ABSTRACT
Background: Previous meta-analytical research examining cocaine and methamphetamine separately suggests potentially different neuropsychological profiles associated with each drug. In addition, neuroimaging studies point to distinct structural changes that might underlie differences in neuropsychological functioning. Objectives: This meta-analysis compared the effect sizes identified in cocaine versus methamphetamine studies across 15 neuropsychological domains. Method: Investigators searched and coded the literature examining the neuropsychological deficits associated with a history of either cocaine or methamphetamine use. A total of 54 cocaine and 41 methamphetamine studies were selected, yielding sample sizes of 1,718 and 1,297, respectively. Moderator analyses were conducted to compare the two drugs across each cognitive domain. Results: Data revealed significant differences between the two drugs. Specifically, studies of cocaine showed significantly larger effect-size estimates (i.e., poorer performance) in verbal working memory when compared to methamphetamine. Further, when compared to cocaine, methamphetamine studies demonstrated significantly larger effect sizes in delayed contextual verbal memory and delayed visual memory. Conclusion: Overall, cocaine and methamphetamine users share similar neuropsychological profiles. However, cocaine appears to be more associated with working memory impairments, which are typically frontally mediated, while methamphetamine appears to be more associated with memory impairments that are linked with temporal and parietal lobe dysfunction.
Psychostimulants, such as cocaine and methamphetamine, are among the most commonly abused substances in the United States. A recent review reported prevalence rates for lifetime use of cocaine and methamphetamine as high as 14.7% and 8.5%, respectively (Citation1). At low doses, both substances lead to increased vigilance, arousal, and attention (Citation2). However, repeated and prolonged use of each substance has been associated with physiological consequences resulting in vascular and central nervous systems changes (Citation2,Citation3). While both drugs increase extracellular dopamine concentrations, they have been found to differ in their pharmacological mechanism (Citation4) and therefore their potential impact on the brain. These distinctions are likely to translate into differences in neuropsychological functioning between the two groups of users (Citation5,Citation6).
Animal studies present strong evidence for the distinct pharmacologic effects of cocaine versus methamphetamine. Those studies demonstrated that, compared to cocaine, methamphetamine was associated with a significantly longer half-life and had a more substantial neurotoxic effect on striatal dopamine transporters (Citation7,Citation8). Additionally, Fleckenstein and colleagues (Citation9) showed that multiple high doses of methamphetamine significantly decreased the efficiency of striatal dopamine transporters, while high-dose cocaine administrations had little to no impact. Similarly, using functional magnetic resonance imaging (fMRI), Taheri and colleagues (Citation10) found that cocaine and methamphetamine induced opposite changes in the striatum of rats. The authors reported that methamphetamine resulted in a prolonged negative blood-oxygen-level-dependent signal, while cocaine yielded a positive signal.
Pharmacological distinctions between cocaine and methamphetamine have also been noted in humans. A positron emission tomography study found that compared to cocaine, methamphetamine showed a significantly prolonged rate of clearance in the striatum (Citation4). Winhusen and colleagues (Citation11) reported that compared to cocaine, methamphetamine had an accelerated and more widespread distribution across brain regions. The authors suggested that pharmacokinetic differences play a role in the observed neurotoxic impact on dopamine cells. These distinct patterns of brain changes are likely to be associated with different patterns in neuropsychological functioning.
There are many studies that have examined cognition in cocaine and methamphetamine users separately compared to their respective controls. Previous single-drug meta-analyses, such as the ones conducted by Scott and colleagues (Citation12) on methamphetamine and Potvin and colleagues (Citation13) on cocaine, have reported on the different cognitive domains impacted by each drug. Although these studies provided clarification as to the cognitive weaknesses associated with cocaine and methamphetamine use separately, they do not offer direct comparisons. Subsequently, these analyses do not provide insight on how the two drugs may differ in relation to cognitive deficits. Qualitatively reviewing the differences in effect-size calculations between these two meta-analyses suggests that each stimulant may be associated with a somewhat distinct cognitive profile.
There have been few primary studies that compare the neurocognitive differences associated with cocaine and methamphetamine (or amphetamine) dependence directly, and the results from those studies have been inconsistent. For example, Ersche and colleagues (Citation5) found that cocaine users made significantly more perseverative errors than did amphetamine users on the Probabilistic Reversal Learning Task. These findings differed from Simon and colleagues (Citation6) who found that methamphetamine users made significantly more perseverative errors than did cocaine users on the Wisconsin Card Sorting Test (WCST). In contrast, van der Plas and colleagues (Citation14) found no differences between cocaine and methamphetamine users on WCST perseverative errors. In addition, several studies have suggested that both cocaine-dependent and methamphetamine-dependent individuals struggle significantly on measures of decision-making ability, as measured by the Iowa Gambling Task (IGT). Bechara and Martin (Citation15) showed that methamphetamine users were more impaired than cocaine users on the IGT. However, van der Plas and colleagues (Citation14) reported that the two drug groups did not differ significantly on IGT performance, despite both drug groups differing significantly from their respective control groups. Discrepant findings have also been demonstrated in working memory. Simon and colleagues (Citation6) concluded that cocaine users showed unique difficulties on Digit Span-Backward that were not apparent in the methamphetamine sample. This finding differs with van der Plas and colleagues (Citation14) who showed that methamphetamine users made more errors on the Tic-Tac-Toe working memory task than did cocaine users. Similarly, Bechara and Martin (Citation15) found that methamphetamine users performed worse on a Delayed Non-Match to Sample task. However, Verdejo-Garcia and colleagues (Citation16) found no differences between cocaine and methamphetamine users on a N-Back working memory task. Although these findings are inconsistent, they suggest the potential for distinct substance-related neurocognitive weaknesses in addition to overlapping deficits.
The potential for unique neuropsychological profiles is also supported by a recent structural neuroimaging meta-analysis on gray matter differences between cocaine and methamphetamine use conducted by Hall and colleagues (Citation17). Results showed that while cocaine and methamphetamine users shared similar structural profiles, each substance was associated with unique regions of gray matter abnormalities. More specifically, cocaine users displayed greater gray matter reductions in the right insula and left frontal gyrus when compared to methamphetamine users. In contrast, methamphetamine users were shown to have significantly larger reductions in gray matter in the right inferior parietal lobe and the left temporal gyrus. Differences between the two drugs have also been observed in other neuroimaging studies. For example, research has shown significantly lower global cerebral blood flow in methamphetamine-dependent versus cocaine-dependent alcoholics (Citation18). Cocaine and methamphetamine use has also been associated with regional differences in gray matter volumes (Citation19–Citation21). A recent qualitative article reviewing structural differences between cocaine and methamphetamine users suggested that cocaine users may show more reductions in gray matter in the temporal cortex when compared to methamphetamine users (Citation22). Bartzokis and colleagues (Citation23), who conducted a structural comparison between cocaine and methamphetamine reported temporal lobe changes only with cocaine users. These differences further suggest that each substance may be associated with unique neuropsychological distinctions, as structural brain changes have been shown to correlate with cognitive ability (Citation24,Citation25).
