Abstract
In this study, capillary alginate gel (Capgel™), a collagen and alginate based self-assembling biomaterial, was used as a cellular scaffold for the treatment of ischemic, full-thickness dermal wounds in mice. Capgel™ was synthesized using copper sulfate to form the initial sol before rinsing and stabilizing the patent capillary structures with carbodiimide chemistry. This crosslinked hydrogel was then injected into ischemic, full-thickness dermal wounds and analyzed via histology after 7 and 10 days to assess wound contracture, granulation bed tissue and vascular structures. Capgel™ showed good resorbability and was well-invested with infiltrating host cells and vascular structures during and after resorption.
GRAPHICAL ABSTRACT
1. Introduction
Chronic wounds affect millions of Americans at tremendous cost to the healthcare system, with an estimated cost of $33 billion in 2016.[Citation1–3] The resolution of full thickness dermal wounds requires a sequence of events orchestrated by several different cell types. Macrophages, keratinocytes, dermal fibroblasts and endothelial cells must migrate, repopulate and restructure the injury site to allow for the regeneration of functional dermis and to limit the formation of a scar.[Citation4] This process of wound healing can be broken down into three distinct steps: inflammation, proliferation and repair.[Citation5] Each of these steps is defined by the presence of particular subsets of the aforementioned cell types. During the early, inflammatory stage of healing, cells of the myeloid lineage, such as macrophages and neutrophils, migrate into the wound bed to initiate the clearing of debris and pathogens.[Citation6,Citation7] Following this, a proliferative phase ensues whereby the macrophages that have infiltrated during the inflammatory phase provide biochemical queues to migrating keratinocytes[Citation8] to help re-epithelialize the epidermis, and to dermal fibroblasts and endothelial cells for the de novo formation of blood vessels[Citation9] Finally, a reparative remodeling stage helps to shape newly deposited extracellular matrix proteins (ECM) into functional mature tissue akin to uninjured tissue.[Citation10]
Obstacles to the reparation and functional maturation of full thickness wounds include contracture of the wound site, desiccation of the tissue and insufficient granulation bed formation during the inflammatory phase. Further exacerbating these defects in normal reparative function are conditions that pertain to ischemic and diabetic tissue, including lack of oxygen and defects in the inflammatory response.[Citation10] The endothelium and response to growth factors may also be perturbed in diabetic patients.[Citation11]
The use of a flowable, dermal support matrix comprised of a resorbable biomaterial could help circumvent these obstacles by limiting contracture, aiding guidance of infiltrating immune cells and supporting de novo formation of vasculature in the healing wound bed. Injectable synthetic and natural substrates have been used previously to provide an extracellular support matrix allowing for both the study of cellular processes and clinical wound healing applications. Base substrates such as collagen, fibrin, hyaluronic acid, polyacrylamide, polyethylene glycol and others have previously been studied for wound healing applications. Unfortunately, many poorly resorbable materials remain in tissue for long durations, having undesirable side effects such as late-onset inflammation.[Citation12] Another challenge presented is the lack of negative space within the bulk of injectable materials which limits the formation of granulation tissue even if reepithelialization occurs.[Citation13,Citation14] Therefore, a resorbable material may be better suited for therapeutic applications. The current gold standard for flowable wound care matrices is the INTEGRA® Flowable Wound Matrix, which has been shown to improve wound healing in patients.[Citation15] More recently, materials such as hydrogels decorated with functional peptides and microporous annealed matrix scaffolds consisting of poly (ethylene) glycol-vinyl sulfone (PEG-VS) spheres with RGD peptides have shown great promise in the wound healing field.[Citation16] The regenerative environment provided by the biomaterial would help not only to improve clinical outcomes but also may enhance the cosmetic appearance of healed skin by limiting scar formation. Optimizable aspects of these materials include, but are not limited to, tuning of the material’s modulus and adjusting the extent of negative space available within the scaffold. Both will help to promote cellular infiltration and ultimately, regenerative tissue formation.
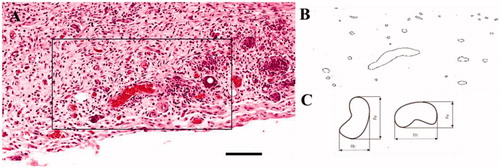
Capillary Alginate Gel (Capgel™) is a self-assembling, flowable hydrogel made from alginate and oligomeric gelatin that consists of many patent capillaries running parallel to each other throughout the length of the gel.[Citation17] Capgel™ is a stable matrix that is crosslinked via carbodiimide chemistry, forming peptide bonds throughout the hydrogel network. Capillary diameters can be tuned from 10 μm to over 100 μm (), allowing a single cell or several to infiltrate individual capillaries. This tailored material has proven useful in applications such as injectable tissue scaffolding systems for cell delivery, infiltration, and host integration of newly formed tissue. Capgel™ as a flowable slurry () has previously been injected into the left ventricle (subepicardial) to assess possible therapeutic applications in the heart. After one month, Capgel™ was observed as being well-received by the host tissue, having been infiltrated by host cells, blood vessels and containing a collagen matrix.[Citation18]
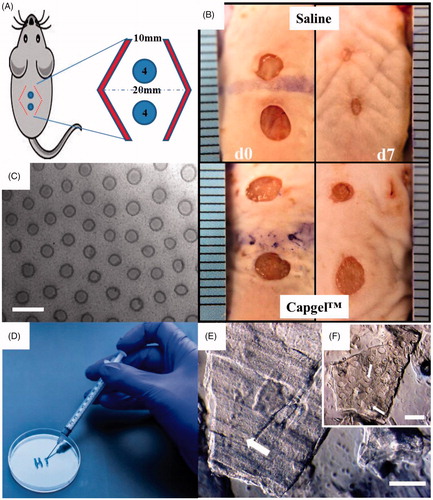
Figure 1. Overview of wound model generation and treatment methods. (A&B) Mice had 4 mm wounds created with a biopsy punch and a bipedical flap (red chevrons) was created to mimic ischemia. Mice received either saline or were treated with Capgel™. (C) Phase micrograph showing the microcapillary structure of Capgel™. (D) Capgel™ flowed in a controllable fashion from a syringe and (E&F) retained open capillaries (arrows) as entangled microparticles. Scale Bar = 200 µm.
