ABSTRACT
Background: Finding an effective, non-pharmacological approach to treat opioid withdrawal could remove some of the barriers associated with pharmacotherapy. The BRIDGE® is a noninvasive, percutaneous electrical nerve field stimulator developed to target pain. Objectives: This pilot study aimed to determine (1) the effects of the BRIDGE on withdrawal scores during the induction phase of opioid withdrawal therapy, (2) the percentage of subjects who successfully transitioned to medication assisted therapy (MAT). Methods: Adult patients treated with the BRIDGE during medically supervised withdrawal were included in this open label, uncontrolled, and retrospective study. The clinical opioid withdrawal scale (COWS) scores were prospectively recorded at different intervals (20, 30, and 60 min) and analyzed retrospectively. A subset of patients had scores recorded 5-days post-BRIDGE. Those who returned to the clinic and received their first dose of maintenance medication were considered to be successfully transitioned. Results: In this cohort (n=73), 65% were male. The mean COWS score prior to BRIDGE placement was 20.1 (±6.1). Twenty minutes after BRIDGE placement, the mean score was reduced to 7.5 (±5.9) (62.7% reduction, p<0.001). The scores further decreased after 30 minutes 4.0 (±4.4) and 60 minutes 3.1 (±3.4) (84.6% reduction, p<0.001). No rescue medications were administered during this period. The mean withdrawal score on day 5 was 0.6 (97.1% reduction, p<0.001) (n=33). Overall, 64/73 patients (88.8%) successfully transitioned to MAT. Conclusions: Neurostimulation with the BRIDGE is associated with a reduction in opioid withdrawal scores. This effect persisted during the induction period and allowed for effective transition to MAT.
Introduction
Opioid addiction is a serious public health issue that negatively impacts many communities around the world. There are as many as 36 million people misuse opioids worldwide.(Citation1) In 2013, it was estimated that in the United States, approximately 1.9 million people suffered from substance use disorders related to prescription opioid pain relievers and close to 517,000 were addicted to heroin.(Citation2) Addressing strategies for addiction treatment and recovery has become a major priority for government agencies given the substantial impact on health, social, and economic welfare. The challenges associated with treatment are many, including access, variations in patient needs, education and available treatment options for both the initial phase of treatment and maintenance.(Citation3–Citation6)
Abrupt discontinuation of heroin or an opioid receptor agonist in order to transition to an opioid antagonist, such as naltrexone, requires an “induction phase” with medical supervision to properly control symptoms. Successful completion of the induction phase is critical, since it allows for transition to long term, medication-assisted therapy (MAT), as well as behavioral interventions. These are key components of the long-term recovery process. The initial treatment can take place in the inpatient or outpatient setting, each presenting its own set of challenges.(Citation7) In general, however, it appears that patients are more likely to leave treatment prematurely when opioid withdrawal symptoms are not appropriately managed. Pain associated with withdrawal is often a major reason for patients opting out of treatment.(Citation8)
Until now, pharmacotherapy has been the mainstay for the induction phase of treatment of opioid withdrawal with varying degrees of success.(Citation9–Citation15) A recent strategy in healthcare reform has been to expand access by integrating drug treatment into primary care settings.(Citation16) This has been challenging for many reasons including limited availability of licensed physicians that can prescribe pharmacotherapy.(Citation17) Also, certain treatment options, such as opioid receptor antagonists, cannot be prescribed until patients have successfully completed medically supervised withdrawal due to the risk of inducing a precipitated withdrawal.(Citation18) Currently, an estimated 80% of individuals with an opioid use disorder are not able to get treatment.(Citation19) Thus, finding an effective, non-pharmacological approach that would: 1) require minimal training, 2) be available to physicians and advanced care providers, 3) have minimal to no side-effects, and 4) remove the fear of inducing a precipitated withdrawal would be critical in improving and expanding treatment for opioid addiction. Anecdotal evidence exists for the rapid and effective attenuation of signs and symptoms associated with opioid withdrawal through neurostimulation using the BRIDGE® device. This FDA-cleared device is a percutaneous, auricular field stimulator (Innovative Health Solutions, Versailles, IN, USA) developed to alleviate pain through stimulation of peripheral cranial neurovascular bundles in the external ear that could potentially gain access to brain areas involved in fear, pain, and nociception.(Citation20,Citation21).
