ABSTRACT
Background: The psychoactive indole alkaloid ibogaine has been associated with encouraging treatment outcomes for opioid dependence. The legal status of ibogaine in New Zealand provides a unique opportunity to evaluate durability of treatment outcomes. Objective: To examine longitudinal treatment effects over a 12-month period among individuals receiving legal ibogaine treatment for opioid dependence. Method: This observational study measured addiction severity as the primary outcome in 14 participants (50% female) over 12 months post-treatment using the Addiction Severity Index-Lite (ASI-Lite) following a single ibogaine treatment by either of two treatment providers. Secondary effects on depression were assessed via the Beck Depression Inventory-II (BDI-II). The Subjective Opioid Withdrawal Scale (SOWS) was collected before and immediately after treatment to measure opioid withdrawal symptoms. Results: Nonparametric comparisons via Friedman Test between baseline and 12-month follow-up for participants completing all interviews (n = 8) showed a significant reduction for the ASI-Lite drug use (p = 0.002) composite score. Reductions in BDI-II scores from baseline to 12-month follow-up were also significant (p < 0.001). Significant reductions in SOWS scores for all participants (n = 14) were also observed acutely after treatment (p = 0.015). Patients with partial data (n = 4) also showed reductions in ASI-Lite drug use scores and family/social status problems. One patient enrolled in the study died during treatment.
Conclusion: A single ibogaine treatment reduced opioid withdrawal symptoms and achieved opioid cessation or sustained reduced use in dependent individuals as measured over 12 months. Ibogaine’s legal availability in New Zealand may offer improved outcomes where legislation supports treatment providers to work closely with other health professionals.
Introduction
Opioid dependence is a debilitating condition associated with increased morbidity and mortality, limited treatment response, and high relapse rate (Citation1). Patients suffer from fractured relationships, depression, inability to maintain employment, diminished cognitive and psychosocial functioning, and high healthcare costs (Citation2). Annual prevalence of opioid dependence was estimated in 2007 to be 0.4% of the world population and 0.325% of the New Zealand population aged 15–64 (Citation3). Overprescribing and insufficient monitoring of opioids, including those approved to treat substance use disorders, have contributed to increased prevalence in recent years in the United States (U.S.) (Citation4). Reducing opioid-related overdoses and deaths requires a comprehensive effort combining detoxification, behavioral, psychoanalytic, and counseling therapies with all available pharmacotherapies (Citation5). Nonetheless even with combined medication-assisted therapies, consistently achieving remission is difficult due to lack of adherence, underutilization, and limited or ineffective adoption by treatment providers (Citation5,Citation6). Collectively these phenomena add urgency to the search for solutions to opioid dependence and its accompanying risks.
The present epidemic of opioid dependence justifies consideration of novel therapeutic options. Ibogaine treatment, associated with reduced opioid use, attenuation of withdrawal symptoms, and cessation of cravings (Citation7), offers an underutilized yet promising option in response to the limitations of available treatments. Ibogaine is a psychoactive indole alkaloid with stimulatory and hallucinogenic effects that is derived from the root bark of the West African shrub Tabernanthe iboga. Iboga’s powerful psychedelic properties remain a central component of ceremonial use in the Bwiti religion among the Gabonese Fang people of West Africa, who still incorporate iboga in religious ritual, with lower doses of the root bark used as a stimulant and appetite suppressant (Citation8). Purified ibogaine hydrochloride (HCl) was previously marketed, at 5–8 mg doses used 3–4 times per day, under the trade name Lambarene in France (1939–1970) as an antidepressant and enhancer of mental and physical ability (Citation9).
Ibogaine’s potential for treating opioid dependence was discovered in 1962 by Howard Lotsof, based on personal experience and anecdotal reports. Doses up to 19 mg/kg were associated with attenuation of opioid withdrawal and craving, as well as cessation of use (Citation10,Citation11). In contrast to most pharmacotherapies which require ongoing maintenance doses, ibogaine is typically administered as a single-dose treatment on a few occasions as an adjunct to a detoxification treatment model. This treatment regimen helps to mitigate risk of adverse events associated with ibogaine. In two Phase 1 studies using very low doses, single 20 mg doses of ibogaine were well tolerated (N = 21), with no effect on vital signs and no adverse effects (Citation9,Citation12); however, typical doses used for opioid dependence treatment in New Zealand are much higher. Based on in vitro studies and one case report, the principle risk associated with ibogaine is cardiotoxicity resulting from blockade of repolarizing potassium channels and retarded repolarization of the ventricular action potential simultaneous with QT interval prolongation (Citation13). This sequence may lead to life-threatening torsades de pointes (TdP) arrhythmias and sudden death in rare instances.
