ABSTRACT
Background: Discovery of modifiable factors influencing subjective withdrawal experience might advance opioid use disorder (OUD) research and precision treatment. This study explores one factor – withdrawal catastrophizing – a negative cognitive and emotional orientation toward withdrawal characterized by excessive fear, worry or inability to divert attention from withdrawal symptoms.
Objectives: We define a novel concept – withdrawal catastrophizing – and present an initial evaluation of the Withdrawal Catastrophizing Scale (WCS).
Methods: Prospective observational study (n = 122, 48.7% women). Factor structure (exploratory factor analysis) and internal consistency (Cronbach’s α) were assessed. Predictive validity was tested via correlation between WCS and next-day subjective opiate withdrawal scale (SOWS) severity. The clinical salience of WCS was evaluated by correlation between WCS and withdrawal-motivated behaviors including risk taking, OUD maintenance, OUD treatment delay, history of leaving the hospital against medical advice and buprenorphine-precipitated withdrawal.
Results: WCS was found to have a two-factor structure (distortion and despair), strong internal consistency (α = .901), and predictive validity – Greater withdrawal catastrophizing was associated with next-day SOWS (rs (99) = 0.237, p = .017). Withdrawal catastrophizing was also correlated with risk-taking behavior to relieve withdrawal (rs (119) = 0.357, p < .001); withdrawal-motivated OUD treatment avoidance (rs (119) = 0.421, p < .001), history of leaving the hospital against medical advice (rs (119) = 0.373, p < .001) and buprenorphine-precipitated withdrawal (rs (119) = 0.369, p < .001).
Conclusion: This study provides first evidence of withdrawal catastrophizing as a clinically important phenomenon with implications for the future study and treatment of OUD.
Introduction
Withdrawal is known to play a central role in the neuropathogenesis of opioid addiction (Citation1–4). Individuals with opioid use disorder (OUD) experience cycles of withdrawal which come to drive compulsive opioid use (Citation5). This process is characterized by negative reinforcement. Over time, the removal of withdrawal becomes the primary motive for opioid use (Citation4,Citation6–8). Repeated episodes of withdrawal are believed to gradually sensitize brain-stress-systems – an effect known as hyperkatifeia (Citation9–11). Hyperkatifeia involves the progressive worsening of emotional symptoms of withdrawal. Such negative emotions are also thought to worsen the subjective experience of physical withdrawal symptoms, including pain (Citation12). Indeed, hyperkatifeia and pain are linked in the brain (Citation10,Citation13–17). The originators of this concept have called hyperkatifeia the “emotional corollary of hyperalgesia,” because the neural substrates of pain perception and hyperkatifeia are so significantly overlapping (Citation13,Citation18,Citation19).
Despite extensive literature characterizing the role of withdrawal in addiction neurobiology, less is known about individual difference factors that might modulate subjective withdrawal experience or withdrawal motivated behavior. Prior research has observed substantial between-person variability in withdrawal severity, unaccounted for by pharmacologic or physiologic factors (Citation20–23). Studies of modifiable factors influencing the subjective experience of opioid withdrawal are lacking. One such factor might be “catastrophizing” (Citation24–26).
Catastrophizing describes a tendency to magnify or exaggerate the threat value or severity of aversive stimuli and is associated with excessive fear, worry or an inability to divert attention from such stimuli (Citation27). Catastrophizing has been conceptualized as a “maladaptive cognitive style” involving cognitive distortions that contribute to the development and maintenance of negative affectivity (Citation28). This phenomenon has been well studied in multiple psychopathologies and chronic pain (Citation24,Citation27,Citation29–33). Catastrophizing is related to symptom burden in depression and anxiety and contributes to emotional dysregulation in borderline personality disorder (Citation34–41). In chronic pain, catastrophizing has been linked to a variety of negative clinical outcomes and is a target for psychosocial pain management interventions (Citation42,Citation43). Pain catastrophizing has even been offered as a potential therapeutic target to improve substance use treatment outcomes (Citation26). However, to our knowledge, no prior study has described withdrawal-specific catastrophizing – hereafter referred to as “withdrawal catastrophizing” - or sought to measure withdrawal catastrophizing among individuals with OUD.
Given withdrawal catastrophizing might plausibly modulate withdrawal-related negative reinforcement in OUD, the development of an instrument to measure withdrawal catastrophizing could be of considerable importance (Citation44). Such an instrument might inform new OUD treatments or serve as a control variable in future OUD clinical trials (Citation11). Therefore, the present work intends to fill this gap in the literature. Described is a novel instrument, the Withdrawal Catastrophizing Scale (WCS) adapted from the reference standard psychometric tool for measuring pain catastrophizing – the Pain Catastrophizing Scale (PCS) (Citation45,Citation46). The theoretical basis for adapting a withdrawal catastrophizing instrument from a pain catastrophizing instrument is informed by the overlapping neurobiology of hyperkatifeia and chronic pain. For example, the central nucleus of the amygdala (CeA) – a key structure dysregulated in hyperkatifeia – is also known as the “nociceptive amygdala” for its role in the emotional processing of pain (Citation47).
The present study aimed to introduce the concept of withdrawal catastrophizing and provide initial psychometric evidence for WCS including factor structure, internal consistency, and predictive validity. Additionally, the clinical salience of WCS was evaluated by correlation, with questions pertaining to withdrawal-motivated behaviors and buprenorphine-precipitated withdrawal. Specifically assessed were withdrawal-motivated risk-taking behaviors, OUD maintenance, OUD treatment delay, history of leaving the hospital against medical advice and buprenorphine-precipitated withdrawal.
Materials and methods
This was a prospective observational study with a cross-sectional and a longitudinal component. Approval was obtained prior to study initiation from the Ohio State University Wexner Medical Center (OSUWMC) Institutional Review Board. Participants provided verbal consent and were monetarily compensated.
