ABSTRACT
Background: Response disinhibition plays an important role in addictive behaviors. However, results of studies on the performance on response inhibition tasks of individuals evidencing potentially problematic levels of alcohol drinking are mixed. Objectives: We assessed conditions under which persons with a relatively high risk of alcohol dependence show inhibition deficits in such tasks and investigated the nature of those deficits. Methods: Fifty-eight male undergraduate students, 27 of which were high-risk drinkers according to the Alcohol Use Disorders Identification Test, performed a go/no-go inhibition task with differing percentages of no-go trials (50% vs. 25%), stimulus presentation times (600 vs. 200 ms), and types of go and no-go stimuli (alcohol related vs. -unrelated). Response inhibition was indexed by response time (RT) to go trials and response accuracy on go and no-go trials. Results: There were no differences between low- and high-risk drinkers on any of the three outcome measures under the 600-ms stimulus presentation condition. Under the 200-ms condition, the high-risk drinkers showed faster RTs to go stimuli, and more errors on both go- and no-go trials than the low-risk drinkers, irrespective of type and percentage of no-go stimuli. However, the accuracy differences between the two groups disappeared after controlling for the RT on go trials, suggesting a speed-accuracy trade-off. Conclusion: High-risk drinkers’ response inhibition deficits are not restricted to alcohol-related cues and are especially likely to occur under conditions prompting fast responding. These findings could be used to inform treatment, suggesting the promotion of strategies aimed at preventing high-risk alcohol users from making quick decisions.
Introduction
It is generally assumed that substance abuse and addiction are associated with deficits in response inhibition (Citation1). Either inhibition deficits contribute to the development of substance use disorders, substance use results in response inhibition deficits, or both (e.g., 2). However, for alcohol abuse, evidence for response inhibition deficits as measured by common laboratory tasks is mixed (Citation1). Moreover, corresponding studies contrasting performance of individuals with and without alcohol use disorder tend to reveal only low-to-moderate effect sizes (Citation3).
One of the most commonly used task to experimentally study response inhibition is the go/no-go task (Citation4). This task requires the participant to respond as fast as possible to one stimulus or class of stimuli (go trials), but not respond to another stimulus or class of stimuli (no-go trials). Whether or not finding deficits using this task may be dependent on experimental factors such as percentage of no-go trials used, presentation time of go and no-go stimuli, type of participants, and type of stimuli used as no-go stimuli. For example, differences in task performance between groups of participants might only be reliably detectable when contrasting individuals with extreme scores on the clinical feature of interest under the most challenging task conditions. Such conditions may be characterized by relatively few no-go trials, implying the presence of a strong “go” bias, short stimulus presentation times, encouraging fast responding, and the use of stimuli that are relevant to the clinical condition of interest, such as the use of alcohol-related cues in the case of alcohol use disorder. This is a relatively unexplored issue in research on response inhibition deficits in psychopathology in general, and addiction in particular, although knowledge of the effect of these parameters is essential for increasing the sensitivity and specificity of the go/no-go paradigm (Citation5).
Another factor potentially important for differences in task sensitivity concerns the dependent measure(s) used to index response inhibition. In principle, the go/no-go task provides three measures: mean response time (RT) to go stimuli (MRT), errors of omission (OEs, not responding to go stimuli), and errors of commission (CEs, responding to no-go stimuli). Although CEs are most commonly used as index of (failing) response inhibition, it is important to evaluate the three measures in combination. True impaired response inhibition is reflected in a high number of CEs in the absence of differences in OEs and MRTs, whereas a high number of CEs in combination with a low MRT may reflect a speed-accuracy trade-off favoring speed over accuracy (Citation6). Fast approach responses to addiction-relevant cues have also been linked in the literature to the concept of attentional bias. Accordingly, addiction-relevant cues might be particularly able to grab the addicted individual’s attention (Citation7). Such attentional bias may in turn be associated with fast approach responses toward relevant cues (e.g., 8). In the context of go/no-go inhibition tasks, using addiction-relevant stimuli on no-go trials would lead to faster responses and more approach errors (CEs) in addicted individuals than would be the case for non-addiction-relevant no-go stimuli. These considerations further highlight the importance of a joint evaluation of the different outcome measures.