Putative anti-addiction properties of ibogaine led to extensive studies of acute effects in dependent human volunteers with hazardous opioid use to explore the risk/benefit profile, summarized in . These studies support reproducible indications of effectiveness and an acceptable risk/benefit profile of ibogaine in the treatment of opioid dependence and withdrawal, as opioid dependence has a pooled relative mortality risk (RR) of 2.38 (95% CI: 1.79–3.17) even while in treatment. Out of treatment mortality risk was much greater (Citation14). Ibogaine and its active metabolite noribogaine were found to have numerous direct and indirect functional targets with complex pharmacology in studies aiming to elucidate mechanism of action (Citation15). Most recently, noribogaine was found to be the principal active moeity responsible for interrupting psychological and physiological effects of opiate dependence in rats, with profound implications for effects in humans (Citation16).
Table 1. Published reports of Ibogaine administrations to opioid-dependent patients.
The present study describes a prospective observational case series of 14 participants seeking ibogaine treatment for opioid dependence. The study aimed to contribute information on durability of ibogaine treatment outcomes covering 12-month post-treatment, which was lacking from prior studies. Undertaking this research in New Zealand received further impetus with the 2009 scheduling of ibogaine, by New Zealand’s medical regulatory body Medsafe, as a non-approved medicine (Citation17). Thus, unlike many other regulatory environments, ibogaine is available via legal prescription in New Zealand. The location of the study was chosen to encourage participants to honestly report outcomes without concern of legal consequence and to take advantage of the ability of treatment providers to share information about the patient when covered by appropriate release forms. This study evaluated durable effects of ibogaine on severity of opioid dependence. Acute withdrawal symptoms and long-term depression symptoms were also evaluated as potential contributors to treatment response. Evaluation of safety was beyond the scope of this non-interventional study. Results are intended to support design of future studies on prevention of relapse of opioid dependence after single-dose ibogaine treatment.
Methods
Ethical review, treatment providers, and participants
All participants were treated in accordance with ethical guidelines for health and disability research in New Zealand to ensure it met or exceeded established ethical standards. The study was evaluated and approved by the Health and Disabilities Multi-region Ethics Committee in February 2012 (Ethics Reference # MEC/11/11/095). Per the International Conference of Harmonization (ICH) definition, this was a non-interventional/observational study on the effects of ibogaine prescribed in accordance with regulatory authorization in a manner clearly separated from the decision of including participants in the study.
Participants who independently sought treatment were recruited through two ibogaine providers offering treatments on a fee-for-service basis (hereafter Provider 1 and Provider 2). Provider 1 offered ibogaine treatment at a clinic located in the far north of New Zealand’s North Island utilizing a medically qualified physician. Provider 2 was a registered addictions counselor who offered ibogaine treatment in a private practice setting, in collaboration with the treating physician of each client and a community health psychiatrist. Both providers offered a period of post-treatment supervision extending beyond the typical three days of treatment, during which food intake, exercise, and sleep was monitored. Patients of Provider 1 remained in care for periods extending beyond a week post-treatment. Provider 2’s patients usually left their direct care within four days post-treatment. Despite Provider 1’s greater treatment volume, they contributed only one participant due to limited engagement with this study. Although Provider 2 treated fewer patients concurrent with the study period, they ultimately contributed 13 of the 14 participants described in the present study.
Study participants were required to meet the following inclusion criteria: They had to voluntarily seek treatment without coercion; had independently contacted treatment providers seeking treatment; were over the age of 18 years; able to communicate in English; provided contact information for a close affiliate whom the researcher would contact for corroborating data; and committed to regular contact for twelve-month post-treatment via phone or Skype. Prospective participants meeting any of the following criteria were excluded: those seeking ibogaine treatment for any reason other than opioid dependence; had received ibogaine treatment on a previous occasion; in the opinion of the investigators participants had any personal, situational, health, social, or other issue that would prevent full adherence to study requirements; and those unable to give informed consent.
Drug
All participants were orally administered staggered doses of ibogaine HCL (200 mg capsules). Initially, both providers imported ibogaine HCL (98.5%) from a European manufacturer through a registered New Zealand pharmaceutical importer. Subsequently Provider 2 switched to using Remogen™, a Canadian product, assessed by HPLC as 99.5% pure ibogaine HCl. Of 14 participants, 42.9% received Remogen™.
