ABSTRACT
Background: TNF-α and S100B are important signaling factors that are involved in many aberrant conditions of the brain. Chronic morphine exposure causes aberrant modifications in the brain. Objectives: We examined the consequences of chronic morphine consumption by parents before mating on hippocampus TNF-α and S100B levels in the parents and their offspring. Methods: A total of 12 adult female and 12 adult male Wistar rats were used as parents. Each gender was divided randomly into two groups: control and morphine consumer. Morphine consumer groups received morphine sulfate dissolved in drinking water (0.4 mg/ml) for 60 days. Control groups received water. Thirty days before mating, morphine was replaced with water. All offspring also received water. The hippocampus of both parental and offspring groups was extracted to measure TNF-α and S100B levels using an ELISA. Results: Hippocampus TNF-α levels were significantly increased due to chronic morphine use in both male and female parents compared to those of control parents (P < 0.01). Moreover, both male and female offspring of morphine-exposed parents showed a significant increase in hippocampus TNF-α levels compared to those of control offspring (P < 0.01). Hippocampus levels of S100B were significantly decreased in male (P < 0.05) but not female morphine consumer parents relative to control parents. Both male and female offspring of morphine-exposed parents showed significant decreases in hippocampus S100B levels (P < 0.05) compared to those of control offspring. Conclusions: The consequences of chronic morphine use by parents, even when it is stopped long before mating and pregnancy, could induce modifications in the hippocampus of the next generation.
Introduction
Morphine and other opioids are common medications for moderate-to-severe pain (1,Citation2). In addition, because of their narcotic effects, use of and addiction to opioids are increasing in some populations (Citation3,Citation4). The long-term presence of opiates in the neuronal environment produces various permanent modifications in neural functions of the brain (Citation5–Citation7). The hippocampus is an important part of the brain, especially for learning and memory (Citation8). Due to the presence and distribution of opioid receptors in the hippocampus, chronic exposure to opiates has been reported to produce modifications in neural assignments, synaptic transmission, and behavioral tasks of the hippocampus (Citation9–Citation11). Despite these effects, no evidence exists to show that opiate consumption causes DNA alteration; however, recent studies have shown that chronic exposure to opiates affects chromatin-related proteins and therefore induces modifications in gene expression patterns. In fact, chronic morphine exposure causes epigenetic modifications (Citation12–Citation16).
The S100 protein family includes a group of calcium-binding proteins that are involved in a variety of intracellular and extracellular functions, including regulation of cell growth and differentiation, apoptosis, calcium homeostasis, and inflammation (Citation17). S100B (brain) was the first discovered protein of this family and is mainly expressed in astrocytes, certain neuronal populations, and Schwann cells. This protein plays a key role in neuroinflammation as one of the markers of inflammatory diseases of the nervous system, such as Alzheimer’s disease and Parkinson’s disease (Citation18–Citation20). Studies have shown that S100B acts on receptors, such as the receptor for advanced glycation end products (RAGE), and its downstream pathways cause the production and secretion of inflammatory cytokines through the activation of NF-KB (Citation21,Citation22). In pathophysiological conditions, astrocytes and especially microglia release large amounts of TNF-α (Citation23). An increased level of TNF-α has been reported in disorders such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis (Citation24,Citation25). Morphine binds to μ-opioid receptors in microglia and increases the secretion of inflammatory factors such as TNF-α (Citation26,Citation27). Moreover, TNF-α and S100B exist in different regions of the hippocampus and are involved in hippocampal physiology and pathophysiology (Citation28,Citation29). An elevated level of TNF-α in the hippocampus produces behavioral symptoms (Citation29). Additionally, blocking TNF-α or S100B in the hippocampus reduces the symptoms produced by these two factors (Citation28–Citation30).
Because chronic morphine consumption has shown epigenetic effects and causes durable abnormal activities in the hippocampus (Citation9,Citation16,Citation31,Citation32), epigenetic modifications are inheritable from parents to their offspring (Citation12), and TNF-α and S100B are present in the hippocampus and involved in anomalous hippocampal functions (Citation18,Citation23). In the current research study, we investigate the consequences of chronic consumption of morphine by both male and female parents before mating and gestation on the hippocampus TNF-α and S100B levels of the parents themselves and their offspring.
