ABSTRACT
Background: Medications for opioid use disorder (MOUD) reduce risks for overdose among correctional populations. Among other barriers, daily dosing requirements hinder treatment continuity post-release. Extended-release buprenorphine (XR-BUP) may therefore be beneficial. However, limited evidence exists.
Objectives: To conduct a systematic review examining the feasibility and effectiveness of XR-BUP among correctional populations.
Methods: Searches were carried out in Pubmed, Embase, and PsychINFO in October 2023. Ten studies reporting on feasibility or effectiveness of XR-BUP were included, representing n = 819 total individuals (81.6% male). Data were extracted and narratively reported under the following main outcomes: 1) Feasibility; 2) Effectiveness; and 3) Barriers and Facilitators.
Results: Studies were heterogeneous. Correctional populations were two times readier to try XR-BUP compared to non-correctional populations. XR-BUP was feasible and safe, with no diversion, overdoses, or deaths; several negative side effects were reported. Compared to other MOUD, XR-BUP significantly reduced drug use, resulted in similar or higher treatment retention rates, fewer re-incarcerations, and was cost-beneficial, with a lower overall monthly/yearly cost. Barriers to XR-BUP, such as side effects and a fear of needles, as well as facilitators, such as a lowered risk of opioid relapse, were also identified.
Conclusion: XR-BUP appears to be a feasible and potentially effective alternative treatment option for correctional populations with OUD. XR-BUP may reduce community release-related risks, such as opioid use and overdose risk, as well as barriers to treatment retention. Efforts to expand access to and uptake of XR-BUP among correctional populations are warranted.
Introduction
Opioid use disorder (OUD) is highly prevalent among correctional populations, although recent estimates are scarce or imprecise. In North America, between 80% and 90% of samples of incarcerated men in United States (US) county jails had a substance use disorder (Citation1), and over two-thirds (73%) had an OUD, with nearly half (42%) being classified on the severe end of the spectrum (Citation2). In Canada, available information indicates a dramatic increase in the proportion of individuals with OUD who were involved in British Columbia’s provincial correctional system between 2009 and 2017 (from 11% to 26%) (Citation3). Extant prevalence data also predate the recent escalation of the North American opioid overdose crisis where rates of OUD and related harms have been steadily increasing (Citation3,Citation4).
Clinical guidelines recommend pharmacological management of OUD with medications for OUD (MOUD), which include both opioid agonist and antagonist treatments (Citation5). However, the availability of specific MOUD medications varies, depending on which formulations have been approved for the clinical management of OUD by national health regulatory organizations (Citation6). For instance, in Canada and the US, the opioid agonists buprenorphine/naloxone and methadone have traditionally been considered gold-standard MOUD formulations (Citation7,Citation8). The US has also approved and utilizes the opioid antagonist naltrexone (Citation8).
Providing MOUD to correctional populations is particularly important and beneficial given that they face a significantly increased risk of drug-related death upon release from incarceration (Citation9,Citation10). For instance, a seminal, albeit outdated, US study found that the risk of drug overdose death within the first two weeks of release from incarceration was 129 times that of the general public (Citation11). In the US (Citation12) and Canada (Citation13,Citation14), opioid overdose is a leading cause of both fatal and non-fatal overdoses post-release. However, screening for OUD and the provision of MOUD among correctional populations is rare, although there are large variations by country, state/province and county/community (Citation15–17). For instance, several US-based studies suggest that MOUD provision is severely limited and processes vary considerably (Citation4,Citation15,Citation18,Citation19). This is detrimental considering MOUD is associated with reductions in cravings and withdrawal, as well as illicit drug use and overdoses among correctional populations with OUD (Citation9,Citation20). Recent US data suggest that prerelease MOUD provision is associated with an 80% reduction in overdose mortality risk within the first month post-release (Citation21,Citation22).