Participants ingested their last dose of opioids between 12 and 33 hours (mean 20.2, s.d. 9.6) before ibogaine treatment. All participants were fasted prior to dosing. Participants received 25–55 mg/kg (mean 31.4, s.d. 7.6) of ibogaine with concomitant benzodiazepine and sleep aids in most cases. Treatments typically commenced in the early evening and involved multiple doses over 24–96 hours (mean 57 hours). Initial dosage was selected based on patient characteristics (psychological and physical health, age, fitness, drug use) and provider experience. Dosage was adjusted based on patient response and provider assessment through observation and questioning (SOWS scores, changes in proprioception, interoception, mood). A “test” dose of 200 mg, administered when the provider determined the patient was sufficiently in withdrawal, was followed between 1 and 4 hours by a larger dose (typically 400–600 mg), then more rapidly by smaller doses (e.g., 200 mg at 20 minute intervals) until the provider determined the appropriate level of dosing had been achieved. Administration of ibogaine was within the purview of the providers, as the investigator (GN) was solely involved in an observational capacity and was not present during treatments. Per New Zealand regulations, providers were responsible for selection and determining medical eligibility of participants. All aspects of medical care were documented in provider medical records.
Data collection and outcome measures
Data were collected over 14 interviews. These included pretreatment baseline (interview 1); an interview immediately post-treatment (interview 2); and twelve-month interviews (interviews 3–14, corresponding to post-treatment months 1–12). lists the schedule of interviews, along with the outcome measures administered during each interview.
Table 2. Schedule of subject interviews (Citation14) and data collection, with outcome measures to 12-months post-tx.
The New Zealand-based investigator (GN), who was trained in administration of the Addiction Severity Index Lite (ASI-Lite) and Beck Depression Inventory-II (BDI-II), conducted all the interviews at treatment sites in person, at baseline and immediately post-treatment. Baseline data were collected as close to the treatment time as feasible, typically either the preceding day or on the day of treatment. For subsequent visits, the same investigator collected data in person for 28.6% of participants, with the remaining participants assessed during Skype or phone call interviews. Two participants preferred to complete mailed responses.Footnote1 Verification interviews with affiliates were conducted by phone. At baseline and post-treatment interviews (except interview 2), the investigator would attempt contact with participants’ affiliates to independently verify responses, for example, for current substance use, aftercare, mood, and level of social support. Additional interviews where the outcome measures were not administered comprised brief conversations between the investigator and participants at interviews 4, 6, 7, 9, 10, and 13. Attempts were also made to contact participants’ affiliates at these times.
The primary outcome measure was the ASI-Lite (Citation18). This was administered pretreatment at baseline and at months 1, 3, 6, 9, and 12 post-treatment. The instrument uses a 40-minute clinical interview to indicate problem severity in seven life areas commonly affected by substance use disorders: medical status, employment, alcohol use, other drug use, legal status, family and social relationships, and psychiatric status. Symptoms and problems are measured over the preceding 30 days, with higher scores representing greater severity (Citation19).
The BDI-II was administered for the assessment of depression symptoms (Citation20) at baseline, immediately post-treatment, and at 3-month intervals, generally corresponding with administration of the ASI. The Subjective Opioid Withdrawal Scale (SOWS) was administered as close as feasible pre- and post-treatment by the investigator to determine participants’ experience of withdrawal symptoms (Citation21). It was also administered by each provider subsequent to the baseline data collection, to determine level of subject withdrawal immediately prior to administration of ibogaine, and up to 72 hours post-initial dosing to assess subject response during treatment.
Biological verification of drug use data post-treatment involved two random urine screens during the follow-up period, with a third final screen at the time of their last interview (interview 14). Participants were asked to complete screens within 24 hours of administration of the ASI-Lite. Arrangements were made with various testing facilities and laboratories accessible to participants, and all expenses associated with testing were met. Participants received a $10 gift voucher for each follow-up interview they participated in, up to a maximum of $120 for all twelve post-treatment interviews. Study oversight was provided on behalf of the Multidisciplinary Association for Psychedelic Studies by the third author (BY).
