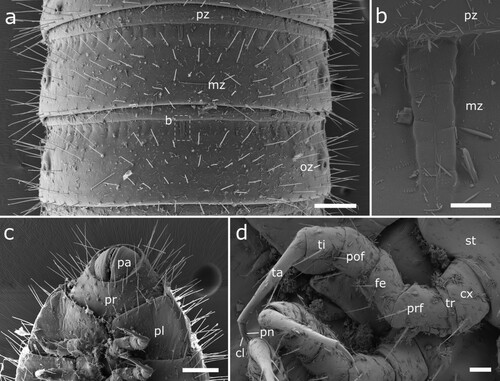
Figure 17. Male (a-d) and female (e) sexual characters of Siphonethus obtusus sp. nov. (NZAC03038954) from New Zealand, male, SEM images. (a) Coxa of leg pair 2 with gonopore, ventral view. (b-d) Gonopods. (b) Ventral view. (c) Posterior view. (d) Lateral view. (e) Female, second leg pair with gonopore, ventral view. Scale: a, e = 20 µm, b-d = 100 µm. Abbreviations: aI-aV = podomeres of anterior gonopod, cx = coxa, fe = femur, gp = gonopore, pe = penis, pI-pVI = podomeres of posterior gonopod, pof = postfemur, prf = prefemur, st = sternite, ta = tarsus, ti = tibia.
Material examined: Holotype: Male (NZAC03038954), NEW ZEALAND, Poor Knights Island, Tawhiti Rahi, Shag Bay, 40 m, 12.ix.1980, leg. J.C. Watt, Litter 80/73. Paratypes: 3 males, 1 female.
Etymology: The species name obtusus is derived from the Latin word obtusis = obtuse/blunt, and refers to the obtuse head of the species compared to the pointed head of many other Siphonotidae. Noun in apposition.
Diagnoses: S. obtusus sp. nov. differs from S. dudleycookeorum sp. nov. and S. coxaespinosus sp. nov. by the presence of elevated ozopores. Differs from S. dudleycookeorum sp. nov. by a stouter head, which lacks a sculptured labrum, as in S. coxaespinosus sp. nov.. S. obtusus sp. nov. differs from S. dudleycookeorum sp. nov. and S. coxaespinosus sp. nov. by the shape of the apical podomere of the anterior gonopod, this carries a mesal lobe covered by spines on the apical podomere. Differs from S. coxaespinosus sp. nov. by the absence of sternal protuberances behind the gonopods, the absence of special setae on the apical podomeres of the anterior gonopod and the absence of coxal protuberances.
Description:
Colouration in ethanol: Specimens pale, whitish, no colour pattern could be observed ().
Measurements: Ca. 10 times as long as wide. Length: 8 mm, Width: 0.7 mm. Up to 28 Tergites and 46 leg pairs.
Head: Head small, conical, stout (a, b). Labrum split anteriorly with triangular field lined anteriorly by short setae. Labrum with single pair of setae projecting beyond its margin (c). Two pairs of ommatidia and one pair of macrosetae positioned between ommatidia. Head covered by setae. Mandibles not visible externally (b).
Gnathochilarium: Stout triangular plate with anterior incision. Apically with two pairs of short setae. Covered by few setae, especially along anterior margin (d).
Antennae: Consisting of 7 antennomeres. Antennomeres wider than long. Antennomere 3–4 widest. Antennomeres 5–7 with row of sensilla basiconica. Apical disc with 4 sensory cones (e).
Collum: Collum large, saddle shaped, concealing posterior part of head, reaching to posterior ommatidia. Ca. as wide as following tergites. Covered by setae (a).
Body-rings: Up to 28 tergites + preanal ring. Body-rings semicircular, tergites well separated from pleurites and sternites. Tergites divided into prozonite and metazonite with distinct margin. Metazonite with faint longitudinal lines on anterior margin (a) and mesal rectangular structure (b). Metazonite with long setae arranged in 5 irregular rows. Ozopores starting on body-ring 5, laterally on metazonite, slightly elevated (a). Pleurites almost rectangular, with lateral marginal row of setae (a, c). Sternites carrying tracheal openings lateral of legs, also present lateral of the gonopods. Last body-ring anterior of anal ring apodous, pleurites overlapping.