Taken together, the extant literature suggests that these two psychostimulants may be associated with distinctions in neurocognitive functioning, yet definitive conclusions regarding these differences in cognitive profiles remain elusive due to inconsistencies in the research. Disparities in the primary research may also be significantly influenced by differences in sample and methodological characteristics such as test selection, comorbid substance use, duration of use, and length of abstinence. Therefore, the objective of this study is to meta-analytically investigate the neurocognitive performance associated with cocaine versus methamphetamine use. It was postulated that a meta-analysis would demonstrate that cocaine- and methamphetamine-use is associated with neuropsychological profiles comprised of both shared and unique neurocognitive weaknesses compared to controls. Fully exploring the profiles associated with the two drugs may possibly aid clinicians in tailoring treatment according to the cognitive difficulties unique to users of each drug, thus potentially improving treatment outcomes.
Method
Search strategies and data acquisition
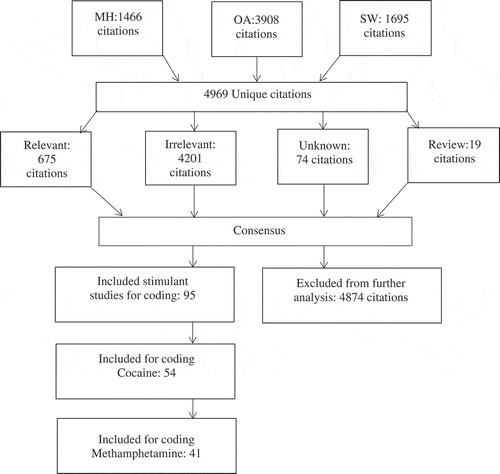
The methodology of this study was conducted following the Meta-Analysis Reporting Standards (MARS) guidelines (Citation26). Searches covered the content of databases up to June 2016. Two researchers (MH, SW) conducted independent searches in nine databases: 1) Academic Search Complete E-Journals, 2) ArticlesFirst, 3) CINAHL PLUS, 4) PapersFirst, 5) ProceedingsFirst, 6) ProQuest Dissertation and Theses, 7) PsycINFO, 8) PUBMED, and 9) Web of Science. In an attempt to conduct a comprehensive search of the literature with a special emphasis on finding tests associated with standard neuropsychological testing batteries (Citation27), clinically relevant neuropsychological search terms were determined by the two independent searchers in consultation with clinical experts and in accordance with Neuropsychological Assessment and the Compendium of Neuropsychology Tests (Citation27,Citation28). Search strategy and terms were independently determined by searchers to minimize potential study collection bias. Databases were searched using truncated terms such as “neuropsychology,” “neurocognitive,” “cognitive,” combined with terms related to neurocognitive domains such as “attention,” “motor,” “processing speed,” “memory,” “visuospatial,” or “executive functioning.” Databases were then searched using the truncated and related terms combined with substance related terms such as “crack,” “cocaine,” “methamphetamine,” or “amphetamine.” A specialist with professional experience in database searching (OA) created a third independent and extensive search based on the PsycINFO database algorithm to identify all abstracts included in PsycSCAN: Neuropsychology. Searches were presented to the PsycINFO staff for potential amendments, and the feedback was incorporated. Terms included controlled vocabulary (e.g., The Thesaurus of Psychological Index Terms) combined with free-text words from the title and abstract. A complete example of the search strategy and terms is published in Stephan and colleagues (Citation29), however, cocaine and methamphetamine terms were used instead of alcohol terms. Results were combined to remove duplicates, and sorted into four categories: 1) Relevant (studies that were highly likely to be included), 2) Irrelevant (studies unrelated based upon title and abstract, such as animal or case studies), 3) Unknown (studies that required full-text examination before inclusion), and 4) Reviews (articles that may be utilized to identify additional sources of data; see ).
Inclusion and exclusion criteria
Studies were included based on the following a priori inclusion criteria: 1) the participants were identified in the primary studies as being current or lifetime psychostimulant abusing or dependent patients, 2) psychostimulant groups were compared to an appropriate control group (i.e., drug naive or limited use) and matched on at least age, education, and/or IQ, 3) data were provided on neuropsychological tests, 4) sufficient information was provided to calculate effect sizes, and 5) the article was published in English. Studies were excluded if 1) a significant proportion (>25%) of the control or psychostimulant group had comorbid conditions known to impact cognitive functioning (e.g., human immunodeficiency virus, hepatitis C, or traumatic brain injury), 2) the neuropsychological assessment was conducted using unstandardized procedures, 3) the majority of either group had current poly-substance dependence (other than nicotine or caffeine), and/or 4) the authors characterized their psychostimulant group as meeting criteria for major psychiatric disorder (e.g., psychotic disorder). Finally, it is important to note that studies were not excluded if the stimulant users had a history or current diagnosis of abuse of substances other than the stimulants.
Coding procedures
Fifty-four cocaine articles (Citation5,Citation6,Citation14,Citation30–Citation80) and 41 methamphetamine articles (Citation5,Citation6,Citation14,Citation81–Citation118) were selected for coding. Two independent reviewers coded all articles for the necessary neuropsychological data, as well as relevant demographic and drug-related variables. Prior to data being entered into the Comprehensive Meta-analysis Version 2 (CMA), a third independent reviewer assessed the coding for accuracy by randomly selecting and recoding five articles and examining potential outliers in the data.
Effect-size calculations and measures of heterogeneity
CMA was used to calculate effect-size estimates for Hedges’ g (Citation119). Similar to other neuropsychological meta-analyses (Citation13,Citation120), the current study utilized Cohen’s effect-size ranges to describe magnitude of effect (Citation121). The Q and I2 statistics were used to assess for homogeneity of effect-size estimates across studies, while Τ2 was utilized to estimate the variance of the true effect size across the population.
When multiple tests measuring the same construct were reported in a single study, composites were constructed in order to avoid violating the assumption of independence. The choice of domain in which to place a test consisted of a two-step process. Two independent coders examined the manuscripts for the author’s categorization of the tests in relation to the domain of functioning. If no such information was located or if different articles placed tests in different domains, then the coders independently consulted two references, the Compendium of Neuropsychology Tests and Neuropsychological Assessment (Citation27,Citation28) in order to classify the test into the appropriate domain.
All analyses were conducted using the random-effects model approach. In order to more accurately assess verbal memory, studies were separated into those that measured non-contextual memory (i.e., list-learning tests) and contextual verbal memory (i.e., story memory tests). Additionally, a subsample of the cocaine studies (k = 9) identified the participants as crack or crack/cocaine users. Similarly, a small proportion (k = 8) of the methamphetamine studies reported that the patients were amphetamine users. Each subsample was compared to their respective cocaine or methamphetamine samples. This revealed no differences in effect sizes and therefore, these subsamples were included in their respective group. Studies were classified as acute-users versus non-acute users based on the description of the participant’s last use by the primary authors. In order to assess potential differences between the acuteness (one week or less since last use or positive toxicology) and cognitive functioning, subgroup analyses were conducted comparing the two groups (acute versus non-acute). Additionally, length of use and length of abstinence were coded as potential moderating variables to analyze heterogeneity when the number of studies was greater than ten (Citation122). Finally, publication bias was evaluated for each cognitive construct assessed using funnel plots and Duval and Tweedie’s Trim and Fill method (Citation123).
Results
A total of 95 studies were included in this analysis (see and ). Cocaine and methamphetamine users were compared on 15 different neuropsychological domains (see ).
Table 1. Demographic and clinical characteristics of included cocaine studies.
Table 2. Demographic and clinical characteristics of the included methamphetamine studies.
Table 3. Breakdown of the measures included for each construct assessed.