Statistical methods
Descriptive statistics including means, standard deviations, ranges, frequencies, and percentages were used to summarize data. Friedman’s nonparametric ANOVA was used to test for significant patterns of change over time for the ASI-Lite subscales, BDI-II, and SOWS scores. Where significant effects were identified with these analyses incorporating all assessment times, these were further explored using Wilcoxon signed rank tests to compare individual assessment times with those at baseline. The sample size was too small to usefully explore any differential sub-group effects by site. An alpha level of 0.05 was used for all tests. Statistical analysis was performed by the statistician and second author (CF) using IBM SPSS v23.
Results
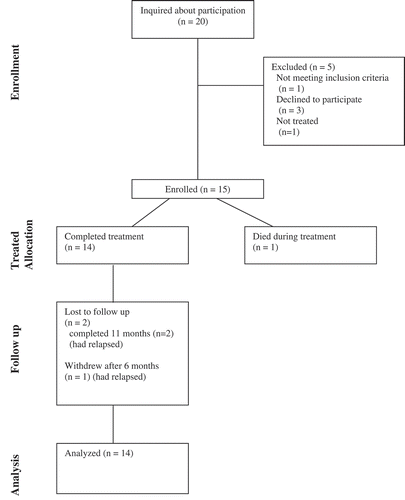
Twenty people who sought ibogaine treatment with the two providers indicated interest during the enrollment period (). Three declined to participate in the study, and one person was declined treatment by their provider due to concerns about post-treatment safety. Sixteen participants signed the study Information and Consent Form and 15 were enrolled into the study. One person died during their treatment, and a second person was disqualified from the study upon review of their treatment, due to leaving the treatment before it had been completed to the satisfaction of the Provider. The data reported here describes post-treatment outcomes for 14 participants administered ibogaine for opioid dependence.
Participants’ age ranged between 28 and 47 years (mean 38; s.d. 4.8), 50% were female, and all identified as Caucasian. Participants had moderate comorbid depression symptoms at baseline (mean 22.1; s.d. 10.8). They had previously received an average of 4.7 treatments for substance dependence (range 0–20), with 58% of these being detoxification. At the time of treatment, 71% (n = 10) were receiving methadone maintenance treatment (MMT; see Supplementary Table S1). Data from the ASI-Lite show that in the thirty-day period prior to baseline interviews participants had on average used opioids for approximately 28.8 days. Methadone was most commonly reported as the primary drug of dependence (n = 10), followed by codeine (dihydrocodeine) (n = 3) and poppy seeds (n = 1). Despite all those receiving MMT considering methadone as their most problematic drug, six also reported using other opioids in the preceding thirty days, that is, morphine sulfate (n = 3),Footnote2 dihydrocodeine (n = 2), and buprenorphine (n = 1).
During treatment 10 of Provider 2’s 13, subjects were administered ondansetron (4–8 mg); 5 received diazepam 5–25 mg; and 1 received zopiclone (7.5 mg). Provider 1 also administered diazepam (30 mg) and zopiclone (15 mg) to their single participant in the study during treatment. Overall, two participants were enrolled via Provider 1, with one subsequently lost to treatment at 11-months and a second disqualified from the study immediately following treatment as their Provider revealed they had not completed treatment. A third patient of Provider 1 died during treatment before they were formally enrolled. Of 13 participants enrolled through Provider 2, one voluntarily left the study at eight months and a second was lost to follow up at 11 months post-treatment. The fatality was the subject of two investigations, a coronial inquiry and the second involving New Zealand’s Health and Disability Commissioner (HDC). The latter, completed first, described the treatment provider as being in breach of their duty of care but did not offer a medical explanation for the death (Citation22). The coroner’s ruling generally supported the HDC’s findings.
SOWS assessments showed a significant reduction in withdrawal symptoms from baseline, that is, pre-administration up to 24 hours post-administration assessment (p = 0.015). Although this reduction was slightly greater at the second post-administration SOWS assessment (≥ 42 hours), it did not reach statistical significance, likely due to sample size limitations (). The SOWS was administered multiple times by providers during treatment to determine the need for further dosing of patients.
Table 3. SOWS scores comparing pre-administration baseline with post-administration 12–24 hours, and baseline with post-administration 42–84 hours.
Of the seven ASI-Lite subscales, only the Drug component showed a statistically significant decrease over time (p = 0.002). As seen in , there was a decrease in excess of 80% in the score from 0.32 to 0.06 from baseline to 12 months (p = 0.004). Of the remaining composite score categories, the majority show nonsignificant decreases over twelve months, with the notable exception of the Medical component, which actually increased significantly from 0.00 to 0.34 (p < 0.05), suggesting an increase in participants’ reported health problems or motivation to seek medical care.