The patent capillaries in Capgel™ provide a reservoir for soluble factors and wound exudate as well as surface area for host cell adhesion, migration and differentiation. The volumizing ability of Capgel™ aids in limiting wound contracture, thus providing a larger granulation bed more consistent with the original volume of the affected site. The steady-state elastic modulus of Capgel™ has previously been recorded at 3.16 ± 0.60 kPa, which is well below the stiffness for fibrotic tissue formation by fibroblasts of 20 kPa.[Citation19,Citation20] Limiting formation of fibrotic tissue may also serve to further limit wound site contracture. Altogether, this favorable mechanical environment, along with the resorbable nature of the peptide linkages in the Capgel™, allows the material to be well-received by the host tissue with little material being excluded from the wound bed and most material being resorbed by 10 days after wounding. This study sought to utilize Capgel’s™ characteristics to improve clinically relevant outcomes including the limiting of contracture, the increase in granulation bed volume and the revascularization of the wound bed within an ischemic wound model mimicking the difficult healing environment found in diabetic patients.
2. Methods
2.1. Generation of capillary alginate gel (Capgel™)
Capgel™ was prepared as previously described.[Citation21] Briefly, 300 g bloom gelatin (G1890, Sigma-Aldrich) was prepared at 10% gelatin by dissolution in water and degraded to oligomeric form by heating the solution at 80 °C for 72 h. After allowing the gelatin solution to return to room temperature, sodium alginate powder (Protanal CR8133, FMC Biopolymer) was added to achieve final concentrations of 2% alginate and 2.6% gelatin. A glass petri dish coated with baked on layers of 4% alginate solution was placed inside a larger glass container and the parent solution was poured into the petri dish. A Kimwipe® soaked in 0.5 M copper sulfate was pulled taut over a plastic needle point ring, laid over top of the petri dish containing the parent solution and 0.5 M copper sulfate was dripped onto the gel covering portion of the Kimwipe® for 10 min. The kimwipe was then removed and the larger dish filled with 0.5 M copper sulfate as to cover the Capgel™ forming inside the petri dish. After 72 h, the formed Capgel™ was removed from the petri dish, sectioned into strips and allowed to rinse in cold distilled, deionized water for 4 days, rinsing once every day. After rinsing, the strips were cut into ∼5 × 5×3 mm blocks and cross-linked via carbodiimide chemistry (Sulfo-NHS 24525, ThermoFisher; EDC, E7750, Sigma-Aldrich). After cross-linking, the blocks were rinsed in saline and citrate until all remaining copper sulfate and carbodiimide chemistry reagents were removed. The blocks were then autoclaved in saline and stored at 4 °C until use.
2.2. Surgery
All animal experiments were approved by the University of Central Florida Institutional Animal Care and Use Committee, and animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals. A minimum of three 8–11 week old C57Bl/6J adult male mice (Jackson Laboratories, Bar Harbor, ME) per treatment option underwent bipedicle dermal wound surgery. Two 4 mm wounds were made with a biopsy punch (Acuderm, Ft. Lauderdale, FL) by folding the skin perpendicular to the spine below the thoracic hump and above the hips. A bipedical flap was created using micro-serrated dissecting scissors in a rounded, football shape with 1 cm pedicles on the edges and up to 2 cm at the middle, widest point. The skin was separated from the muscle and any blood vessels outside of the pedicles were severed (). The flap was closed with either Vetbond (3M, St. Paul, MN) or Wound Strips (CVS, Woonsocket, RI). Wound punches were treated with saline or Capgel™ slurries (i.e., fragments) injected through a 26G needle. Mice were maintained in an environment with a temperature of 21–23 °C, 40–60% relative humidity and access to ad libitum water and PicoLab rodent diet 5053 pellets (LabDiet, St. Louis, MO).
2.3. Dressing and maintenance
Wounds were occlusively dressed with multiple dressing options due to the behavior of the mice (e.g., gnawing, scratching or grooming) though there were no quantifiable statistical differences in study outcomes between dressing options. Primary dressing options included Tegaderm®, Adaptic® (Johnson and Johnson, New Brunswick, NJ) with petroleum or sealing the wound with Vetbond® (3M). Secondary dressings included Elastikon® vet wrap (Johnson and Johnson), 0.5 mm thick silicone sheets (TP, Lawrenceville, GA) sutured on or silicone skin adhesive (Uro-Bond III 5000, Urocare Products, Pomona, CA). Dressings were monitored daily and changed if compromised. Animals with dressings that failed due to animal behaviors and allowed wounds to desiccate were excluded from analysis. Animals were anesthetized with isoflurane for final wound assessment and digitally photographed prior to euthanasia by overdose of isoflurane and aortic transection.