Preanal ring: Preanal ring narrow cylindrical, forming completely fused ring around paraprocts. Covered by long setae, setae shorter ventrally of paraproct. Paraproct devoid of setae (c).
Legs: Up to 46 leg pairs. Leg 1 and 2 with 6 podomeres, from leg 3 onwards 7 podomeres (with trochanter). 1st leg pair slightly smaller than following legs, but not reduced in segmentation. Coxa carrying eversible sacs (coxal pouches) from leg pair 3 onwards (a), except for ultimate leg pairs. Tarsus long and narrow with apical claw and paronychium, paronychium ca. twice as long as claw (d).
Male sexual characters: Male gonopore positioned on coxa 2, on an elongated flattened/fan-shaped lobe (gonapohysis or penis) (a). Leg pairs 9 and 10 modified to well-developed gonopods (b–d).
Anterior gonopod consisting of 5 podomeres (segmentation mainly visible in posterior view), podomere 2 largest, podomere 2–4 each with single row of long setae. Podomere 5, basally with group of larger setae, apically with large plate-like outer lobe and small spoon shaped inner lobe, covered by small spines. Sternite with mesal pair of setae.
Posterior gonopod leg-like with 6 podomeres, devoid of setae. Apical podomere longest, draw out into long flagellum, ca as long as podomere 1–5 combined (b–d).
Female sexual characters: Coxae of second leg pair free, not touching. Gonopores (vulvae) on coxae of second leg pair, buldged, no division visible (e).
Siphonethus sp. 1
Material examined: DNA-Vouchers from Drummond et al. (Citation2015): Female (NZAC03011232) (a–c), NEW ZEALAND, Little Barrier Island, xii.2010, Plot 9 L (CM30C30), litter. Juvenile, (NZAC03012795) (d, e), NEW ZEALAND, Little Barrier Island, xii.2010, Plot 3 N, litter.
Figure 18. Siphonethus sp., specimens sequenced by Drummond et al (Citation2015). (a-c) Female (NZAC03011232). (a) Habitus, lateral. (b) Details of head. (c) Label. (d & e) Juvenile (NZAC03012795). (d) Habitus, dorsal. (e) Label.
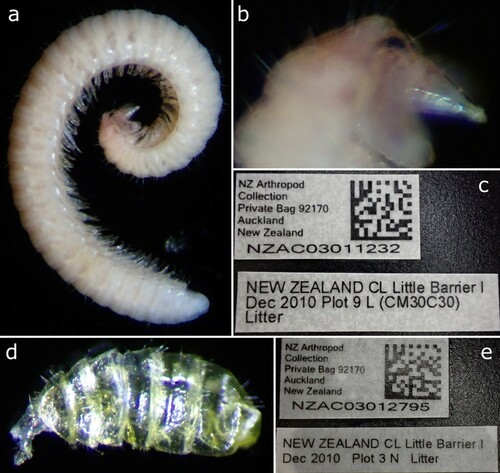
Remarks: The DNA-Voucher NZAC03011232 is a female with 35 tergites + preanal ring and is ca. 7 mm in length. The specimen is of uniform pale whitish colour, with partly transparent cuticle (a), which is probably the result of the lysis for DNA extraction (see Dummond et al. Citation2015). The head is pointed and slightly constricted anterior of the antennae as in Siphonethus dudleycookeorum sp. nov.. The specimen has two pairs of ommatidia and long macrosetae on the head (b). The body is densely covered by long setae. The anterior margin of the metazonite carries a median rectangular structure, as well as fine longitudinal striae as in S. dudleycookeorum sp. nov.. As the specimen is a valuable DNA-Voucher it was not dissected for further examination.
The DNA-Voucher NZAC03012795 is a small juvenile with 6 tergites and 6 leg pairs. The animal is hollow and the cuticle transparent (d) as a result of lysis for DNA extraction. The body is densely covered by setae and the head is pointed as in the female specimen.
Siphonethus sp. 2
Material examined: Female (NZAC03038956) (a, b), NEW ZEALAND, Poor Knights Island, Tawhiti Rahi, 10 Dec. 1980. G. Kuschel, sifted litter from dry water channel 80/148.