Because of a lack of studies simultaneously and systematically manipulating at least some of these experimental parameters within one study, while measuring and relating all three outcome measures, it is impossible to draw any clear conclusion about which of these factors (if any) are most crucial for obtaining go/no-go task performance deficits and about the nature of such deficits in alcohol use disorder and addiction. To help fill this gap, we used a go/no-go task while manipulating percentage of no-go trials, stimulus presentation time, and type of no-go stimuli in a group of students with either a low or high risk of (developing) alcohol dependence (hereafter termed low- and high-risk drinkers), as defined using the Alcohol Use Disorders Identification Test (AUDIT; 9). Based on a previous study employing a similar design in tobacco smokers and nonsmokers (Citation10), we expected faster responding and more CEs and OEs for high-risk drinkers than low-risk drinkers, especially under the condition of a short stimulus-presentation time, whilst type of no-go stimuli (alcohol related vs. not alcohol related) and percentage of no-go stimuli (high or low) were not expected to play a significant role in these differences.
Replication of this pattern of results in the present study would support the notion that go/no-go response deficits may be a marker of some general inhibition deficit underlying multiple types of addiction, and perhaps even other disorders characterized by impulsive behavior, such as attention deficit hyperactivity disorder, obsessive compulsive disorder, and schizophrenia (e.g., 5). More specifically, this general inhibition deficit would mainly be characterized by speeded responding prompted by environmental demands, which in turn would be associated with low behavioral response accuracy. If confirmed, these results could motivate treatments that are specifically directed at preventing the individual concerned from encountering environmental circumstances implicating a necessity of rapid decision-making, or training techniques to slow down behavioral responding (e.g., “stop-and-think” training; 11).
Method
Participants
Sixty-two male undergraduate students were recruited via university advertisements. We explicitly included only male participants for the purpose of comparison with the study reported by Zhao et al. (Citation10), and to prevent introducing more variables in addition to the already manipulated three task variables (see below). Data from four participants were excluded due to a misunderstanding of the instructions. The participants were divided in two groups according to their score on the AUDIT (maximum score = 40), using the commonly used cutoff score of 8 to separate low-risk from high-risk drinkers (Citation9). The group of 31 low-risk drinkers had a mean age of 19.74 years (SD = 1.21; range = 18–23) and a mean AUDIT score of 2.35 (SD = 2.23; range = 0–6). The group of 27 high-risk drinkers had a mean age of 20.22 years (SD = 1.85) and a mean AUDIT score of 12.00 (SD = 3.49; range = 8–19). Next to the AUDIT, all participants completed a Chinese version of the Beck Depression Inventory (BDI; 12) and the Barratt Impulsiveness Scale-11 (BIS-11; 13) before the task. The mean score for the high-risk drinkers was 9.23 (SD = 5.55) and 59.33 (SD = 5.52) for the BDI and BIS-11, respectively. The corresponding means for the low-risk drinkers were 8.77 (SD = 5.94) and 59.48 (SD = 8.15) and there was no difference between the groups on either measure, Fs < 1. All participants had a normal or corrected-to-normal vision and no neuropsychological disease. They participated voluntarily and signed a consent form after being informed about the experimental procedures. The procedures were approved by the local ethics committee and were performed in accordance with the approved guidelines. Participants received a small financial remuneration at the end of the study.
