ABSTRACT
Background: Extended-release (ER) morphine formulations are commonly manipulated for non-oral routes of administration, particularly via injection. Morphine ARER, an abuse-deterrent formulation of ER morphine, has both physical and chemical properties that deter abuse. Objectives: To assess the syringeability of morphine ARER using in vitro laboratory studies. Methods: Intact, cut, or crushed morphine ARER tablets were incubated in 1, 5, or 10 mL of room temperature or 90°C water for 1, 5, 10, or 30 min of agitation. Crushed ER morphine tablets were assessed in 10 mL room temperature water. The difficulty to draw the mixture into a syringe was assessed on a scale of 1 (very easy) to 10 (impossible). If the prepared mixture was syringeable, released morphine was measured analytically. Results: Crushed and cut morphine ARER tablets formed a viscous material when subjected to a liquid environment and were rated as “impossible to syringe” in ≤5 mL water and were slightly syringeable in 10 mL water. In contrast, all syringeability tests of crushed ER morphine were rated as “very easy to syringe”. After 30 min in room temperature water, crushed ER morphine released 75% morphine whereas intact, cut, and crushed morphine ARER tablets released a maximum of 12%, 12%, and 5% of the total morphine content. Heating extractions resulted in increased morphine release. Conclusion: The difficulty to syringe morphine ARER when manipulated suggests that morphine ARER has abuse-deterrent properties that may deter intravenous abuse.
Introduction
Misuse, abuse, and diversion of prescription opioids are major public health concerns. The Centers for Disease Control and Prevention has defined prescription drug abuse as an epidemic in the United States (Citation1), and the US Food and Drug Administration (FDA) has encouraged the development of abuse-deterrent formulations (ADFs) of opioids and has included the expanded access of opioid ADFs as component in their Opioid Action Plan (Citation2). Abusers of prescription opioids commonly manipulate tablets and attempt to extract the active ingredient from extended-release (ER) formulations to bypass the intended slow-release characteristics and increase the opioid’s bioavailability for oral administration, snorting (intranasal administration), injecting, or smoking (Citation3–Citation6). However, intentional manipulation to induce dose dumping of the entire ER dose by oral or other routes of administration can lead to serious consequences including overdose and death.
Regulatory approval of abuse-deterrent opioids is based on in vitro and in vivo studies that test the abuse potential via routes of administration commonly used by recreational drug users (Citation2). Data from epidemiologic studies suggest that ER morphine formulations are commonly manipulated and then abused via the oral, intranasal, and intravenous (IV) routes of administration; morphine is more likely to be abused via injection than any other prescription opioid, except hydromorphone (Citation6,Citation7). In addition to concerns for overdose and death, abusers who inject opioids are at increased risk for contracting blood-borne infections (e.g., HIV, hepatitis C) (Citation8–Citation11), and developing bacterial and fungal skin infections (Citation12) and cardiovascular complications (e.g., endocarditis) (Citation13).
Morphine ARER, an FDA-approved ADF of ER morphine (MorphaBond™ ER, Daiichi Sankyo, Inc., Basking Ridge, NJ) is formulated with SentryBond™ technology that has both physical and chemical properties that contribute to abuse deterrence. First, the active ingredient is contained within a polymer matrix of inactive ingredients and the active ingredient is difficult to visibly distinguish or physically separate from the polymer matrix. In addition, physically manipulated tablets (crushing, cutting, grating, grinding, etc.) form a viscous material in aqueous environments (Citation14); this is one of the features incorporated into SentryBond technology that is designed to make abuse via injection difficult. The intranasal abuse potential of morphine ARER was previously assessed in non-dependent recreational opioid abusers (Citation15). Subjects in that study reported significantly lower drug liking for crushed intranasal morphine ARER compared with crushed intranasal ER morphine (p< .0001) (Citation15). Subjects also reported significantly lower scores for drug high (p< .0001), overall drug liking (p= .007), good effects of the drug (p= .0004), and desire to take the drug again (p= .0341) when comparing crushed intranasal morphine ARER with crushed intranasal ER morphine (Citation15). To assess the abuse deterrence by the IV route of administration, in vitro extraction and syringeability studies of morphine ARER tablets were conducted.