Figure 19. Siphonethus sp. (a & b) NZAC03038956. (a) Habitus, lateral. (b) Label. (c & d) NZAC3038957. (c) Habitus, lateral. (d) Label.

Remarks: Body uniform pale, with 48 tergites + preanal ring, ca. 9 mm in length. Head pointed, tapering anterior of antennae and with two ommatidia (a) as in Siphonethus dudleycookeorum sp. nov.. Body densely covered by long setae and metazonite with median rectangular structure and fine striae on anterior margin as in S. dudleycookeorum sp. nov.. As only a single female is present we hesitate to assign it to Siphonethus dudleycookeorum sp. nov.. The vial also includes a single Polyxenida specimen.
Siphonethus sp. 3
Material examined: Female (NZAC03038957) (c, d), NEW ZEALAND, Poor Knights Island, Tawhiti Rahi, ix.1980, pit trap 80/75
Remarks: Body uniform pale, with 25 tergites + preanal ring, ca. 5 mm in length. Head stout and with two pairs of ommatidia (c) as in Siphonethus coxaespinosus sp. nov. and Siphonethus obtusus sp. nov.. Body sparsely covered by short setae as in S. coxaespinosus sp. nov.. As only a single female is present we hesitate to assign it to Siphonethus coxaespinosus sp. nov..
Genetic analyses
Our distance analyses including all available COI barcodes for the Polyzoniida, showed a p-distance from the New Zealand specimens of 14.3% (NZAC 03012795) and 13.9% (NZAC 03011232) to Siphonethus dudleycookeorum sp. nov. (ZFMK MYR10095) from Great Britain. The genetic distance between the two specimens from New Zealand (KP421302.1; KP421754.1) was 0.2%. The genetic p-distance of S. dudleycookeorum sp. nov. to the only other members of the Siphonotidae for which sequence data are available, viz. Rhinotus purpureus and Eumillipes persephone was between 27.7% and 28%. The genetic distance to representatives of the families Hirudisomatidae and Polyzoniidae was comparatively high, 28% (Polyzonium germanicum, ZFMK-MYR1351, ZSM-MYR00428) and 31.1% (Hirudisomatidae sp., BIOUG32547-D08) (). Phylogenetic reconstruction () retrieved S. dudleycookeorum sp. nov. from Great Britain as a sister-group to the two unidentified specimens from New Zealand with a high bootstrap support (100%). A monophyletic Siphonotidae was retrieved with the three specimens from New Zealand as the sister-group to a clade comprising Eumillipes persephone and the two Rhinotus purpureus. Siphonotidae was supported by a bootstrap of 100%, Eumillipes persephone + Rhinotus purpureus was supported by a bootstrap of 98%. While Polyzoniida + Hirudisomatidae and a monophyletic Hirudisomatidae were supported by a bootstrap of 100%, a monophyletic Polyzoniidae only retrieved low support (44%).
Figure 20. Maximum likelihood tree of Polyzoniida based on the COI sequence after 1000 bootstrap replicates analyzed with the Hasegawa Kinshino Yano Model. Numbers on branches indicate bootstrap support. Codon positions included were 1st+2nd+3rd. All positions with less than 5% site coverage were eliminated. The tree is drawn to scale, with branch length indicating genetic distance. Specimens from New Zealand in green box.
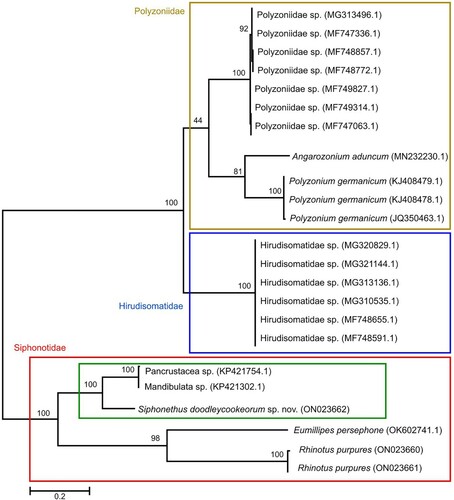
Table 3. Distance matrix. Numbers in parenthesis indicate the GenBank accession number and the voucher number.