Go/no-go task
Fifty-four pictures from the international affective picture system (IAPS; 14) and internet were selected, including 27 beer-related pictures and 27 non-beer-related pictures consisting of photos from everyday, neutral objects. The beer-related pictures were taken from the internet and each only displayed one or more glasses of different shapes that were filled with beer except for one picture that displayed a bottle on ice that was filled with beer. The neutral pictures each displayed an everyday object, such as a towel, fork, umbrella, clock, or chair (e.g., IAPS pictures 7002, 7080, 7150, 7190, and 7235). The pictures had a colored frame, a blue one for beer-related pictures and a yellow one for beer-unrelated pictures. After the experimental task (see below), participants rated all pictures on a scale of 1–9 (1 low; 9 high) on valence, arousal, dominance, and beer relatedness. The go/no-go task was identical to that described in Zhao et al. (Citation10). It included four tasks, manipulating stimulus presentation time (600 or 200 ms) and percentage no-go trials (50% or 25%): Task 1: 600 ms/50%; Task 2: 600 ms/25%; Task 3: 200 ms/50%; Task 4: 200 ms/25%. The frame color indicated whether the trial concerned a go or a no-go trial. Each block of 100 trials started with a 1000-ms presentation of a fixation cross, to focus the participant’s attention. Thereafter, on each trial, a picture was presented for 600 or 200 ms, or until a response was made (whichever came first), immediately followed by a gray screen that was presented for 1000 ms. The next trial was presented immediately thereafter. Participants were instructed to respond to each go stimulus by pressing the letter “J” on the keyboard as quickly as possible, and to withhold responding to no-go stimuli, while maintaining accuracy throughout. Participants first received practice trials involving alcohol-related and -unrelated pictures that were not used in the remainder of the task. Participants proceeded with the four main tasks once their practice accuracy rate, based on both go- and no-go trials, exceeded 85%. Each of the four tasks comprised four 100-trial blocks. Counterbalanced across participants, during the first two blocks, go trials were indicated by the beer-related or beer-unrelated pictures; on each of the last two 100-trial blocks, go-trials were indicated by the other type of stimulus (beer-unrelated or beer-related pictures, respectively). Participants had a short break between tasks. The four tasks were presented in a random order for each participant and each task lasted approximately 10 min.
Data analysis
The participants’ rating of the beer-related and neutral pictures on valence, arousal, dominance, and beer relatedness was analyzed using analyses of variance (ANOVAs) with group (low- vs. high-risk drinkers) as between-subject variable. Concerning go/no-go task performance, trials with a MRT exceeding two standard deviations were excluded (<15% of each participant’s trials). MRT on go trials, percentage of OEs, and percentage of CEs were each subjected to a repeated measures ANOVA (RM-ANOVA), with group (low- vs. high-risk drinkers) as between-subject factor, and no-go percentage (50% vs. 25%) and picture type (alcohol related vs. not alcohol related) as within-subject factors. Significant interactions were followed up by simple main effect analyses. The RM analyses were performed separately for the two stimulus-presentation duration trial blocks because preliminary RM-ANOVAs with presentation time as additional within-group factor revealed a significant group × presentation time × percentage × picture type interaction for MRT, F(Citation1, 56) = 5.10, p = 0.028, η2 = 0.08. Moreover, these separate ANOVAs were also motivated by the floor effect which was observed for OEs and CEs during the 600-ms presentation time trials (precluding a meaningful analysis) but not for the OEs and CEs observed in the 200-ms task condition. We also performed analyses of covariance (ANCOVAs), controlling for overall MRT on go trials (pooled over no-go percentage and picture type conditions of the respective presentation-time condition), on the OEs and CEs. The purpose of these analyses was to examine whether any potential significant accuracy differences between groups and/or conditions would survive a control for MRT. If not, this would implicate that the error differences were significantly driven by MRT differences. A p value of <.05 was adopted as criterion for statistical significance throughout and effect sizes were expressed as partial eta-squared.
Results
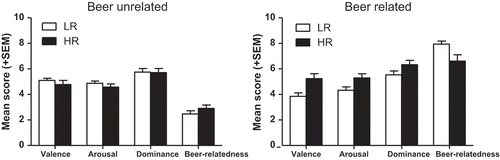
displays the mean valence, arousal, dominance, and beer-relatedness scores of each group, separately for each of the two picture types. Concerning the beer-unrelated pictures, the groups did not differ in the score on any of the scales, Fs(Citation1, 56) < 1.41, ps > 0.23, η2s < 0.03. However, compared to the low-risk drinkers, the high-risk drinkers rated the beer-related pictures as having a significantly higher valence and arousal, but being less beer related, Fs(Citation1, 56) > 5.44, ps < 0.03, η2s > 0.08. The groups did not differ on the dominance ratings.
Figure 1. Groups’ mean (+SEM) score on valence, arousal, dominance, and beer-relatedness of the beer-related and -unrelated pictures used in the go/no-go task. LR: Low-risk drinkers; HR: high-risk drinkers.