Table 4. ASI summary statistics at baseline and 12-month follow-up.
The BDI-II scores decreased significantly over time (p < 0.001) with a significant reduction seen at 1-month post-treatment (mean = 22.1 v 9.3) and continuing to the final 12-month assessment (mean = 4.4) (), indicating a reduction in depression severity.
Table 5. BDI-II scores at baseline; immediately post-treatment; and at 1-, 3-, 6-, 9-, and 12-month follow-up.
Researchers were unable to consistently collect urine drug test data corroborating ASI-Lite reported drug use. For participants providing samples testing negative for opioids, percentages were recorded for some participants at three months (n = 8), six months (n = 7), and 12 months (n = 8) post-treatment. Over these periods only small percentages of participants tested positive for opioids: one subject each at three and six months (12.5% and 14.3% of observed cases, respectively), and two participants (25.0% of observed cases) at 12 months. ASI-Lite data for participants reporting positive opioid use in the preceding 30 days exceeded the incidence of positive urine drug findings and were 43% (three months; n = 14), 50% (six months; n = 14) and 45% (12 months; n = 11). Reductions both in other drug use and alcohol use were reported by 21% of participants (n = 14) each, at three and six months. Of the 11 participants remaining at twelve months, 55% reported reduced other drug use and 36% reduced alcohol use.
Discussion
The present study reports treatment outcomes for opioid dependent participants during 12 months following single-dose ibogaine administration and extends earlier work identifying ibogaine’s effectiveness in treating opioid dependence. Consistent with preceding studies, evidence showed significant attenuation of withdrawal, sustained reduction in drug craving/use, and cessation of use in some cases.
These outcomes, particularly the sustained reduction and/or cessation of opioid use reported by 12 of 14 participants, are comparable with the success of currently accepted treatments, including those reported from wide-ranging analyses and combined interventions reviewed previously (Citation6). This analysis, covering 28 trials (n = 2945 participants) of 12 psychosocial interventions combined with the pharmacological treatments, showed an advantage for treatments in combination for abstinence only (RR 1.15, 95% CI [1.01–1.32]). Measures showing nonsignificant outcomes included retention in treatment, adherence, psychiatric symptoms, and depression.
Where interventions aim for detoxification and cessation (i.e., as with ibogaine), outcomes are even more modest. This was evident in a multi-site US study measuring buprenorphine stabilization and tapering to cessation of opioids, by opioid-free urine tests for two outpatient groups, a 7-day taper group (n = 255) and a 28-day taper group (n = 261) (Citation23). There were no differences in abstinence rates between 7- and 28-day taper groups at 1-month (18% both groups) and 3-month follow-ups (12% 7-day vs. 13% 28-day taper). While the present study’s small numbers preclude conclusive comparison, by contrast, outcomes described above in , indicating that a consistently higher proportion of participants returned negative urine drug screens at three (87.5%), six (85.7%), and 12 months (75%), respectively.
Table 6. Percentages of participants in observed cases reporting urine screen data for opioid use at 3, 6, and 12 months post-ibogaine treatment.
Evidence from the current study showing attenuation of withdrawal substantiates earlier experimental research referencing ibogaine’s “significant pharmacologically mediated effect” on opioid withdrawal (Citation24). This is particularly relevant in a country like New Zealand, where methadone, a long acting synthetic opioid with a correspondingly lengthy withdrawal period, is the most commonly injected opioid (Citation25). That 71% of the present study’s participants sought ibogaine treatment for dependence on methadone perhaps also explains the increase observed in the ASI-Lite Medical composite score from baseline to 12-months (0.00 to 0.34, p < 0.05) described in . This reflects higher reporting of physical discomfort post-treatment, with methadone cessation likely exacerbating preexisting medical conditions such as chronic pain, a phenomenon described elsewhere (Citation26). The observed significant effect may also act as a proxy measure further substantiating cessation of opioid use in lieu of some participants’ missing drug test data.