Discussion
Siphonethus Chamberlin, Citation1920
The family Siphonotidae comprises 12 genera and ca. 30 species, of which the majority is in need of revisionary work, as genus specific characters are only poorly understood, and their taxonomy and classification is rather chaotic. Even the diagnostic characters of the family seem not fully clear as discussed by Shear (Citation2016). Problems arise from the poor quality of descriptions, and the rarity and small size of the Polyzoniida (Verhoeff Citation1924; Hoffman Citation1977). Most species descriptions are rather short, lacking detailed accounts on the species-specific gonopods and illustrations. As stated by Shear (Citation2016) future research on the Siphonotidae needs to include examinations of males of all nominal genera. We provisionally place the new species in Siphonethus Chamberlin, Citation1920, the only genus known from New Zealand, to avoid creating further chaos in the current classification of the Siphonotidae. The genus Siphonethus is a ‘source of considerable difficulty’ (Hoffman Citation1977: 431), as it might be a senior or a junior synonym of another siphonotid genus.
We suggest that the species currently classified as Siphonethus Chamberlin, Citation1920 constitute two distinct genera, with S. enotatus Chamberlin Citation1920 as the type species for Siphonethus. But detailed examinations of S. enotatus and S. bellus are needed for clarification. From the available data we conclude that Siphonethus enotatus Chamberlin, Citation1920 and Siphonethus dudleycookeorum sp. nov. may belong to a group with the following characters: head pointed and tapering anterior of antennae; antennae long, cylindrical, widening distally; head excavated at insertion of antennae, with transverse depression. The second group comprises S. bellus Chamberlin, Citation1920, S. coxaespinosus sp. nov. and S. obtusus sp. nov. and is characterised by the following characters: head stout, anteriorly rounded; antennae stout, moderately clavate. Setation and colouration seems to be variable, and the latter is probably impacted by the preservation and storage. Furthermore, S. dudleycookeorum sp. nov. has an anterior gonopod consisting of six podomeres, while in S. coxaespinosus sp. nov. and S. obtusus the anterior gonopod consists of five podomeres, and the state is unknown for S. enotatus and S. bellus (both are only known from females). While the shape and position of the male gonopore is used to distinguish polyzoniid families (Hoffman Citation1982; Enghoff et al. Citation2015), the female gonopore (vulva) remains largely unstudied and only view studies (e.g. Kurnik & Thaler Citation1989) use it as a diagnostic character. The shape and position of the female gonopore varies greatly between the studied specimens, although it has to be taken into account that it is unknown how the structure of the vulva changes during development. More detailed studies of the female gonopore might yield more taxonomically relevant characters to distinguish species, genera or even families.
Siphonethus dudleycookeorum sp. nov., S. coxaespinosus sp. nov. and S. obtusus sp. nov share characters with the genus Siphonotus Brandt, Citation1837 as the metazonite mesally carries a demarcated rectangular area at its anterior margin. However, this character has only been described for Siphonotus brasiliensis Brandt, Citation1837 (Hoffman Citation1977) and it is unknown if it is present in all Siphonotus species, or even common among the Siphonotidae in general. A similar structure has been described from the posterior margin of tergites 1–4 of Siphonotus latus Verhoeff, Citation1924 (fig. 97) from Australia. All species described here differ from undescribed species from the South Island of New Zealand, which will be described at a later date, by the absence of the rectangular structure on the anterior margin of the metazonite.
Diversity of the Polyzoniida on New Zealand
The genus Siphonethus Chamberlin, Citation1920 has previously been known from the two species Siphonethus enotatus Chamberlin, Citation1920 and Siphonethus bellus Chamberlin, Citation1920, both from the North Island of New Zealand. Not a single species has currently been described from the South Island. The siphonotid Rhinotus bivitattus (Pocock, Citation1894), which is also known from New Caledonia and the Loyalty Islands, is recorded from Tauranga, New Zealand (Carl Citation1926). However, Dawson (Citation1958) argues that the species might be introduced to the islands. It is also likely that the single female specimen, which was collected from New Zealand (leg. Dr. Thilenius) and was subsequently lost (Dawson Citation1958), was actually a representative of a different genus and/or species.
