ABSTRACT
Background
Pharmacologic treatment is recommended for many individuals with opioid use disorder (OUD). For patients who select opioid antagonist treatment, effective management of opioid withdrawal symptoms during transition to antagonist treatment requires consideration of the patient experience.
Objectives
To compare patterns of opioid withdrawal between those withdrawing from untreated opioid use and those withdrawing from buprenorphine.
Methods
We performed a post hoc, cross-study comparison of the temporal pattern of opioid withdrawal during 1-week induction onto extended-release naltrexone by similar protocols enrolling two participant populations: participants with OUD entering a study with untreated opioid use (N = 378, NCT02537574) or on stable buprenorphine (BUP) treatment (N = 101, NCT02696434).
Results
The temporal pattern of withdrawal from induction day 1 through day 7 differed between the two participant populations for Clinical Opiate Withdrawal Score (COWS) and Subjective Opiate Withdrawal Score (SOWS): participants with untreated OUD prior to study entry were more likely to experience an earlier relative peak in opioid withdrawal followed by a gradual decline, whereas participants on stable BUP treatment prior to study entry were more likely to experience a relatively later, though still mild, peak opioid withdrawal. The peak COWS was reached at a mean (standard deviation) of 1.9 (1.5) days for participants with untreated OUD and 5.0 (1.5) days for participants on stable BUP. Daily peak cravings were generally higher for participants with untreated OUD than participants on stable BUP.
Conclusion
Awareness of population-specific variations in the patient experience of opioid withdrawal may help clinicians anticipate the expected course of withdrawal.
Introduction
Pharmacologic treatment is recommended for many individuals with opioid use disorder (OUD) because it can reduce cravings and the subsequent risk of continued use or relapse. Recommended medications include methadone, buprenorphine (BUP), and the monthly naltrexone extended-release injectable suspension (XR-NTX). These medications have demonstrated effects in reduction of opioid use as well as risk of overdose and other opioid-related problems during active treatment (Citation1). Before initiating XR-NTX, a µ-opioid receptor antagonist, withdrawal management is necessary to avoid precipitation of severe opioid withdrawal (Citation2); the time course and severity of withdrawal symptoms during this period may vary according to management strategy and patient characteristics. Effective management of withdrawal signs and symptoms is important for a positive clinical outcome, as the intensity of withdrawal has been shown to be correlated with drop-out from treatment with medications for OUD (Citation3).
Withdrawal management and induction onto XR-NTX has been evaluated in a number of clinical studies. Two phase 3 randomized clinical trials have evaluated induction onto XR-NTX using similar 1-week transition protocols with the same standing ancillary medications and psycho-educational counseling. In participants with OUD entering a study at baseline with either untreated, active opioid use (henceforth referred to as Untreated OUD) or on stable BUP treatment (henceforth referred to as Stable BUP), low-dose oral naltrexone (NTX) and a BUP taper did not increase the rate of induction onto XR-NTX above the rate observed with placebo (Citation4,Citation5). We performed a post hoc, cross-study comparison of the temporal pattern of opioid withdrawal during induction onto XR-NTX between these two participant populations using the combined treatment groups. Understanding the temporal pattern of opioid withdrawal may assist clinicians with medical management, particularly as avoidance of withdrawal symptoms is a powerful motivation for continued drug-seeking behavior in patients with OUD (Citation6).
Materials and methods
This was a post hoc analysis of two phase 3, multicenter, randomized, double-blind, placebo-controlled trials conducted in the United States (Clinicaltrials.gov identifiers, NCT02537574 and NCT02696434) (Citation4,Citation5). Both trials were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice principles. For both trials, the protocol, amendments, and informed consent were reviewed and approved by a qualified institutional review board for each site, and all participants completed informed consent prior to study participation.
Untreated OUD to XR-NTX study
Study design
In a study of participants transitioning from Untreated OUD at baseline to XR-NTX, 378 participants were randomized on day 1 in a 1:1:1 ratio to receive one of the following transition regimens administered in an outpatient clinic: (i) oral NTX plus sublingual BUP (N = 126), (ii) oral NTX plus sublingual placebo-BUP (N = 126), or (iii) oral placebo-NTX plus sublingual placebo-BUP (N = 126) (Citation4). During a transition period carried out over seven days (days 1–7), participants received their assigned transition regimen with standing ancillary medications () and psychoeducational counseling; then on day 8, participants who had a negative naloxone challenge received a first injection of XR-NTX ().