Materials and methods
Animals
The experiments were performed on male and female Wistar rats (parents: 220–250 g; offspring: 100–120 g) housed under a 12-h light–dark cycle and controlled temperature (22 ± 2°C). Food and water were available ad libitum. All research and animal care procedures were performed according to the Guide for the Care and Use of Laboratory Animals (8th edition; National Academies Press; 2011) and approved by the Review Board and Ethics Committee of Arak University of Medical Sciences. All efforts were made to minimize the number of animals used and their suffering.
Experimental groups and morphine consumption
Twelve 5-week-old female and twelve 5-week-old male rats were randomly selected as parents. Each gender was divided randomly into two groups: control and morphine consumer. Males were kept separated from females in cages until mating. In the morphine consumer groups (male and female groups), rats received morphine sulfate (TEMAD Co., Karaj, Alborz, IR) dissolved in drinking water (0.4 mg/ml) for 60 days (Citation33,Citation34). The control groups (male and female groups) received water. Thereafter, morphine use was stopped, and all animals in the control and experimental groups received water (). Subsequently, morphine-treated males and females were randomly selected and mated as a pair in each cage for 4 days. Mating was confirmed when the vaginal plug was observed. Females were then separated from males and kept in individual cages. The same procedure was performed for the males and females of the control group. After gestation (20–22 days) and weaning (4 weeks), male and female offspring and parents were divided and kept in separate groups as listed below:
Offspring: (1) Five postnatal males from control parents (Cnt-Moffs), (2) five postnatal females from control parents (Cnt-Foffs), (3) five postnatal males from morphine parents (Mor-Moffs), and (4) five postnatal females from morphine parents (Mor-Foffs). All offspring were approximately the same age and received water until they were sacrificed (at 5 weeks old) for hippocampus extraction.
Parents: (1) Five male control parents (Cnt-MP), (2) six female control parents (Cnt-FP), (3) five male morphine parents (Mor-MP), and (4) six female morphine parents (Mor-FP). Parental groups were approximately at same age. After they had finished nursing their offspring, the animals were sacrificed (26–27 weeks old) for hippocampus extraction.
Sample preparation and measurement of TNF-α and S100B
All animals were deeply anesthetized using diethyl ether and decapitated. Brains were removed in chilled PBS (pH = 7.4; 100 mM), and the right hippocampus was quickly dissected. All samples were stored at −80°C. Hippocampus tissue was homogenized in phosphate buffer containing protease inhibitor cocktail (Sigma-Aldrich, USA) and centrifuged (14,000 rpm, 40 min), and the supernatant fraction was then isolated. TNF-α (Diaclone, France) and S100B (ZellBio GmbH, Germany) ELISA kits were used for assessments. Assays were performed according to the manufacturer’s instructions. In brief, for S100B assessment, 10 µl of S100B antibody and 50 µl of streptavidin-HRP were added to 50 µl of samples and incubated at 37°C for 60 min. The plates were then washed five times using the 1X wash buffer. For color development, 50 µl of chromogen solutions A and B were added to the plates and incubated at 37°C for 10 min. Reactions were stopped by adding 50 µl of stop solution to the plates, and optical density (OD) values were read (ELx800 plate reader, BioTek, USA) within 10 min at 450 nm. A standard curve was prepared using the standard solutions of S100B protein. S100B levels were calculated in samples according to the OD of samples and using the standard curve.
Briefly, for TNF-α measurement, 50 μl of anti-rat TNF-α was added to 100 μl of samples or standard solutions and incubated at room temperature for 3 h. Subsequently, plates were washed three times, and 100 μl of streptavidin-HRP was added to the plates and incubated at room temperature for 30 min. The plates were washed three times again, and 100 μl of chromogen solution (3,3ʹ,5,5ʹ-tetramethylbenzidine) was added to the plates, after which they were protected from light and incubated for 15 min. Reactions were stopped by adding stop solution consisting of H2SO4. The absorbance was read at 450 nm. A standard curve was prepared using the standard solutions of TNF-α protein, and the TNF-α level was calculated in samples accordingly.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. Fisher’s exact test was used to analyze the mortality rate for pups of morphine parents versus control parents. Two-way ANOVA was used with morphine exposure (morphine-exposed, control) and sex (male, female) as factors and hippocampus TNF-α/S100B levels as the dependent variable. Subsequently, Bonferroni post hoc tests were used to evaluate statistical differences between experimental groups. Statistical significance was accepted when P < 0.05. Data are presented as the mean ± SEM.