Methods
Tablet preparation
Morphine ARER 100-mg tablets were left intact, cut with a knife, or crushed for 60 s using a household tool shown to produce the greatest reduction in particle size in previous in vitro studies (Citation14). The comparator, ER morphine (MS Contin®, Purdue Pharma LP, Stamford, CT) 100-mg tablets, was crushed with a glass pestle on wax paper. Intact, cut, or crushed tablets of morphine ARER were placed in glass vials containing 1, 5, or 10 mL of water. Crushed ER morphine tablets were assessed in 10 mL of water; other volumes were not assessed because of the known dose-dumping of ER morphine. The vials were incubated for 1, 5, 10, or 30 min at room temperature (morphine ARER and ER morphine) or elevated temperatures (90°C; morphine ARER only) with agitation in a laboratory shaker at 100 revolutions per minute (rpm).
Syringeability and extraction
After incubation in the various conditions (room temperature or 90°C water, with agitation, for up to 30 min), attempts were made to draw each prepared tablet mixture into a 10-cc syringe fitted with a 27-, 24-, or 18-gauge needle. Water was chosen as the solvent for this study because it is the most commonly utilized solvent by IV drug users and it was the solvent that resulted in the most efficient extraction of morphine in large volume extraction studies (Citation16). Tests were completed in an iterative fashion; samples that were easily syringeable with the smallest needle (27 gauge) were not tested with larger-bore needles. Difficulty to syringe was assessed on a scale of 1 (very easy to syringe) to 10 (impossible to syringe). If the prepared tablet mixture was syringeable (i.e., able to draw liquid into the syringe, regardless of whether any portion of the tablet mixture is drawn into the syringe), the volume was recorded, and the percentage of morphine released was measured analytically using a validated, high-performance liquid chromatography (HPLC) assay method. Morphine ARER tests were repeated 5 times and ER morphine tests were repeated 3 times. The difficulty ratings and the morphine concentration detected in syringeable volumes were reported as means.
Results
This study assessed two outcomes: the difficulty to pull water-immersed intact and manipulated tablets into a syringe (syringeability), and the detection of any morphine that may have been drawn into the syringe (extraction).
Syringeability
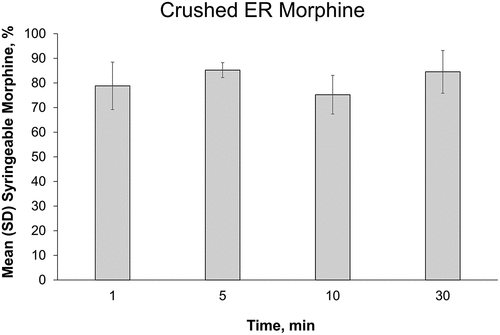
When assessing the ability to draw samples into a syringe, crushed ER morphine passed easily through the smallest needle (27 gauge) when agitated in 10 mL of water for up to 30 min; the average difficulty rating was 3.3 and 10 mL of liquid could be easily drawn into the syringe in these tests (see ). Crushed and cut morphine ARER tablets immediately formed a viscous material when placed in liquid that was difficult or impossible to draw into a syringe (see ). In 1-mL and 5-mL water, all syringeability tests with crushed or cut morphine ARER tablets were rated with a difficulty of 10, regardless of liquid volume or time of incubation, even with larger 18-gauge needles. In 10-mL water, crushed and cut morphine ARER tablets were somewhat syringeable, although 6.2 was the lowest average difficulty rating for any condition and the largest volume that could be drawn into the syringe was 4 mL. The liquid surrounding the intact tablets was easily syringeable (mean difficulty ratings ranged between 1 and 3.6 for all conditions) and were assessed for the amount of morphine extracted (see below).
Table 1. Mean difficulty ratings and syringeable volume for syringeability tests on a scale of 1 (very easy to draw into a syringe) to 10 (impossible to draw into a syringe).
Figure 1. Representative samples of (a) intact, (b) cut, and (c) crushed morphine ARER tablets after a 10-min incubation in 5 mL room temperature water with agitation.
Notes: Panels on the left show the resulting mixture; panels on the right illustrate the attempts to draw the resulting solution into a syringe.

Morphine extraction in syringeable material
Crushed ER morphine
After up to 30 min of incubation in 10 mL of room temperature water, as much as 85% of the morphine from crushed ER morphine tablets was detected in the syringeable volume (see ). Additional extraction experiments were not performed for crushed ER morphine tablets because of the dose dumping that occurred under the least rigorous conditions.