Figure 1. Study designs. BUP = buprenorphine; COWS = Clinical Opiate Withdrawal Scale; NTX = naltrexone; OUD = opioid use disorder; PBO = placebo
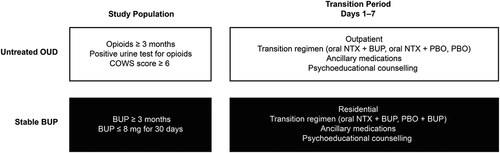
Table 1. Ancillary medications administered during the transition period (days 1–7)
Oral NTX was administered twice daily in ascending doses: days 1 and 2, 0.25 mg; day 3, 0.5 mg; day 4, 1.5 mg; day 5, 3 mg; day 6, 7.5 mg; and day 7, 15 mg. BUP was administered in tapering doses in the transition regimen: day 1, 4 mg; days 2 and 3, 2 mg; and days 4 to 7, no dose. The key outcome measure in this trial was the overall rate of induction onto XR-NTX (i.e., the proportion of participants who received the first dose of XR-NTX).
Study population
Participants aged 18 to 60 years of age who were voluntarily seeking transition to antagonist treatment with XR-NTX were eligible if they had a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of moderate-to-severe OUD. In this study, participants were additionally required to have: (i) reported consistently using opioids for ≥3 months (excluding BUP and methadone), (ii) a positive urine drug test for opioids at screening (excluding BUP and methadone), and (iii) at least mild withdrawal symptoms on the Clinical Opiate Withdrawal Score (COWS) scale as indicated by a score of ≥6 on day 1 (Citation4). The sample was recruited through print, radio, and digital advertising, and through word-of-mouth referral at research sites that offered clinical treatment for OUD.
Stable BUP to XR-NTX study
Study design
In a study of participants transitioning from Stable BUP at baseline to XR-NTX, 101 participants received up to 4 mg of sublingual BUP once daily for 5 days (on an outpatient basis for the first 3 days) to establish a consistent daily dose. Following this lead-in period, participants were randomized on day 1 in a 1:1 ratio to receive one of the following transition regimens administered in a residential setting: oral NTX plus sublingual BUP (N = 50) or oral placebo-NTX plus sublingual BUP (N = 51) (Citation5). The study design was similar to the Untreated OUD to XR-NTX study, with participants undergoing a transition period (days 1–7) where they received the assigned transition regimen with standing ancillary medications () and psychoeducational counseling (). Participants with a negative naloxone challenge then received the first XR-NTX injection on day 8 (). The dosing and administration of oral NTX and BUP in the transition period followed the same regimen as the Untreated OUD to XR-NTX study. Similarly, the key outcome measure was the overall rate of induction onto XR-NTX.
Study population
Eligible participants included those 18 to 60 years of age with a DSM-5 diagnosis of moderate-to-severe OUD and voluntarily seeking transition to XR-NTX. Additional eligibility criteria in this study were a history of prescribed BUP, either alone or in a combined formulation with naloxone, for the past ≥3 months and currently maintained on a daily BUP dose of ≤8 mg for ≥30 days prior to initiating the BUP lead-in period. Participants with consistently positive urine drug screens for opioids (morphine, oxycodone, or methadone) at screening and those seeking outpatient-only treatment were excluded (Citation5). The recruitment for this study was carried out using similar strategies to those employed in the Untreated OUD to XR-NTX study.
Outcome measures
For the present analysis, given that outcomes in both studies did not significantly differ according to treatment arm (Citation4,Citation5) (see Results, Participant characteristics for details), the treatment arms were combined within each study and the pattern of withdrawal was compared during transition (days 1–7), on the day of first XR-NTX injection (day 8), and on the day following XR-NTX injection (day 9). Withdrawal was assessed by the COWS scale and the Subjective Opiate Withdrawal Score (SOWS) scale. Withdrawal symptom scores of ≤12 on the COWS scale and ≤10 on the SOWS scale were considered mild. Severity of craving for opioids was assessed on a visual analog scale (VAS).
COWS, SOWS, and opioid cravings VAS were collected as follows: on days 1 to 7 in the morning (and evening for the Untreated OUD study), 30 minutes before treatment (as outlined in Materials and methods, Study design), at treatment, and at 30, 60, 90, and 120 minutes after treatment; on day 8, before XR-NTX injection and at 30 minutes (in the Stable BUP study only) and 60 minutes after injection; and on day 9, at the outpatient visit.