Results
The mean number of offspring of morphine-exposed parents was 8.8 ± 0.9 (n = 53), while the mean number of offspring of control parents was 10 ± 0.7 (n = 60). The mortality rate for pups of morphine-exposed parents was 5 of 53 rats (9.4%), while it was 2 of 60 rats (3.3%) for the offspring of control parents; no significant difference was observed between the groups according to Fisher’s exact test. At the end of nursing, five male and five female pups were randomly selected from all offspring of the control mothers. In addition, five male and five female pups were randomly selected from all offspring of the morphine mothers. These selected offspring were sacrificed, and their hippocampus was extracted for an ELISA study. No mortality occurred in the parental groups; however, the extracted hippocampus from two of the male rats (one from the control group and one from the morphine group) was lost during the tissue preparation for the ELISA; therefore, the male parental groups included five animals, while in the female parental groups included six animals. The following results were assessed approximately 3 months after stopping morphine administration in the parental groups.
Hippocampus TNF-α level
Two-way ANOVA revealed a significant main effect of chronic morphine consumption on the hippocampus TNF-α levels of parents [F(1, 18) = 51; P < 0.001], while no significant effect of sex was detected. Bonferroni post hoc tests showed significant increases in the TNF-α levels of male (n = 5) and female (n = 6) morphine-exposed parents compared to those of male (n = 5) and female (n = 6) control parents (P < 0.001; ).
Figure 2. Evaluation of the mean TNF-α values in the experimental groups. Hippocampus TNF-α levels were significantly increased in both male and female parents compared to those in control parents due to chronic use of morphine (A). Moreover, both male and female offspring of morphine-exposed parents showed a significant increase in hippocampus TNF-α levels compared to those of control offspring (B). Two-way ANOVA followed by Bonferroni post hoc tests was used. A value of P < 0.05 was considered to indicate statistical significance. All data are presented as the mean ± SEM; n = 5 for all groups except the female parental groups, where n = 6; Cnt-MP: male control parents; Cnt-FP: female control parents; Mor-MP: male morphine-exposed parents; Mor-FP: female morphine-exposed parents; Cnt-Moffs: male offspring from control parents; Cnt-Foffs: female offspring from control parents; Mor-Moffs: male offspring from morphine-exposed parents; Mor-Foffs: female offspring from morphine-exposed parents.
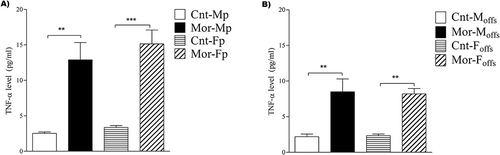
In addition, prepregnancy parental morphine exposure showed a significant main effect on the hippocampus TNF-α level of offspring when analyzed by two-way ANOVA [F(1, 16) = 37; P < 0.001]. Meanwhile, no significant difference was observed between males and females. Bonferroni post hoc analyses revealed a significant increase in the TNF-α levels of male and female offspring of morphine-exposed parents compared to those of male and female offspring of control parents, respectively (P < 0.001 for male offspring and P < 0.01 for female offspring; n = 5; ).
Hippocampus S100B level
Analysis of the mean values of the hippocampus S100B level by two-way ANOVA revealed no significant effect of parental morphine exposure, although a significant effect of sex [F (1, 18) = 5; P < 0.001] and an interaction between sex and morphine treatment [F (1, 18) = 6.1; P < 0.05] were observed. As demonstrated in , a subsequent Bonferroni post hoc analysis detected a significant decrease in the S100B levels in male morphine-exposed parents compared to those in male control parents (P < 0.05; n = 5), while no significant differences were observed between female offspring of morphine-exposed and control parents.