Participants
A total of 124 participants were recruited between May 8, 2022, and December 16, 2022, from the Ohio State University Wexner Medical Center (OSUWMC) Talbot Hall. Talbot Hall provides comprehensive addiction care, including admission for medically supervised withdrawal. At Talbot Hall, admitted patients receive 24-hour nursing care, group counseling, case management and are evaluated daily by an Addiction Medicine specialist physician. Withdrawal management is protocolized. Per protocol, all patients are offered clonidine, acetaminophen, magnesium hydroxide, promethazine, ondansetron, bisacodyl, dicyclomine, hydroxyzine and melatonin to ease symptoms of withdrawal. Patients may choose to be initiated on buprenorphine/naloxone with a standard dose and frequency of 4/1 mg every 6 hours. Patients may also elect to receive tramadol for myalgia and arthralgia with a tapering schedule as follows: 100 mg every six hours for 12 doses, 100 mg every eight hours for nine doses, 100 mg every twelve hours for 4 doses. Recruitment was conducted by trained staff during a routine admission assessment.
Participants were adults presenting for medically supervised withdrawal from opioids. All participants were diagnosed with OUD at the time of recruitment. OUD was defined by the presence of at least 2 of 11 OUD criteria during the past 12 months as described in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (Citation48). Exclusion criteria included concurrent use of or withdrawal from alcohol, benzodiazepines, or other central nervous system depressants, inability to provide informed consent, read or comprehend survey items. Eight individuals declined participation. Two were excluded for concurrent alcohol withdrawal. The final sample was 122 (n = 122).
Participants completed an initial survey at the time of admission (hospital day 1) which included demographic information, WCS, and seven additional original items. Beginning the following day (hospital day 2) they were asked to rate their withdrawal using the Subjective Opiate Withdrawal Scale (SOWS) once daily for up to four days (hospital days 2–5). Survey responses were collected using REDCap (Citation49). Participants privately accessed the surveys on tablet computers and did not interact with one another or view others’ responses.
Measures
Subjective Opiate Withdrawal Scale (SOWS)
Participants rated the severity of their withdrawal using SOWS (Citation20,Citation50). SOWS is a 16-item self-report instrument (range 0–64) designed to assess the symptoms of opiate withdrawal. Individual items are scored (0) Not at all (1), A little (2), Moderately (3), Quite a bit (4), Extremely. SOWS has previously been shown to have high internal consistency, good construct validity and moderate-to-high test–retest reliability (Citation20,Citation50).
Withdrawal Catastrophizing Scale (WCS)
WCS is a 13-item scale (range 0–52) intended to measure catastrophizing about withdrawal. WCS was adapted from the widely used Pain Catastrophizing Scale (PCS), which has been referred to as the reference standard psychometric tool for measuring pain catastrophizing and is one of the nine core data elements required by the National Institutes of Health (NIH) Helping End Addiction Long-Term (HEAL) Initiative (Citation45,Citation46). The rationale for creating WCS was based on the fact that both pain and withdrawal are aversive conditions, and their temporary relief by opioids may be negatively reinforcing for individuals with OUD (Citation4,Citation8). WCS responses were scaled as not at all (0), to a slight degree (1), to a moderate degree (2), to a great degree (3) all the time (4). WCS is displayed in the results (see ).
Table 1. Sample characteristics.
Table 2. Withdrawal catastrophizing scale (WCS).
Seven original items pertaining to withdrawal-motivated behavior and buprenorphine-precipitated withdrawal
Seven original questions captured withdrawal-related risk-taking behaviors, OUD maintenance, treatment delay, and buprenorphine precipitated withdrawal. These questions were not part of WCS but instead were written in order to evaluate the potential clinical salience of WCS. Items 1–3 asked how frequently participants engaged in risk-taking behaviors to escape opioid withdrawal and were coded on a six-option Likert scale from (0) never, to (5) very often. These items were based on prior research identifying accidental injury, shame, and arrest or incarceration as relevant risks to individuals who use substances (Citation51–56). Item 4 assessed history of leaving the hospital against medical advice due to uncontrolled withdrawal (Citation57). This item was informed by literature suggesting opioid withdrawal might motivate premature patient-directed discharge (Citation58). Items 5 and 6 measured aversion to withdrawal as a motive for continuing opioid use and delaying OUD treatment. These questions were adapted from the Pain-related OUD Exacerbation Scale (PrOUD-ES) (Citation59,Citation60). Item 7 inquired about whether participants had ever experienced buprenorphine precipitated withdrawal. Buprenorphine-precipitated withdrawal is considered a clinically significant barrier to successful initiation of this treatment for OUD (Citation61,Citation62). Items 4–7 were coded strongly disagree (0), disagree (1), neutral (2), agree (3), strongly agree (4). Original items are presented in the results ().
Table 3. Correlations between the withdrawal catastrophizing scale (WCS) and original items.
Data analysis
An exploratory factor analysis (EFA) was run on WCS (n = 122). Although WCS adapted from PCS, the structure of this new scale was uncertain. Therefore, exploratory, not confirmatory, factor analysis was appropriate (Citation63). Common factor analysis was chosen over principal components analysis as the goal of this study was to assess WCS’s latent structure (Citation64). Extraction was achieved by principal axis factoring due to the relative tolerance of multivariate nonnormality of principal axis factoring, and its superior recovery of weak factors (Citation65). Minimum average partial analysis and parallel analysis, complemented by screen plot inspection, determined factor retention (Citation66–69). Promax rotation was selected (Citation70). To optimize the practical (10% variance explained) and the statistical significance (p < .05), salience threshold, or the minimum factor loading to be considered meaningful, was predetermined as 0.32 (Citation71). Also predetermined was a maximum acceptable factor correlation of 0.8. Kaiser–Meyer–Olkin (KMO) was used to evaluate sampling adequacy. Bartlett’s test of sphericity was conducted to ensure that the correlation matrix differed significantly from an identity matrix.
The predictive validity of WCS was evaluated using Spearman’s rank order correlations between WCS (measured at the time of admission) and SOWS (collected on hospital day 2) (Citation72). Due to the large amounts of missing SOWS data on hospital day 3 and beyond, assessment of relationships between WCS and subjective withdrawal experience on subsequent hospital days was not possible (see section Subjective Withdrawal & Opioid Medication Use below for further detail). Spearman’s correlations between the seven original items and WCS assessed the clinical salience of withdrawal catastrophizing. All correlations were two-tailed and deemed significant at α < 0.05. Statistical analyses were performed using SPSS software (Version 28.0, SPSS. Inc).