The development of withdrawal symptoms such as dysphoria, emotional distress, pain, and sleep disturbances has been proposed to result from several potential mechanisms. A recent study involving brain imaging during acute precipitated opioid withdrawal demonstrated increased neural activity in certain brain regions including right pregenual cingulate, putamen, and bilateral caudate.(Citation22) The aversive and anxiety-like state during withdrawal is thought to involve stress-related systems such as noradrenergic pathways. Thus, drugs such as clonidine that inhibit noradrenergic release are often effective in the treatment of heroin withdrawal.(Citation23) HPA axis activation with elevated adrenocorticotropin hormone, corticosterone, and CRF in limbic brain regions has also been documented during withdrawal.(Citation24,Citation25) Similarly, decreases in serotonergic transmission in the nucleus accumbens and decreases dopamine activity in the mesolimbic system have been reported during withdrawal in animal models.(Citation26,Citation27).
The external ear contains branches of cranial nerves that project to the nucleus tractus solitarius (NTS) (Citation28,Citation29) which communicates with other brain structures involved in autonomic control and pain including the amygdala.(Citation30,Citation31). The extended amygdala has been shown to play an important role in not only fear conditioning and pain processing, but also in processing the negative emotional state of withdrawal.(Citation32–Citation34). Unpublished data from our laboratory using extracellular recordings from single cells in the rat amygdala before and during neurostimulation with the BRIDGE device showed a 65% reduction in the baseline firing of neurons in the central nucleus of the amygdala and a 56% decrease in response to somatic stimulation after just 15 minutes.(Citation35) This suggests a possible mechanism involving neuromodulation of limbic structures that could help alleviate symptoms of withdrawal and offer a noninvasive, drug free alternative.
Thus, we aimed to investigate the effects of the BRIDGE device during the induction phase of opioid withdrawal therapy in an outpatient setting. We hypothesized that treatment with this device would decrease withdrawal scores effectively and rapidly and that this would translate into a high rate of success in transitioning patients to MAT.
Methods
Adult patients (≥18 years old) who met DSM-IV criteria for opioid dependence and voluntarily presented to outpatient drug treatment clinics between June 2015 and July 2016 were included in this retrospective study. Inclusion criteria included a minimum age of 18 y/o and a history of dependence on heroin or other opioids including prescription narcotics, methadone, and buprenorphine/naloxone. Patients were excluded if they reported a history of dependence on alcohol, pregnancy, or inability to consent to the treatment. A history of benzodiazepines use was not a contraindication to BRIDGE placement. Participating clinics located across five states provided data from patients treated with the BRIDGE: St. Louis, MO,(Citation27) Liberty, IN,(Citation13) Florence, KY,(Citation12) Anchorage, AK,(Citation9) Rising Sun, IN,(Citation6) Richmond, IN,(Citation2) Dayton, OH,(Citation2) and Indianapolis, IN.(Citation2) Six of the eight clinics were private clinics, and all of them were chosen to participate because the BRIDGE had been utilized as the standard of clinical care in the initial treatment of opioid withdrawal (). The participating treatment centers provided individualized evaluation, stabilization, and treatment with a team of physicians, nurses, counselors, and case managers. Variations exist among the eight outpatient clinics with an approximate daily census ranging from 4 to 25 patients.
Table 1. Patient demographics and drug use characteristics.
Seventy-three medical records were reviewed, reflecting every opioid-dependent individual who was voluntarily treated with the BRIDGE. Data obtained from all subjects such as demographics and history that included age, gender, approximate length of addiction, and substances used were obtained from chart review (). Urine analysis to screen for concomitant use of other substances was performed prior to start of treatment in all patients ().