The significant, sustained reductions in BDI-II scores (; p < 0.001), similarly supports earlier research identifying reductions in depressive symptoms post-ibogaine treatment (Citation27). These results are interesting as they incorporate all available data from 12 months (n = 11) and not only the eight completers (). Thus, it was notable during follow-up that even participants who did not cease opioid use entirely described their ibogaine experience in positive terms. A typical comment was that treatment had provided participants with insight into their situation. Baseline BDI-II data suggested that eight participants would have met criteria for moderate or worse depression and four participants for mild depression. Nonetheless, at baseline, only three participants were taking prescribed antidepressants (Venlafaxine, Citalopram). Of these, two ceased use post-treatment. Following treatment four participants intermittently reported antidepressant use (prescribed), with three of these ultimately being unsuccessfully treated for their opioid use. Future studies should include a measure of anxiety in addition to depression to assess the contributions of these symptoms to treatment response, remission, and relapse.
Finally, with five participants reporting benzodiazepine use in the 30 days preceding treatment (one prescribed), the research team considered the possibility that pretreatment anxiety might have inflated baseline BDI-II scores. This concern was mitigated, however, by the understanding that analyses of the BDI-II suggest it is capable of differentiating between depression and anxiety (Citation28,Citation29). Interestingly, at one month, post-treatment six participants (43%) reported benzodiazepine use (five prescribed), while at 12 months, only one of the remaining 11 participants reported this.
Despite the study’s evidence of positive outcomes, it remains that there are also specific risks associated with ibogaine. The most salient of these concerns is mortality temporally associated with treatment. Given the death during treatment of one subject pre-enrolled in the present study, this issue is of particular significance. As described above, the New Zealand death was the subject of two investigations, with a coronial inquiry supporting the earlier ruling of a failed duty of care by the treatment provider (Citation22). The coroner, however, also noted a lack of Post-Mortem and forensic evidence indicating any significant cardiac pathology or history, or other definable cause of death. Consequently, report suggests that the death was very likely “related to ibogaine ingestion and most probably related to a cardiac arrhythmia.” Nonetheless, given the positive outcomes reported in this study and in a recent study of treatments in Mexico that both suggest that treatments are likely to continue (Citation30), it is appropriate to discuss what is clearly a risk.
Ibogaine-related fatalities in treatment have been reported in detail for 19 individuals from 1990 to 2008, known to have died within 1.5–76 hours of taking ibogaine (Citation31). This thorough review of all available autopsy, toxicological, and investigative reports did not suggest a characteristic syndrome of neurotoxicity. Rather, it suggested that advanced preexisting medical comorbidities, primarily cardiovascular, and/or the misuse of a range of substances explained or contributed to 12 of the 14 cases for which there was adequate postmortem data. Seizures from alcohol and benzodiazepine withdrawal and the uninformed use of ethnopharmacological forms of ibogaine were considered other apparent risk factors.
The metabolism of ibogaine by cytochrome P450 enzyme CYP2D6 into noribogaine through the first-pass process has implications for clinical safety (Citation12), with 5–10% of Caucasians lacking the gene required for the enzyme’s synthesis (Citation32). In poor CYP2D6 metabolizers, active moiety (ibogaine plus noribogaine) is projected to be approximately two-fold higher than in individuals having standard metabolic function. Noribogaine’s long half-life, recently reported as 28–49 hours (Citation9), also suggests the potential for high plasma levels of noribogaine with multiple ibogaine treatments over a period of several days as observed in the present study. Although clinical pharmacology studies in patients with impaired hepatic function are yet to be conducted, it may be prudent to genotype potential ibogaine patients and to reduce the intended dose in cases of hepatic impairment or concomitant medications with CYP2D6 inhibition (Citation12).
Regarding New Zealand treatments, inquiries subsequent to the conclusion of the study revealed that although official reporting on New Zealand treatments is voluntary and, therefore, data are incomplete, the two providers collectively reported treating 83 patients (Provider 1, 53 patients; Provider 2, 30 patients). It seems likely, however, that more than 100 treatments occurred during the time of the study, that is, between 2012 and 2015. Notwithstanding the death reported here, in New Zealand ibogaine treatment currently, remains legal and has not been subject to any specific sanctions as a response to the fatality.
Despite the fatality, due to ibogaine being available by prescription in New Zealand, structural mechanisms within the treatment context exist to reduce ibogaine’s potential risks in that country. These are promoted through the legal availability of ibogaine, for example, where patients, ibogaine providers, and other health professionals are all able to openly engage with the treatment process. This process is clearly facilitated by legal access to ibogaine, an approach emphasized by Provider 2 in their treatment of participants in the present study. The possibility of improved treatment safety through regulation, however, is most likely to occur where there is a will amongst all stakeholders to develop a set of robust clinical guidelines or preferably national standards. These must apply to and be adhered to by all treatment providers, regardless of putative experience, skill, or qualification. In this regard, it is interesting to note that the New Zealand fatality occurred at a clinic run by a qualified medical practitioner with considerable emergency medicine experience who nonetheless was adjudged to have failed in their duty of care.