2.4. Histology and Immunohistochemistry
Wounds were excised from the margins of the ischemic flap, affixed to aluminum foil with a pin to keep them flat and fixed in 10% neutral buffered formalin overnight. Tissues were processed routinely and embedded in paraffin wax. Sections (Citation5 µm) were cut and affixed to Superfrost™ Plus slides (Thermo Fisher Scientific Inc., Waltham, MA). Paraffin was removed with xylene and the tissue hydrated with water before being stained with hematoxylin and eosin (H&E), Masson’s trichrome (kit) and for immunohistochemistry. Endogenous peroxidase was blocked with 3% peroxide for 10 min, followed by 15 min each of avidin and biotin (Vector Biolabs, CA) and then 5% horse serum for 20 min. Macrophages were characterized with anti-F4/80 antibody (1:50 dilution, Santa Cruz, Dallas, TX) 1 h RT with DAB (Vector Biolabs) and counterstained with hematoxylin.
2.5. Wound histopathology
Wounds were evaluated by light microscopy 7 and 10 days post-surgery using a semi-quantitative grading scheme adapted by a veterinary pathologist who is board certified by the American College of Veterinary Pathologists (author KMDL). Clinically relevant outcomes that were assessed include: re-epithelialization (0: none, significant epidermal defect; 1: partial, incomplete, discontinuous; 2: complete with epidermis of normal thickness; 3: complete with hyperplastic epidermis); acute (neutrophils) and chronic (lymphocytes, plasma cells, macrophages) inflammation (0: none; 1: scant; 2: moderate; 3: abundant); granulation tissue formation (0: none; scant, partial across wound gap or depth, immature and loose; 2: moderate, partial across wound gap or depth; immature and loose; 3: abundant, complete across wound gap and entire depth, mature and dense); and, neovascularization (0: none; 1: up to 5 vessels/high power (40×) field (hpf); 2: 6–10 vessels/hpf; 3: >10 vessels/hpf).
2.6. Quantification of microvasculature, neovascularization and granulation tissue
Size and density of blood vessels were analyzed from H&E stained histology slides. Criteria for identifying a vessel included the presence of a red blood cell(s) within the structure, lining of the structure by a cell with flattened morphology and the overall shape of the structure (i.e., round in shape). All three criteria must have been met before inclusion of the vessel. Tracing and quantification of the blood vessels was performed in ImageJ (). Vessel size was quantified using the minimum Ferret’s diameter ().[Citation22,Citation23] Regions of interest (ROIs) in a minimum of eleven stained slides per condition were chosen to quantify the wound area. Briefly, boundaries for the lateral margin were chosen by extending a line 250 µm from the wound edge, denoted by a change in collagen density and the edge of the panniculus carnosus muscle. This lateral margin extended from the top to the bottom of the wound. Granulation tissue area was quantified using ImageJ with an ROI being drawn within the wound bed.
2.7. Statistical analysis
t-tests were used to evaluate differences in mean wound area, granulation tissue and the diameter of new blood vessels in the wound. Differences in means were evaluated according to dressing type by evaluation day (d7 or d10). Differences in means within dressing type by day were also evaluated. Statistical significance was defined as P < .05.
3. Results
Mice, after having undergone bipedicle dermal wound surgery, had their wound sites treated with either Capgel™ slurries or saline alone and were sacrificed and assessed via histology at d7 and d10. Photomicrographs of Capgel™ samples at d7 show a flowable matrix replete with infiltrating neutrophils, macrophages and some vasculature with Capgel™ along the lateral margins of the wound bed beginning to be resorbed (). This dermal support matrix was quantified and compared to saline controls to characterize the wound site for infiltration of neutrophils and macrophages, the presence of blood vessels, contracture of the wound bed and formation of granulation tissue.
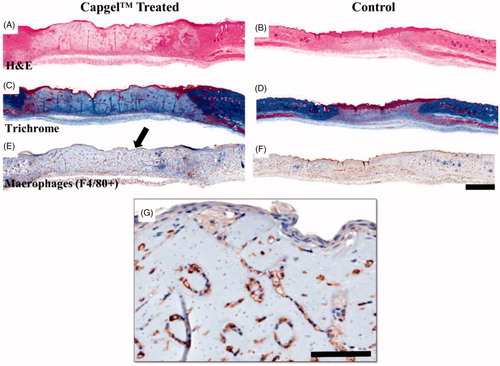
Figure 3. Representative d7 histological images of control (saline) and well-integrated Capgel™-treated wounds. (A, B) H&E staining, (C, D) Trichrome staining and (E-G) immunohistochemical F4/80 staining; Capgel™-treated wounds showed a larger wound bed that resisted contracture compared to saline controls as evident by the edges of the panniculus carnosus muscle. Arrows indicate location of photo inset. (G) Inset, lining of Capgel™ with infiltrating host cells. Scale bar =1 mm (whole tissue), 100 µm (inset).
3.1. Capgel™ modulates the inflammatory environment and prolongs initial inflammatory phases
Wound sites were assessed via H&E in conjunction with Masson’s trichrome and F4/80 immunostaining to assess ECM content and macrophage infiltration, respectively (). Histology of Capgel™ treated wounds showed the patent capillaries of the hydrogel as well as the surfaces between the injected Capgel™ particles were lined with host cells. At d7, F4/80 labelled cells filled and lined the gel in addition to many neutrophils (). This infiltration and lining of our novel biomaterial is, to our knowledge, the first time a flowable material has been used to facilitate the beginnings of capillary-like structures in vivo using the existing microarchitecture inherent to the material.