Results
Sample characteristics
Demographics were obtained from 119 (97.5%) participants. summarizes sample characteristics.
Subjective withdrawal & opioid medication use
Participants were offered SOWS by the attending Addiction Medicine physician once daily during rounds on hospital days two through five (hospital day 2 was the following day after WCS was completed). One hundred and one participants (N = 101) completed SOWS on day 2, 82 on day 3, 44 on day 4, and 11 on day 5. Twenty-one participants completed no SOWS assessments. One participant declined SOWS. The remaining 20 were lost to premature discharge (transfer to acute care hospital for COVID-19, unstable medical comorbidity or left against medical advice). There were no substantive differences between those who did not complete SOWS and those who completed SOWS based on age (completed SOWS Mdn = 35, did not complete SOWS Mdn = 35), U = 1016.0, z = −0.091, p = .928; OUD severity (completed SOWS Mdn = 11, did not complete SOWS Mdn = 11), U = 1025.0, z = −0.435, p = .663 or degree of withdrawal catastrophizing (completed SOWS Mdn = 34.0, did not complete SOWS Mdn = 37.5), U = 917.5, z = −0.971, p = .332. The sample mean, minimum, and maximum day 2 SOWS score was 28.2 (SD = 15.6), 0 and 64, respectively.
Data regarding buprenorphine/naloxone and tramadol use were missing from the 21 participants who completed no SOWS assessments. Of those who completed at least one SOWS (N = 101), 58 (57.4%) participants were recorded to have received buprenorphine/naloxone and 45 (44.6%) were recorded to have received tramadol at any point during admission. Eleven (11, 10.9%) received both tramadol and buprenorphine/naloxone. At the time of the day 2 SOWS assessment, 25 (24.8%) participants were recorded to have received buprenorphine/naloxone and 42 (41.6%) tramadol. The day 2 SOWS scores of participants who received buprenorphine/naloxone did not differ from those who had not received buprenorphine/naloxone at the time SOWS was measured (received buprenorphine/naloxone Mdn = 29, had not received buprenorphine/naloxone SOWS Mdn = 27), U = 907.5, z = −0.335, p = .738. Similarly, day 2 SOWS did not differ by receipt of tramadol (received tramadol Mdn = 24.5, had not received tramadol SOWS Mdn = 30), U = 1142.5, z = −0.665, p = .506. Degree of withdrawal catastrophizing also did not differ between those who did or did not receive buprenorphine/naloxone (received buprenorphine/naloxone WCS Mdn = 34, had not received buprenorphine/naloxone WCS Mdn = 38.5), U = 807.5, z = −1.122, p = .262; or tramadol (received tramadol WCS Mdn = 36.5, had not received tramadol WCS Mdn = 37), U = 1232.5, z = −0.045, p = .964.
Seven original items pertaining to withdrawal-motivated behavior and buprenorphine-precipitated withdrawal
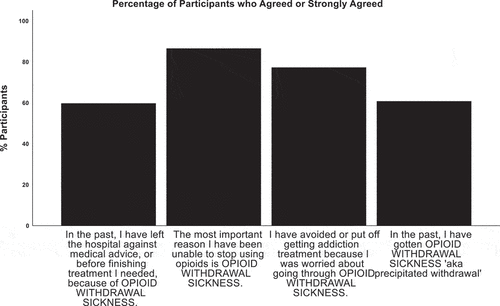
The seven original items had a good internal consistency as determined by a Cronbach’s alpha of 0.78. Responses to items 1–3 revealed variability in the frequency with which participants endorsed engaging in risk-taking behaviors to relieve opioid withdrawal. A small minority of participants reported they never engaged in risk-taking behaviors to relieve withdrawal: 20 (16.5%) never put themselves in danger of being hurt; 16 (13.2%) never did things that made them feel ashamed; and 23 (19.0%) never broke the law. A larger percentage indicated they often or very often risked their safety 45 (36.9%), performed shameful 38 (31.1%), or illegal acts 46 (37.7%) to relieve opioid withdrawal sickness. The majority of participants agreed or strongly agreed with items 4, 5, and 6 indicating that most participants had left the hospital before completing needed medical care due to uncontrolled withdrawal (61, 60.4% of the 101 who had ever been hospitalized); found withdrawal to be the most important reason they had struggled to stop using opioids (105, 86.1%); and had delayed accessing OUD treatment because of opioid withdrawal (93, 76.2%). A majority also reported they had previously experienced buprenorphine-precipitated opioid withdrawal (69, 61.1% of 113 participants who had tried buprenorphine). Agreement with items 4–7 is illustrated by .
Psychometric analysis of WCS
All 122 participants (100%) completed WCS. Responses to WCS were non-normally distributed per Shapiro–Wilk test (p = .006) and skewed toward greater withdrawal catastrophizing −.276 (standard error = .219). The median, minimum and maximum total WCS scores were 35.5, 11.0 and 52.0, respectively. Total WCS scores reflected prevalent catastrophizing about opioid withdrawal in this sample of individuals with OUD. Nearly 70% (85, 69.7%) scored 30 or above on WCS – the threshold for clinically significant catastrophizing on PCS, the measure on which WCS was based (Citation73).
Factor structure and internal consistency
Exploratory factor analysis (EFA) was run on the 13 item WCS (n = 122). EFA appropriateness was assessed prior to extraction. A correlation matrix showed all 13 variables had correlation coefficients greater than 0.3. Kaiser–Meyer–Olkin (KMO) was meritorious (0.87) for WCS as a whole and individual questionnaire item KMO measures were all greater than 0.8. Bartlett’s test of sphericity indicated that the correlation matrix differed significantly from an identity matrix (p < .001). Due to multivariate non-normality, extraction was achieved via principal axis factoring.