While this study has provided further evidence supporting ibogaine’s effectiveness in reducing opioid withdrawal, cravings and use over an extended period, it nonetheless has a number of weaknesses. Chief among these is the study’s method, with its reliance on a small (n = 14) convenience sample already intending treatment. Additionally, this group was also filtered by the treatment providers prior to indicating interest in participation to the investigator. The small sample size further decreased with attrition and partial datasets. Finally, while attempts were made to ensure accuracy of drug use data through random testing and interviewing significant others, these efforts were not achieved consistently with all participants. Consequently, the sample is not representative, which limits generalizability.
Despite noted limitations, this study has demonstrated that for some opioid-dependent individuals, ibogaine treatment can be effective in significantly reducing opioid withdrawal, craving and depressed mood, and reducing or ceasing opioid use. Given the modest success of existing treatments, some of which involve extensive, repeated administration and considerable risk, and the significant increase in opioid dependence globally, it seems prudent to more seriously examine the place of ibogaine in the context of treating this intractable problem. Therefore, support for further research into non-traditional options such as ibogaine is urgently needed to improve clinical outcomes of opioid dependence.
Financial disclosures
Geoffrey E. Noller: Independent researcher receiving payment for study conduct over three years from funding sources listed below.
Chris M. Frampton: Reports no relevant financial conflicts
Berra Yazar-Klosinski: Employee receiving full time salary support from MAPS over three years, a tax-exempt charity funding research and education
IADA_A_1310218_Supplementary_File.docx
Download MS Word (82.6 KB)Acknowledgments
The authors wish to acknowledge Kenneth Alper, M.D., Associate Professor of Psychiatry and Neurology New York University School of Medicine New York; Professor Paul Glue, Chair of Psychological Medicine, School of Medical Sciences, University of Otago, Dunedin, New Zealand; and Allison Feduccia, Ph.D., MAPS Clinical Trial Leader, for comments on earlier drafts of the manuscript. Ben Shechet and Colin Hennigan (MAPS) provided data management support.
Funding
Research was supported by grants from the Multidisciplinary Association of Psychedelic Studies (MAPS; a tax-exempt charity funding research and education) and The Star Trust.
Supplemental data
Supplemental data for this article can be accessed on the Publisher’s website.
Additional information
Funding
Notes
1 The researchers were aware of the potential validity problems recognised to be associated with self-completion of the ASI-Lite [see 34]. In both cases, however, participants firmly expressed their preference for this approach. One subject identified a lack of time to complete interviews, primarily due to his demanding job. The second subject expressed concerns about anonymity regarding supplying drug use-related information by phone, which he had done up to interview 8. Both of these participants were amongst the few who provided full drug screens to corroborate their data.
2 There is very limited street heroin in New Zealand, with only 16% of regular Needle Exchange attendees reporting its use in the preceding month [40]. For this reason New Zealand users of morphine sulphate (“misties” or “MST’s”) typically combine (“double”) it with acetic anhydride, thereby producing diamorphine (aka heroin).
References
- Cornish R, Macleod J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ 2010;341:c5475.
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health 2015;36:559–574.
- Adamson SJ, Deering DE, Sellman JD, Sheridan J, Henderson C, Robertson R, et al. An estimation of the prevalence of opioid dependence in New Zealand. Int J Drug Policy 2012;23:87–89.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths–United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–1382.
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies–tackling the opioid-overdose epidemic. N Engl J Med 2014;370:2063–2066.
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev 2008:CD004147.
- Popik P, Layer RT, Skolnick P. 100 years of ibogaine: neurochemical and pharmacological actions of a putative anti-addictive drug. Pharmacol Rev 1995;47:235–253.
- Fernandez JW, Fernandez RL. “Returning to the path”: the use of iboga[ine] in an equatorial African ritual context and the binding of time, space, and social relationships. Alkaloids Chem Biol 2001;56:235–247.
- Glue P, Lockhart M, Lam F, Hung N, Hung CT, Friedhoff L. Ascending-dose study of noribogaine in healthy volunteers: pharmacokinetics, pharmacodynamics, safety, and tolerability. J Clin Pharmacol 2015;55:189–194.