600-ms presentation time condition
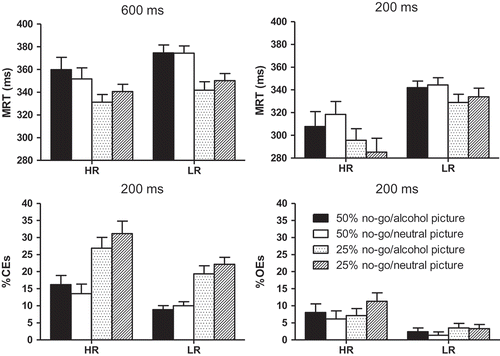
The MRT for each group, picture type, and no-go trial percentage is displayed in (top-left). ANOVA (see for statistical details) revealed a main effect of percentage, reflecting slower responding on 50% (M = 365.83, SD = 44.69) than 25% no-go trial blocks (M = 341.33, SD = 34.77), and of the percentage × picture type interaction, reflecting faster responding to alcohol-related pictures (M = 336.88, SD = 38.77) than neutral pictures (M = 345.77, SD = 34.30) in the 25% no-go condition, F(Citation1, 57) = 8.76, p = 0.004, η2 = 0.13, but not the 50% no-go condition (M = 367.78, SD = 48.10, and M = 363.89, SD = 43.97, respectively), F(Citation1, 57) = 1.74, p = 0.19, η2 = 0.03. All other main and interaction effects were not significant. The overall percentage of CEs and OEs was close to zero, 3.3% and 0.1% for CEs and OEs, respectively, and ANOVA did not reveal any significant effects for these outcome measures.
Table 1. Results of statistical analysis of go/no-go performance measures.
Figure 2. Mean value (+SEM) of the high-risk drinkers (HR) and low-risk drinkers (LR) on the (Citation1) go RT measure on the 600-ms stimulus presentation time trial blocks (top-left), (Citation2) go RT measure on the 200-ms stimulus presentation time trial blocks (top-right), (Citation3) percentage CEs on the 200-ms stimulus presentation time trial blocks (bottom-left), and (Citation4) percentage OEs on the 200-ms stimulus presentation time trial blocks (bottom-right). Values are presented separately for each no-go stimulus percentage and stimulus type condition.
200-ms presenting time condition
ANOVA using the MRTs of the 200-ms presentation time trials (: top-right) revealed main effects for group, reflecting overall faster responding for the high-risk drinkers (M = 301.84, SD = 54.84) compared to the low-risk drinkers (M = 337.31, SD = 32.84), and percentage. The MRT in the 50% condition (M = 329.16, SD = 50.05) was longer than in the 25% condition (M = 312.44, SD = 50.11). All other main and interaction effects were not significant.
ANOVA on percentage CEs (: bottom-left) revealed a main effect of group. The high-risk drinkers had a higher overall percentage of CEs (M = 21.97, SD = 12.69) than the low-risk drinkers (M = 15.10, SD = 8.21). However, this group effect disappeared when controlling for MRT, ANCOVA, F(Citation1, 55) = 1.49, p = 0.23, η2 = 0.03, while the effect of MRT was highly significant, F(Citation1, 55) = 11.83, p < 0.001, η2 = 0.18. The main effect of percentage was also significant, as was the percentage × picture type interaction. The interaction reflected fewer CEs on the 25% no-go trials condition for the alcohol-related pictures (M = 22.90, SD = 14.93) than the neutral pictures (M = 26.36, SD = 15.85), F(Citation1, 57) = 6.20, p = 0.02, η2 = 0.10, but no difference as a function of no-go picture type in the 50% no-go trials condition (M = 12.28, SD = 11.12, and M = 11.66, SD = 11.09, F < 1). The interaction became insignificant too after controlling for MRT. All other main and interaction effects were not significant.