Immunohistochemical analysis by an independent pathologist (author KMDL) of the d10 wound healing samples reports the delay in acute inflammation in Capgel™ samples. This delay was marked by lesser scores for acute inflammation (AI) and chronic inflammation (CI) for saline (d7: AI = 1, CI = 1.6; d10: AI = 0.8, CI = 1) compared to Capgel™ (d7: AI = 2.3, CI = 1.3; d10: AI = 3, CI = 2) over time. There were few multinucleated giant cells and lymphocytes observed at d7 and d10 for saline controls compared to Capgel™ treated conditions which showed many neutrophils and foamy, F4/80+ macrophages within the scaffold provided by the material. By d10, most of the Capgel™ was resorbed into the tissue with very little or none being found outside the most central portion of the wound bed. The abundance of neutrophils may be correlated to the chronic wound healing such as that found in diabetic tissue/ulcers. Of note, intralesional bacteria were not apparent in any histology sections.
3.2. Capgel™ provides for greater degrees of revascularization and vascular density
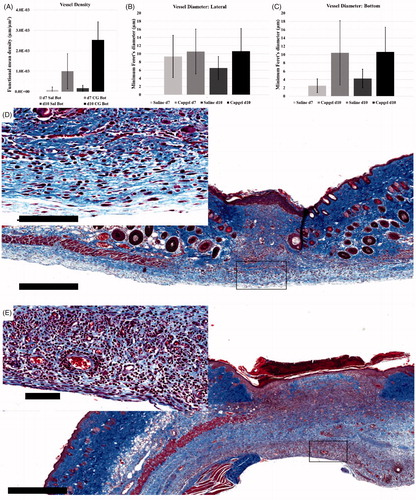
The functional microvasculature density (FMD) of lateral ROIs was found to be similar between saline controls and Capgel™ treated groups at d7 and d10 though there were more capillaries present overall in the less contracted Capgel™ treated wounds. However, differences in FMD at the bottom of the wound bed were noted with saline groups having lower vascularization compared to Capgel™ treated animals (). The mean diameter of new blood vessels in the wound bed was compared according to dressing type, location in the wound and day of measurement. Comparing lateral wound areas on d7 of observation, mice with Capgel™ had significantly higher mean vessel diameter (mean difference = 1.21, std. dev. = ±5.37, p = .02). This difference was larger at d10 of observation (mean difference = 4.12, std. dev. = ±5.10, p < .01). Comparing bottom wound areas (i.e., deepest portion of wound) on d7 of observation, vessels with Capgel™ dressing had significantly larger diameter (mean difference = 7.93, std. dev. = ±7.16, p < .01) but this difference narrowed by d10 of observation (mean difference = 6.40, std. dev. = ±5.87, p < .01). (Note: very few observations for saline with bottom wounds: n = 13 at d7, n = 12 at d10). For lateral wounds, there was no difference in vessel diameter according to day of observation for Capgel™. However, for saline, there were significantly larger vessels on day 7 of observation versus day ten (mean difference = 2.83, std. dev. = ±4.55, p < .01). For bottom wound areas, again, there was no difference according to day of observation for Capgel™. For saline, there was a difference, but for this wound location, vessels at day 10 were significantly larger than day 7 (mean difference = 1.72, std. dev. = ±1.95, p = .04) (sample size, i.e., the number of total vessels counted within control, was again low in this comparison).
Figure 4. Quantification and corresponding representative micrographs of control (saline) and Capgel™-treated wound microvasculature. (A) Comparison of vessel density between control and Capgel™-treated wounds; minimum Feret’s diameter analysis of lateral (B) and (C) bottom wound ROIs. Micrographs of Trichrome staining from d10 mice: (D) control, (E) Capgel™. Capgel™-treated wounds (E) had more microvessels present than saline controls. Scale bars = 500 µm (full image), 100 µm (inset). Sal = Saline.
3.3. Wound contracture is prevented by the addition of a Capgel™ dressing
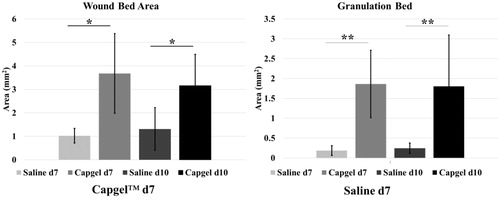
Granulation tissue (GT) analysis by an independent pathologist (author KMDL) showed similar scores for saline (d7: GT = 1.4; d10: GT = 2) compared to Capgel™ treated conditions (d7: GT = 1.3; d10: GT = 2.3) when assessing for maturity of the GT bed and the density of collagen. Photomicrographs and pathology scores indicated a loose collagen matrix forming in both dressing conditions. Differences between dressing conditions were more apparent when assessed for area and, subsequently, wound contracture (). The mean wound area at d7 for mice with Capgel™ dressing was 3.68 mm2 (std. dev. = ±1.70 mm2) compared to 1.02 mm2 (std. dev = ±0.31 mm2) for mice with saline. This resulted in a statistically significant difference in means (mean difference = 2.65, std. dev. = ±1.28, p = .02). Similar results were obtained for d10. There was no significant difference in the wound area by day of observation according to dressing type. The mean area of granulation tissue at d7 was 1.86 mm2 (std. dev. = ±0.85 mm2) for mice with Capgel™ dressing compared to 0.19 mm2 (std. dev = ±0.12 mm2) for saline. This resulted in a statistically significant difference in the amount of granulation tissue between the two groups (mean difference = 1.67, std. dev. = ±0.66, p < .01). Similar results were obtained for d10. There was no significant difference in the amount of granulation tissue by day of observation according to dressing type (). By d10, most of the gel was resorbed though remnants could be appreciated in the center of the wound via Masson’s trichrome staining.