It can be expected that the assessment of natural history collections from New Zealand and Oceania, as well as more sampling efforts in the area, will lead to the discovery and description of numerous siphonotid species new to science. Fundamental for such an effort would be the detailed redescription of S. enotatus and S. bellus based on type material and freshly collected material (preferably males) from the type localities, including molecular analyses. We were able to study specimens from the South Island of New Zealand, which are distinct and clearly differ in somatic and gonopod characters from the species described here. These specimens await description. For Australia it is known that a great variety of siphonotid millipedes await description (Marek et al. Citation2021).
Six siphonotid species from Australia, placed in the three genera Siphonotus Brandt, Citation1837, Rhinotus Cook, 1896 and Eumillipes Marek, 2021 are described: Siphonotus brevicornis Pocock, Citation1903, Siphonotus flavimarginatus Attems, Citation1911, Siphonotus latus Verhoeff, Citation1924, Rhinotus michaelseni (Attems, Citation1911), Rhinotus mjöbergi Verhoeff, Citation1924, Eumillipes persephone Marek, 2021. However, in unpublished works, several more species and siphonotid genera, which await description, have been recorded (Shear Citation2016; Marek et al. Citation2021) and an unpublished revision of the Australian Polyzoniida has been undertaken (Black Citation1997). These Oceanian millipedes can be found mainly in wet forests (Black Citation1997). We suggest that especially the wet forests of Oceania and less accessible habitats, such as deeper soil layers (Marek et al. Citation2021), will harbour a great variety of unknown polyzoniidan species.
Introduction of Siphonethus dudleycookeorum sp. nov. to Geat Britain
Reports of alien species introduced into New Zealand are frequent with over 2,200 species of alien invertebrates alone, which cause considerable damage (Barlow & Goldson Citation2002). The opposite, the introduction of New Zealand species to other parts of the world, are rare. Siphonethus dudleycookeorum sp. nov. was probably transported to Great Britain by the transportation of plants from New Zealand. Hence, the specimens were encountered between tree ferns from New Zealand and Australia in Lamorran House Gardens. The blast search of the genetic barcode recovered unidentified Panarthropoda and Manidbulata from Little Barrier Island in New Zealand (Drummond et al. Citation2015) as closest match, and the species is morphologically highly similar to specimens from Tawhiti Rahi (Poor Knights Island, New Zealand). However, it seems unlikely that Siphonethus dudleycookeorum sp. nov has been directly introduced from Tawhiti Rahi, a small island, to which the access is highly restricted due to nature conservation efforts (Coulston Citation2002). We suggest rather that Siphonethus dudleycookeorum sp. nov. was introduced from the mainland of New Zealand to Great Britain and to Tawhiti Rahi as well, as the island was extensively cultivated over a long period, which has heavily impacted the native flora and fauna (Hayward Citation1993). The introduction of Siphonethus dudleycookeorum sp. nov. is probably facilitated by the similar mild, maritime climate in northern New Zealand and the southern part of the British Isles. The unique climatic conditions of Lamorran House Gardens, without frost since 1987, allow the growth of many subtropical and southern hemisphere flora (Lamorran House Gardens).
Another example of an alien soil invertebrates introduced by human activity from New Zealand to the British Isles is the New Zealand flatworm (Arthurdendyus triangulatus (Dendy, 1894)). As a predator of earthworms this flatworm has a negative effect on earthworm populations across its introduced range, and thereby on the nutrient cycle and also on native predators of earthworms (Murchie & Gordon Citation2013). As a non-predatory millipede, which is probably highly specialized to feed on more or less liquid food (Moritz et al. Citation2022), Siphonethus dudleycookeorum sp. nov. is unlikely to cause severe problems. The preferred food source of Siphonethus dudleycookeorum sp. nov. is unknown but for other Polyzoniida it is assumed that they feed on algae, bacterially degraded substances and fungal hyphae (Dunger Citation1993). Therefore, Siphonethus dudleycookeorum sp. nov. might be in competition with other species that graze on algae, bacteria and fungi. Unlike Arthurdendyus tringulatus, S. dudleycookeorum sp. nov. has not been recorded outside the botanical garden, as is also the case for Rhinotus purpureus and other alien millipedes, centipedes, and woodlice, which are only known from greenhouses in Europe (Decker et al. Citation2014; Stoev et al. Citation2010; Gregory & Lugg Citation2020).