ANOVA on the percentage OEs (: bottom-right) revealed a main group effect, reflecting more OEs for the high-risk drinkers (M = 8.16, SD = 10.49) than the low-risk drinkers (M = 2.65, SD = 5.09). This effect disappeared when controlling for MRT, ANCOVA, F(Citation1, 55) < 1, while the effect of MRT was highly significant, F(Citation1, 55) = 74.72, p < 0.001, η2 = 0.58. The main effect of percentage was significant, as was the percentage × picture type interaction. The interaction, which became insignificant when controlling for MRT, reflected a significantly higher percentage of OEs in the 25% (M = 7.05, SD = 10.71) compared to the 50% (M = 3.60, SD = 9.51) condition for trials with neutral go stimuli, F(Citation1, 57) = 10.40, p = 0.002, η2 = 0.15, but not for trials with alcohol-related go stimuli (M = 5.19, SD = 9.16, and M = 5.02, SD = 10.37, respectively), F < 1. All other main and interaction effects were insignificant.
Discussion
The high-risk alcohol drinkers, which were defined by having an AUDIT score of ≥8, responded faster to go stimuli and made more OEs and CEs compared to the low-risk drinkers (AUDIT score < 8). These differences only occurred under the condition of a 200-ms stimulus presentation time, whilst the percentage and type of no-go stimuli did not modulate the group effects. Interestingly, the accuracy differences disappeared when controlling for MRT, which is suggestive of a speed-accuracy trade-off. Specifically, under the 200-ms presentation conditions, the high-risk drinkers were particularly prone to respond quickly, which in turn was not only associated with making relatively many CEs but also many OEs. The latter type of error may reflect occasional more-or-less strategic slowing of responding, triggered by frequently experiencing CEs, to the extent of missing on-time responses to go stimuli. Support for this possibility was found in additional analyses that we performed on a subset of the data (data not shown). Specifically, we further examined the data from Task 4, pooling the RT data across picture type and inserting a RT of 1200 ms for trials with no response. When comparing the RT on the first go-trial that immediately followed each CE and that immediately followed each (correct) no response on a no-go trial, we observed a longer RT on the first compared to latter trial type for each participant. This result suggests a post-error slowing triggered by CEs. In any case, the overall pattern of results is very similar to that found in a previous study comparing go-/no-go inhibition performance in smokers and nonsmokers (Citation10) and is suggestive of a more general deficit underlying all substance use addictions.
Although the majority of previous studies on alcohol use disorder using some laboratory response inhibition task provide details on all three major outcome measures (MRTs, OEs, and CEs; see 1, for an overview), to the best of our knowledge, none of these studies evaluated CEs in the context of RTs and OEs. Studies reporting RTs provide a mixed picture, with most studies finding no significant RT differences as a function of alcohol use status (e.g., 15–26), some finding longer RTs for individuals with alcohol use disorder relative to controls (e.g., 27), and some finding the reverse (e.g., 28). None of these studies used identical samples (also in terms of measurement instrument for defining alcohol use disorder), response inhibition task (e.g., differing in modality or nature of go and no-go stimuli, and including or not including response feedback), and/or experimental parameters within these tasks, which makes it very difficult to pinpoint the source of these different results. However, most of the studies finding no group difference in go RTs used longer stimulus presentation times than the presently used 200 ms that proved to be sensitive for detecting such difference.
Previous studies found that, especially in individuals with alcohol use disorder, alcohol-related cues evoke attentional biases and reduced response inhibition capacity (as reflected in CEs) compared to non-alcohol-related cues (e.g., 7, 27, 25, but see 22). In our study, we found that, compared to the low-risk drinkers, high-risk drinkers rated the beer-related pictures as more positive and more arousing, but also as less “beer related.” The latter rating may perhaps reflects a stronger generalization of the beer stimuli to other alcohol-related stimuli in the high-risk compared to low-risk group. However, importantly, the valence and arousal differences might have been expected to result in a stronger attentional bias and approach response, and hence even stronger impaired inhibition, in the high-risk compared to low-risk group. However, concerning the effect of picture type, we only observed interaction effects with percentage of no-go trials, primarily reflecting faster responding and less OEs and CEs for alcohol-related then neutral no-go stimuli under low-frequency but not high-frequency no-go trial conditions. One possible reason for these effects is that the alcohol-related pictures constituted a clear, homogeneous set of stimuli (all beer-related stimuli), which may have encouraged quick and accurate stimulus processing. However, importantly, these effects did not interact with the group factor. One possible reason for the absence of a differential effect of picture type, for example in the sense of displaying shorter RTs, and more CEs and OEs for alcohol-related than alcohol-unrelated no-go stimuli in the high-risk but not low-risk drinkers, might be the simultaneous presence of a color frame that indicated whether the stimulus had to be responded to or not (see also, e.g., 29, who examined inhibitory control in smokers using a similar task). Hence, in principle, the participants could have performed the task without paying any attention to the pictures. However, the significant interaction effects that we did find with respect to the picture type variable speak against this possibility, suggesting that the specific content of the pictures was actually processed during the task. Another possibility is that the alcohol-related pictures that were presented during the practice phase and during the first trial block evoked a general craving effect that affected the high-risk drinkers’ inhibitory capacity on all remaining trials.