4. Discussion
For the resolution of a wound to occur, several processes must be carried out in succession. Wounds move from an inflammatory phase to a proliferative phase and finally a repair phase that leaves the tissue as close to its original state as possible.[Citation4] First among these phases, the inflammatory phase, is characterized by the infiltration of neutrophils which aid in wound healing by the secretion of cytokines, the phagocytosis of pathogens and the clearing of debris.[Citation5] A biomaterial which prevents the premature closure of a wound (i.e., contracture) while also allowing for the infiltration of cells during the inflammatory phase would prove, overall, to be an effective therapeutic option for full-thickness dermal wounds. In order to elucidate the performance of Capgel™ in such a scenario, an ischemic wound model was chosen as this model can mimic one of the major factors that contributes to the impaired healing environment found in diabetic patients.
In this study, Capgel™ has shown potential as a dermal wound support matrix that aids in the infiltration of reparative inflammatory cells and also promotes granulation tissue formation, neovascularization and possibly the reduction of scar tissue by prolonging the initial inflammatory phase. The patent capillaries (i.e., negative space) inherent to Capgel’s™ morphology allows for this immune cell infiltration as well as possibly the beginnings of tissue formation all without relying upon the material degradation or resorption, such as with biomaterials with no vacancies throughout their bulk.[Citation24] This is readily apparent via histology which shows the capillary structures of the gel lined with both neutrophils and F4/80+ macrophages (). This initial infiltration of the capillary structures helped create a granulation bed of maximum volume all while potentially paving the way for increased neovascularization, as more vessels overall were present in wounds treated with Capgel™.
Traditionally, degradation of a biomaterial scaffold or implant has proven paramount to allowing new tissue development in skin wounds as many models have lacked negative space.[Citation25] Previous studies with PEG macromers and polysaccharide-based scaffolds have provided reepithelization in burn and diabetic wound models but granulation bed tissue formation and deposition of extracellular matrix proteins within the wound bed was prevented due to what is effectively a monolith of material precluding the infiltration of cells prior to material resorption.[Citation13,Citation14] Available materials such as INTEGRA® Flowable have microporous and fibrous characteristics though the extent of negative space within these injected scaffolds are generally smaller and more heterogeneous than that of Capgel. While the pore sizes of matrices such as INTEGRA® are typically large enough to allow cellular infiltration (25 µm), recent studies observed only peripheral granulation tissue and neovascularization and only single cells being able to penetrate into the interior of the implant INTEGRA® matrix.[Citation26] Similarly, a study investigating dextran-based hydrogels noted that the slower resorption time of INTEGRA limited neutrophil infiltration and subsequent neovascularization of the wound bed.[Citation27] In contrast, the negative space provided by the Capgel™ capillaries may promote more cellular infiltration as Capgel™ was replete with cells prior to material resorption (). The maintenance of the microstructure architecture within Capgel™ following injection () makes this biomaterial unique compared to current products such as INTEGRA®. Further, Capgel™ presents a more consistent and homogeneous biomaterial scaffold across the wound bed from the injected particles that includes the regularly spaced capillary microstructures inherent to Capgel™. More recently, injectable materials have been explored that polymerize in vivo upon injection and retain tunable negative spaces that allow for the ready infiltration of host immune cells.[Citation16] Similarly, Capgel™ provides void spaces for host cells to form new vasculature in the absence of material degradation via capillary structures inherent to the material’s structure (). Comparatively, constructed hydrogels that were crosslinked extensively have seen minimal to no cell invasion after 20 days post injection,[Citation28] lending further credence to the need for negative space within a scaffold.
Figure 6. Micrograph of Trichrome-stained Capgel™-treated full-thickness dermal wound, d7. Capgel™ presented in varying degrees of resorption moving from the lateral wound edge (left) to the center (right); injected Capgel™ material towards the lateral edge was more resorbed and contained numerous microvessels by d7 (arrows). Scale bar: 150 µm.
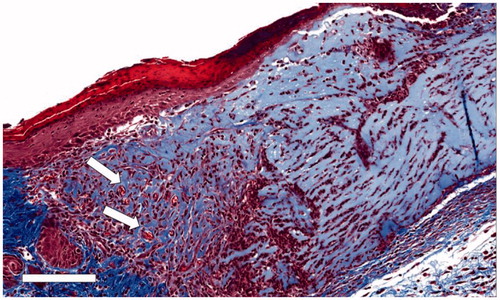
Vascularization is generally more prevalent at wound edges due to the proximity of angiogenic chemokine releasing cells.[Citation29,Citation30] Typically, a gradation of maturation is exhibited in a wound bed with the edges being exhibiting a more advanced wound healing process compared to the inflammatory phase of more interior portions of the wound bed.[Citation31] Thus, more angiogenic activity is present in lateral regions. This natural progression was also seen in Capgel™ treated animals with vascular structures being seen in the lateral and deeper wound margins of d7 mice. Of note, the deeper margins of Capgel™ treated wounds had significantly higher vascularization compared to controls, especially since Capgel™ treated wounds resisted contracture (i.e., retained a larger volume) ( and ). This resistance to contracture and associated increase in overall vessel density may help to resist scar formation in difficult to heal wounds.[Citation32,Citation33] It is unclear whether the structure of the Capgel™ itself is initiating blood vessel formation or if the negative space in the hydrogel allows for infiltrating host cells to first populate and then promote vasculogenesis and angiogenesis in the wound bed by migrating endothelial cells.