As part of the treatment protocol in using the BRIDGE device, initial Clinical Opioid Withdrawal Scale (COWS) scores are recorded at baseline and at three other time intervals (20, 30, and 60 minutes) in all patients. These scores were extracted from the chart along with scores recorded at 5-day post-BRIDGE placement when available. The scores range from 0 to 48 and are rated as mild,(Citation5–Citation12) moderate,(Citation13–Citation24) moderately severe,(Citation25–Citation36) or severe (>36).(Citation36)
BRIDGE stimulating protocol
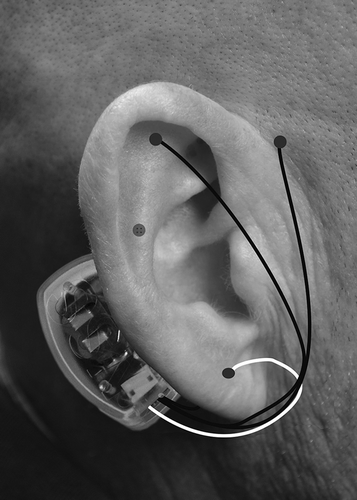
All providers received training regarding BRIDGE placement through an online module and then through a site visit from I-H-S training representatives. The device is packaged with all the necessary supplies for proper placement. In all participating clinics, the BRIDGE was placed as per standard protocol. In brief, the ear was cleaned with alcohol wipes and transilluminated to identify neurovascular bundles that are to be avoided during needle implantation. The generator was attached with adhesive to the skin behind the ear just over the mastoid process. There are four leads that come off the generator, each with sterile 2mm, titanium needles that are inserted into the dorsal and ventral aspects of the ear. The array was place in four general areas within 1–1.5mm of the visible arterial branches to create a field effect (see ). The BRIDGE delivers 3.2V with alternating frequencies of stimulation and has a battery life of 5 days.
The majority of patients were observed after BRIDGE placement and sent home after symptoms of withdrawal were relieved, usually after the first hour. The use of any rescue medications during the first hour of neurostimulation with the BRIDGE, including antipsychotics, narcotics, or benzodiazepines, was also recorded. Part of the discharge instructions given to patients emphasizes that the BRIDGE is not a cure, but rather a tool to treat the pain associated with opiate withdrawal. Patients were instructed to follow-up within 1–5 days, depending on the clinic, and to leave the device on for the entire 5-day period, since removal of the device prior to this time usually results in recurrence of symptoms. Patients were also instructed to return the device once removed so that is could be properly disposed. Any adverse outcome associated with the device was also recorded including localized pain, infections, or neurological complaints not related to withdrawal. Informed consent was not obtained due to the retrospective study design. The human research Institutional Review Board (IRB) at Medical College of Wisconsin approved this study (PRO00027502).
Data analysis
The main outcome measured was reduction in withdrawal scores as measured by the COW scale. Although the primary outcome of this study was to assess improvement in symptoms during withdrawal from opioids, successful transition to MAT was also investigated in a subset of patients. Those subjects returning to clinic on day 5 post-BRIDGE placement and receiving their first dose of maintenance medication with the opioid receptor antagonist, naltrexone, were considered to be successfully transitioned.
Statistical analysis was performed with SPSS version 19. Basic descriptive statistics were used for participant demographics. Nonparametric analysis with repeated measures ANOVA was used to evaluate effectiveness in reducing withdrawal scores across all time periods measured. Missing COWS scores were encountered at 20 min (11/73) and at 60 min (2/73). Imputing of missing data was not performed, and analysis was made only with available data since the missing values were considered missing at random. Data were presented as mean (±standard deviation), and p<0.05 was considered statistically significant.