Individual drug effect sizes
Most effect sizes within each of the drug classes were statistically significant (see ). The only effect size that did not reach statistical significance was manual motor speed (g = .147, p = .111) for cocaine.
Table 4. Effect size estimates and subgroup analyses comparing cocaine and methamphetamine users.
Neuropsychological profile overlap
The following subgroup comparisons assessing various aspects of executive functioning were not statistically significantly different between the two drugs: mental flexibility, as measured by the Trail Making Test-B (TMT-B); inhibition, as measured by Stroop; decision-making, as measured by the IGT and the Decision Making Test; and perseveration, measured by the WCST. In addition, the Controlled Oral Word Association Test, which comprised the verbal fluency domain, was not significantly different between the two types of drug studies. The non-contextual verbal memory domain (i.e., list learning tasks) was not significantly different between the groups. Processing speed measures not statistically different were: Stroop Color Naming and Word Reading (CNWR), Trail Making Test-A (TMT-A), and the Digit Symbol Test (DST). Measures of attention were also found to not differ significantly between the two drug using groups. Finally, coordinated motor speed and manual motor speed were also not statistically different between the two groups of drug studies (All ps > .05).
Differences between methamphetamine and cocaine domains
Verbal working memory
Subgroup analysis revealed a significant difference between cocaine and methamphetamine users (Q = 7.736, df = 1, p = .005) on verbal working memory. More specifically, results indicated a significant and medium effect size for cocaine g = 0.650 (95% CI: [.421 to .879], z = 5.567, df = 15, p = .000), with high and significant heterogeneity I2 = 68.642% (Q = 47.835, p = .000, Τ2 = .146) and a significant and small effect for methamphetamine g = 0.226 (95% CI: [.033 to .418], z = 2.297, df = 9, p = .022), without significant heterogeneity I2 = 28.729% (Q = 12.628, p = .180, Τ2 = .027).
Contextual delayed verbal memory
Subgroup analysis for contextual delayed verbal memory revealed a significant difference between the groups (Q = 4.565, df = 1, p = .033). A significant and small effect for cocaine was observed g = .305 (95% CI: [.051 to .558], z = 2.356, df = 6, p = .018), without significant heterogeneity I2 = 52.008% (Q = 12.502, p = .052, Τ2 = .058) and a significant and large effect for methamphetamine was found g = 0.726 (95% CI: [.434 to 1.017], z = 4.884, df = 3, p = 0.000), without significant heterogeneity I2 = 0.00% (Q = 1.413, p = .703, Τ2 = 0.000).
Delayed visual memory
Subgroup analysis revealed a significant difference between cocaine and methamphetamine users (Q = 4.622, df = 1, p = .032) on measures of delayed visual memory. Results indicated a significant small effect for cocaine g = .302 (95% CI: [.162 to .442], z = 4.220, df = 12, p = 0.000), without significant heterogeneity I2 = 12.786% (Q = 13.759, p = .316, Τ2 = .008) and a significant and medium effect for methamphetamine g = .550 (95% CI: [.373 to .728], z = 6.078, df = 8, p = 0.000), without significant heterogeneity I2 = 18.766% (Q = 9.848, p = .276, Τ2 = .014).
Moderator analyses
Length of use and length of abstinence were not found to significantly predict any effect sizes in meta-regression analyses. Subgroup analyses results indicated that there were no differences between acute and non-acute users except on verbal fluency (Q = 15.9, df = 1, p < .000). Specifically, acute cocaine users were found to have a significantly larger effect-size estimate when compared to non-acute cocaine users.
Risk of publication bias
The Trim and Fill method (Citation123) revealed possible overestimation of effect size in four domains: non-contextual delayed verbal memory (biased estimate g = .506 and unbiased estimate g = .427), mental flexibility (biased estimate g = .405 and unbiased estimate g = .284), decision-making (biased estimate g = .526 and unbiased estimate g = .447), and manual motor speed (biased estimate g = .211 and unbiased estimate g = .135). Funnel plots for each cognitive construct are available in supplemental material.
Discussion
The current study is the first to meta-analytically compare the neuropsychological functioning of cocaine versus methamphetamine users. Findings from this study suggest that cocaine and methamphetamine users share a similar neuropsychological profile. This is expected since both drugs impact monoamines, affect reuptake transporters, and release acetylcholine in the brain’s reward pathway. Additionally, both substances result in the downregulation of dopaminergic receptors (Citation1). Users of both substances included in this meta-analysis differed significantly from their respective controls on all but one domain (manual motor speed for cocaine). Both cocaine and methamphetamine studies were found to have large effect-size estimates (i.e., poorer performance compared to controls) in the domain of decision-making, comprised primarily of the IGT. The IGT has been found to have high ecological validity and has also been shown to be predictive of real-life decision-making among substance-dependent individuals (Citation124). This is consistent with research that has demonstrated that stimulant users struggle with areas of daily functioning that require intact decision-making skills related to obtaining employment, medical care, and continued sobriety (Citation125). Users of both substances displayed significant and moderate effect-size estimates on the domains of executive functioning, which is generally consistent with previous single-drug meta-analyses (Citation12,Citation13). These findings may have important implications for clinicians. Patients may benefit from cognitive rehabilitation strategies focused on recovery and/or acquisition of skills related to executive functioning. Such rehabilitation has been shown to help patients with executive dysfunction following traumatic brain injuries (Citation126). Specifically, in a sample of brain injured patients, Levine and colleagues (Citation127) found that seven two-hour sessions of Goal Management Training (GMT) led to improvements on various neuropsychological tests measuring working memory, attentional ability, behavioral consistency, and problem-solving. Future research should investigate the possibility of improving treatment outcomes by utilizing cognitive remediation techniques such as those used in patients with traumatic brain injury.
Difficulties in processing speed have also been previously demonstrated meta-analytically in both methamphetamine and cocaine users (Citation12,Citation13). Specifically, Scott and colleagues (Citation12) reported a medium effect size (e.g., d = .52) for the domain of processing speed, which included TMT-A, Stroop CNWR, and the Digit Symbol Test. Similarly, Potvin and colleagues (Citation13) reported a comparable effect size in the speed of processing domain (e.g., d = .45), which included TMT-A, Stroop CNWR, DST, and Grooved Pegboard. In the current meta-analysis, each test was examined separately. By reducing heterogeneity, a better picture of the relationships between these different components of processing speed emerged. The results suggest that performance on DST may have had the largest impact on the summary effect size for processing speed in previous meta-analyses. In the current study, both cocaine and methamphetamine studies were shown to have similar and substantial effect sizes on the DST (g = .57 and g = .69, respectively), while the effect sizes for TMT-A and Stroop CNWR speed were minimal. It appears that the DST might be more sensitive to the impact of cocaine and methamphetamine than tests that are typically used to assess other aspects of processing speed. This sensitivity is likely due to the complex nature of this test. Therefore, complex tasks that require sustained and/or divided attention are likely to be challenging for patients with a history of stimulant abuse. One potential practical implication of this finding is that clinicians may consider providing information in smaller chunks in order to allow ample time for the processing and integration by patients.
The current analysis also found that cocaine and methamphetamine studies did not significantly differ on tasks designed to measure attention. Cocaine users were found to have a medium-to-large effect size (g = .61), a result consistent with Potvin and colleagues (Citation13), who reported an effect size of d = .59 for their attention domain. Similarly, the effect size of methamphetamine users was relatively smaller (g = .35), a finding consistent with Scott and colleagues (Citation12), who reported an effect size of d = .39. Research has indicated that attentional ability significantly impacts the success of treatment interventions (Citation128). It has been suggested that adequate attentional capacity is essential to encode and process information and therefore, when information is inadequately processed, patients’ abilities to transform therapeutic information into behavioral change may be negatively impacted (Citation128).