Capgel™ appears to change the wound healing kinetics by extending the acute inflammatory and proliferative stages and attenuating the chronic inflammatory and remodeling/resolution phases as evidenced by the remnants of acute inflammatory cells such as neutrophils through d10 in some Capgel™ treated animals. Though prolonged wound healing brought on by an extended inflammatory phase is generally viewed as deleterious to the overall regenerative process,[Citation34] this extension of the inflammatory phase and associated attenuation of the chronic portion of wound resolution is potentially beneficial via reduction of events such as excessive wound contracture associated with chronic inflammation.[Citation35–38] This augmented inflammation supports the use of Capgel™ in full excisional wounds as Capgel helped to prevent contraction of the wound bed (), which could lead to reductions in pathological contracture, improving overall skin quality and cosmesis of repaired scars. Extension of the wound resolution and regenerative phases may increase the extent of functional tissue regeneration. For example, wounds in older humans or aged animals exhibit slowed wound healing (as defined by the rate of reepithelialization) but interestingly yield improved end results that are likely due to decreases in key cytokines (e.g., TGF-β) and mechanical forces within the wound bead that lead to decreases in scar formation and increases in total neovascularization.[Citation35,Citation36] Future work will assess if this attenuation is what is occurring in Capgel™-treated animals; the resisted contracture () and increased neovascularization () over saline controls is promising.
One of the most clinically important factors in wound healing is the formation of a protective epithelium to prevent water loss and provide a barrier against bacterial invasion.[Citation25] This re-epithelialization process is initiated by the formation of an epithelial tongue comprised of migrating keratinocytes moving across the wound bed.[Citation39] Unfortunately, behavior of the mice used for the experimentation hindered any meaningful quantification of re-epithelialization as many of the wound dressings, both control and Capgel™ treated, were gnawed or scratched away, leading to wound desiccation and scab formation. In the few instances where the dressing remained in place throughout the experimental timeframe, an epithelial tongue can be seen with a multilayer, stratified epithelium present behind the leading edge (). The lack of a complete mature epithelium in our study may have an effect on the wound healing process due to the altered phenotype and protein expression seen in keratinocytes during proliferation and migration.[Citation39] For instance, activated and migrating keratinocytes are known to release a complex mixture of factors such as vascular endothelial cell growth factor.[Citation40] Previously, Capgel™ has been used in conjunction with a micro patterned gelatin-based construct, Sharklet.[Citation41] Using a similar grading scheme for wound outcome, the Sharklet dressing did not yield any statistical differences in rates of re-epithelization, though this may be due to the mouse model, which, unlike humans or pigs, relies primarily on contracture to close a dermal wound. Further experimentation to increase sample size, possibly in a different, more cooperative animal model (e.g., porcine), would allow for improved analysis. Interrogation of keratin expression may lead to further validation of epithelial stratification in our model.[Citation42,Citation43]
5. Conclusion
Our self-assembling, novel biomaterial, Capgel™, was able to act alone as a dermal support matrix in a hypoxic skin model in mice. Capgel™-treated mice showed less wound contracture and also an increased granulation bed volume. Most importantly, Capgel™ aided in neovascularization and resulted in a greater number of total vessels present in the wound bed, a crucial aspect in wound healing. Future experiments will further explore Capgel™, especially with different Capgel™ synthesis conditions, surface derivatizations and cell delivery modalities. Capgel™ and other tunable biomaterials have the potential to facilitate more functional wound healing within full-thickness dermal wounds such as the de novo morphogenesis of epithelial appendages (e.g., sebaceous glands and hair follicles) which are not normally regenerated.
Disclosures/conflict of interest statement
BJW has a 66% ownership stake in Saisijin Biotech, LLC; the company has an IP arrangement with the University of Florida around the Capgel technology. BJW receives no financial or material support from Saisijin and no financial (salary or research) or material support was provided by Saisijin for the reported work.
Acknowledgments
The authors would like to acknowledge John Shelley of the Sanford Burnham Prebys Medical Discovery Institute in Lake Nona, FL for assistance with slide-scan imaging, and Alexey Goloubev, Nicholas Giel and Mario Pita for assistance with Cagpel™ generation and animal procedures.
References
- Rice, J. B.; Desai, U.; Cummings, A. K. G.; Birnbaum, H. G.; Skornicki, M.; Parsons, N. B., Burden of Diabetic Foot Ulcers for Medicare and Private Insurers (vol 37, pg 651, 2014). Diabetes Care. 2014, 37(9), 2660–2660. DOI:10.2337/dc14-er09
- Rice J. B.; Desai, U.; Cummings, A. K. G.; Birnbaum, H. G.; Skornicki, M.; Parsons, N. Burden of Venous Leg Ulcers in the United States. J. Med. Econ. 2014, 17(5), 347–356. DOI: 10.3111/13696998.2014.903258
- Russo, C. A.; Spector, W. Hospitalizations Related to Pressure Ulcers, 2006. HCUP Statistical Brief #64. December 2008. Agency for Healthcare Research and Quality, Rockville, MD.
- Rodero, M. P.; Khosrotehrani, K. Skin Wound Healing Modulation by Macrophages. Int. J. Clin. Exp. Pathol. 2010, 3(7), 643–653.
- Gurtner, G. C.; Werner, S.; Barrandon, Y.; Longaker, M. T. Wound Repair and Regeneration. Nature. 2008, 453(7193), 314–321. DOI: 10.1038/nature07039
- Leibovich, S. J.; Ross R. The Role of the Macrophage in Wound Repair. A Study with Hydrocortisone and Antimacrophage Serum. Am. J. Pathol. 1975, 78(1), 71–100.