Safety endpoints included the incidence of treatment-emergent adverse events (TEAEs) during transition (days 1–7); TEAEs were summarized as preferred terms using the Medical Dictionary for Regulatory Activities, version 19.1.
Sensitivity analysis
To ensure that our findings were not influenced by the presence of oral NTX used in one of the treatment arms in each study, the pattern of opioid withdrawal was analyzed for the placebo arms only (placebo for oral NTX) and compared with the withdrawal pattern for the combined groups (presented in Results, Temporal pattern of withdrawal).
Results
Participant characteristics
Participant characteristics were similar for both studies. For participants transitioning from Untreated OUD (Citation4) (total group; N = 378): median age was 36.0 years (range 19–60), 65.9% were male, and 73.8% were white; history of primary opioid use was 64.0% for heroin and 36.0% for prescription opioids; and duration of opioid use was a median of 5.0 years (range 0–42 years) for heroin and 6.0 years (range 1–40 years) for prescription. For participants transitioning from Stable BUP (Citation5) (total group; N = 101), median age was 34.0 years (range 20–57 years), 70.3% were male, and 92.1% were white; history of primary opioid use was 49.5% for heroin and 51.5% for prescription opioids; and duration of opioid use was 42.6% for longer than 5 years, 19.8% for 3 to 5 years, and 37.6% for less than 3 years.
The overall rate of induction onto XR-NTX did not significantly differ between treatment groups in either study (for participants transitioning from Untreated OUD: oral NTX plus BUP = 46.0%, oral NTX plus placebo-BUP = 40.5%, oral placebo-NTX plus placebo-BUP = 46.0% (Citation4); for participants transitioning from Stable BUP: oral NTX plus BUP = 68.6%, oral placebo-NTX plus BUP = 76.0% (Citation5). For participants transitioning from Untreated OUD, 44% of participants completed induction onto XR-NTX (Citation4). For participants transitioning from Stable BUP, 72% of participants completed induction onto XR-NTX (Citation5).
Temporal pattern of withdrawal
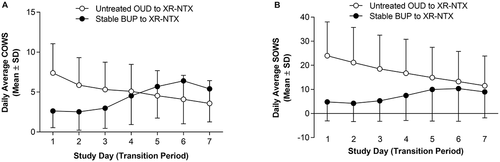
Mean COWS scores (daily average, daily peak, and maximum daily peak) were uniformly mild (≤12) during transition, at XR-NTX injection, and following XR-NTX injection in both studies (). For participants transitioning from Untreated OUD, SOWS scores were in the moderate-to-severe range (20–30) during transition (days 1–7) and the mild-to-moderate range (0–20) at and following XR-NTX injection (days 8 and 9). For participants transitioning from Stable BUP, SOWS scores were generally mild (≤10) overall.
Table 2. Summary of withdrawal symptom scores
The temporal pattern of withdrawal from induction days 1 through 7 differed between the two studies for COWS and SOWS. Most participants transitioning from Untreated OUD experienced peak COWS scores on day 1 (N = 232/378; 61.4%), compared with participants transitioning from Stable BUP, for whom the most common day for peak COWS scores was day 6 (N = 31/101; 30.7%). The peak COWS score was reached at a mean (standard deviation [SD]) of 1.9 (1.5) days for participants transitioning from Untreated OUD and 5.0 (1.5) days for participants transitioning from Stable BUP. SOWS scores followed a similar pattern: the peak SOWS score was reached at a mean (SD) of 2.0 (1.6) days for participants transitioning from Untreated OUD and 4.4 (2.0) days for participants transitioning from Stable BUP. Daily average COWS and SOWS followed a similar pattern (): for participants transitioning from Untreated OUD, scores decreased over time during the transition period, and for participants transitioning from Stable BUP, scores increased modestly during the transition period over time, and the mean values remained in the mild range.