Results
A total of 73 subjects were included. In this cohort, the mean age was 32.9 (9.4) years old and 65% were male. The mean length of drug use was 70 (Citation55) months and 90.5% of the subjects had been using opioids for at least 2 years.
Immediately prior to BRIDGE placement, the majority of patients in this study, 53/73 (72.6%), fell into the moderate withdrawal range, while 16/73 (21.9%) were in the moderately severe range and 4/73 (5.4 %) were in the mild range. In the entire cohort, the mean (standard deviation) COWS score prior to BRIDGE placement was 20.1 (±6.1). The BRIDGE decreased scores to a mean of 7.5 (±5.9) by 20 minutes (62.7% reduction p<0.001), 4.0 (±4.4) by 30 minutes (80% reduction, p<0.001), and 3.1 (±3.4) after 60 minutes (84.6% reduction, p<0.001) (). Overall, 73/73 (100%) subject had a reduction in COW scores by 60 minutes with a minimum decrease in at least 36.4%. At 60 minutes 57/73 (78.0%) had withdrawal scores of ≤3. No rescue medications were used in any subject during the first 60 minutes after device placement. During the entire 5-day period, no antipsychotic, narcotic or benzodiazepine medications were given. Thirty eight percent (28/73) of patients received an antiemetic.
Figure 2. Opioid withdrawal scores before and after BRIDGE device placement. A significant decrease in scores was seen after just 20 minutes with an 84.6% reduction from baseline by 60 minutes and 97% after 5 days (*p<0.001 vs. baseline scores).
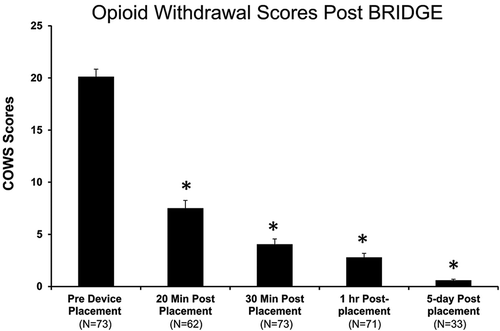
A subset of patients also had withdrawal scores recorded 5 days after BRIDGE placement and prior to transitioning to MAT (n=33). In this group, the average withdrawal score prior to receiving the first dose of naltrexone was 0.6 (97.1% reduction). In the entire cohort of 73 patients, 64 (88.8%) successfully transitioned to MAT after BRIDGE placement. No adverse events were recorded in any subject during the entire time of neurostimulation with the BRIDGE.
Discussion
This pilot study is the first to demonstrate that the signs and symptoms of opioid withdrawal can be rapidly and effectively attenuated without the use of pharmacotherapy. The effects on decreasing withdrawal scores were extremely robust and lasted throughout the induction phase. Within 20 minutes of BRIDGE device application, there was a 62.7% reduction in withdrawal scores and 84.6% by 60 minutes. Five-day post-BRIDGE placement, withdrawal scored had been reduced by 97% in a subgroup of patients. Overall, 88.8% of patients in the entire cohort successfully transitioned to MAT.
These properties of percutaneous electrical nerve field stimulation (PENFS) and the technique used in this study make it very different from other therapies, including acupuncture. Unlike acupuncture, this type of treatment is designed to be used continuously in the outpatient setting. Also, electroacupuncture delivers very different electrical current and unlike PENFS with the BRIDGE, and it requires precise placement of needles in specific “acupoints” in the ear by a trained expert. A study that investigated the effects of electroacupuncture on opioid withdrawal symptoms showed a delay of up to two days to demonstrate any efficacy in alleviating withdrawal scores, even with the concomitant use of opioid receptor agonists.(Citation37) In this study, no side-effects were reported with the BRIDGE. This is consistent with a previous report of over 1,200 patients that reported minimal to no side-effects using percutaneous nerve field stimulation for other indications.(Citation38) However, more studies are needed to investigate the exact mechanism responsible for the clinical effects seen.