- Lotsof HS, Alexander NE. Case studies of ibogaine treatment: implications for patient management strategies. Alkaloids Chem Biol 2001;56:293–313.
- Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J. Treatment of acute opioid withdrawal with ibogaine. Am J Addict 1999;8:234–242.
- Glue P, Winter H, Garbe K, Jakobi H, Lyudin A, Lenagh-Glue Z, et al. Influence of CYP2D6 activity on the pharmacokinetics and pharmacodynamics of a single 20 mg dose of ibogaine in healthy volunteers. J Clin Pharmacol 2015;55:680–687.
- Koenig X, Kovar M, Boehm S, Sandtner W, Hilber K. Anti-addiction drug ibogaine inhibits hERG channels: a cardiac arrhythmia risk. Addict Biol 2014;19:237–239.
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 2011;106:32–51.
- Brown TK. Ibogaine in the treatment of substance dependence. Curr Drug Abuse Rev 2013;6:3–16.
- Mash DC, Ameer B, Prou D, Howes JF, Maillet EL. Oral noribogaine shows high brain uptake and anti-withdrawal effects not associated with place preference in rodents. J Psychopharmacol 2016;30:688–697.
- Medsafe. Minutes of the 42nd Meeting of the Medicines Classification Committee. Available from: http://www.medsafe.govt.nz/profs/class/mccmin03nov2009.htm [last accessed February 2016]. 2009 [cited 2016]; Minutes from NZ organizaiton meeting].
- McLellan T, Cacciola J, Carise D, Coyne TH. Addiction Severity Index Lite-CF. Clinical/Training Version 1999;Available from: Sept 2014 http://m.breining.edu/ASILite112909.pdf.
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis 1985;173:412–423.
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation 1996;1:82.
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 1987;13:293–308.
- Health and Disability Commissioner. A Report by the Health and Disability Commissioner. Available from: http://www.hdc.org.nz/media/289826/13hdc00966.pdf [last accessed 18 February 2016]. Coroner’s report: New Zealand Health and Disability Commissioner. 2015. Report No.: 13HDC00966.
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction 2009;104:256–265.
- Alper KR, Lotsof HS, Kaplan CD. The ibogaine medical subculture. J Ethnopharmacol 2008;115:9–24.
- Noller G, Henderson C. Report of the National Needle Exchange Blood-borne Virus Seroprevalence Survey. [BBVNEX2013] to the New Zealand Ministry of Health. Christchurch, New Zealand: National Office of the New Zealand Needle Exchange Programme; 2014.
- Winstock AR, Lintzeris N, Lea T. “Should I stay or should I go?” Coming off methadone and buprenorphine treatment. Int J Drug Policy 2011;22:77–81.
- Mash DC, Kovera CA, Pablo J, Tyndale R, Ervin FR, Kamlet JD, et al. Ibogaine in the treatment of heroin withdrawal. Alkaloids Chem Biol 2001;56:155–171.
- Mathew RJ, Swihart AA, Weinman ML. Vegetative symptoms in anxiety and depression. Br J Psychiatry 1982;141:162–165.
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. Philadelphia, PA: University of Pennsylvania Press; 1967.
- Brown T. Results from the MAPS Mexico-based Ibogaine Study. 5th Global Ibogaine Conference; 2016 March 14–16; Tepoztlan, Mexico.
- Alper KR, Stajic M, Gill JR. Fatalities temporally associated with the ingestion of ibogaine. J Forensic Sci 2012;57:398–412.
- Gonzalez FJ, Meyer UA. Molecular genetics of the debrisoquin-sparteine polymorphism. Clin Pharmacol Ther 1991;50:233–238.
- Sheppard SG. A preliminary investigation of ibogaine: case reports and recommendations for further study. J Subst Abuse Treat 1994;11:379–385.
- Luciano D. Observations on treatment with ibogaine. Am J Addict 1998;7:89–90.
- Mash DC, Kovera CA, Pablo J, Tyndale RF, Ervin FD, Williams IC, et al. Ibogaine: complex pharmacokinetics, concerns for safety, and preliminary efficacy measures. Ann N Y Acad Sci 2000;914:394–401.
- Bastiaans E. Life after ibogaine: an exploratory study of the long-term effects of ibogaine treatment on drug addicts. Doctorandus thesis Vrije Universiteit Amsterdam, Faculty of Medicine. Available from: https://www.iceers.org/docs/science/iboga/Bastiaans%20E_Life_After_Ibogaine.pdf [last accessed 2004].