EFA revealed two factors with an eigenvalue greater than one, and a third factor with an eigenvalue very near to one (0.95). Potential factors one, two and three explained 46.52%, 10.91% and 7.31% of the total variance, respectively. Parallel analysis showed that the observed eigenvalue of factor 1 (5.59) and factor 2 (0.99) exceeded the 95th percentile mean eigenvalue of factor 1 (0.87) and factor 2 (0.67) obtained from generating 1000 permutations of the original data with preserved distributions (Citation67). However, the observed eigenvalue of potential factor 3 (0.48) was less than the 95th percentile mean eigenvalue (0.52) in this parallel analysis. Therefore, parallel analysis supported retention of two factors. The minimum average partial analysis revealed that the smallest mean squared partial correlation was 0.04 and the smallest mean 4th power partial correlation was 0.003, which corresponded to a two-factor solution. Finally, visual inspection of the scree plot also indicated that two factors should be retained.
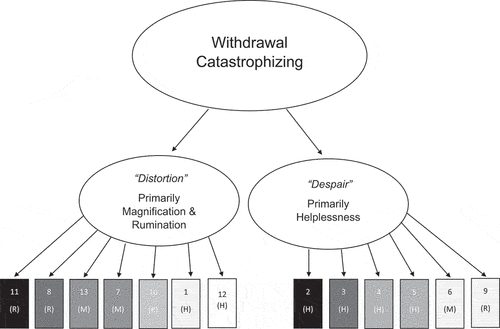
The two-factor solution explained 57.4% of the variance and exhibited a largely simple structure. Cross-loading above the predetermined threshold of salience (.32) was noted on item 6 only. Item 6 loaded slightly higher on factor 2 (hereafter despair). Because of its slightly higher loading and apparent thematic relationship to the other items in factor 2 (despair), item 6 was considered to be a part of factor 2 (despair). As a whole, WCS exhibited a high level of internal consistency with Cronbach’s alpha of .901. Factor 1 (hereafter referred to as distortion) consisted of 7 items primarily reflecting rumination and magnification and had a Cronbach’s alpha of .862. Factor 2 (despair) contained 6 items mostly reflecting helplessness and was found to have a Cronbach’s alpha of .854. Distortion and despair were well correlated, but this relationship did not exceed the predetermined value of 0.8 (rs (119) = 0.747, p < .001) WCS factor loadings, communalities and item means are shown in . is a path diagram illustrating the two-factor solution.
Figure 2. Path diagram illustrating the two-factor solution of the withdrawal catastrophizing scale (WCS) obtained from exploratory factor analysis. Rectangles representing the 13 items of WCS are shaded to represent the strength of their rotated loading on their associated factor (darker = stronger). Items are also labeled magnification (M), rumination (R), or helplessness (H) based on the schema of the pain catastrophizing scale (PCS) from which WCS is derived.
Predictive validity
Spearman’s rank-order correlation was used to assess a hypothesized relationship between baseline withdrawal catastrophizing (WCS measured at the time of admission on hospital day 1) and subjective withdrawal severity (SOWS collected during rounds on hospital day 2). Cases with missing SOWS data were excluded from analysis. The assumption of monotonicity was confirmed by scatterplot inspection. WCS was significantly associated with total SOWS (rs (99) = 0.237, p = .017). In addition to WCS as a whole, WCS factors 1 and 2 were also assessed for predictive validity. WCS factor 1 (distortion) was not significantly correlated with SOWS (rs (99) = 0.192, p = .055). WCS factor 2 (despair) was correlated with SOWS (rs (99) = 0.247, p = .013). Finally, SOWS item 16 “I feel like using now” was correlated with WCS (rs (99) = 0.299, p = .002), factor 1 (distortion) (rs (99) = 0.225, p = .024) and factor 2 (despair) (rs (99) = 0.309, p = .002).
Clinical salience of withdrawal catastrophizing
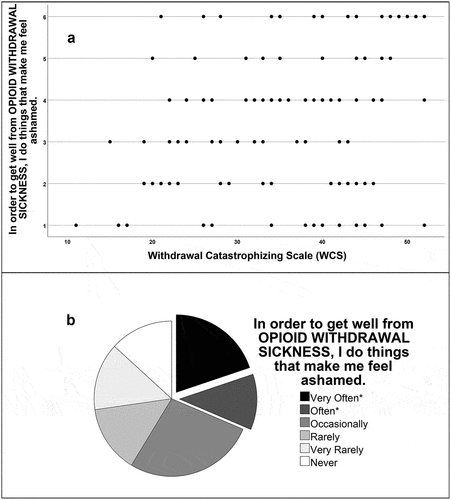
Clinical salience was assessed by Spearman’s rank-order correlations between WCS and a seven-item battery of original questions () regarding withdrawal motivated risk-taking behaviors (items 1–4), withdrawal motivated OUD maintenance and treatment delay (items 5–6) and self-report of prior buprenorphine precipitated withdrawal (item 7). WCS and its two factors were significantly correlated with all original items. presents a) a scatterplot of the relationship between risk-taking (shameful acts) and WCS and b) a pie chart of how often participants resorted to performing shameful acts to relieve withdrawal. lists the original items and presents the correlation coefficients of this analysis.
Figure 3. (a) Scatterplot of the relationship between risk-taking (shame) and WCS, and (b) pie chart representing how often participants found themselves doing things that made them feel ashamed to relieve withdrawal. Asterisks (*) in the key correspond to the two exploded pie pieces in the pie chart.
Discussion
This study introduces a novel concept to addiction science - withdrawal catastrophizing – which we define as a negative cognitive and emotional orientation toward withdrawal characterized by excessive fear, worry or inability to divert attention from withdrawal symptoms. We present WCS, an instrument we have adapted to measure withdrawal catastrophizing. WCS exhibited promising psychometric properties including a two-factor solution, high internal consistency, and predictive validity. Greater withdrawal catastrophizing was associated with more severe next-day withdrawal by self-report. Withdrawal catastrophizing was also related to a history of buprenorphine-precipitated withdrawal and leaving the hospital against medical advice due to uncontrolled withdrawal. Finally, withdrawal catastrophizing was associated with greater agreement among participants that withdrawal had motivated them to continue using opioids; avoid OUD treatment and perform acts that were dangerous, illegal, or made them feel ashamed. Together, these early exploratory findings suggest withdrawal catastrophizing might be an important, previously unappreciated individual difference factor related to withdrawal experience and withdrawal-motivated behavior among individuals with OUD.