Figure 3. Comparison of the mean S100B values in the experimental groups. Two-way ANOVA followed by Bonferroni post hoc tests verified that hippocampus S100B levels were significantly decreased in male but not female parents compared to those in control parents due to chronic use of morphine (A). However, both male and female offspring of morphine-exposed parents showed a significant decrease in hippocampus S100B levels compared to those in control offspring (B). A value of P < 0.05 was considered statistically significant. All data are presented as the mean ± SEM; n = 5 for all groups except the female parental groups, where n = 6; Cnt-MP: male control parents; Cnt-FP: female control parents; Mor-MP: male morphine-exposed parents; Mor-FP: female morphine-exposed parents; Cnt-Moffs: male offspring from control parents; Cnt-Foffs: female offspring from control parents; Mor-Moffs: male offspring from morphine-exposed parents; Mor-Foffs: female offspring from morphine-exposed parents.
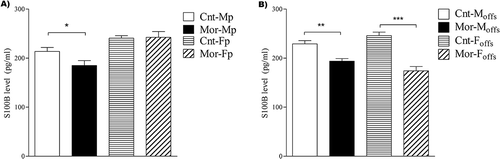
Furthermore, two-way ANOVA showed that prepregnancy parental morphine exposure had a significant main effect on the hippocampus S100B levels of offspring [F(1, 16) = 59; P < 0.001]. While no significant effect of sex was observed, the interaction between parental morphine exposure and sex was significant [F(1, 16) = 9.6; P < 0.01]. Bonferroni post hoc analysis revealed a significant decrease in the S100B levels of both male and female offspring of morphine-exposed parents compared to those of male and female offspring of control parents, respectively (P < 0.01 for male offspring and P < 0.001 for female offspring; n = 5; ).
Discussion
The results of this study revealed for the first time that chronic morphine consumption by male and female parents before mating and pregnancy affects the hippocampus levels of TNF-α and S100B in their offspring. Hippocampus levels of TNF-α were significantly increased, while the levels of S100B were significantly decreased in the offspring of parents with chronic morphine exposure. In addition, consequences were detectable in the morphine consumer parents, even 3 months after ceasing morphine use. The hippocampus TNF-α levels of morphine consumer parents were significantly higher than those in control parents, while S100B levels were significantly higher in male control parents.
Recently, chronic use of opiates by parents has been reported to produce consequences in children with no direct contact with these substances (Citation35–Citation37). Epigenetic mechanisms are suggested to be responsible for this phenomenon (Citation38,Citation39). Although investigations of the effects of these types of opiates are scarce, some recent studies in animal models have shown that the offspring of parents with a background of morphine use demonstrate differences in the expression pattern of opioid receptors in different brain areas (Citation36). They also exhibited alterations in the level of endogenous opioids in the nervous system (Citation36,Citation40).
Chronic administration of opioids induces functional interactions between the immune and nervous systems (Citation41–Citation44). Some factors of the immune system (such as TNF-α, IL-1β, IL-6, and NF-KB) show modifications due to chronic morphine exposure, and these modifications affect hippocampus function (Citation45,Citation46). In our study, TNF-α levels in parents that consumed morphine were measured 3 months after withdrawal. Despite this delay, TNF-α levels in the hippocampus of these parents were significantly higher than those in control parents maintained under the same conditions. Consistent with our study, previous research has shown that chronic morphine administration during the early postnatal period changed hippocampus biochemical factors such as TNF-α. Interestingly, these alterations were detectable even 2 months after morphine treatment. TNF-α expression and its role in hippocampus physiology and pathophysiology have been shown previously (Citation29,Citation47). Chronic morphine exposure has also been shown to alter memory performance in rats, which has been associated with modification of TNF-α levels in the hippocampus (Citation48). µ-Type opioid receptors are present in neural cells, and they increase the production of the transcription factor NF-KB through Mitogen-activated protein kinase (MAPK) intracellular pathways and transfer NF-KB into the nucleus (Citation46,Citation49). NF-KB binds to the TNF-α gene promoter and increases the expression of the TNF-α protein (Citation41). However, no study has investigated TNF-α or other cytokine levels in offspring of parents that were exposed to morphine. Several studies have revealed that opioids and opioid receptor expression may be altered in the offspring of parents with a background of morphine consumption (Citation36,Citation40,Citation50). In fact, some consequences of chronic opioid consumption could be transgenerationally transmitted from parents to offspring. Because we measured TNF-α and S100B levels in parents 3 months after morphine withdrawal and in offspring 1 month after birth and because alterations of these factors were still detectable, it is possible that the consequences of chronic morphine consumption in the brain of parents and even their offspring induce stable conditions that might be due to structural changes.