In addition to substantial overlap, this study also suggests distinctions between cocaine and methamphetamine users, which appear to correspond to their respective brain structural differences. Cocaine users exhibited a significantly larger effect-size estimate on verbal working memory when compared to methamphetamine users. Hall and colleagues (Citation17) found that compared to methamphetamine users, cocaine users showed significantly more reduced gray matter in the left inferior and superior frontal gyri, which are important regions in the network system for working memory. Research has shown that scores on Digit Span backward significantly correlated with gray matter volume in the left inferior frontal gyrus (Citation129). This region has been shown to exhibit increased cerebral blood flow during the rehearsal process, which is a component of working memory procedures (Citation130). Research has also shown an association between the left superior frontal gyrus and working memory performance (Citation131). Du Boisgueheneuc and colleagues (Citation131) concluded that the left superior frontal gyrus appears more involved in higher order aspects of working memory performance, such as monitoring and manipulation. Similarly, Chen and colleagues (Citation132) reported reduced activation of the left inferior frontal gyrus under a low-load working memory condition and reduced activation of the left superior frontal gyrus under the high-load working memory condition in patients with diabetes. These findings could help explain the verbal working memory weaknesses observed in cocaine-dependent individuals, which may have important clinical implications. Performance on verbal working memory tasks have been shown to correlate with treatment goals and relapse in substance users (Citation128,Citation133). Given that working memory processes have been postulated to be related to the regulation of unwanted craving (Citation134), providers should be mindful that cocaine users may be especially susceptible to craving-induced relapse. Cognitive remediation tools such as Work Therapy and Neurocognitive Enhancement Therapy have been shown to improve working memory performance in psychiatric populations (Citation135). Additional rehabilitation strategies such as GMT have been shown to be effective at improving working memory and other cognitive functions in substance using populations. Specifically, Alfonso and colleagues (Citation136) demonstrated that compared to treatment as usual, seven weeks of GMT led to significant improvements in working memory, response inhibition, and decision-making in a population of alcohol and polysubstance abusers. Implementing these and other interventions may aid cocaine-using individuals with difficulties in working memory.
When compared to cocaine, methamphetamine users performed significantly worse on measures of delayed contextual verbal memory. Hall and colleagues (Citation17) reported that when compared to cocaine users, methamphetamine users showed significantly more reductions in gray matter in the left superior temporal gyrus (L-STG), particularly Heschl’s gyrus, a region that might be considered part of a network system that is correlated with contextual verbal memory. Functional and structural studies have reported negative associations between activation and volume in the L-STG and performance on Logical Memory (Citation137,Citation138). Additionally, reduced cerebral perfusion in areas adjacent to the L-STG has been positively correlated with performance on the Babcock Story Memory Test (Citation139). While these studies provide evidence for the involvement of the L-STG in contextual verbal memory, they do not specify the regions of the superior temporal gyrus that may be more or less involved during contextual verbal memory performance. However, by manually segmenting the L-STG, researchers have shown the left Heschl gyrus to correlate with Logical Memory performance (Citation140). Interestingly, the researchers reported that performance on the California Verbal Learning Test (CVLT) did not correlate with this region. The latter finding may provide some clarification as to why the current analysis did not find significant differences between the drugs on non-contextual verbal memory tests. These findings suggest that while users of both drugs perform poorly on non-contextual verbal measures, methamphetamine users appear to perform significantly worse on measures of contextual verbal memory compared to cocaine users. This finding is important for treatment providers, since it appears that methamphetamine users may be less likely to benefit from the context of information presented in a verbal format. Additionally, research has shown contextual verbal memory performance to correlate with the amount of treatment-related information learned by patients at discharge (Citation141). Therefore, providers may help patients, especially methamphetamine users, by implementing strategy training techniques designed to compensate for verbal memory difficulties (Citation142). Specifically, Kaschel and colleagues (Citation142) conducted a small study of brain-injured patients and found that 30 sessions of imagery-based mnemonics improved delayed recall of everyday relevant verbal material such as stories and appointments. Additionally, a recent study by Rupp and colleagues (Citation143) compared the effectiveness of cognitive behavioral therapy (CBT) and CBT supplemented with cognitive rehabilitation in an alcohol dependent population. The authors found that CBT supplemented with 12 sessions of computer-assisted cognitive rehabilitation led to significant improvements in delayed verbal memory as well as other cognitive abilities. Finally, it is important for providers to be aware of the necessity of considering alternatives to verbally mediated instructions for both drug groups, but potentially more so for methamphetamine-dependent patients.
Methamphetamine users also performed significantly worse on measures of delayed visual memory when compared to cocaine users. This finding appears consistent with Hall and colleagues (Citation17) who demonstrated that methamphetamine users have significantly greater reductions in gray matter in the right inferior parietal gyrus (particularly the supramarginal gyrus), which is a region potentially involved in the visual memory system. Specially, these regions have been shown to be involved in the processing of visually presented stimuli and the retrieval of spatial location (Citation144,Citation145). Melrose and colleagues (Citation144) found that the right supramarginal gyrus was correlated with cerebral metabolism during performance of the Rey–Osterrieth Complex Figure Test (Rey-O). Further, a voxel-based lesion mapping study has shown these regions to be associated with Rey-O performance (Citation146). Evidence from these studies suggests that the right inferior parietal lobe, particularly the supramarginal gyrus, appears to play a role in the processing of visually presented information. Importantly, visual memory performance has been shown to correlate with treatment engagement, as well as substance relapse (Citation147). Cicerone and colleagues (Citation148) suggested that memory strategy training, such as internalized strategies (e.g., visual imagery) and external memory compensations (e.g., notebooks) may improve and compensate for memory difficulties in individuals with acquired brain injury. It is possible that methamphetamine users may also benefit from such interventions.
Previous research has demonstrated that length of substance use and abstinence are significant predictors of level of cognitive functioning (Citation149,Citation150). However, results from the present analysis indicated that neither length of use nor length of abstinence were significant predictors of effect sizes. It is possible that the limited number of studies included in each analysis resulted in insufficient statistical power to detect moderating effects.
Despite careful control over the studies that were included, the current meta-analysis has several limitations. Specifically, given that some of the primary studies included participants with current or past substance abuse, it is difficult to rule out the confounding effect of additional substances. Additionally, this meta-analysis included only articles that were published in English. Considering the high rate of stimulant abuse in non-English-speaking countries (Citation151), this inclusion criterion may limit generalizability. Females were significantly underrepresented which may limit generalizability across genders. Finally, considering the observed heterogeneity in the verbal working memory analysis and the limited number of methamphetamine studies included in the contextual verbal and visual memory analyses, significant differences between groups of studies should be interpreted with caution.
There are several important considerations to be made for future directions based on findings from this study. First, it is important to acknowledge that diverse neural networks, as opposed to single neuroanatomical structures, subserve the cognitive functions noted in this study. Therefore, future research may benefit from adopting a network-based approach to assess potential differences in functional connectivity between the two drugs. Second, given that the majority of studies examining cognitive remediation approaches have involved subjects with acquired brain injury, the discussion of their efficacy and possible application to cocaine and methamphetamine users requires empirical evaluation. Finally, although the subgroup analyses examining differences between crack versus cocaine and methamphetamine versus amphetamine did not reveal differences, these analyses were underpowered due to the limited number of studies available. The field of stimulant research would benefit from direct comparisons between crack and cocaine users, as well as amphetamine and methamphetamine users.