Genetic analyses
As only very few COI sequences are available for Polyzoniida, with a total of 20 sequences from GenBank plus 3 sequences from this study covering 8 putative species, and as the COI sequence is only of limited value for inferring phylogenetic relationship, the phylogenetic hypothesis presented here has to be taken with caution. Based on the low genetic distance of 0.2% we suggest that the two unidentified Polyzoniida from Little Barrier Island, New Zealand (NZAC03012795 and NZAC03011232) are representatives of the same species, while the moderate genetic distance of 13.9% between these two specimens and Siphonethus dudleycookeorum sp. nov. suggests that the two species are congeneric. This is also supported by morphological data, as they share several somatic characters like the shape of the pointed head, which narrows anterior of the antennae, the presence of two ommatidia, the long and dense setation of the body and the rectangular structure and striation on the metazonites. We hesitate to describe the species from Little Barrier Island, as males are unavailable and a detailed examination with dissection of the valuable voucher specimens is not possible.
Acknowledgements
We thank Robert and Maria-Antoinette Dudley-Cooke for allowing free access to Lamorran House Gardens to survey for invertebrates and Jo Clark for assistance with the collection of specimens. We thank Darren Ward from the New Zealand Arthropod Collection (NZAC) for access to specimens from the collection of the NZAC and Laura Leibensperger (MCZ, Harvard, USA) for photographs of the types of Siphonethus enotatus and Siphonethus bellus in the collection of the MCZ. Furthermore, we thank Claudia Etzbauer (ZFMK) for DNA extraction and PCR. We are grateful to John Marris and two anonymous reviewers for their valuable comments and suggestions, which greatly improved the quality of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Affeld K, Worner S, Didham RK, Sullivan J, Henderson R, Olarte JM, Thorpe S, Clunie L, Early J, Emberson R. 2009. The invertebrate fauna of epiphyte mats in the canopy of northern rata (Myrtaceae: Metrosideros robusta A. cunn.) on the west coast of the South Island, New Zealand. New Zealand Journal of Zoology. 36:177–202.
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 25(17): 3389–3402.
- Astrin JJ, Stüben PE. 2008. Phylogeny in cryptic weevils: molecules, morphology and new genera of western palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebrate Systematics. 22(5): 503–522.
- Attems CG. 1911. Myriapoda exkl. Scolopendridae. In: Ergebnisse Der hamburger südwest-australischen forschungsreise 1905 (eds. W Michaelsen, R Hartmeyer) pp. 147–204. Verlag von Gustav Fischer. Jena.
- Barlow ND, Goldson S. 2002. Alien invertebrates in New Zealand. In: Biolological invasions: economic and environmental cost of alien plant, animal and microbe species (ed. D Pimentel) pp. 195–216. CRC Press. Boca Raton.
- Black DG. 1997. Diversity and biogeography of Australian millipedes (Diplopoda). Memoirs of Museum Victoria. 56:557–561.
- Blower JG. 1985. Millipedes: keys and notes for the identification of the species. London: Brill. 242 p.
- Brandt JF. 1837. Note sur un ordre nouveau de la classe des myriapodes et sur 1’éstablissement des sections de cette classe d’animaux en général. Bulletin Scientifique de L’Académie Impériale des Sciences de Saint-Pétersbourg. 1:178–179.
- Carl J. 1926. Diplopoden von Neu-caledonien und den loyalty-inseln. In: Nova caledonia, Zoologie Vol. IV, L. III (eds. F Sarasin, J Roux) pp. 369–462. Kreidel’s Verlag. München.
- Chamberlin RV. 1920. The myriopoda of the Australian region. Bulletin of the Museum of Comparative Zoology. 64:1–269.
- Cook OF, Collins GN. 1895. The craspedosomatidae of North America. Annals of the New York Academy of Sciences. 9:1–100.
- Coulston GJ. 2002. Control of invasive plants on the Poor Knights Islands, New Zealand. In: Turning the tide: The eradication of invasive species (eds. CR Veitch, MN Clout) pp. 79–84. IUCN. Gland.