Figure 2. Temporal pattern of withdrawal. (a) Daily average COWS during the transition period; (b) Daily average SOWS during the transition period. For participants transitioning from Untreated OUD, the mean (SD) of daily average COWS scores decreased over time during the transition period, from 7.4 (3.7) on day 1 to 3.6 (2.9) on day 7. Similarly, the mean (SD) of daily average SOWS scores decreased from 24.0 (14.1) on day 1 to 11.5 (12.4) on day 7. For participants transitioning from Stable BUP, the mean (SD) of daily average COWS scores increased modestly over time during the transition period, from 2.6 (2.1) on day 1 to 5.4 (4.1) on day 7. The mean (SD) of daily average SOWS scores also increased, from 4.8 (7.8) on day 1 to 9.0 (10.8) on day 7. BUP = buprenorphine; OUD = opioid use disorder; SD = standard deviation; XR-NTX = extended-release naltrexone injectable suspension
Opioid cravings
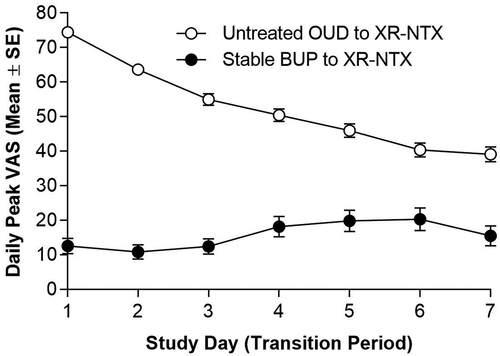
As expected, daily peak cravings VAS scores were generally lower in participants transitioning from Stable BUP compared with participants transitioning from Untreated OUD ( and ).
Figure 3. Opioid cravings during transition. Daily VAS scores were generally lower in participants transitioning from Stable BUP compared with participants transitioning from Untreated OUD. For participants transitioning from Stable BUP, the mean (SE) of daily peak VAS scores increased slightly from 12.6 (2.2) on day 1 to 15.5 (2.9) on day 7. For participants transitioning from Untreated OUD, the mean (SE) of daily peak VAS scores decreased from 74.4 (1.3) on day 1 to 39.1 (2.1) on day 7. BUP = buprenorphine; OUD = opioid use disorder; SE = standard error; VAS = visual analog scale; XR-NTX = extended-release naltrexone injectable suspension
Adverse events during the transition period
Transition to XR-NTX was well tolerated in both studies, with the large majority of TEAEs during days 1 to 7 being mild to moderate in severity. For participants transitioning from Untreated OUD, the TEAEs occurring in ≥5% of the combined treatment groups were diarrhea (5.8%) and anxiety (5.0%). For participants transitioning from Stable BUP, the TEAEs occurring in ≥5% of the combined treatment groups were anxiety (29.7%), insomnia (25.7%), diarrhea (24.8%), nausea (13.9%), abdominal pain upper (12.9%), constipation (8.9%), restlessness (8.9%), headache (7.9%), pain (6.9%), back pain (5.9%), drug withdrawal syndrome (5.0%), dyspepsia (5.0%), fatigue (5.0%), and hypotension (5.0%). The reports of adverse events may have been higher for participants transitioning from Stable BUP because they were in a residential setting, which affords greater opportunity to capture events.
Sensitivity analysis
The temporal patterns of withdrawal, opioid cravings, and TEAEs during the transition period for the placebo group of each study (Supplementary Material) were similar to the combined treatment groups reported here. Given this consistency, we can confirm that the inclusion of oral NTX treatment during the transition period did not confound the main results of this analysis.
Discussion
To our knowledge, this is the first analysis to directly compare temporal patterns of opioid withdrawal from different treatment-seeking populations. In this post hoc, cross-study comparison, distinct patterns of opioid withdrawal were observed depending on whether participants were transitioning from untreated OUD or stable BUP treatment to XR-NTX. Our findings, in combination with a recent study that found that SOWS (compared with COWS) was associated with earlier detection of withdrawal symptoms and prediction of opioid taper completion (Citation7), may help to inform withdrawal management, including ancillary treatments and patient expectations. Findings with direct clinical application include the difference in time of peak withdrawal, suggesting that clinical visits or closer monitoring should occur in the first few days of withdrawal management for patients transitioning from untreated OUD, whereas for patients withdrawing from BUP, it is important to offer closer clinical monitoring later in the course of the transition. Further, the comparatively lower mean SOWS scores observed in the BUP-to-XR-NTX transition may recommend this route of initiation for some patients seeking to begin XR-NTX treatment. Finally, we observed that the SOWS demonstrated greater sensitivity at detecting increases in opioid withdrawal symptoms compared with the COWS, which recommends the use of the SOWS instrument during withdrawal management, particularly in settings in which daily clinic visits are not feasible.