Finally, several unique cognitive weaknesses have been linked with the use of cocaine versus methamphetamine. Such distinctions may result in different behavior patterns that could impact treatment planning and triggers for relapse. Treatment success rates are similar between cocaine and methamphetamine users (Citation152,Citation153), however, regardless of the drug, a noteworthy percentage return to frequent use by the first posttreatment year (Citation154,Citation155). Individuals with compromised neuropsychological functioning are less likely to benefit from traditional treatments since cognitive difficulties limit their ability to engage in change (Citation128). Tailoring behavioral treatment plans based on the unique neurocognitive difficulties associated with cocaine and methamphetamine use may aid in reducing relapse rates.
Declaration of interest
The authors report no relevant financial conflicts.
Supplementary Materials
Download Zip (69.9 KB)Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Funding
This research was supported by NIDA award DA 026306 (Dr. Igor Grant) and seed grant from the California School of Professional Psychology (Dr. Alexander O. Hauson).
Additional information
Funding
References
- * = included in meta-analysis
- Ciccarone D. Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Prim Care. 2011;38(1):41–58, v-vi. doi:10.1016/j.pop.2010.11.004.
- Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2014;66(1):193–221. doi:10.1124/pr.112.007054.
- Enevoldson TP. Recreational drugs and their neurological consequences. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 3):iii9–15. doi:10.1136/jnnp.2004.045732.
- Fowler JS, Volkow ND, Logan J, Alexoff D, Telang F, Wang GJ, Wong C, Ma Y, Kriplani A, Pradhan K, et al. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. Neuroimage. 2008;43(4):756–63. doi:10.1016/j.neuroimage.2008.07.020.
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl). 2008;197(3):421–31. doi:10.1007/s00213-007-1051-1.
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21(1):61–74. doi:10.1300/J069v21n01_06.
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296(2):520–27.
- Yeh SY, De Souza EB. Lack of neurochemical evidence for neurotoxic effects of repeated cocaine administration in rats on brain monoamine neurons. Drug Alcohol Depend. 1991;27(1):51–61. doi:10.1016/0376-8716(91)90086-E.
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 2000;406(1):1–13. doi:10.1016/S0014-2999(00)00639-7.
- Taheri S, Xun Z, See RE, Joseph JE, Reichel CM. Cocaine and methamphetamine induce opposing changes in BOLD signal response in rats. Brain Res. 2016. doi:10.1016/j.brainres.2016.04.040.
- Winhusen T, Walker J, Brigham G, Lewis D, Somoza E, Theobald J, Somoza V. Preliminary evaluation of a model of stimulant use, oxidative damage and executive dysfunction. Am J Drug Alcohol Abuse. 2013;39(4):227–34. doi:10.3109/00952990.2013.798663.
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–97. doi:10.1007/s11065-007-9031-0.
- Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8(5):368–76. doi:10.1097/ADM.0000000000000066.
- Van Der Plas EA, Crone EA, Van Den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31(6):706–19. doi:10.1080/13803390802484797.
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18(1):152–62. doi:10.1037/0894-4105.18.1.152.
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12(3):405–15. doi:10.1017/S1355617706060486.
- Hall MG, Alhassoon OM, Stern MJ, Wollman SC, Kimmel CL, Perez-Figueroa A, Radua J. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2015;41(4):290–99. doi:10.3109/00952990.2015.1044607.
- Alhassoon OM, Dupont RM, Schweinsburg BC, Taylor MJ, Patterson TL, Grant I. Regional cerebral blood flow in cocaine- versus methamphetamine-dependent patients with a history of alcoholism. Int J Neuropsychopharmacol. 2001;4(2):105–12. doi:10.1017/S1461145701002334.
- Barros-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56(3):1021–26. doi:10.1016/j.neuroimage.2011.02.035.
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162(8):1461–72. doi:10.1176/appi.ajp.162.8.1461.
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, Verdejo-Garcia A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125(3):208–14. doi:10.1016/j.drugalcdep.2012.02.012.
- Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev. 2013;33(3):300–16. doi:10.1016/j.neubiorev.2012.12.003.
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98(2):93–102. doi:10.1016/S0925-4927(99)00052-9.
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, Bilder RM. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am J Psychiatry. 2002;159(2):217–26. doi:10.1176/appi.ajp.159.2.217.
- Christian CJ, Lencz T, Robinson DG, Burdick KE, Ashtari M, Malhotra AK, Betensky JD, Szeszko PR. Gray matter structural alterations in obsessive-compulsive disorder: relationship to neuropsychological functions. Psychiatry Res. 2008;164(2):123–31. doi:10.1016/j.pscychresns.2008.03.005.
- APA. Publication manual of the American psychological association. Washington, DC: American Psychological Association; 1994.
- Lezak MD. Neuropsychological assessment: Oxford University Press. New York, NY; 2004.
- Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford University Press, New York, NY; 2006.
- Stephan RA, Alhassoon OM, Allen KE, Wollman SC, Hall M, Thomas WJ, Gamboa JM, Kimmel C, Stern M, Sari C, et al. Meta-analyses of clinical neuropsychological tests of executive dysfunction and impulsivity in alcohol use disorder. Am J Drug Alcohol Abuse. 2016; 43:1–20.
- Abi-Saab D, Beauvais J, Mehm J, Brody M, Gottschalk C, Kosten TR. The effect of alcohol on the neuropsychological functioning of recently abstinent cocaine-dependent subjects. Am J Addict. 2005;14(2):166–78. doi:10.1080/10550490590924854.
- Adinoff B, Devous MD Sr., Cooper DB, Best SE, Chandler P, Harris T, Cervin CA, Cullum CM. Resting regional cerebral blood flow and gambling task performance in cocaine-dependent subjects and healthy comparison subjects. Am J Psychiatry. 2003;160(10):1892–94. doi:10.1176/appi.ajp.160.10.1892.
- Albein-Urios N, Martinez-Gonzalez JM, Lozano O, Clark L, Verdejo-Garcia A. Comparison of impulsivity and working memory in cocaine addiction and pathological gambling: implications for cocaine-induced neurotoxicity. Drug Alcohol Depend. 2012;126(1–2):1–6. doi:10.1016/j.drugalcdep.2012.03.008.
- Albein-Urios N, Martinez-Gonzalez JM, Lozano-Rojas O, Verdejo-Garcia A. Executive functions in cocaine-dependent patients with Cluster B and Cluster C personality disorders. Neuropsychology. 2014;28(1):84–90. doi:10.1037/neu0000007.
- Balconi M, Finocchiaro R, Campanella S. Reward sensitivity, decisional bias, and metacognitive deficits in cocaine drug addiction. J Addict Med. 2014;8(6):399–406. doi:10.1097/ADM.0000000000000065.
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend. 1995;37(3):247–53. doi:10.1016/0376-8716(94)01072-S.
- Berry J, Van Gorp WG, Herzberg DS, Hinkin C, Boone K, Steinman L, Wilkins JN. Neuropsychological deficits in abstinent cocaine abusers: preliminary findings after two weeks of abstinence. Drug Alcohol Depend. 1993;32(3):231–37. doi:10.1016/0376-8716(93)90087-7.