Withdrawal catastrophizing appeared common and consequential. Nearly 70% of the participants scored 30 or above on WCS. While further studies are needed to determine meaningful cut scores for WCS, 30 is considered to be the threshold for clinically significant catastrophizing on WCS’s parent measure, PCS (Citation19). A plurality of participants reported they were often or very often motivated by withdrawal to do things that were physically dangerous, illegal or made them feel ashamed. Accidental injury, incarceration and diminished self-esteem are known detrimental consequences of OUD (Citation74–79). Sixty percent of participants who had previously been hospitalized indicated they had left the hospital against medical advice because of opioid withdrawal. Leaving the hospital against medical advice exposes patients to an increased risk of adverse outcomes, including death (Citation80,Citation81). Withdrawal catastrophizing was robustly correlated with all four of these high-risk withdrawal-motivated behaviors, suggesting withdrawal catastrophizing might possibly have an unrecognized relationship to some of the worst consequences of OUD.
Withdrawal catastrophizing was also correlated with treatment avoidance. Seventy-six percent of participants agreed or strongly agreed they had avoided or delayed OUD treatment for fear of going through opioid withdrawal. In 2020, only 11.2% of the 2.5 million adolescents and adults living with OUD in the United States received medication treatment (buprenorphine, methadone, or naltrexone) (Citation82). Given the increasing pervasiveness of illicitly manufactured fentanyl and associated loss of life, treatment delay may have lethal consequences for individuals with OUD (Citation83–86). Additional studies are needed to determine if interventions targeting withdrawal catastrophizing might improve OUD treatment engagement.
Over 60% of participants who had previously tried buprenorphine reported having experienced buprenorphine-precipitated withdrawal. Buprenorphine-precipitated withdrawal is considered a significant barrier to buprenorphine treatment and initiation protocols intended to circumvent this barrier are an area of active inquiry (Citation61,Citation62). This inquiry has been based on the premise that buprenorphine-precipitated withdrawal is a pharmacological problem accounted for by the partial opioid agonism of buprenorphine and exacerbated by the lipophilicity of fentanyl (Citation87). However, our finding that withdrawal catastrophizing was correlated with buprenorphine-precipitated withdrawal strongly suggests psychological involvement. To our knowledge, no prior research has controlled for withdrawal catastrophizing when investigating withdrawal treatments or medications for OUD like buprenorphine. WCS might one day be considered a useful control variable in clinical trials of emerging treatments for opioid withdrawal and OUD (Citation88). Additional research is needed to determine if WCS might possibly predict treatment response or assist with mechanism identification (i.e. hyperkatifeia).
WCS may also provide insight into the withdrawal/negative affect stage of the brain disease model of addiction. Eighty-six percent of participants agreed or strongly agreed withdrawal was the most important reason they kept using opioids. WCS was correlated with agreement with this statement, suggesting withdrawal catastrophizing might relate to the refractoriness of OUD as a chronic health condition. Our findings are consistent with the observations of Koob in describing hyperkatifeia and the withdrawal/negative affect stage of addiction (Citation4,Citation8,Citation9,Citation11,Citation89). We build upon this literature by demonstrating a relationship between withdrawal catastrophizing and withdrawal as a key motive for continued opioid use in OUD. As a measure of emotional orientation to withdrawal, WCS might aid community and epidemiological studies of hyperkatifeia, a recently identified research area of high interest to the NIH National Institute on Alcohol Abuse and Alcoholism (Citation19).
Study strengths included adequate sample size and low levels of missing data on the day 1 survey (WCS, demographics). There were also notable limitations. Approximately 17% of day 2 SOWS responses were missing, requiring those cases to be removed from the predictive validity analysis. However, participants excluded from predictive validity analysis were not different from included participants on age, OUD severity or degree of withdrawal catastrophizing. Missing data precluded assessment of predictive validity on day-three and beyond. SOWS was collected once daily during physician rounds. We did not control for the timing of WCS in relation to last use of opioids or other substances or medications. Collection of SOWS by ecological momentary assessment might have yielded more complete data. Additional research is needed to further establish the reliability and validity of WCS.
The present study defined an original concept withdrawal catastrophizing – a negative cognitive and emotional orientation toward withdrawal characterized by excessive fear, worry or inability to divert attention from withdrawal symptoms – and proposed a new scale (WCS) to measure withdrawal catastrophizing among individuals with OUD. WCS exhibited promising initial psychometric properties including: a two-factor solution, internal consistency and predictive validity. Withdrawal catastrophizing was associated with withdrawal-motivated risk-taking, including the performance of dangerous, illegal, or shameful acts to relieve withdrawal and leaving the hospital against medical advice. Withdrawal catastrophizing was also related to next-day withdrawal severity, history of buprenorphine-precipitated withdrawal and OUD treatment avoidance. Taken together, these findings provide first evidence of withdrawal catastrophizing as a clinically important phenomenon with implications for the future study of hyperkatifeia and withdrawal-motivated behavior in OUD.
Acknowledgments
The authors would like to thank Kirk Carruthers, MD; Bradley Lander, PhD; Mamie Martin, RN, MSN, APRN-CNP, DNP; Leah Brown, MS, MPH, APRN-CNP; Jessica Belser, MSW, MS, PMHNP-BC, CARN-NP and Megan Ackley APRN-CNP for their support in conducting this study.
Disclosure statement
Dr. Hall provided expert opinion regarding the opioid overdose crisis to Lumanity and Emergent BioSolutions.
Additional information
Funding
References
- Volkow ND, Koob GF, McLellan AT, Longo DL. Neurobiologic advances from the brain disease model of addiction. N Engl J Med. 2016;374:363–71. doi:10.1056/NEJMra1511480.
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. doi:10.1016/S2215-0366(16)00104-8.
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi:10.1038/npp.2009.110.
- Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44–53. doi:10.1016/j.biopsych.2019.05.023.
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62. doi:10.1038/npp.2013.261.
- George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacol (Berl). 2014;231:3911–17. doi:10.1007/s00213-014-3623-1.
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–900 . doi:10.1523/JNEUROSCI.0740-06.2006.
- Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–63. doi:10.1016/j.conb.2013.03.011.