One study showed that chronic morphine use produces an increase in TNF-α levels in the cerebrospinal fluid caused by the increased permeability of the blood–brain barrier to this factor (Citation51), possibly due to an increase of TNF-α transporter activity, which transfers TNF-α from the plasma to the cerebrospinal fluid. Increases in TNF-α levels in the hippocampus in our study might have originated from the plasma. In fact, the activity of the brain TNF-α transporter might be altered by parental morphine consumption, and this new property might be transferred to offspring. The mechanisms of this transgenerational effect could be considered in future studies.
S100B autoantibody is increased in the brain of rats following chronic morphine exposure, and this was associated with changes in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and μ-opioid receptor autoantibodies (Citation52). The increase in S100B antibodies may represent an augmented level of S100B protein and its entrance into the plasma, which is caused by chronic exposure of astrocytes and neural cells to opioids. In our study, the hippocampus levels of S100B in morphine-exposed mothers were reduced compared to those in control mothers, while no change was observed in morphine-exposed male parents compared to control males. This observed decrease might be a secondary consequence due to an increase in autoantibodies against S100B, and thereby increased binding to the protein, which may reduce detectable S100B levels in tissue. Correspondingly, our results revealed a decreased S100B level in the hippocampus of male and female offspring of parents with a history of chronic morphine exposure compared to that in offspring of control parents. In this case, despite the decreased hippocampus levels of S100B in the female parents, its presence was discernible in both male and female offspring even 2 months after birth. Parental morphine consumption and its effect on hippocampus S100B levels in mothers and their offspring provides evidence for possible transgenerational transmission of the consequences of morphine (Citation53). However, the mechanisms of this phenomenon should be investigated in the future.
The S100B levels in hippocampus tissue and cerebrospinal fluid increase during brain damage or pathophysiological situations, and the consequent behavioral symptoms (Citation28,Citation30,Citation54–Citation57) might be induced by glial cell activation through the RAGE-dependent pathway (Citation22,Citation58). According to these studies, a close relationship exists between TNF-α and S100B levels in brain tissues, and typically, these levels are altered accordingly (Citation22,Citation55,Citation56). In our study, the hippocampus TNF-α levels were increased while the S100B levels were inversely reduced as a result of chronic morphine exposure. Because no previous reports have investigated S100B levels following chronic morphine exposure, future studies could determine whether this inverse alteration of S100B and TNF-α is interrelated or whether each factor is independently altered due to chronic morphine use.
Conclusion
Our findings provide evidence for the hypothesis that chronic morphine use by parents, even long before mating and pregnancy, could induce long-lasting modifications in some chemical factors in the hippocampus of their offspring. Epigenetic mechanisms might be responsible for these transgenerational effects of morphine.
Declaration of interest
The authors declare no relevant financial conflicts and no other conflicts of interests.
Acknowledgments
The authors thank Victoria Barkley and Dr. M. Elahdadi Salmani for significant English language editing. They also thank the anonymous reviewers for their helpful comments and suggestions that improved the first version of this article.
Funding
This work was supported by Arak University of Medical Sciences (funding No: 2644).
Additional information
Funding
References
- Sehgal N, Colson J, Smith HS. Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev Neurother 2013;13:1201–1220.
- Salsitz EA. Chronic pain, chronic opioid addiction: a complex nexus. J Med Toxicol 2016;12:54–57.
- Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev 2013;37:2597–2607.
- Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug Alcohol Rev 2011;30:300–305.
- Madsen HB, Brown RM, Lawrence AJ. Neuroplasticity in addiction: cellular and transcriptional perspectives. Front Mol Neurosci 2012;5:99.
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 2001;81:299–343.
- Bodnar RJ. Endogenous Opiates and Behavior: 2015. Peptides 2017;88:126–188.
- Eichenbaum H. Conscious awareness, memory and the hippocampus. Nat Neurosci 1999;2:775–776.