Study limitations
The present study has a number of limitations that are identical or similar to those reported in the study performed by Zhao et al. (Citation10). First, we do not know whether the critical parametric feature determining the sensitivity of the current go/no-go task concerns the stimulus presentation time (600 vs. 200 ms) or the maximal response window (1600 vs. 1200 ms). Second, we had no measure of alcohol consumption prior to the participant performing the task so that we cannot be sure whether the group differences are driven by alcohol use status per se or by alcohol intake (e.g., see 15, for a study on the effects of alcohol intake on response inhibition). Third, all problem drinkers had an AUDIT score below 19, which means that persons with the highest risk level were not represented (Citation9). Moreover, we only included male undergraduate participants. These selections obviously limit the generalizability of the present results (e.g., see 22, for possible sex differences). Finally, the present study does not address the issue of causality, that is, whether the inhibition problems were caused by alcohol use or constituted a causal factor in the initiation and continuation of alcohol use.
Conclusions
The results of the present study, combined with those from Zhao et al. (Citation10), support the notion that substance use disorder and addiction are associated with impaired behavioral inhibition. They further suggest that this impairment is specifically prone to occur under conditions prompting quick responding and, at least under these conditions, is of a general nature, not specifically related to addiction-relevant stimuli. Future studies should replicate these findings and further examine the effect of other factors that were not systematically manipulated in the present study, such as magnitude of alcohol intake and abstinence. If replicated, these results could be used to inform and improve treatment, for example, by promoting strategies aimed at preventing individuals with a substance use disorder, or those at risk of developing such disorder, from making quick decisions.
Financial disclosure
The authors report no relevant financial conflicts.
Funding
This work was supported by The National Natural Science Foundation of China (grant number 31560283).
Additional information
Funding
References
- Smith JL, Mattick RP, Jamadar S, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend 2014;145:1‒33. doi: 10.1016/j.drugalcdep.2014.08.009
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 2008;34:777–810. doi: 10.1016/j.neubiorev.2007.11.003
- Stephan RA, Alhassoon OM, Allen KE, Wollman SC, Hall M, Thomas WJ, Grant I, et al. Meta-analyses of clinical neuropsychological tests of executive dysfunction and impulsivity in alcohol use disorder. Am J Drug Alcohol Abuse 2016. doi: 10.1080/00952990.2016.1206113
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. “Oops!: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 1997;35:747‒58. doi:10.1016/S0028-3932(97)00015-8
- Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, Schachar R. Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. J Abnormal Psychol 2014;123:429–439. doi: 10.1037/a0036295
- Seli P, Jonker TR, Cheyne JA, Smilek D. Enhancing SART validity by statistically controlling speed-accuracy trade-offs. Front Psychol 2013;4:1‒8. doi: 10.3389/fpsyg.2013.00265
- Field M, Cox WM. Attentional bias in addictive behaviours: a review of its development, causes, and consequences. Drug Alcohol Depend 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030
- Field M, Kiernan A, Eastwood B, Child R. Rapid approach responses to alcohol cues in heavy drinkers. J Behav Therapy Exp Psychiatry 39:209‒218. doi: 10.1016/j.jbtep.2007.06.001
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT. The alcohol use disorder identification test. Guidelines for use in primary care, 2nd ed. Geneva, Switzerland: World Health Organization, 2001.