- Dawson E. 1958. Exotic millipedes (Diplopoda) in New Zealand. New Zealand Entomologist. 2:1–5.
- Decker P, Reip HS, Voigtländer K. 2014. Millipedes and centipedes in German greenhouses (myriapoda: diplopoda. Chilopoda). Biodiversity Data Journal e. 1066:1–43.
- Derraik JGB, Barratt BIP, Sirvid P, Macfarlane RP, Patrick BH, Early J, Eyles AC, Johns PM, Fraser PM, Barker GM, et al. 2001. Invertebrate survey of a modified native shrubland, brookdale covenant, rock and pillar range, otago, New Zealand. New Zealand Journal of Zoology. 28:273–290.
- Drummond AJ, Newcomb RD, Buckley TR, Xie D, Dopheide A, Potter BCM, Heled J, Ross HA, Tooman L, Grosser S, et al. 2015. Evaluating a multigene environmental DNA approach for biodiversity assessment. GigaScience. 4(1): 1–19.
- Dunger W. 1993. Antennata. In: Lehrbuch der speziellen zoologie, band I: wirbellose tiere, 4. teil: arthropoda (ohne insecta) (ed. H-E Gruner. Gustav Fischer Verlag. Jena. pp. 1031-1214.
- Enghoff H, Golovatch S, Short M, Stoev P, Wesener T. 2015. Diplopoda — taxonomic overview. In: Treatise on zoology - anatomy, taxonomy, biology. The myriapoda, Volume 2 (ed. A Minelli) pp. 363–453. Brill. Leiden.
- Felsenstein J. 1985. Phylogenies and the comparative method. The American Naturalist. 125(1): 1–15.
- Golovatch SI. 2014. On some new or poorly-known millipedes from Chile and Argentina (diplopoda). Russian Entomological Journal. 23(4): 249–281.
- Golovatch SI, Kime RD. 2009. Millipede (Diplopoda) distributions: A review. Soil Organisms. 81:565–597.
- Gregory SJ, Lugg K. 2020. Some recent observations of woodlice (isopoda: oniscidea), millipedes (Diplopoda) and centipedes (Chilopoda) from artificially heated glasshouses. Bulletin of the British. Myriapod and Isopod Group. 32:35–43.
- Hall T. 1999. Bioedit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series. 41:95–98.
- Hasegawa M, Kishino H, Yano TA. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution. 22(2): 160–174.
- Hayward BW. 1993. Prehistoric archaeology of the Poor Knights Islands, northern New Zealand. Tane. 34:89–105.
- Hebert PD, Ratnasingham S, De Waard JR. 2003. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society of London. Series B: Biological Sciences. 270:96–99.
- Hoffman RL. 1977. On the status of Siphonotus brasiliensis brandt, and of the diplopod family Siphonotidae (Polyzoniida) (diplopoda). Deutsche Entomologische Zeitschrift. 24:425–431.
- Hoffman RL. 1982. Diplopoda. In: Synopsis and classification of living organisms (ed. SP Parker) pp. 689–724. McGraw-Hill. New York City.
- Jeekel CAW. 1971. Nomenclator generum et familiarum diplopodorum: A list of the genus and family-group names in the Class Diplopoda from the 10th edition of linnaeus 1758, to the end of 1957. Monografieën van de Nederlandse Entomologische Verenigung. 5:1–412.
- Johns P. 1962. Introduction to the endemic and introduced millipedes of New Zealand. New Zealand Entomologist. 3:38–46.
- Kime D, Enghoff H. 2011. Atlas of European Millipedes (Class Diplopoda), Volume 1. Order Polyxenida, Glomerida, Platydesmida, Siphonocryptida, Polyzoniida, Calipodida, Polydesmida. Pensoft, Sofia-Moscos. 282p.
- Korsós Z, Johns PM. 2009. Introduction to the taxonomy of Iulomorphidae of New Zealand, with descriptions of two new species of Eumastigonus Chamberlin, 1920 (Diplopoda: Spirostreptida: Epinannolenidea). Zootaxa 2065: 1–24.
- Kurnik I, Thaler K. 1989. Über verbreitung und taxonomie von Colobognatha der alpen (diplopoda, polyzoniida). Mitteilung der Schweizerischen Entomologischen Gesellschaft. 62:183–198.