A common central link between a state of chronic pain and opioid dependence appears to be the limbic region.(Citation34,Citation39–Citation43). The amygdala, in particular, is a brain region which is involved in the emotional component of pain processing, and activation of this area has also been associated with the negative emotional state of withdrawal to opioids and drug craving.(Citation44) The auricular branches of four cranial nerves (V, VII, IX, and X) in the external ear have projections to the brainstem, in particular, the NTS.(Citation20,Citation28,Citation29) Neurostimulation with the BRIDGE is thus likely to modulate the NTS, which acts as a “relay station” to higher brain structures involved in autonomic control and nociception, including the rostral ventral medulla, hypothalamus, and amygdala.(Citation30,Citation31) The negative emotional state of withdrawal primarily involves the amygdala, which processes the emotional component of pain and fear conditioning. Preclinical data in an animal model of pain suggest not only a significant attenuation of amygdala neuronal firing after 15 minutes of neurostimulation with the BRIDGE device, but also a 47% reduction in spontaneous activity of lumbar spinal neurons.(Citation35) This suggests that in addition to the modulatory effects of the amygdala on spinal cord neurons, other areas may be involved since nociceptive drives entering the spinal cord are subject to modulation from pontomedullary nuclei such as the periaqueductal gray (PAG) and rostro ventral medulla (RVM). These areas also get input from the NTS.(Citation45–Citation49) Further studies are needed to investigate the effects of neurostimulation on other brain regions as well as the effects on neurotransmitters known to be deranged during opioid withdrawal including norepinephrine, ACTH, cortisol, and endorphins.(Citation50,Citation51) Improvement in withdrawal symptoms during neurostimulation could also theoretically result from improvement in dopamine levels in limbic structures that have been shown to be significantly low in during withdrawal.(Citation52,Citation53)
In this study, the COW scale was used as a tool to evaluate the signs and symptoms experienced during withdrawal. This scale has been clinically validated and is commonly used in drug treatment protocols to assess the severity of opioid withdrawal.(Citation54) It includes assessment of heart rate, sweating, restlessness, pupil size, somatic pain, runny nose, GI upset, tremors, yawning, anxiety or irritability, and gooseflesh skin. Because it includes subjective symptoms along with objective signs, it limits the possibility of feigned responses. The majority of patients in this study fell into the moderate range of withdrawal, which is consistent with other studies using pharmacotherapy as initial treatment.(Citation9,Citation10,Citation15) A recent study that compared treatment outcomes in opioid-dependent patients with different buprenorphine/naloxone induction doses found that baseline COWS scores influence treatment retention rates.(Citation8) A different study that investigated predictors of outcomes using methadone and buprenorphine therapy concluded that higher withdrawal scores are associated with poorer retention.(Citation13) Thus, having a treatment that offers rapid and effective improvement could lead to improved compliance and better treatment outcomes. The results of the study may therefore have significant clinical implications.
To address the opioid epidemic, the US Congress passed the DRUG Addiction Treatment Act (Citation55) to expand treatment options beyond specialized, addiction treatment centers.(Citation56,Citation57) This allows office-based physicians who complete training to obtain a waiver, allowing them to prescribe the opioid receptor agonist, buprenorphine to a limited number of subjects.(Citation58) Unfortunately, a recent study suggests that only about 2.2% of American physicians have obtained a waiver and 90% of those were practicing in urban counties.(Citation59) Some of the challenges reported by physicians include lack of time, inadequate staff support, concerns about DEA investigations, and paperwork requirements.(Citation17) While it is beyond the scope of this study to compare outcomes of neurostimulation with the BRIDGE to those of commonly used pharmacotherapies, it seems logical that this type of therapy could play an important role as a therapeutic option since it could help overcome some of the current barriers encountered with pharmacotherapy.(Citation60,Citation61) The BRIDGE requires minimal training, can be used by physician extenders without limitations on the number of subjects treated, and can be implemented without the fear of inducing a precipitated withdrawal. At a cost of approximately $500 per device, which requires a one-time use, it is also likely to be cost effective and easily accessible. It is important to keep in mind that pharmacotherapy with opioid receptor agonists and/or antagonists plays an indispensable role not only in long-term maintenance therapy, but also during the induction phase in certain patients.