Intact morphine ARER
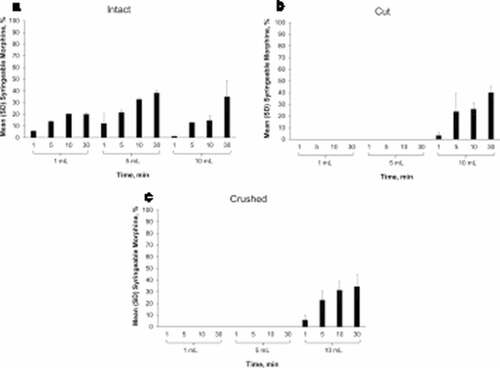
The liquid surrounding the intact (non-manipulated) morphine ARER tablets was easily syringeable; however, only a negligible amount of morphine was detected in syringeable material after intact morphine ARER tablets were placed in 1 mL of room temperature water for 1 min (see ). Up to 12% of morphine from an intact morphine ARER tablet was detected in syringeable volumes after 30 min in 10 mL of room temperature water with agitation. At elevated temperatures, the amount of morphine detected in syringeable material was low relative to ER morphine extractions. Intact morphine ARER released a maximum of 38% of the nominal morphine dose after 30 min with agitation in 5 mL of 90°C water (see ).
Figure 3. Mean percentage of morphine released after 1, 5, 10, and 30 min in 1 mL, 5 mL, or 10 mL room temperature water with 100 rpm agitation for (a) intact, (b) cut, or (c) crushed morphine ARER tablets. Notes: Error bars = standard deviation; ER = extended-release; SD = standard deviation.
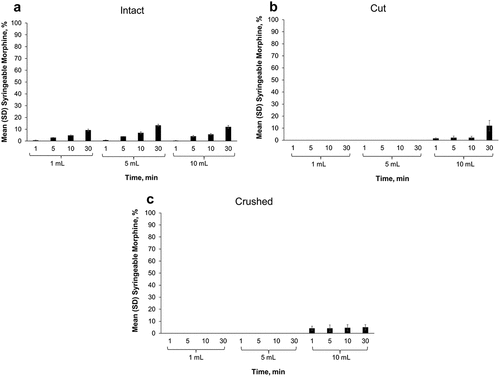
Figure 4. Mean percentage of morphine released after 1, 5, 10, and 30 min in 1 mL, 5 mL, or 10 mL 90°C water with 100 rpm agitation for (a) intact, (b) cut, or (c) crushed morphine ARER tablets. Notes: Error bars = standard deviation; ER = extended-release; rpm = revolutions per minute; SD = standard deviation.
Cut and crushed morphine ARER
When manipulated SentryBond tablets are subjected to a liquid environment, they form a viscous material that makes it difficult or impossible to draw small volumes of manipulated tablets through a needle. Because cut and crushed morphine ARER were impossible to syringe (difficulty rating of 10) in 1 mL and 5 mL of room temperature water, for up to 30 min, there was no syringeable volume to assess for morphine content. Minimal amounts of morphine (up to 12%) were detected in syringeable volumes from cut or crushed morphine ARER in 10 mL water (see , ). No syringeable liquid was obtained from cut or crushed morphine ARER tablets placed in 1 mL or 5 mL of 90°C water (see , ). In 10 mL of 90°C water, the most morphine detected from cut or crushed morphine ARER tablets was 40.3%, which was similar to the amount detected in the liquid surrounding intact tablets.
Discussion
In June 2017, the FDA requested the removal of an ER opioid formulation with theoretical abuse-deterrent properties from the market (Citation17) due to post-marketing data that demonstrated a dangerous shift in the route of abuse from snorting to injection (Citation17,Citation18). This shift was associated with a subsequent outbreak of HIV and hepatitis C infections (Citation17,Citation18), thus highlighting the need for opioid ADFs that deter abuse via injection.
Intravenous administration of opioids is associated with serious health risks and is a reported route of abuse for ER morphine formulations; thus, it is necessary to evaluate the potential for abusing morphine ARER by this route of administration (Citation7). In 2015 the FDA provided guidance on three categories of premarket studies required to demonstrate abuse-deterrent properties for unique opioid formulations. These categories include laboratory-based in vitro studies (Category 1), pharmacokinetic studies (Category 2), and human abuse-potential studies (Category 3) (Citation2). In vitro studies investigate the physical manipulation, large-volume extraction, and small-volume extraction/syringeability of an abuse-deterrent opioid formulation and are important for identifying the investigational product’s potential for abuse by all routes of administration. In this in vitro, small-volume extraction and syringeability study, crushed or cut morphine ARER tablets immediately formed a viscous material in liquid and were difficult to draw into a syringe after up to 30 min of agitation (at 100 rpm) in small volumes, even when large 18-gauge needles were used. When manipulated morphine ARER tablets are subjected to a liquid environment, they form a viscous material that makes it difficult or impossible to draw through a needle. In addition, any syringeable material contained visible particulate that would not be safe for injection. A potential limitation of this study is the use of the inherently subjective scale to rate difficulty of syringeability; however, the difficulty rating system was utilized by design so the analyst could provide qualitative but comparative information about the ease or difficulty of abuse of IR morphine relative to morphine ARER from a drug abuser’s point of view. Nonsubjective measures of evaluating the difficulty of syringeability have been utilized elsewhere (Citation19,Citation20); however, scientific evaluation of viscosity and the force required to compress a syringe can be difficult to relate to real-world abuse potential without including a concurrent subjective comparison. Indeed, the development of a validated scientific method to evaluate the difficulty of syringeability should be considered an important goal.