- Zhao X, Liu X, Zan X, Jin G, Maes JHR. Male smokers’ and non-smokers’ response inhibition in go/no-go tasks: effect of three task parameters. PloS One 2016;11:e0160595. doi: 10.1371/journal.pone.0160595
- McMurran M, Fyffe S, McCarthy L, Duggan C, Latham A. “Stop & Think!”: social problem-solving therapy with personality-disordered offenders. Crim Behav Mental Health 2001;11:273‒285. doi: 10.1002/cbm.401
- Shek DTL. Reliability and factorial structure of the chinese version of the Beck Depression Inventory. J Clin Psychol 1990;46:35–43. DOI: 10.1002/1097-4679(199001)
- Zhou L, Xiao SY, Xiao-Yan HE, Jie LI. Reliability and validity of Chinese version of Barratt Impulsiveness Scale-11. Chin J Clin Psychol 2006;14:343–344.
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): affective ratings of pictures and instruction manual (Report No. A-6). Gainesville, FL: University of Florida, 2005.
- Adams S, Ataya AF, Attwood AS, Munafò MR. Effects of alcohol on disinhibition towards alcohol-related cues. Drug Alcohol Depend 2013;127:137–142. doi: 10.1016/j.drugalcdep.2012.06.025
- Fallgatter AJ, Wiesbeck GA, Weijers H-G, Boening J, Strik WK. Event-related correlates of response suppression as indicators of novelty seeking in alcoholics. Alcohol Alcohol 1998;33:475–481.
- Goudriaan AE, Oosterlaan J, De Beurs E, Van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cognit Brain Res 23, 137–151. doi: 10.1016/j.cogbrainres.2005.01.017
- Heitzeg MM, Nigg JT, Yau W-YW, Zucker RA, Zubieta J-K. Striatal dysfunction Marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020
- Henges AL, Marczinski CA. Impulsivity and alcohol consumption in young social drinkers. Addict Behav 2012;37:217–220. doi: 10.1016/j.addbeh.2011.09.013
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Begleiter H, et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a go/no-go task. Biol Psychol 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004
- Karch S, Jäger L, Karamatskos E, Graz C, Stammel A, Flatz W, Mulert C, et al. Influence of trait anxiety on inhibitory control in alcohol-dependent patients: simultaneous acquisition of ERPs and BOLD responses. J Psychiatric Res 2008;42:734–745. doi: 10.1016/j.psychires.2007.07.016
- Kreusch F, Vilenne A, Quertemont E. Response inhibition towards alcohol-related cues using an alcohol go/no-go task in problem and non-problem drinkers. Addict Behav 38:2520–2528. doi: 10.1016/j.addbeh.2013.04.007
- López-Caneda E, Cadaveira F, Crego A, Gómez-Suárez A, Corral M, Parada M, Caamaño-Isorna, F, et al. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction 2012;107:1796–1808. doi: 10.1111/j.1360-0443.2012.03908.x
- Oddy BW, Barry RJ. The relationship of N2 and P3 to inhibitory processing of social drinkers in a Go/NoGo task. Int J Psychophysiol 2009;72:323–330. doi: 10.1016/j.ijpsycho.2009.02.002
- Petit G, Kornreich C, Noël X, Verbanck P, Campanella S. Alcohol-related context modulates performance of social drinkers in a visual go/no-go task: a preliminary assessment of event-related potentials. Plos One 2012;7:e37466. doi: 10.1371/journal.pone.0037466
- Rossiter S, Thompson J, Hester R. Improving control over the impulse for reward: sensitivity of harmful alcohol drinkers to delayed reward but not immediate punishment. Drug Alcohol Depend 2012;125:89–94. doi: 10.1016/j.drugalcdep.2012.03.017
- Noël X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, Verbanck P. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology 2007;192:291–298. doi: 10.1007/s00213-006-0695-6
- Pandey AK, Kamarajan C, Tang Y, Cholian DB, Roopesh BN, Manz N, Porjesz B, et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol 2012;89:170–182. doi: 10.1016/j.biopsycho.2011.10.009
- Luijten M, Little M, Franken IHA. Deficits in inhibitory control in smokers during a go/nogo task: an investigation using event-related brain potentials. Plos One 2011;6:e18898. doi: 10.1371/journal.pone.0018898
- Lezak MD. Neuropsychological assessment, 3rd ed. New York: Oxford University Press, 1995.