Limitations of the current study included the uncontrolled, retrospective study design, and the relatively small sample size. While randomized, placebo controlled trials have the highest level of validity, most individuals in the current study presented in moderate to severely moderate withdrawal. The concern of using a placebo or sham device in such a vulnerable population must be taken into consideration in future studies. While the data in the current study were collected retrospectively, the objective and subjective COWS scores were all recorded prospectively during intervals of clinical care. This eliminates recall bias and helps establish the temporal relationship of the results. Another weakness of the study was that the potential presence of psychiatric disorders other than opioid dependence and more precise drug use patterns could not be included in the analysis. Studies suggest that perhaps the types of drugs used or the presence of other psychiatric disorders could influence treatment outcomes.(Citation62,Citation63) Although a large percentage of subjects successfully transitioned to MAT, the study took place in an outpatient setting and thus did not allow us to record withdrawal scores or the use of adjunct medications between day 1 and day 5. Finally, the study design did not allow for long-term follow-up, which is also an important limitation.
In summary, percutaneous nerve field stimulation with the BRIDGE device is associated with a reduction in opioid withdrawal scores and allows for an effective transition to secondary therapy. With the urgent need to expand access to opioid addiction treatment, this approach could become an important treatment option and help eliminate some of the barriers to increasing access in the primary care setting. While this approach can improve the symptoms associated with opioid withdrawal, it is likely to be an adjunct to currently available therapies that include MAT and counseling which are key components of the recovery process. The majority of patients in this cohort were treated with the BRIDGE device with the intent to transition to long-acting injectable naltrexone. While this seems like a logical target population given the battery life of the device which coincides with the abstinence time required to initiate treatment, improving symptoms of withdrawal and providing comfort to patients would be beneficial in almost any setting. Additionally, it seems reasonable to consider that removing the fear of experiencing severe symptoms associated with opioid withdrawal may motivate and encourage more individuals to seek treatment in the future.
Conflict of Interest
Neither author has a commercial or financial relationship to disclose with Innovative Health Solutions (IHS). The company did not provide funding for this study. Dr. Taca is a consultant to Alkermes, Inc.
Dr. Taca reports that he filed a patent application in 2015 for a method of treating drug or alcohol abuse, addiction or dependency comprising to the use of auricular stimulation with devices like the BRIDGE that may be affected by the research reported in the enclosed paper.
This patent has not been granted and he otherwise has no financial interests in the device manufactures, Innovative Health Solutions, Inc. (IHS). He has not received any monetary compensation or funding from IHS and the company is not part of this patent application.
Acknowledgments
The authors would like to thank Kim Kline, RN, Andrea Keane, FNP-BC, Katrina Lock, FNP-BC, Paul Finch, PA-C, Barb Davis, MD, Ashley Halker, MHA, Jordan Blankmann, BSN RN, Jamie Strobel, MSW, Carissa Hoffman, and John Cavanagh for their assistance with this project.
References
- UNODC, World Drug Report 2012. Available at: http://www.unodc.org/unodc/en/data-and-analysis/WDR-2012.html.
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf.
- Arfken CL, Johanson CE, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J Subst Abuse Treat 2010;39:96–104.
- Li X, Shorter D, Kosten TR. Buprenorphine in the treatment of opioid addiction: opportunities, challenges and strategies. Expert Opin Pharmacother 2014;15:2263–2275.
- Markowitz JD, Francis EM, Gonzales-Nolas C. Managing acute and chronic pain in a substance abuse treatment program for the addicted individual early in recovery: a current controversy. J Psychoactive Drugs 2010;42:193–198.