- Yang SN, Liu CA, Chung MY, Huang HC, Yeh GC, Wong CS, Lin WW, Yang CH, Tao PL. Alterations of postsynaptic density proteins in the hippocampus of rat offspring from the morphine-addicted mother: Beneficial effect of dextromethorphan. Hippocampus 2006;16:521–530.
- Sadegh M, Fathollahi Y. Repetitive systemic morphine alters activity-dependent plasticity of Schaffer-collateral-CA1 pyramidal cell synapses: involvement of adenosine A1 receptors and adenosine deaminase. J Neurosci Res 2014;92:1395–1408.
- Sadegh M, Fathollahi Y, Naghdi N, Semnanian S. Morphine deteriorates spatial memory in sodium salicylate treated rats. Eur J Pharmacol 2013;704:1–6.
- Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology 2014;76:259–268.
- Higgins GA, Allyn-Feuer A, Athey BD. Epigenomic mapping and effect sizes of noncoding variants associated with psychotropic drug response. Pharmacogenomics 2015;16:1565–1583.
- Ciccarelli A, Calza A, Santoru F, Grasso F, Concas A, Sassoe-Pognetto M, Giustetto M. Morphine withdrawal produces ERK-dependent and ERK-independent epigenetic marks in neurons of the nucleus accumbens and lateral septum. Neuropharmacology 2013;70:168–179.
- Ebrahimi G, Asadikaram G, Akbari H, Nematollahi MH, Abolhassani M, Shahabinejad G, Khodadadnejad L, Hashemi M. Elevated levels of DNA methylation at the OPRM1 promoter region in men with opioid use disorder. Am J Drug Alcohol Abuse 2017;1–7.
- Ruffle JK. Molecular neurobiology of addiction: what’s all the (Delta)FosB about? Am J Drug Alcohol Abuse 2014;40:428–437.
- Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med 2013;13:24–57.
- Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech 2003;60:614–632.
- Astrand R, Unden J, Romner B. Clinical use of the calcium-binding S100B protein. Methods Mol Biol 2013;963:373–384.
- Kleindienst A, Hesse F, Bullock MR, Buchfelder M. The neurotrophic protein S100B: value as a marker of brain damage and possible therapeutic implications. Prog Brain Res 2007;161:317–325.
- Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 2009;1793:1008–1022.
- Bianchi R, Kastrisianaki E, Giambanco I, Donato R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J Biol Chem 2011;286:7214–7226.
- Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal 2010;22:977–983.
- Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol 2012;7:42–59.
- Olmos G, Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014;2014:861231.
- Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, Fink DJ. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 2011;36:664–676.
- Tian X, Hua F, Sandhu HK, Chao D, Balboni G, Salvadori S, He X, Xia Y. Effect of delta-opioid receptor activation on BDNF-TrkB vs. TNF-alpha in the mouse cortex exposed to prolonged hypoxia. Int J Mol Sci 2013;14:15959–15976.
- Himeda T, Kanbara S, Oki C, Kato H, Araki T. Effects of chronic administration with nilvadipine against immunohistochemical changes related to aging in the mouse hippocampus. Metab Brain Dis 2005;20:141–153.
- Martuscello RT, Spengler RN, Bonoiu AC, Davidson BA, Helinski J, Ding H, Mahajan S, Kumar R, Bergey EJ, Knight PR, Prasad PN, Ignatowski TA. Increasing TNF levels solely in the rat hippocampus produces persistent pain-like symptoms. Pain 2012;153:1871–1882.
- Krawczyk A, Jaworska-Adamu J, Rycerz K. Immunohistochemical evaluation of hippocampal CA1 region astrocytes in 10-day-old rats after monosodium glutamate treatment. Pol J Vet Sci 2015;18:767–774.
- Mithbaokar P, Fiorito F, Della Morte R, Maharajan V, Costagliola A. Chronic maternal morphine alters calbindin D-28k expression pattern in postnatal mouse brain. Synapse 2016;70:15–23.
- Sadegh M, Fathollahi Y, Semnanian S. The chronic treatment in vivo of salicylate or morphine alters excitatory effects of subsequent salicylate or morphine tests in vitro in hippocampus area CA1. Eur J Pharmacol 2013;721:103–108.
- Hosseinmardi N, Fathollahi Y, Naghdi N, Javan M. Theta pulse stimulation: a natural stimulus pattern can trigger long-term depression but fails to reverse long-term potentiation in morphine withdrawn hippocampus area CA1. Brain Res 2009;1296:1–14.