- Koob GF. Anhedonia, hyperkatifeia, and negative reinforcement in substance use disorders. In: Pizzagalli D, editor. Anhedonia: preclinical, translational, and clinical integration [internet]. Cham: Springer International Publishing; 2022 [accessed 2022 Dec 8]. p. 147–65. (Current Topics in Behavioral Neurosciences; vol. 58). doi:10.1007/7854_2021_288.
- Koob GF, Powell P, White A. Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID-19. Am J Psychiatry [Internet]. 2020 Nov 1 [accessed 2022 Sep 28];177:1031–37. doi:10.1176/appi.ajp.2020.20091375.
- Koob GF, Dantzer R. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev. 2021;73:163–201. doi:10.1124/pharmrev.120.000083.
- Carcoba LM, Contreras AE, Cepeda-Benito A, Meagher MW. Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. J Addict Dis [Internet]. 2011 Jul 1 [accessed 2023 Aug 5];30:258–70. doi:10.1080/10550887.2011.581985.
- Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med [Internet]. 2010 Jul [accessed 2022 Dec 8];11:1092–98. doi:10.1111/j.1526-4637.2010.00881.x.
- Case A, Hansen E, Goforth H, Koob G. The dark side of opioids: the neurobiological cycle of pain, opioids, tapering, hyperkatifeia, and despair. A conversation with George Koob, NIH addictions director (VF201). J Pain Symptom Manage [Internet]. 2022 May [accessed 2022 Dec 8];63:840–41. doi:10.1016/j.jpainsymman.2022.02.317.
- Hall OT, Entrup P, King A, Vilensky M, Bryan CJ, Teater J, Niedermier J, Kaplan CM, Turner JA, Gorka S, et al. Central sensitization in alcohol use disorder: correlates of pain, addiction and health-related quality of life. J Addict Dis [Internet]. 2023 Jul 22 [accessed 2023 Jul 22]:1–12. doi:10.1080/10550887.2023.2237396.
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S. Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav. 2013;112:34–41.
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev [Internet]. 2012 Nov [accessed 2022 Dec 8];36:2179–92. doi:10.1016/j.neubiorev.2012.07.010.
- Koob GF, Dantzer R. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Dantzer R, editor. Pharmacol Rev [Internet]. 2021 Jan 1 [accessed 2022 Dec 23];73:163–201. doi:10.1124/pharmrev.120.000083.
- Patterson JT, Koob GF, Anderson RI. Understanding hyperkatifeia to inform treatment for alcohol use disorder: an assessment of the National Institute on Alcohol Abuse and Alcoholism Research Portfolio.Biol Psychiatry [Internet]. 2022 Jun 15 [accessed 2022 Dec 23];91:e53–e9. doi:10.1016/j.biopsych.2022.02.011.
- Dijkstra BAG, Krabbe PFM, Riezebos TGM, van der Staak CPF, de Jong CAJ. Psychometric evaluation of the dutch version of the Subjective Opiate Withdrawal Scale (SOWS). Eur Addict Res [Internet]. 2007 [accessed 2023 Feb 6];13:81–88. doi:10.1159/000097937.
- Dunn KE, Saulsgiver KA, Miller ME, Nuzzo PA, Sigmon SC. Characterizing opioid withdrawal during double-blind buprenorphine detoxification. Drug Alcohol Depend. 2015;151:47–55. doi:10.1016/j.drugalcdep.2015.02.033.
- Lopatko OV, White JM, Huber A, Ling W. Opioid effects and opioid withdrawal during a 24 h dosing interval in patients maintained on buprenorphine. Drug Alcohol Depend [Internet]. 2003 Apr 1 [accessed 2023 Mar 3];69:317–22. doi:10.1016/S0376-8716(02)00322-8.
- Cohen AJ, Klett CJ, Ling W. Patient perspectives of opiate withdrawal. Drug Alcohol Depend. 1983;12:167–72. doi:10.1016/0376-8716(83)90041-8.
- Martel MO, Jamison RN, Wasan AD, Edwards RR. The association between catastrophizing and craving in patients with chronic pain prescribed opioid therapy: a preliminary analysis. Pain Med. 2014;15:1757–64. doi:10.1111/pme.12416.
- Martel MO, Wasan AD, Jamison RN, Edwards R. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug Alcohol Depend. 2013;132:335–41. doi:10.1016/j.drugalcdep.2013.02.034.
- Kneeland ET, Griffin ML, Taghian N, Weiss RD, McHugh RK. Associations between pain catastrophizing and clinical characteristics in adults with substance use disorders and co-occurring chronic pain. Am J Drug Alcohol Abuse. 2019;45:488–94. doi:10.1080/00952990.2019.1581793.
- Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524. doi:10.1037/1040-3590.7.4.524.
- Tapia MAS, Ponce EEO. Catastrophizing-quality of life and pain. Int J Recent Adv Multidiscip Res. 2020;7:5687–89.
- Van Loey NE, Klein‐König I, De Jong AEE, Hofland HWC, Vandermeulen E, Engelhard IM. Catastrophizing, pain and traumatic stress symptoms following burns: a prospective study. Eur J Pain. 2018;22:1151–59. doi:10.1002/ejp.1203.
- Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi:10.1016/j.pain.2005.11.008.
- Keogh E, Asmundson GJ. Negative affectivity, catastrophizing, and anxiety sensitivity. Underst Treat Fear Pain. 2004;91:115.
- Hazlett-Stevens H, Craske MG. The catastrophizing worry process in generalized anxiety disorder: a preliminary investigation of an analog population. Behav Cogn Psychother. 2003;31:387–401. doi:10.1017/S1352465803004016.
- Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain. 2005;21:439–45. doi:10.1097/01.ajp.0000135236.12705.2d.
- Özdel K, Taymur I, Guriz SO, Tulaci RG, Kuru E, Turkcapar MH, Seedat S. Measuring cognitive errors using the cognitive distortions scale (CDS): psychometric properties in clinical and non-clinical samples. PLoS ONE [Internet]. 2014 Aug 29 [accessed 2023 Apr 24];9:e105956. doi:10.1371/journal.pone.0105956.