- Kim, M. H.; Liu, W.; Borjesson, D. L.; Curry, F. R. E.; Miller, L. S.; Cheung, A. L.; Liu, F. T.; Isseroff, R. R.; Simon, S. I. Dynamics of Neutrophil Infiltration During Cutaneous Wound Healing and Infection Using Fluorescence Imaging. J. Invest. Dermatol. 2008, 128(7), 1812–1820. DOI: 10.1038/sj.jid.5701223
- Schurmann, C.; Seitz, O.; Sader, R.; Pfeilschifter, J.; Goren, I.; Frank, S. Role of Wound Macrophages in Skin Flap Loss or Survival in an Experimental Diabetes Model. British J. Surg. 2010, 97(9), 1437–1451. DOI: 10.1002/bjs.7123
- Schultz, G.S.; Grant, M. B. Neovascular Growth Factors. Eye (Lond). 1991, 5(Pt 2), 170–180. DOI: 10.1038/eye.1991.31
- Schultz, G. S.; Wysocki, A. Interactions Between Extracellular Matrix and Growth Factors in Wound Healing. Wound Rep. Regeneration. 2009, 17(2), 153–162. DOI: 10.1111/j.1524-475X.2009.00466.x
- Kolluru, G. K.; Bir, S. C.; Kevil, C. G., Endothelial Dysfunction and Diabetes: Effects On Angiogenesis, Vascular Remodeling, and Wound Healing. Int. J. Vasc. Med. 2012, 2012, 918267.
- Alijotas-Reig, J.; Fernandez-Figueras, M. T.; Puig, L. Late-Onset Inflammatory Adverse Reactions Related to Soft Tissue Filler Injections. Clin. Rev. Allergy Immunol. 2013, 45(1), 97–108. DOI: 10.1007/s12016-012-8348-5
- Li, Z. Y.; Yuan, B. M.; Dong, X. M.; Duan, L. J.; Tian, H. Y.; He, C. L.; Chen, X. S., Injectable Polysaccharide Hybrid Hydrogels As Scaffolds for Burn Wound Healing. RSC Adv. 2015, 5(114), 94248–94256. DOI: 10.1039/C5RA16912G
- Xu, Q.; Sigen, A.; Gao, Y. S.; Guo, L. R.; Creagh-Flynn, J.; Zhou, D. Z.; Greiser, U.; Dong, Y. X.; Wang, F. G.; Tai, H. Y.; et al. A Hybrid Injectable Hydrogel from Hyperbranched PEG Macromer as a Stem Cell Delivery and Retention Platform for Diabetic Wound Healing. Acta Biomaterialia. 2018, 75, 63–74. DOI: 10.1016/j.actbio.2018.05.039
- Clayman, M. A.; Clayman, S. M.; Mozingo, D. W., The Use of Collagen-Glycosaminoglycan Copolymer (Integra) for the Repair of Hypertrophic Scars and Keloids. J. Burn Care Res. 2006, 27(3), 404–409. DOI: 10.1097/01.BCR.0000216749.72080.89
- Griffin, D. R.; Weaver, W. M.; Scumpia, P. O.; Di Carlo, D.; Segura, T., Accelerated Wound Healing by Injectable Microporous Gel Scaffolds Assembled From Annealed Building Blocks. Nature Mater. 2015, 14(7), 737–+. DOI: 10.1038/nmat4294
- Willenberg, B. J.; Hamazaki, T.; Meng, F. W.; Terada, N.; Batich, C., Self-Assembled Copper-Capillary Alginate Gel Scaffolds with Oligochitosan Support Embryonic Stem Cell Growth. J. Biomed. Mater. Res. Part A. 2006, 79A(2), 440–450. DOI: 10.1002/jbm.a.30942
- Della Rocca, D. G.; Willenberg, B. J.; Qi, Y. F.; Simmons, C. S.; Rubiano, A.; Ferreira, L. F.; Huo, T.; Petersen, J. W.; Ruchaya, P. J.; Wate, P. S.; et al. An Injectable Capillary-Like Microstructured Alginate Hydrogel Improves Left Ventricular Function After Myocardial Infarction in Rats. Int. J. Cardiol. 2016, 220, 149–154. DOI: 10.1016/j.ijcard.2016.06.158
- Goffin, J. M.; Pittet, P.; Csucs, G.; Lussi, J. W.; Meister, J. J.; Hinz, B. Focal Adhesion Size Controls Tension-Dependent Recruitment of Alpha-Smooth Muscle Actin to Stress Fibers. J. Cell Biol. 2006, 172(2), 259–268. DOI: 10.1083/jcb.200506179
- Pelton, J. C.; Wright, C. E.; Leitges, M.; Bautch, V. L., Multiple Endothelial Cells Constitute the Tip of Developing Blood Vessels and Polarize to Promote Lumen Formation. Development. 2014, 141(21), 4121–4126. DOI: 10.1242/dev.110296
- Willenberg, B. J.; Zheng, T.; Meng, F. W.; Meneses, J. C.; Rossignol, C.; Batich, C. D.; Terada, N.; Steindler, D. A.; Weiss, M. D., Gelatinized Copper-Capillary Alginate Gel Functions as an Injectable Tissue Scaffolding System for Stem Cell Transplants. J. Biomater. Sci. Polym. Ed. 2011, 22(12), 1621–1637. DOI: 10.1163/092050610X519453
- Rendell M. S.; Milliken, B. K.; Finnegan, M. F.; Finney, D. E.; Healy, J. C.; Bonner, R. F. The Microvascular Composition of the Healing Wound Compared At Skin Sites with Nutritive Versus Arteriovenous Perfusion. J. Surg. Res. 1998, 80(2), 373–379. DOI: 10.1006/jsre.1998.5463