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11(3):361–69. doi:10.1176/jnp.11.3.361.
- Browndyke JN, Tucker KA, Woods SP, Beauvals J, Cohen RA, Gottschalk PC, Kosten TR. Examining the effect of cerebral perfusion abnormality magnitude on cognitive performance in recently abstinent chronic cocaine abusers. J Neuroimaging. 2004;14(2):162–69. doi:10.1111/j.1552-6569.2004.tb00234.x.
- Cheney CW Memory performance in abstinent chronic cocaine abusers [ Unpublished doctoral dissertation]. New York(NY): New School for Social Research; 1995.
- Cunha PJ, Bechara A, De Andrade AG, Nicastri S. Decision-making deficits linked to real-life social dysfunction in crack cocaine-dependent individuals. Am J Addict. 2011;20(1):78–86. doi:10.1111/j.1521-0391.2010.00097.x.
- Cunha PJ, Goncalves PD, Ometto M, Dos Santos B, Nicastri S, Busatto GF, De Andrade AG. Executive cognitive dysfunction and ADHD in cocaine dependence: searching for a common cognitive endophenotype for addictive disorders. Front Psychiatry. 2013;4:126. doi:10.3389/fpsyt.2013.00126.
- De Oliveira LG, Barroso LP, Silveira CM, Sanchez ZV, De Carvalho Ponce J, Vaz LJ, Nappo SA. Neuropsychological assessment of current and past crack cocaine users. Subst Use Misuse. 2009;44(13):1941–57. doi:10.3109/10826080902848897.
- Di Sclafani V, Clark HW, Tolou-Shams M, Bloomer CW, Salas GA, Norman D, Fein G. Premorbid brain size is a determinant of functional reserve in abstinent crack-cocaine and crack-cocaine-alcohol-dependent adults. J Int Neuropsychol Soc. 1998;4(6):559–65. doi:10.1017/S1355617798466049.
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66(2):161–71. doi:10.1016/S0376-8716(01)00197-1.
- Elman I, Chi WH, Gurvits TV, Ryan ET, Lasko NB, Lukas SE, Pitman RK. Impaired reproduction of three-dimensional objects by cocaine-dependent subjects. J Neuropsychiatry Clin Neurosci. 2008;20(4):478–84. doi:10.1176/jnp.2008.20.4.478.
- Fernandez-Serrano MJ, Perales JC, Moreno-Lopez L, Perez-Garcia M, Verdejo-Garcia A. Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology (Berl). 2012;219(2):673–83. doi:10.1007/s00213-011-2485-z.
- Gillen RW, Kranzler HR, Bauer LO, Burleson JA, Samarel D, Morrison DJ. Neuropsychologic findings in cocaine-dependent outpatients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(7):1061–76. doi:10.1016/S0278-5846(98)00057-8.
- Goldstein RZ, Woicik PA, Lukasik T, Maloney T, Volkow ND. Drug fluency: a potential marker for cocaine use disorders. Drug Alcohol Depend. 2007;89(1):97–101. doi:10.1016/j.drugalcdep.2006.12.001.
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl). 2011;218(4):681–92. doi:10.1007/s00213-011-2360-y.
- Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, Wang GJ, Volkow N. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60(2–3):167–76. doi:10.1016/0165-1781(96)02758-8.
- Kalapatapu RK, Vadhan NP, Rubin E, Bedi G, Cheng WY, Sullivan MA, Foltin RW. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228–39. doi:10.1111/j.1521-0391.2011.00128.x.
- Kelley BJ, Yeager KR, Pepper TH, Beversdorf DQ. Cognitive impairment in acute cocaine withdrawal. Cogn Behav Neurol. 2005;18(2):108–12. doi:10.1097/01.wnn.0000160823.61201.20.
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, Swann AC, Moeller FG. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res. 2010;178(2):299–304. doi:10.1016/j.psychres.2009.11.024.
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am J Drug Alcohol Abuse. 2007;33(5):717–26. doi:10.1080/00952990701522724.
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5(7):e11591. doi:10.1371/journal.pone.0011591.
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85(3):205–12. doi:10.1016/j.drugalcdep.2006.04.008.
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2011;37(2):117–22. doi:10.3109/00952990.2010.543204.
- Madoz-Gurpide A, Blasco-Fontecilla H, Baca-Garcia E, Ochoa-Mangado E. Executive dysfunction in chronic cocaine users: an exploratory study. Drug Alcohol Depend. 2011;117(1):55–58. doi:10.1016/j.drugalcdep.2010.11.030.
- Meade CS, Towe SL, Skalski LM, Robertson KR. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend. 2015;149:128–35. doi:10.1016/j.drugalcdep.2015.01.034.
- Mittenberg W, Motta S. Effects of chronic cocaine abuse on memory and learning. Arch Clin Neuropsychol. 1993;8(6):477–83. doi:10.1093/arclin/8.6.477.
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68(1):105–11. doi:10.1016/S0376-8716(02)00106-0.
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610–17. doi:10.1038/sj.npp.1300617.
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133(Pt 5):1484–93. doi:10.1093/brain/awq066.
- O’Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18(2):131–44. doi:10.3109/00952999208992826.
- Patzelt EH, Kurth-Nelson Z, Lim KO, Macdonald AW 3rd. Excessive state switching underlies reversal learning deficits in cocaine users. Drug Alcohol Depend. 2014;134:211–17. doi:10.1016/j.drugalcdep.2013.09.029.
- Ranlett SG Attention and verbal memory deficits in abstaining cocaine abusers [ Unpublished doctoral dissertation]. San Diego(CA): California School of Professional Psychology; 1995.
- Robinson JE, Heaton RK, O’Malley SS. Neuropsychological functioning in cocaine abusers with and without alcohol dependence. J Int Neuropsychol Soc. 1999;5(1):10–19. doi:10.1017/S1355617799511028.
- Rosselli M, Ardila A. Cognitive effects of cocaine and polydrug abuse. J Clin Exp Neuropsychol. 1996;18(1):122–35. doi:10.1080/01688639608408268.
- Rosselli M, Ardila A, Lubomski M, Murray S, King K. Personality profile and neuropsychological test performance in chronic cocaine-abusers. Int J Neurosci. 2001;110(1–2):55–72. doi:10.3109/00207450108994221.
- Selby MJ, Azrin RL. Neuropsychological functioning in drug abusers. Drug Alcohol Depend. 1998;50(1):39–45. doi:10.1016/S0376-8716(98)00002-7.
- Smelson DA, Roy A, Santana S, Engelhart C. Neuropsychological deficits in withdrawn cocaine-dependent males. Am J Drug Alcohol Abuse. 1999;25(2):377–81. doi:10.1081/ADA-100101867.
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11(4):742–47. doi:10.3758/BF03196629.
- Tucker KA Relationship between neuropsychological deficits and cerebral perfusion abnormalities in cocaine abusers [ Unpublished doctoral dissertation]. Louisiana State University, Louisiana; 2003.
- Vadhan NP, Hart CL, Haney M, Van Gorp WG, Foltin RW. Decision-making in long-term cocaine users: effects of a cash monetary contingency on Gambling task performance. Drug Alcohol Depend. 2009;102(1–3):95–101. doi:10.1016/j.drugalcdep.2009.02.003.