Participants with untreated OUD prior to study entry were more likely to experience an earlier relative peak in opioid withdrawal followed by a gradual decline, whereas participants on stable BUP treatment were more likely to experience a relatively later, though still mild, peak opioid withdrawal. The observed differences in patterns of withdrawal, and the reported adverse events in each trial, likely reflect differences in duration of action of the opioids (short-acting illicit/non-prescribed opioids vs BUP) used by these two patient populations. Of note, the pattern of delayed withdrawal we observed in participants transitioning from Stable BUP to XR-NTX is a novel finding, with direct relevance for clinicians assisting patients who request discontinuation from BUP and initiation of antagonist therapy for the prevention of relapse. In addition, although the intensity of withdrawal was generally similar between the studies, opioid cravings were consistently higher in participants transitioning from untreated OUD, likely related to (i) low baseline craving in participants on stable BUP treatment and (ii) higher likelihood of exposure to opioids and drug stimuli for participants transitioning from untreated OUD.
Withdrawal is a critical manifestation of cessation of opioid use, or opioid agonist/partial agonist use, in individuals with OUD. Type and severity of withdrawal varies widely and can include both somatic (muscle aches, stomach cramps, insomnia, and sweating) and behavioral (anxiety, irritability, agitation, and malaise) symptoms (Citation8), and many patients worry about withdrawal symptoms when considering opioid discontinuation (Citation9). The use of psychosocial support and ancillary medications during transition to XR-NTX is an important component of withdrawal management during a 7- to 10-day transition period (Citation10). In addition, given the observed association between lower levels of craving/withdrawal and remaining opioid-free longer (Citation11), our study highlights the importance of consistently monitoring craving and withdrawal during opioid discontinuation and shows that patients on stable BUP treatment may experience milder withdrawal in the transition to XR-NTX than those transitioning from untreated OUD. However, even though XR-NTX is a recommended treatment following opioid with-drawal (Citation12), there are currently no published guidelines available for induction onto XR-NTX. Recent studies have explored the role of XR-NTX in the management of opioid discontinuation, withdrawal, and relapse (Citation13–15), and future studies are expected to examine the role of treatment setting (outpatient vs residential) for the management of opioid withdrawal, which remains an important (Citation16), although unclarified, issue (Citation17).
This analysis is inherently limited because of its post hoc nature and because it includes two studies with different clinical populations (including type and number of participants), contrasting treatment settings, and different standing ancillary medication regimens. The study lacks information on frequency of use of additional ancillary medications during the transition period. In addition, the patterns of withdrawal reported here occurred in participants receiving standing ancillary medications for the treatment of symptoms and are not representative of untreated individuals undergoing unassisted opioid withdrawal. However, the analysis used data from randomized controlled trials with similar transition regimens and detailed, robust withdrawal assessments. Of note, there has been a recent increase in the use of potent synthetic opioids, such as fentanyl. The degree to which the findings apply to patients using novel synthetic opioids is unknown. Despite the limitations, this analysis describes distinct patterns of opioid withdrawal and may help tailor withdrawal management to the specific experiences of individuals. Further studies and shared clinical experience will confirm (i) whether short-acting opioid use requires robust medication assistance to treat withdrawal and craving early in the transition phase, (ii) whether individuals discontinuing BUP could benefit from intensive monitoring before XR-NTX administration owing to the risk of lingering withdrawal symptoms, and (iii) a possible role for a staged transition to short-term BUP maintenance for certain individuals (for example, patients with high-severity OUD or a history of severe opioid withdrawal) seeking to transition from untreated OUD to XR-NTX.
In conclusion, the findings from our post hoc, cross-study analysis show that, although distinct patterns of opioid withdrawal were observed for two different patient populations using similar XR-NTX induction regimens, the transition procedures were generally well tolerated and effective. Awareness of variations in the patient experience of withdrawal, based on the substances used or the treatments received, may help clinicians anticipate the expected course of symptoms and signs of withdrawal and thereby enable provision of better guidance and support for patients. In addition, our findings may assist with the identification of appropriate and timely paths for XR-NTX administration.
Credit author statement
All authors participated in the interpretation of study results and in the drafting, critical revision, and approval of the final version of the manuscript. PM and ABD were study investigators; SCA, AZ, MAS, and AL were involved in the study design and data analyses. AL conducted the statistical analyses.
Role of the funding source
Alkermes, Inc. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Acknowledgements
Medical writing support was provided by Janelle Keys, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Alkermes, Inc.
Data availability statement
The data collected in this study are proprietary to Alkermes, Inc. Alkermes, Inc. is committed to public sharing of data in accordance with applicable regulations and laws.
Disclosure statement
PM has received consultation fees and grants from Alkermes, Inc. and other pharmaceutical companies. ABD has participated in advisory boards and received grants from Alkermes, Inc. SCA and MAS are employees and shareholders of Alkermes, Inc. AZ and AL are former employees of Alkermes, Inc., and may own stock or options in the company. MAS previously received study medication from Alkermes, Inc. for a NIDA-funded investigation.