- Parrino MW, Maremmani AG, Samuels PN, Maremmani I. Challenges and opportunities for the use of medications to treat opioid addiction in the United States and other nations of the world. J Addict Dis 2015;34:255–262.
- Amato, L, Davoli, M, Ferri, M, Gowing, L, Perucci, CA. Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug Alcohol Depend 2004; 73:219–226.
- Kamali-Sarvestani F, Motiallah T, Ghaffarinejad F. The prevalence of musculoskeletal pain and forward head posture among heroin users during their withdrawal with methadone. Addict Health 2014;6:30–35.
- Gunderson EW, Sumner M. Efficacy of Buprenorphine/Naloxone Rapidly Dissolving sublingual Tablets (BNX-RDT) after switching from BNX sublingual film. J Addict Med 2016;10:122–128.
- Jacobs P, Ang A, Hillhouse MP, Saxon AJ, Nielsen S, Wakim PG, Mai BE, Mooney LJ, S Potter J, Blaine JD. Treatment outcomes in opioid dependent patients with different buprenorphine/naloxone induction dosing patterns and trajectories. Am J Addict 2015;24:667–675.
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS Jr, Kintaudi P, Wesson DR, McNicholas L, Tusel DJ, Malkerneker U, Renner JA Jr, Santos E, Casadonte P, Fye C, Stine S, Wang RI, Segal D. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction 1998;93:475–486.
- Oreskovich MR, Saxon AJ, Ellis ML, Malte CA, Reoux JP, Knox PC. A double-blind, double-dummy, randomized, prospective pilot study of the partial mu opiate agonist, buprenorphine, for acute detoxification from heroin. Drug Alcohol Depend 2005;77:71–79.
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol 2008;11:641–653.
- Umbricht A, Hoover DR, Tucker MJ, Leslie JM, Chaisson RE, Preston KL. Opioid detoxification with buprenorphine, clonidine, or methadone in hospitalized heroin-dependent patients with HIV infection. Drug Alcohol Depend 2003;69:263–272.
- Webster L, Hjelmström P, Sumner M, Gunderson EW. Efficacy and safety of a sublingual buprenorphine/naloxone rapidly dissolving tablet for the treatment of adults with opioid dependence: a randomized trial. J Addict Dis 2016;7:1–14.
- Fiellin DA, O’Connor PG. Clinical practice. Office-based treatment of opioid-dependent patients. N Engl J Med 2002;347:817–823. Review.
- Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Ann Fam Med 2014;12:128–133.
- Fishman M. Precipitated withdrawal during maintenance opioid blockade with extended release naltrexone. Addiction 2008;103:1399–1401.
- Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004–2013. JAMA 2015;314:1515–1517.
- Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 2015;8:624–636.
- Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal - a pilot study. Brain Stimul 2013;6:798–804.
- Chu LF, Lin JC, Clemenson A, Encisco E, Sun J, Hoang D, Alva H, Erlendson M, Clark JD, Younger JW. Acute opioid withdrawal is associated with increased neural activity in reward-processing centers in healthy men: a functional magnetic resonance imaging study. Drug Alcohol Depend 2015;153:314–322.
- Gowing L, Farrell M, Ali R, White JM. Alpha₂-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev 2016;5:CD002024.
- Koob GF. A role for brain stress systems in addiction. Neuron 2008;10:59:11–34.
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 2007;164:1149–1459. Review.
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol 1992;221:227–234.
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res 1992;593:314–318.
- Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst 1982;6:303–322.
- Zhang LL, Ashwell KW. The development of cranial nerve and visceral afferents to the nucleus of the solitary tract in the rat. Anat Embryol (Berl) 2001;204:135–151.
- Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol 1985;242:511–534.
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 1984;224:1–24.
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 2004;10:221–234.
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature 2008;454:642–645.
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–238.
- Miranda A, Babygirija R. Neurostimulation with IBStim attenuates amygdala neurons and prevents post-inflammatory visceral and somatic hyperalgesia in rats. World Congress of pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr 2016;63 Suppl 2:S1–S415.