- Vadhan NP, Myers CE, Rubin E, Shohamy D, Foltin RW, Gluck MA. Stimulus-response learning in long-term cocaine users: acquired equivalence and probabilistic category learning. Drug Alcohol Depend. 2008;93(1–2):155–62. doi:10.1016/j.drugalcdep.2007.09.013.
- Van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, Plotkin D. Declarative and procedural memory functioning in abstinent cocaine abusers. Arch Gen Psychiatry. 1999;56(1):85–89. doi:10.1001/archpsyc.56.1.85.
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90(1):2–11. doi:10.1016/j.drugalcdep.2007.02.004.
- Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, Bolla KI, Quednow BB. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry. 2013;203(1):35–43. doi:10.1192/bjp.bp.112.118091.
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34(5):1112–22. doi:10.1038/npp.2008.60.
- Woicik PA, Urban C, Alia-Klein N, Henry A, Maloney T, Telang F, Wang GJ, Volkow ND, Goldstein RZ. A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin Card Sorting Test. Neuropsychologia. 2011;49(7):1660–69. doi:10.1016/j.neuropsychologia.2011.02.037.
- Ahn WY, Vasilev G, Lee SH, Busemeyer JR, Kruschke JK, Bechara A, Vassileva J. Decision-making in stimulant and opiate addicts in protracted abstinence: evidence from computational modeling with pure users. Front Psychol. 2014;5:849. doi:10.3389/fpsyg.2014.00849.
- Al-Zahrani MA, Elsayed YA. The impacts of substance abuse and dependence on neuropsychological functions in a sample of patients from Saudi Arabia. Behav Brain Funct. 2009;5:48. doi:10.1186/1744-9081-5-48.
- Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, Guttman M, Saint-Cyr JA, Wilson AA, Kish SJ. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28(39):9850–56. doi:10.1523/JNEUROSCI.3008-08.2008.
- Bousman CA Genes, neurocognition, and HIV risk behaviors in the context of methamphetamine and HIV [ Unpublished doctoral dissertation]. San Diego(CA): Univerisity of California; 2009.
- Carter BC Examining the relationship between methamphetamine use, Iowa gambling task, and the awareness of social interference test [ unpublished doctoral dissertation]. IBoise, daho: Idaho State Univerisity; 2011.
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57(9):967–74. doi:10.1016/j.biopsych.2005.01.039.
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, Group HNRC. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend. 2010;106(2):154–63. doi:10.1016/j.drugalcdep.2009.08.010.
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10(6):765–75. doi:10.1017/S1461145706007395.
- Dean AC, Sevak RJ, Monterosso JR, Hellemann G, Sugar CA, London ED. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943–53. doi:10.15288/jsad.2011.72.943.
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31(5):1036–47. doi:10.1038/sj.npp.1300889.
- Gonzalez R, Bechara A, Martin ME. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clin Exp Neuropsychol. 2007;29(2):155–59. doi:10.1080/13803390600582446.
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Cherner M, Grant I. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend. 2004;76(2):181–90. doi:10.1016/j.drugalcdep.2004.04.014.
- Han DH, Yoon SJ, Sung YH, Lee YS, Kee BS, Lyoo IK, Renshaw PF, Cho SC. A preliminary study: novelty seeking, frontal executive function, and dopamine receptor (D2) TaqI A gene polymorphism in patients with methamphetamine dependence. Compr Psychiatry. 2008;49(4):387–92. doi:10.1016/j.comppsych.2008.01.008.
- Hekmat S, Mehrjerdi AZ, Moradi A, Hamed. E, Bakhshi S. Cognitive flexibility, attention and speed of mental processing in opioid and methamphetamine addicts in comparison with non-addicts. Auton Neurosci. 2010;2:1–19.
- Henry BL, Minassian A, Van Rhenen M, Young JW, Geyer MA, Perry W. Translational Methamphetamine ARCG. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology (Berl). 2011;215(4):697–707. doi:10.1007/s00213-011-2170-2.
- Henry JD, Mazur M, Rendell PG. Social-cognitive difficulties in former users of methamphetamine. Br J Clin Psychol. 2009;48(Pt 3):323–27. doi:10.1111/j.2044-8260.2009.tb00487.x.
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl). 2006;188(2):162–70. doi:10.1007/s00213-006-0494-0.
- Hosak L, Preiss M, Bazant J, Tibenska A, Cermakova R, Cermakova E. Comparison of Wisconsin Card Sorting Test results between Czech subjects dependent on methamphetamine versus healthy volunteers. Psychiatr Danub. 2012;24(2):188–93.
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl). 2006;185(3):327–38. doi:10.1007/s00213-006-0330-6.
- Kalechstein AD, Fong T, Rosenthal RJ, Davis A, Vanyo H, Newton TF. Pathological gamblers demonstrate frontal lobe impairment consistent with that of methamphetamine-dependent individuals. J Neuropsychiatry Clin Neurosci. 2007;19(3):298–303. doi:10.1176/jnp.2007.19.3.298.
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci. 2003;15(2):215–20. doi:10.1176/jnp.15.2.215.
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9(2):221–28. doi:10.1017/S1461145705005699.
- Kim YT, Kwon DH, Chang Y. Impairments of facial emotion recognition and theory of mind in methamphetamine abusers. Psychiatry Res. 2011;186(1):80–84. doi:10.1016/j.psychres.2010.06.027.
- Kim YT, Lee SW, Kwon DH, Seo JH, Ahn BC, Lee J. Dose-dependent frontal hypometabolism on FDG-PET in methamphetamine abusers. J Psychiatr Res. 2009;43(14):1166–70. doi:10.1016/j.jpsychires.2009.03.011.
- Marquine MJ, Iudicello JE, Morgan EE, Brown GG, Letendre SL, Ellis RJ, Deutsch R, Woods SP, Grant I, Heaton RK. Translational Methamphetamine ARCG. “Frontal systems” behaviors in comorbid human immunodeficiency virus infection and methamphetamine dependency. Psychiatry Res. 2014;215(1):208–16. doi:10.1016/j.psychres.2013.11.004.
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62(2):91–100. doi:10.1002/(ISSN)1098-2396.
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: comparison with non-drug-using controls. Drug Alcohol Depend. 1998;50(2):181–84. doi:10.1016/S0376-8716(98)00022-2.
- Moon M, Do KS, Park J, Kim D. Memory impairment in methamphetamine dependent patients. Int J Neurosci. 2007;117(1):1–9.
- Rapeli P, Kivisaari R, Kahkonen S, Puuskari V, Autti T, Kalska H. Do individuals with former amphetamine dependence have cognitive deficits? Nord J Psychiatry. 2005;59(4):293–97.
- Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609–16. doi:10.1007/s00213-008-1408-0.
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20(4):322–39. doi:10.1016/S0893-133X(98)00091-8.
- Segala L Relationship between self-reported ADHD symptoms and drug addiction three measures of impulsivity and decision-making [ Unpublished doctoral dissertation]. Chicago(IL): Alder School of Professional Psychology; 2013.
- Sim T, Simon SL, Domier CP, Richardson K, Rawson RA, Ling W. Cognitive deficits among methamphetamine users with attention deficit hyperactivity disorder symptomatology. J Addict Dis. 2002;21(1):75–89. doi:10.1300/J069v21n01_07.
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71(3):335–44. doi:10.15288/jsad.2010.71.335.
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222–31. doi:10.1080/10550490050148053.
- Van Wyk C, Stuart AD. A comparative study of the effects of methamphetamine on memory in existing and recovering addicts south African population. Health SA Gesondheld. 2012;17(1):1–9.