Extant research has thus highlighted many benefits of providing MOUD to incarcerated individuals (Citation23,Citation24), but has also emphasized a number of barriers that hinder treatment engagement and continuity (Citation25,Citation26). For example, stigma associated with opioid use and MOUD as well as negative past experiences with MOUD have been cited as barriers to MOUD engagement among correctional populations (Citation26). Additionally, a lack of adequate discharge planning and fragmented linkages between correctional and community MOUD providers have been associated with treatment disruptions and poor treatment retention (Citation26–29). Treatment disruptions are a particular issue for pre-trial detainees or other individuals who are rapidly or unexpectedly released and require same day or next day appointments with limited discharge planning (Citation29). Other factors such as social support networks, health insurance, housing, transportation, and program structure have also been noted in the literature as key barriers to treatment retention post-release (Citation30–32). One barrier that has been described as particularly deleterious is the requirement for many individuals on MOUD to present to their designated clinic/pharmacy and undergo daily supervised/witnessed medication administration (Citation8,Citation27). Typically this only occurs during the initial weeks/month of MOUD initiation until individuals have demonstrated clinical stability and are gradually allowed to receive “take-home” or “unwitnessed” doses (Citation8,Citation33). However, stipulations regarding who is eligible and the number of doses/time it takes to receive take-home doses varies depending on the specific formulation, and prescribing practices and processes differ between jurisdictions (Citation8,Citation33). Due to the distinctive circumstances that commonly characterize correctional populations and their post-release community life (including precarious housing/social situations, difficulty securing employment, having to abide by stringent correctional release plans that include frequent probation/parole appointments) (Citation27,Citation34), they often have difficulties demonstrating the clinical stability required to receive take-home doses (Citation35). The burden of continued daily clinic visits among this population ultimately increases the likelihood of relapse (Citation31) and treatment discontinuation (Citation36,Citation37).
Given this reality, novel extended-release buprenorphine (XR-BUP) formulations may offer specific advantages for correctional populations over traditional MOUD formulations (Citation38,Citation39). Injectable XR-BUP formulations have specific pharmacodynamics that result in the slow release of buprenorphine into an individual’s system over time (Citation40,Citation41) resulting in reduced opioid withdrawal and cravings, and considerably longer and more flexible dosing windows compared to methadone or buprenorphine/naloxone (Citation41,Citation42). There are two primary injectable XR-BUP formulations: Sublocade (which is a once-monthly injection with two available doses, 100 mg or 300 mg, provided to patients stabilized on sublingual buprenorphine), and Buvidal/Brixadi (a once-weekly or once-monthly buprenorphine injection with multiple doses that can be used for initiation and/or given to those stabilized with sublingual buprenorphine) (Citation43–45). Based on these weekly or monthly dosing formulations compared to daily dosing with traditional MOUD, XR-BUP may be particularly beneficial for individuals who are released unexpectedly or for institutions and communities with limited resources for comprehensive discharge planning (Citation29). XR-BUP could therefore increase treatment retention and may also obviate the need for individuals to attend a MOUD clinic/pharmacy daily upon community release, thus allowing them to focus on post-release goals (Citation46).
Given that XR-BUP is a novel MOUD formulation that may offer advantages to correctional populations over traditional MOUD formulations (Citation47), the current systematic review sought to investigate available evidence supporting the feasibility and effectiveness of XR-BUP among correctional populations. This information has important clinical, correctional, and public health implications as improved MOUD retention among individuals upon release from incarceration can result in enhanced health and social outcomes.
Methods
This systematic review aimed to answer two primary research questions: 1) What is the feasibility of providing XR-BUP pharmacotherapies to correctional populations, and 2) How effective are XR-BUP pharmacotherapies for correctional populations in terms of clinical and other related outcomes. For the purpose of this review, correctional populations were defined as individuals who were currently or recently (within the past year) incarcerated at any correctional institution (e.g., detention center, jail, prison). “Feasibility” included factors such as safety, logistics, operation/administration, cost benefits, interest, willingness, and preferences for XR-BUP. “Effectiveness” included outcomes such as treatment retention, substance use, overdose, healthcare utilization, as well as correctional-specific outcomes such as probation/parole violations and re-incarceration. The review was registered in the international prospective registry for systematic reviews (PROSPERO, registration # CRD42023397786). Research ethics board approval was not required (see TCPS II article 2.2 use of secondary data).
Search strategy
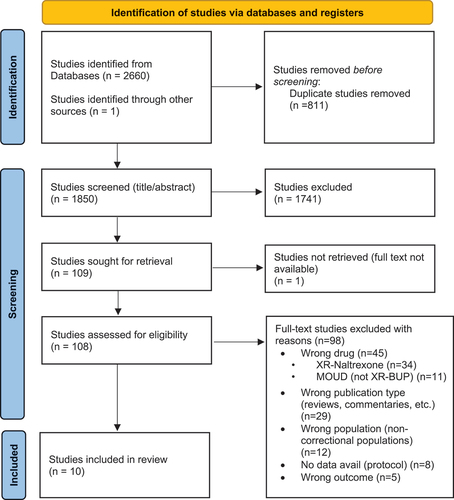
This systematic review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (see for flow diagram of included studies). A comprehensive search was carried out that included all articles from database inception until October 4, 2023, in the following databases: PubMed, Embase, and PsychINFO. The search included medical subject headings (MeSH) and terms related to XR-BUP and correctional populations. The searches were limited to human, peer-reviewed, English language studies, and no date or participant age restrictions were applied (see Online Appendix A for search strategy).
Figure 1. PRISMA flow chart.