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003;35:253–259. Review.
- Ma D, Han JS, Diao QH, Deng GF, Ping XJ, Jin WJ, Wu LZ, Cui CL, Li XD. Transcutaneous electrical acupoint stimulation for the treatment of withdrawal syndrome in heroin addicts. Pain Med 2015;16:839–848.
- Roberts A, Sithole A, Sedghi M, Walker C, Quinn T. Minimal adverse effects profile following implantation of peri-auricular percutaneous electrical nerve field stimulators: a retrospective cohort study. Med devices 2016;9:389–393.
- Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron 2016;89:11–36.
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology 2002;160:425–433.
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res 2007;176:251–258.
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience 2005;134:9–19.
- Rouwette T, Vanelderen P, Roubos EW, Kozicz T, Vissers K. The amygdala, a relay station for switching on and off pain. Eur J Pain 2012;16:782–792.
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–238.
- Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol 2002; 87:2225–2236.
- Helmstetter FJ, Bellgowan PS, Tershner SA. Inhibition of the tail flick reflex following microinjection of morphine into the amygdala. Neuroreport 1993;4:471–474.
- Manning BH, Mayer DJ. The central nucleus of the amygdala contributes to the production of morphine antinociception in the formalin test. Pain 1995;63:141–152.
- Chen LW, Rao ZR, Shi JW. Catecholaminergic neurons in the nucleus tractus solitarii which send their axons to the midbrain periaqueductal gray express Fos protein after noxious stimulation of the stomach: a triple labeling study in the rat. Neurosci Lett 1995;189:179–181.
- Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol 1985;242:511–534.
- Lelevich SV, Lelevich VV, Novokshonov AA. Neurotransmitter mechanisms of morphine withdrawal syndrome. Bull Exp Biol Med 2009;148:184–187.
- Li SX, Shi J, Epstein DH, Wang X, Zhang XL, Bao YP, Zhang D, Zhang XY, Kosten TR, Lu L. Circadian alteration in neurobiology during 30 days of abstinence in heroin users. Biol Psychiatry 2009;65:905–912.
- Acquas E, Di Chiara G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J Neurochem 1992;58:1620–1625.
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol 1992;221:227–234.
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend 2009;105:154–159.
- Drug Addiction Treatment Act of 2000. Public Law No. 106-310, Title XXXV–Waiver authority for physicians who dispense or prescribe certain narcotic drugs for maintenance treatment or detoxification treatment, 2000. Available at: http://www.buprenorphine.samhsa.gov/fulllaw.html [ last accessed 25 Jul 2016].
- Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Subst Abuse Treat Prev Policy 2008;3:17.
- Knudsen HK, Lofwall MR, Havens JR, Walsh SL. States’ implementation of the Affordable Care Act and the supply of physicians waivered to prescribe buprenorphine for opioid dependence. Drug Alcohol Depend2015;157:36–43.
- Stein BD, Gordon AJ, Dick AW, Burns RM, Pacula RL, Farmer CM, Leslie DL, Sorbero M. Supply of buprenorphine waivered physicians: the influence of state policies. J Subst Abuse Treat 2015;48:104–111.
- Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med 2015;13:23–26.
- Hirchak KA, Murphy SM. Assessing differences in the availability of opioid addiction therapy options: rural versus urban and American Indian reservation versus nonreservation. J Rural Health 2017;33:102–109.
- Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, Alford DP. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Intern Med 2008;23:1393–1398.
- Marcovitz DE, McHugh RK, Volpe J, Votaw V, Connery HS. Predictors of early dropout in outpatient buprenorphine/naloxone treatment. Am J Addict 2016 Sep;25(6):472–477.
- Torrington M, Domier CP, Hillhouse M, Ling W. Buprenorphine 101: treating opioid dependence with buprenorphine in an office-based setting. J Addict Dis 2007;26:93–99.