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8(7):e68791. doi:10.1371/journal.pone.0068791.
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I, Group HIVNRC. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19(1):35–43. doi:10.1037/0894-4105.19.1.35.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR Introduction to Meta-analysis. West Sussex, UK: John Wiley & Sons, Ltd; 2009.
- Reger MA, Welsh RK, Watson GS, Cholerton B, Baker LD, Craft S. The relationship between neuropsychological functioning and driving ability in dementia: a meta-analysis. Neuropsychology. 2004;18(1):85–93. doi:10.1037/0894-4105.18.1.85.
- Cohen J Statistical Power Analysis for the Behavioral Sciences. Hillsdale, N.J.: L. Erlbaum Associates; 1988.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons. 2011.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi:10.1111/j.0006-341X.2000.00455.x.
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Decision-making and the Iowa gambling task: ecological validity in individuals with substance dependence. Psychologica Belgica. 2006;46(1–2):55–78. doi:10.5334/pb-46-1-2-55.
- Rawson R, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W. Methamphetamine and cocaine users: differences in characteristics and treatment retention. J Psychoactive Drugs. 2000;32(2):233–38. doi:10.1080/02791072.2000.10400234.
- Chung CS, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database Syst Rev. 2013;4:CD008391.
- Levine B, Schweizer TA, O’Connor C, Turner G, Gillingham S, Stuss DT, Manly T, Robertson IH. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. doi:10.3389/fnhum.2011.00009.
- Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. Int J Neurosci. 2001;106(3–4):253–63. doi:10.3109/00207450109149753.
- Li R, Qin W, Zhang Y, Jiang T, Yu C. The neuronal correlates of digits backward are revealed by voxel-based morphometry and resting-state functional connectivity analyses. PLoS One. 2012;7(2):e31877. doi:10.1371/journal.pone.0031877.
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362(6418):342–45. doi:10.1038/362342a0.
- Du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129(Pt 12):3315–28. doi:10.1093/brain/awl244.
- Chen Y, Liu Z, Zhang J, Xu K, Zhang S, Wei D, Zhang Z. Altered brain activation patterns under different working memory loads in patients with type 2 diabetes. Diabetes Care. 2014;37(12):3157–63. doi:10.2337/dc14-1683.
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106(1):61–64. doi:10.1016/j.drugalcdep.2009.07.020.
- Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16(3):174–80. doi:10.1016/j.tics.2012.01.006.
- Bell M, Bryson G, Wexler BE. Cognitive remediation of working memory deficits: durability of training effects in severely impaired and less severely impaired schizophrenia. Acta Psychiatr Scand. 2003;108(2):101–09. doi:10.1034/j.1600-0447.2003.00090.x.
- Alfonso JP, Caracuel A, Delgado-Pastor LC, Verdejo-Garcia A. Combined Goal Management Training and Mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug Alcohol Depend. 2011;117(1):78–81. doi:10.1016/j.drugalcdep.2010.12.025.
- Nedic S, Stufflebeam SM, Rondinoni C, Velasco TR, Dos Santos AC, Leite JP, Gargaro AC, Mujica-Parodi LR, Ide JS. Using network dynamic fMRI for detection of epileptogenic foci. BMC Neurol. 2015;15:262. doi:10.1186/s12883-015-0514-y.
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42(10):1313–35. doi:10.1016/j.neuropsychologia.2004.02.009.
- Moretti DV. Conversion of mild cognitive impairment patients in Alzheimer’s disease: prognostic value of Alpha3/Alpha2 electroencephalographic rhythms power ratio. Alzheimers Res Ther. 2015;7(1):80. doi:10.1186/s13195-015-0162-x.
- Dickey CC, McCarley RW, Voglmaier MM, Frumin M, Niznikiewicz MA, Hirayasu Y, Fraone S, Seidman LJ, Shenton ME. Smaller left Heschl’s gyrus volume in patients with schizotypal personality disorder. Am J Psychiatry. 2002;159(9):1521–27. doi:10.1176/appi.ajp.159.9.1521.
- Godding PR, Fitterling JM, Schmitz JM, Seville JL, Parisi SA. Discriminative utility of a brief cognitive status assessment with alcoholics and the impact of cognitive status on acquisition of treatment-relevant information. Psychol Addict Behav. 1992;6(1):34–40. doi:10.1037/h0080602.
- Kaschel R, Sala SD, Cantagallo A, Fahlböck A, Laaksonen R, Kazen M. Imagery mnemonics for the rehabilitation of memory: A randomised group controlled trial. Neuropsychol Rehabil. 2002;12(2):127–53. doi:10.1080/09602010143000211.
- Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Fleischhacker WW. Cognitive remediation therapy during treatment for alcohol dependence. J Stud Alcohol Drugs. 2012;73(4):625–34. doi:10.15288/jsad.2012.73.625.
- Melrose RJ, Harwood D, Khoo T, Mandelkern M, Sultzer DL. Association between cerebral metabolism and Rey–Osterrieth Complex Figure Test performance in Alzheimer’s disease. J Clin Exp Neuropsychol. 2013;35(3):246–58. doi:10.1080/13803395.2012.763113.
- Moscovitch C, Kapur S, Kohler S, Houle S. Distinct neural correlates of visual long-term memory for spatial location and object identity: a positron emission tomography study in humans. Proc Natl Acad Sci U S A. 1995;92(9):3721–25. doi:10.1073/pnas.92.9.3721.
- Biesbroek JM, Van Zandvoort MJ, Kuijf HJ, Weaver NA, Kappelle LJ, Vos PC, Velthuis BK, Biessels GJ, Postma A, Utrecht VCISG. The anatomy of visuospatial construction revealed by lesion-symptom mapping. Neuropsychologia. 2014;62:68–76. doi:10.1016/j.neuropsychologia.2014.07.013.
- Desfosses M, Meadows H, Jackson M, Crowe SF. The relationship between neuropsychological functioning and mental health outcomes of chronic alcohol users involved in counselling: prediction of treatment outcome. Aust Psychol. 2014;49(5):287–96. doi:10.1111/ap.12071.
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, Felicetti T, Laatsch L, Harley JP, Bergquist T, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92(4):519–30. doi:10.1016/j.apmr.2010.11.015.
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat. 2009;37(3):292–97. doi:10.1016/j.jsat.2009.03.004.
- Fernández-Serrano MJ, Pérez-García M, Schmidt Río-Valle J, Verdejo-García A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. Journal of Psychopharmacology. 2009;24(9):1317–32. doi:10.1177/0269881109349841.
- Thomas KV, Bijlsma L, Castiglioni S, Covaci A, Emke E, Grabic R, Hernández F, Karolak S, Kasprzyk-Hordern B, Lindberg RH. Comparing illicit drug use in 19 European cities through sewage analysis. Science of the Total Environment. 2012;432:432–39. doi:10.1016/j.scitotenv.2012.06.069.
- Copeland AL, Sorensen JL. Differences between methamphetamine users and cocaine users in treatment. Drug Alcohol Depend. 2001;62(1):91–95. doi:10.1016/S0376-8716(00)00164-2.
- Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263–68. doi:10.1097/YCO.0b013e32832a3b44.
- Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend. 2014;139:18–25. doi:10.1016/j.drugalcdep.2014.02.702.
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD. A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 1999;56(6):507–14. doi:10.1001/archpsyc.56.6.507.