Findings from other studies indicate that morphine ARER tablets resist physical manipulation by most common household tools, making preparation for intranasal and IV administration difficult (Citation14). This is coupled with the fact that it was difficult to prepare morphine ARER tablets for IV administration in small volumes. Even when physical manipulation was not used, intact morphine ARER tablets did not release marked amounts of morphine, despite being subjected to harsh laboratory conditions (incubation for up to 30 min in 90°C water with agitation). The low morphine yield from these conditions would likely dissuade abusers from attempting to abuse morphine ARER via a riskier route of administration (e.g., IV administration). It should be noted that abuse-deterrent opioids are designed to deter abuse and frustrate abusers, but with enough time and effort, abuse by the intranasal, oral, and IV routes is still possible.
Findings from a recent study in which the Internet was monitored for abuse-related discussions indicated that abusers are more likely to switch to non-ADF opioids than attempt to prepare reformulated ER oxycodone for non-oral abuse (Citation21). There was no evidence of dose dumping from morphine ARER during any tested conditions, unlike the dose dumping noted with crushed ER morphine, which does not contain any abuse-deterrent properties. Although the liquid surrounding intact morphine ARER tablets could be easily drawn into a syringe, in room temperature water, up to 12% of the morphine content was detected in the syringeable volume from intact morphine ARER tablets after 30 min of incubation in 10 mL of water. The most morphine detected in the syringeable volumes from intact morphine ARER tablets after incubation in harsher conditions was 38%. In contrast, as much as 85% of the morphine content was released from crushed ER morphine tablets after 5 min of extraction in 10 mL of water.
A limitation of this study is that the “abuse potential” was assessed under controlled laboratory conditions and, although various conditions were investigated, not all conditions that an abuser may try were included in the study. For example, longer extractions or the use of alternative solvents may produce different results. This study was designed to utilize the most efficient solvent identified in large volume extraction studies (water) (Citation16) and evaluate time periods similar to those reported by IV opioid users (Citation22,Citation23). Although adding more time for extraction may result in higher recoveries for intact tablets, we do not anticipate longer times to change the results for manipulated tablets because the viscosity of the samples is not expected to decrease over time. Further, the translation of these data to real-world abuse deterrence is currently unknown. However, it has been previously reported that, when assessed in non-dependent recreational opioid abusers, crushed intranasal morphine ARER received lower scores for drug liking compared with crushed intranasal ER morphine (Citation15). Nonetheless, long-term epidemiological studies are necessary to identify the real-world impact of morphine ARER on rates of abuse, overdose, and death.
In these in vitro studies, morphine ARER was difficult to draw into a syringe and the low concentration of morphine detected in any syringeable volume reported herein suggest that morphine ARER has abuse-deterrent properties that may deter abuse by the IV route of administration.
Data Sharing
For public health concerns, the data used in this manuscript will not be publicly shared.
Acknowledgments
Medical writing support (funded by Daiichi Sankyo, Inc.) was provided by Kelly M Cameron, PhD, ISMPP Certified Medical Publication Professional, of JB Ashtin who, on the behalf of the authors, developed the first draft based on an author-approved outline and assisted in implementing author revisions throughout the editorial process.
Disclosure statement
All authors are full-time employees and/or consultants for Inspirion Delivery Sciences, LLC. All authors were involved in the concept/design of the study and data interpretation, critically reviewed the manuscript, and approved its content for submission. The authors accept overall responsibility for the accuracy of the data, its analysis, and all other information presented in this report.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Press release: opioid painkiller prescribing varies widely among states [Internet]. 2014 [accessed 2019 Feb 25]. www.cdc.gov/media/releases/2014/p0701-opioid-painkiller.html.
- Food and Drug Administration. Abuse-deterrent opioids - evaluation and labeling guidance for industry [Internet]. 2015 [accessed 2019 Feb 25]. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm334743.pdf.
- Webster L. Update on abuse-resistant and abuse-deterrent approaches to opioid formulations. Pain Med. 2009;10(Suppl 2):S124–33. doi:10.1111/j.1526-4637.2009.00672.x.