- Salmanzadeh F, Fathollahi Y, Semnanian S, Shafizadeh M. Long-term potentiation as an electrophysiological assay for morphine dependence and withdrawal in rats: an in vitro study. J Neurosci Methods 2003;124:189–196.
- Byrnes JJ, Johnson NL, Carini LM, Byrnes EM. Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology (Berl) 2013;227:263–272.
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res 2011;218:200–205.
- Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: the effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol 2015;118:21–33.
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med 2008;14:341–350.
- Doehring A, Oertel BG, Sittl R, Lotsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain 2013;154:15–23.
- Cicero TJ, Adams ML, Giordano A, Miller BT, O’Connor L, Nock B. Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring. J Pharmacol Exp Ther 1991;256:1086–1093.
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol 1998;83:63–69.
- Cao F, Gao F, Xu AJ, Chen ZJ, Chen SS, Yang H, Yu HH, Mei W, Liu XJ, Xiao XP, Yang SB, Tian XB, Wang XR, Tian YK. Regulation of spinal neuroimmune responses by prolonged morphine treatment in a rat model of cancer induced bone pain. Brain Res 2010;1326:162–173.
- Borner C, Kraus J. Inhibition of NF-kappaB by opioids in T cells. J Immunol 2013;191:4640–4647.
- Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res 1998;61:190–194.
- Rozisky JR, Laste G, de Macedo IC, Santos VS, Krolow R, Noschang C, Vanzella C, Bertoldi K, Lovatel GA, de Souza IC, Siqueira IR, Dalmaz C, Caumo W, Torres IL. Neonatal morphine administration leads to changes in hippocampal BDNF levels and antioxidant enzyme activity in the adult life of rats. Neurochem Res 2013;38:494–503.
- Sawaya BE, Deshmane SL, Mukerjee R, Fan S, Khalili K. TNF alpha production in morphine-treated human neural cells is NF-kappaB-dependent. J Neuroimmune Pharmacol 2009;4:140–149.
- Kruglov AA, Kuchmiy A, Grivennikov SI, Tumanov AV, Kuprash DV, Nedospasov SA. Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: mouse models. Cytokine Growth Factor Rev 2008;19:231–244.
- Pan J, He L, Li X, Li M, Zhang X, Venesky J, Li Y, Peng Y. Activating autophagy in hippocampal cells alleviates the morphine-induced memory impairment. Mol Neurobiol 2016;54:1710–1724.
- Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A, Borea PA. Morphine mediates a proinflammatory phenotype via mu-opioid receptor-PKCvarepsilon-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol 2013;86:487–496.
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry 2011;2:29.
- Lynch JL, Banks WA. Opiate modulation of IL-1alpha, IL-2, and TNF-alpha transport across the blood-brain barrier. Brain Behav Immun 2008;22:1096–1102.
- Granstrem O, Adriani W, Shumilina M, Izykenova G, Dambinova S, Laviola G. Specific changes in levels of autoantibodies to glutamate and opiate receptors induced by morphine administration in rats. Neurosci Lett 2006;403:1–5.
- Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: physiological and behavioral effects. Neuropharmacology 2014;76:269–275.
- Edwards MM, Robinson SR. TNF alpha affects the expression of GFAP and S100B: implications for Alzheimer’s disease. J Neural Transm (Vienna) 2006;113:1709–1715.
- Hayakata T, Shiozaki T, Tasaki O, Ikegawa H, Inoue Y, Toshiyuki F, Hosotubo H, Kieko F, Yamashita T, Tanaka H, Shimazu T, Sugimoto H. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock 2004;22:102–107.
- Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, Martin HL, Schulte C, Mustafa S, Synofzik M, Vukovic Z, Itohara S, Berg D, Teismann P. S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain 2012;135:3336–3347.
- Somera-Molina KC, Nair S, Van Eldik LJ, Watterson DM, Wainwright MS. Enhanced microglial activation and proinflammatory cytokine upregulation are linked to increased susceptibility to seizures and neurologic injury in a ‘two-hit’ seizure model. Brain Res 2009;1282:162–172.
- Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging 2010;31:665–677.