- Mason CK, Mullins-Sweatt SN. Catastrophizing, negative affectivity, and borderline personality disorder. Personal Ment Health [Internet]. 2021 [accessed 2023 Apr 24];15:283–92. doi:10.1002/pmh.1520.
- Garnefski N, Kraaij V, Spinhoven P. Negative life events, cognitive emotion regulation and emotional problems. Personal Individ Differ [Internet]. 2001 Jun 1 [accessed 2023 Apr 24];30:1311–27. doi:10.1016/S0191-8869(00)00113-6.
- Garnefski N, Boon S, Kraaij V. Relationships between cognitive strategies of adolescents and depressive symptomatology across different types of life event. J Youth Adolesc [Internet]. 2003 Dec 1 [accessed 2023 Apr 24];32:401–08. doi:10.1023/A:1025994200559.
- Garnefski N, Kraaij V, van Etten M. Specificity of relations between adolescents’ cognitive emotion regulation strategies and internalizing and externalizing psychopathology. J Adolesc [Internet]. 2005 Oct 1 [accessed 2023 Apr 24];28:619–31. doi:10.1016/j.adolescence.2004.12.009.
- Garnefski N, Legerstee J, Kraaij V, Van den Kommer T, Teerds J. Cognitive coping strategies and symptoms of depression and anxiety: a comparison between adolescents and adults. J Adolesc [Internet]. 2002 Dec 1 [accessed 2023 Apr 24];25:603–11. doi:10.1006/jado.2002.0507.
- Garnefski N, Teerds J, Kraaij V, Legerstee J, van den Kommer T. Cognitive emotion regulation strategies and depressive symptoms: differences between males and females. Personal Individ Differ [Internet]. 2004 Jan 1 [accessed 2023 Apr 24];36:267–76. doi:10.1016/S0191-8869(03)00083-7.
- Garnefski N, Van Den Kommer T, Kraaij V, Teerds J, Legerstee J, Onstein E. The relationship between cognitive emotion regulation strategies and emotional problems: comparison between a clinical and a non‐clinical sample. Eur J Personal [Internet]. 2002 Sep 1 [accessed 2023 Apr 24];16:403–20. doi:10.1002/per.458.
- Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother [Internet]. 2009 May 1 [accessed 2023 Mar 3];9:745–58. doi:10.1586/ern.09.34.
- Schütze R, Rees C, Smith A, Slater H, Campbell JM, O’Sullivan P. How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. J Pain [Internet]. 2018 Mar 1 [accessed 2023 Mar 3];19:233–56. doi:10.1016/j.jpain.2017.09.010.
- Enos G. Koob: understanding negative reinforcers of opioids could uncover new treatments. Alcohol Drug Abuse Wkly. 2019;31:1–7. doi:10.1002/adaw.32467.
- Leung L. Pain catastrophizing: an updated review. Indian J Psychol Med. 2012;34:204–17. doi:10.4103/0253-7176.106012.
- Wheeler CH, Williams ADC, Morley SJ. Meta-analysis of the psychometric properties of the pain catastrophizing scale and associations with participant characteristics. Pain. 2019;160:1946–53. doi:10.1097/j.pain.0000000000001494.
- Neugebauer V, Li W, Bird GC, Han JS. The Amygdala and Persistent pain. Neuroscientist [Internet]. 2004 Jun 1 [accessed 2023 Aug 5];10:221–34. doi:10.1177/1073858403261077.
- American Psychiatric Association D, Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. 2013. Washington: American Psychiatric Association Publishing.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi:10.3109/00952998709001515.
- Pinedo González R, Palacios Picos A, de la Iglesia Gutiérrez M. “Surviving the violence, humiliation, and loneliness means getting high”: violence, loneliness, and Health of female sex workers. J Interpers Violence [Internet]. 2021 May 1 [accessed 2023 Apr 22];36:4593–614. doi:10.1177/0886260518789904.
- Warf CW, Clark LF, Desai M, Rabinovitz SJ, Agahi G, Calvo R, Hoffmann J. Coming of age on the streets: survival sex among homeless young women in Hollywood. J Adolesc [Internet]. 2013 Dec 1 [accessed 2023 Mar 8];36:1205–13. doi:10.1016/j.adolescence.2013.08.013.
- Syvertsen JL, Agot K, Ohaga S, Bazzi AR. You can’t do this job when you are sober: heroin use among female sex workers and the need for comprehensive drug treatment programming in Kenya. Drug Alcohol Depend [Internet]. 2019 Jan 1 [accessed 2023 Apr 22];194:495–99. doi:10.1016/j.drugalcdep.2018.10.019.
- Sloss CM, Harper GW. When street sex workers are mothers. Arch Sex Behav. 2004;33:329–41. doi:10.1023/B:ASEB.0000028886.95568.2b.
- Bennett T, Holloway K, Farrington D. The statistical association between drug misuse and crime: a meta-analysis. Aggress Violent Behav [Internet]. 2008 Mar 1 [accessed 2023 Apr 22];13:107–18. doi:10.1016/j.avb.2008.02.001.
- Stenbacka M, Leifman A, Romelsjö A. Mortality and cause of death among 1705 illicit drug users: a 37 year follow up. Drug Alcohol Rev [Internet]. 2010 [accessed 2023 Apr 22];29:21–27. doi:10.1111/j.1465-3362.2009.00075.x.
- Nolan NS, Marks LR, Liang SY, Durkin MJ. Medications for opioid use disorder associated with less against medical advice discharge among persons who inject drugs hospitalized with an invasive infection. J Addict Med [Internet]. 2021 Apr 1 [accessed 2023 Apr 22];15:155–58. doi:10.1097/ADM.0000000000000725.
- Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abuse [Internet]. 2020 Oct 1 [accessed 2023 Apr 22];41:519–25. doi:10.1080/08897077.2019.1671942.
- Hall OT, Teater J, Entrup P, Deaner M, Bryan C, Harte SE, Kaplan CM, Phan KL, Clauw DJ. Fibromyalgia predicts increased odds of pain-related addiction exacerbation among individuals with pain and opioid use disorder. Pain. 2022;164:1801–09. doi:10.1097/j.pain.0000000000002878.