- Lamorran House Gardens. About Us. 2022. Retrieved January 27, 2022 from http://lamorrangardens.co.uk/about.html.
- Marek PE, Buzatto BA, Shear WA, Means JC, Black DG, Harvey MS, Rodriguez J. 2021. The first true millipede—1306 legs long. Scientific Reports. 11:1–8.
- Mauriès JP, Silva F. 1971. Colobognathes du chili - I. espèces nouvelles du genre Siphonotus Brandt (diplopoda). Bulletin du Muséum National D’Histoire Naturelle 2e Série, Tome. 43(5): 887–902.
- Moritz L, Borisova E, Hammel JU, Blanke A, Wesener T. 2022. A previously unknown feeding mode in millipedes and the convergence of fluid feeding across arthropods. Science Advances. 8(7): 1–9.
- Murchie AK, Gordon AW. 2013. The impact of the ‘New Zealand flatworm’, Arthurdendyus triangulatus, on earthworm populations in the field. Biological Invasions. 15:569–586.
- Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. New York: Oxford University Press. 348 p.
- Newport G. 1844. [Untitled]. Proceedings of the Linnean Society of London. 1:191–196.
- Pocock RI. 1894. Contributions to our knowledge of the Arthropod fauna of the west indies. part III. Diplopoda and malacopoda, with a supplement on the arachnida of the class pedipalpi. The Journal of the Linnean Society. 24:473–519.
- Pocock RI. 1903. Remarks upon the morphology and systematics of certain chilognathous diplopods. The Annals and Magazine of Natural History; Zoology, Botany, and Geology. 7:515–532.
- Read HJ. 2007. Records of millipedes from Kew Gardens and the eden project, including descriptions of three species. Bulletin British Myriapod and Isopod Group. 23:27–35.
- Rhinotus purpureus (Pocock, 1894). Millibase. Retrieved January 27, 2022 from https://www.millibase.org/aphia.php?p = taxdetails&id = 946605
- Shear WA. 2016. Redescription of the South African millipede cylichnogaster lawrenci verhoeff, 1937 and notes on the family Siphonotidae (diplopoda, polyzoniida). Zootaxa. 4079(1): 119–128.
- Shelley RM. 2000. Annotated checklist of the millipeds of florida (arthropoda: diplopoda). Insecta Mundi. 14:241–251.
- Sierwald P, Bond JE. 2007. Current status of the Myriapod class Diplopoda (millipedes): taxonomic diversity and phylogeny. Annual Review of Entomology. 52:401–420.
- Silvestri F. 1896. Diplopodi Parte I - Sistematica. Annali del Museo civico di storia naturale di Genova, serie 2. 16:121–254.
- Sinclair L, McCartney J, Godfrey J, Pledger S, Wakelin M, Sherley G. 2005. How did invertebrates respond to eradication of rats from kapiti island, New Zealand? New Zealand Journal of Zoology. 32:293–315.
- Stoev P, Zapparoli M, Golovatch S, Enghoff H, Akkari N, Barber A. 2010. Myriapods (myriapoda). chapter 7.2. BioRisk. 4(1): 97–130.
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution. 10(3): 512–526.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 30(12): 2725–2729.
- Verhoeff K. 1924. Results of Dr. E. mjöberg’s Swedish Scientific expeditions to Australia 1910–1913. 34. myriapoda: diplopoda. Arkiv för Zoologi. 16:1–142.
- Watt JC. 1982. Terrestrial arthropods from the Poor Knights Islands, New Zealand. Journal of the Royal Society of New Zealand. 12:283–320.
- Wesener T. 2014. Redescription of Polyzonium malagassum, a new synonym of Rhinotus purpureus (pocock, 1894), with notes about the occurrence of the order Polyzoniida on Madagascar (diplopoda). Zootaxa. 3790:587–594.
- Wesener T. 2015. No millipede endemics north of the Alps? DNA-barcoding reveals glomeris malmivaga verhoeff, 1912 as a synonym of G. ornata koch, 1847 (diplopoda, glomerida, glomeridae). Zootaxa. 3999(4): 571–580.