- Howdieshell, T. R.; Callaway, D.; Webb, W. L.; Gaines, M. D.; Procter, C. D.; Sathyanarayana; Pollock, J. S.; Brock, T. L.; McNeil, P. L. Antibody Neutralization of Vascular Endothelial Growth Factor Inhibits Wound Granulation Tissue Formation. J. Surg. Res. 2001, 96(2), 173–182. DOI: 10.1006/jsre.2001.6089
- O'Brien, F. J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14(3), 88–95. DOI: 10.1016/S1369-7021(11)70058-X
- Zhong, S. P.; Zhang, Y. Z.; Lim, C. T., Tissue Scaffolds for Skin Wound Healing and Dermal Reconstruction. Wiley Interdisciplinary Rev. Nanomed. Nanobiotechnol. 2010, 2(5), 510–525. DOI: 10.1002/wnan.100
- Spater, T.; Frueh, F. S.; Metzger, W.; Menger, M. D.; Laschke, M. W., In Vivo Biocompatibility, Vascularization, and Incorporation of Integra((R)) Dermal Regenerative Template and Flowable Wound Matrix. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2018, 106(1), 52–60. DOI: 10.1002/jbm.b.33813
- Sun, G. M.; Zhang, X. J.; Shen, Y. I.; Sebastian, R.; Dickinson, L. E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J. W.; Gerecht, S. Dextran Hydrogel Scaffolds Enhance Angiogenic Responses and Promote Complete Skin Regeneration During Burn Wound Healing. Proc. Nat. Acad. Sci. USA 2011, 108(52), 20976–20981. DOI: 10.1073/pnas.1115973108
- Lutolf, M. P.; Lauer-Fields, J. L.; Schmoekel, H. G.; Metters, A. T.; Weber, F. E.; Fields, G. B.; Hubbell, J. A. Synthetic Matrix Metalloproteinase-Sensitive Hydrogels for the Conduction of Tissue Regeneration: Engineering Cell-Invasion Characteristics. Proc. Nat. Acad. Sci. USA 2003, 100(9), 5413–5418. DOI: 10.1073/pnas.0737381100
- Hellstrom, M.; Phng, L. K.; Hofmann, J. J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A. K.; Karlsson, L.; Gaiano, N.; et al. Dll4 Signalling Through Notch1 Regulates Formation of Tip Cells During Angiogenesis. Nature. 2007, 445(7129), 776–780. DOI: 10.1038/nature05571
- Gerhardt, H. VEGF and Endothelial Guidance in Angiogenic Sprouting. Organogenesis. 2008, 4(4), 241–246. DOI: 10.4161/org.4.4.7414
- Greenhalgh, D. G. The Role of Apoptosis in Wound Healing. Int. J. Biochem. Cell Biol. 1998, 30(9), 1019–1030. DOI: 10.1016/S1357-2725(98)00058-2
- Shin, D. M.; Minn, K. W. The Effect of Myofibroblast On Contracture of Hypertrophic Scar. Plast. Reconstructive Surg. 2004, 113(2), 633–640. DOI: 10.1097/01.PRS.0000101530.33096.5B
- Pettet, G. J.; Byrne, H. M.; McElwain, D. L. S.; Norbury, J. A Model of Wound-Healing Angiogenesis in Soft Tissue. Mathematical Biosci. 1996, 136(1), 35–63. DOI: 10.1016/0025-5564(96)00044-2
- Guo, S.; DiPietro, L. A., Factors Affecting Wound Healing. J. Dental Res. 2010, 89(3), 219–229.
- Ashcroft, G. S.; Mills, S. J.; Ashworth, J. J. Ageing and Wound Healing. Biogerontology. 2002, 3(6), 337–345.
- Minimas, D. A. Ageing and Its Influence On Wound Healing. Wounds UK. 2007, 3(1), 42–50.
- Demidova-Rice, T. N.; Hamblin, M. R.; Herman, I. M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Skin Wound Care. 2012, 25(7), 304–314.
- Chen, C.; Schultz, G. S.; Bloch, M.; Edwards, P. D.; Tebes, S.; Mast, B. A. Molecular and Mechanistic Validation of Delayed Healing Rat Wounds as a Model for Human Chronic Wounds. Wound Rep. Regen. 1999, 7(6), 486–494.
- Coulombe, P. A. Wound Epithelialization: Accelerating the Pace of Discovery. J. Invest. Dermatol. 2003, 121(2), 219–230.
- Brown, L. F.; Berse, B.; Yeo, K. T.; Yeo, T. K.; Senger, D. R.; Dvorak, H. F.; Vandewater, L. Expression of Vascular-Permeability Factor Vascular Endothelial Growth-Factor (VPF VEGF) by Epidermal-Keratinocytes Durning Wound-Healing. Mol. Biol. Cell. 1992, 3, A333–A333.
- Magin, C. M.; Neale, D. B.; Drinker, M. C.; Willenberg, B. J.; Reddy, S. T.; La Perle, K. M. D.; Schultz, G. S.; Brennan, A. B. Evaluation of a Bilayered, Micropatterned Hydrogel Dressing for Full-Thickness Wound Healing. Exp. Biol. Med. 2016, 241(9), 986–995.
- Moll, R.; Divo, M.; Langbein, L. The Human Keratins: Biology and Pathology. Histochem. Cell Biol. 2008, 129(6), 705–733.
- Pastar, I.; Stojadinovic, O.; Yin, N. C.; Ramirez, H.; Nusbaum, A. G.; Sawaya, A.; Patel, S. B.; Khalid, L.; Isseroff, R. R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care. 2014, 3(7), 445–464.