- Hall OT, Teater J, Rood K, Phan KL, Clauw DJ. Central sensitization in opioid use disorder: a novel application of the 2011 American College of Rheumatology Fibromyalgia survey. PAIN Reports; 2022.
- Spadaro A, Long B, Koyfman A, Perrone J. Buprenorphine precipitated opioid withdrawal: prevention and management in the ED setting. Am J Emerg Med. 2022;58:22–26. doi:10.1016/j.ajem.2022.05.013.
- Greenwald MK, Herring AA, Perrone J, Nelson LS, Azar P. A neuropharmacological model to explain buprenorphine induction challenges. Ann Emerg Med. 2022;80:509–524. doi:10.1016/j.annemergmed.2022.05.032.
- Flora DB. Statistical methods for the social and behavioural sciences: a model-based approach. Stat Methods Soc Behav Sci. 2017;1–472.
- Widaman KF. On common factor and principal component representations of data: implications for theory and for confirmatory replications. Struct Equ Model Multidiscip J. 2018;25:829–47. doi:10.1080/10705511.2018.1478730.
- Briggs NE, MacCallum RC. Recovery of weak common factors by maximum likelihood and ordinary least squares estimation. Multivar Behav Res. 2003;38:25–56. doi:10.1207/S15327906MBR3801_2.
- Velicer WF. Determining the number of components from the matrix of partial correlations. Psychometrika. 1976;41:321–27. doi:10.1007/BF02293557.
- O’connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer’s MAP test. Behav Res Methods Instrum Comput. 2000;32:396–402. doi:10.3758/BF03200807.
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–85. doi:10.1007/BF02289447.
- Cattell RB. The scree test for the number of factors. Multivar Behav Res. 1966;1:245–76. doi:10.1207/s15327906mbr0102_10.
- Tataryn DJ, Wood JM, Gorsuch RL. Setting the value of k in promax: a monte carlo study. Educ Psychol Meas. 1999;59:384–91. doi:10.1177/00131649921969938.
- Norman GR, Streiner DL. Biostatistics: the bare essentials. New Haven: PMPH USA (BC Decker); 2008.
- Streiner DL, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use. USA: Oxford University Press; 2015.
- Sullivan MJ. The pain catastrophizing scale: user manual. Montr McGill Univ. 2009;1:36.
- Larney S, Bohnert ASB, Ganoczy D, Ilgen MA, Hickman M, Blow FC, Degenhardt L. Mortality among older adults with opioid use disorders in the Veteran’s Health Administration, 2000–2011. Drug Alcohol Depend [Internet]. 2015 Feb 1 [accessed 2023 Mar 3];147:32–37. doi:10.1016/j.drugalcdep.2014.12.019.
- Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, Bruneau J, Altice FL, Henderson G, Rahimi-Movaghar A, et al. Global patterns of opioid use and dependence: population harms, interventions, and future action. Lancet Lond Engl [Internet]. 2019 Oct 26 [accessed 2023 Mar 3];394:1560–79. doi:10.1016/S0140-6736(19)32229-9.
- Blatt SJ, Rounsaville B, Eyre SL, Wilber C. The psychodynamics of opiate addiction. J Nerv Ment Dis. 1984;172:342–52. doi:10.1097/00005053-198406000-00005.
- Manganiello JA. Opiate addiction: a study identifying three systematically related psychological correlates. Int J Addict. 1978;13:839–47. doi:10.3109/10826087809039307.
- Nosrati E, Kang-Brown J, Ash M, McKee M, Marmot M, King LP. Economic decline, incarceration, and mortality from drug use disorders in the USA between 1983 and 2014: an observational analysis. Lancet Public Health. 2019;4:e326–e33. doi:10.1016/S2468-2667(19)30104-5.
- Sugarman OK, Bachhuber MA, Wennerstrom A, Bruno T, Springgate BF. Interventions for incarcerated adults with opioid use disorder in the United States: a systematic review with a focus on social determinants of health. PLoS ONE [Internet]. 2020 Jan 21 [accessed 2023 Mar 3];15:e0227968. doi:10.1371/journal.pone.0227968.
- Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health [Internet]. 2015 Dec [accessed 2023 Feb 20];105:e53–e9. doi:10.2105/AJPH.2015.302885.
- Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125:594–602. doi:10.1016/j.amjmed.2011.12.017.
- Abuse S. Key substance use and mental health indicators in the United States: results from the 2020 national survey on drug use and health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56); 2021.
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. Morb Mortal Wkly Rep [Internet]. 2021 Dec 17 [accessed 2023 Mar 3];70:1740–46. doi:10.15585/mmwr.mm7050e3.
- Hall OT, Hall OE, McGrath RP, Haile ZT. Years of life lost due to opioid overdose in Ohio: temporal and geographic patterns of excess mortality. J Addict Med [Internet]. 2020 Apr [accessed 2022 Jul 17];14:156–62. doi:10.1097/ADM.0000000000000554.
- Hall OE, Trent Hall O, Eadie JL, Teater J, Gay J, Kim M, Cauchon D, Noonan RK. Street-drug lethality index: a novel methodology for predicting unintentional drug overdose fatalities in population research. Drug Alcohol Depend [Internet]. 2021 Apr 1 [accessed 2022 Jul 17];221:108637. doi:10.1016/j.drugalcdep.2021.108637.
- Garcia S, Teater J, Trimble C, Entrup P, Hall OE, Hall OT. Years of life lost due to unintentional drug overdose relative to the leading underlying causes of death in the United States: a comparative analysis of excess mortality 2017–2019. J Addict Dis [Internet]. 2023 Mar 6 [accessed 2023 Mar 6];1–5. doi:10.1080/10550887.2023.2173929.
- Antoine D, Huhn AS, Strain EC, Turner G, Jardot J, Hammond AS, Dunn KE. Method for successfully inducting individuals who use illicit fentanyl onto buprenorphine/naloxone. Am J Addict. 2021;30:83–87. doi:10.1111/ajad.13069.
- NIH HEAL initiative [Internet]. Common Data Elements (CDEs) Program; 2021 [accessed 2023 Mar 3]. https://www.heal.nih.gov/data/common-data-elements
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56:18–31. doi:10.1016/j.neuropharm.2008.07.043.