Additional information
Funding
References
- ASAM. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14:1–91. doi:https://doi.org/10.1097/ADM.0000000000000633.
- Alkermes Inc. VIVITROL (naltrexone for extended-release injectable suspension) Prescribing Information; 2020.
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641–53. doi:https://doi.org/10.1017/S146114570700836X.
- Bisaga A, Mannelli P, Yu M, Nangia N, Graham CE, Tompkins DA, Kosten TR, Akerman SC, Silverman BL, Sullivan MA. Outpatient transition to extended-release injectable naltrexone for patients with opioid use disorder: a phase 3 randomized trial. Drug Alcohol Depend. 2018;187:171–78. doi:https://doi.org/10.1016/j.drugalcdep.2018.02.023.
- Comer SD, Mannelli P, Alam D, Douaihy A, Nangia N, Akerman SC, Zavod A, Silverman BL, Sullivan MA. Transition of patients with opioid use disorder from buprenorphine to extended-release naltrexone: a randomized clinical trial assessing two transition regimens. Am J Addict. 2020;29:313–22. doi:https://doi.org/10.1111/ajad.13024.
- Cicero TJ, Ellis MS. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues Clin Neurosci. 2017;19:259–69.
- Dunn K, Bergeria C, Huhn AS, Strain EC. Differences in patient-reported and observer-rated opioid withdrawal symptom etiology, time course, and relationship to clinical outcome. Drug Alcohol Depend. 2020;215:108212. doi:https://doi.org/10.1016/j.drugalcdep.2020.108212.
- Pergolizzi JV Jr, Raffa RB, Rosenblatt MH. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: current understanding and approaches to management. J Clin Pharm Ther. 2020;45:892–903. doi:https://doi.org/10.1111/jcpt.13114.
- Stein MD, Conti MT, Herman DS, Anderson BJ, Bailey GL, Noppen DV, Abrantes AM. Worries about discontinuing buprenorphine treatment: scale development and clinical correlates. Am J Addict. 2019;28:270–76. doi:https://doi.org/10.1111/ajad.12884.
- Bisaga A, Mannelli P, Sullivan MA, Vosburg SK, Compton P, Woody GE, Kosten TR. Antagonists in the medical management of opioid use disorders: historical and existing treatment strategies. Am J Addict. 2018;27:177–87. doi:https://doi.org/10.1111/ajad.12711.
- Northrup TF, Stotts AL, Green C, Potter JS, Marino EN, Walker R, Weiss RD, Trivedi M. Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: a discrete survival and growth mixture model. Addict Behav. 2015;41:20–28. doi:https://doi.org/10.1016/j.addbeh.2014.09.021.
- SAMHSA. 2020. TIP 63: medications for opioid use disorder. [accessed 2021 Jan]. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Full-Document/PEP20-02-01-006.
- Mannelli P, Swartz M, Wu LT. Withdrawal severity and early response to treatment in the outpatient transition from opioid use to extended release naltrexone. Am J Addict. 2018;27:471–76. doi:https://doi.org/10.1111/ajad.12763.
- Nunes EV, Gordon M, Friedmann PD, Fishman MJ, Lee JD, Chen DT, Hu MC, Boney TY, Wilson D, O’Brien CP. Relapse to opioid use disorder after inpatient treatment: protective effect of injection naltrexone. J Subst Abuse Treat. 2018;85:49–55. doi:https://doi.org/10.1016/j.jsat.2017.04.016.
- Sullivan MA, Bisaga A, Glass A, Mishlen K, Pavlicova M, Carpenter KM, Mariani JJ, Levin FR, Nunes EV. Opioid use and dropout in patients receiving oral naltrexone with or without single administration of injection naltrexone. Drug Alcohol Depend. 2015;147:122–29. doi:https://doi.org/10.1016/j.drugalcdep.2014.11.028.
- Williams AR, Nunes EV, Bisaga A, Levin FR, Olfson M. Development of a cascade of care for responding to the opioid epidemic. Am J Drug Alcohol Abuse. 2019;45:1–10. doi:https://doi.org/10.1080/00952990.2018.1546862.
- Day E, Ison J, Strang J. Inpatient versus other settings for detoxification for opioid dependence. Cochrane Database Syst Rev. 2005;CD004580. doi:https://doi.org/10.1002/14651858.CD004580.pub2.