- Budman SH, Grimes Serrano JM, Butler SF. Can abuse deterrent formulations make a difference? Expectation and speculation. Harm Reduct J. 2009;6:8. doi:10.1186/1477-7517-6-8.
- Katz N, Dart RC, Bailey E, Trudeau J, Osgood E, Paillard F. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37(4):205–17. doi:10.3109/00952990.2011.569623.
- Gasior M, Bond M, Malamut R. Routes of abuse of prescription opioid analgesics: a review and assessment of the potential impact of abuse-deterrent formulations. Postgrad Med. 2016;128(1):85–96. doi:10.1080/00325481.2016.1120642.
- Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29. doi:10.1186/1477-7517-8-30.
- Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107(7):1318–27. doi:10.1111/j.1360-0443.2012.03803.x.
- Lankenau SE, Kecojevic A, Silva K. Associations between prescription opioid injection and Hepatitis C virus among young injection drug users. Drugs (Abingdon Engl). 2015;22(1):35–42. doi:10.3109/09687637.2014.970515.
- Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, Hillman D, Hon J, Hoover KW, Patel MR, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone–Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–44.
- Surratt H, Kurtz SP, Cicero TJ. Alternate routes of administration and risk for HIV among prescription opioid abusers. J Addict Dis. 2011;30(4):334–41. doi:10.1080/10550887.2011.609805.
- Del Giudice P. Cutaneous complications of intravenous drug abuse. Br J Dermatol. 2004;150(1):1–10.
- Ghuran A, van Der Wieken LR, Nolan J. Cardiovascular complications of recreational drugs. Bmj. 2001;323(7311):464–66.
- DiFalco R, Kinzler ER, Pantaleon C, Aigner S. Abuse-resistant, extended-release moprhine is resistant to physical manipulation techniques commonly used by opioid abusers. Postgrad Med. 2014;126:S38–9.
- Webster LR, Pantaleon C, Shah MS, DiFalco R, Iverson M, Smith MD, Kinzler ER, Aigner S. A randomized, double-blind, double-dummy, placebo-controlled, intranasal drug liking study on a novel abuse-deterrent formulation of morphine-morphine ARER. Pain Med. 2017;18(7):1303–13. doi:10.1093/pm/pnw213.
- Bianchi R, Kinzler ER, DiFalco R, Shah MS, Aigner S. Extraction testion of a novel extended-release, abuse-deterrent formulation of morphine, Morphine ARER, in common household solvents. J Pain. 2014;15(4):S77(Abstract 404). doi:10.1016/j.jpain.2014.01.315.
- United States Food and Drug Administration. FDA requests removal of Opana ER for risks related to abuse [Internet]. 2017 [accessed 2019 Feb 25]. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm562401.htm.
- Gottlieb S, Woodcock J. Marshaling FDA benefit-risk expertise to address the current opioid abuse epidemic. Jama. 2017;318(5):421–22. doi:10.1001/jama.2017.9205.
- Rahman Z, Yang Y, Korang-Yeboah M, Siddiqui A, Xu X, Ashraf M, Khan MA. Assessing impact of formulation and process variables on in-vitro performance of directly compressed abuse deterrent formulations. Int J Pharm. 2016;502(1–2):138–50. doi:10.1016/j.ijpharm.2016.02.029.
- Rahman Z, Zidan AS, Korang-Yeboah M, Yang Y, Siddiqui A, Shakleya D, Khan MA, Cruz C, Ashraf M. Effects of excipients and curing process on the abuse deterrent properties of directly compressed tablets. Int J Pharm. 2017;517(1–2):303–11. doi:10.1016/j.ijpharm.2016.12.015.
- Vosburg SK, Haynes C, Besharat A, Green JL. Changes in drug use patterns reported on the web after the introduction of ADF OxyContin: findings from the researched abuse, diversion, and addiction-related surveillance (RADARS) system web monitoring program. Pharmacoepidemiol Drug Saf. 2017;26(9):1044–52. doi:10.1002/pds.4248.
- Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Benedek IH, Comer SD. Assessment of a formulation designed to be crush-resistant in prescription opioid abusers. Drug Alcohol Depend. 2012;126(1–2):206–15. doi:10.1016/j.drugalcdep.2012.05.013.
- Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Shapiro DY, Comer SD. A comparison among tapentadol tamper-resistant formulations (TRF) and OxyContin(R) (non-TRF) in prescription opioid abusers. Addiction. 2013;108(6):1095–106. doi:10.1111/add.12114.