Inclusion/exclusion criteria
Any experimental or observational primary study (including both quantitative and qualitative studies) that examined the above-defined outcomes of feasibility or effectiveness of XR-BUP among correctional populations with OUD were included. Economic analyses that focused on the economic feasibility (e.g., cost benefit analyses) of XR-BUP within correctional settings were also included; however, primary studies that drew on samples of criminal justice or related healthcare representatives (correctional officers, correctional healthcare staff, probation/parole officers, medical/administrative staff at community MOUD programs, etc.) were excluded. Studies had to specify the XR-BUP formulation as their primary focus; studies that examined MOUD more generally and did not specify XR-BUP as the formulation of interest were excluded. Secondary data studies such as reviews or opinion pieces (e.g., commentaries and editorials/responses) were excluded, as were dissertations/theses.
Study selection, screening, and data extraction
All studies were imported into Rayyan (an online web application for systematic review screening) (Citation48) where duplicates were removed and screened by the first author (CR) in two phases: first, using titles and abstracts, and second, using full text articles. Data were extracted from all eligible studies by the first author (CR) following a standard data extraction chart that included the study setting, study design and analysis, sample characteristics, and outcomes. A second independent reviewer verified all data extracted for accuracy.
Critical study appraisal
All studies were critically appraised for quality and assessed for risk of bias by the first author (CR) and an independent reviewer using the Joanna Briggs Institute (JBI) Critical Appraisal Tools Checklist for respective study designs (Citation49) (see Online Appendix B for Risk of Bias Assessments).
Data synthesis
Data from included studies were narratively synthesized under the following indicators: Feasibility of XR-BUP: Interest, preferences, and viability, safety, and cost; and Effectiveness of XR-BUP: Drug use, treatment retention, healthcare utilization, and re-incarceration; and Barriers and Facilitators of XR-BUP.
Results
Study characteristics
A total of 2,661 studies were identified by the search, from which 811 duplicates were removed. This left 1,850 studies to be screened using titles and abstracts. 108 studies were screened for full text, from which 98 were excluded with reason. This left ten eligible studies to be included (see for study characteristics and outcomes).
Table 1. Characteristics of included studies
Study designs were heterogeneous and comprised one qualitative study (Citation50), three cost estimation analyses (Citation51,Citation52), one cross-sectional survey (Citation53), two retrospective cohorts using electronic health databases and data linkages (Citation54,Citation55), one case series study (Citation56), and one randomized (Citation57) and one non-randomized (Citation58) comparative effectiveness trial. Study settings were also heterogeneous, with four studies based in the US (two in New York City (Citation50,Citation57), one in Rhode Island (Citation55), and one in California (Citation54)), two in Australia (both in New South Wales) (Citation52,Citation58), and one each in France (Citation53), England (Citation51), Germany (Citation56), and Canada (Citation59). The studies’ samples represented a total of n = 819 individuals (n = 151 [18.4%] female, n = 668 [81.6%] male). Six of the seven studies with participants included both males and females (with a predominately male sample) and one included just males. Due to the novelty of XR-BUP, data collection for all studies occurred recently, beginning in 2018.
Online Appendix B shows the risk of bias for each study. The risk of bias was low in five studies (Citation51–53,Citation58,Citation59), moderate in three studies (Citation50,Citation54,Citation57), and high in two studies (Citation55,Citation56). Among the two studies that were classified as having a high risk of bias, one (Citation55) did not clearly report confounding factors or strategies to deal with them and it was unclear if the appropriate statistical analyses were therefore used and applied; the other (Citation56) lacked clear reporting for key information such as participant demographics and related clinical information, as well as unclear inclusion criteria.
Feasibility of XR-BUP
Interest, preferences, and viability of XR-BUP
Three studies reported on participants’ interest in, preference for, or the viability of XR-BUP. In a chart review of seven incarcerated males who switched from methadone to XR-BUP in Germany (2019–2020), Soyka & Gros (Citation56) reported the viability of rapidly transitioning individuals from methadone to XR-BUP. Six of the participants were able to complete the transition within 48 hours, one within 4 days, with mild to moderate withdrawal symptoms experienced; dose adjustments were needed in four of the seven participants.
Chappuy et al. (Citation53) examined interest in XR-BUP among n = 96 (89.6% male) incarcerated individuals versus n = 221 (69.2% male) non-incarcerated individuals using a cross-sectional survey distributed within 68 addiction clinics and 6 prisons in France (2018–2019). They found that incarcerated individuals were nearly two times readier to try XR-BUP than non-incarcerated individuals (Adjusted Odds Ratio [aOR] = 1.80; 95% Confidence Intervals[CI] = 1.04–3.13). Participants cited reasons that could lead them to choose XR-BUP such as “receiving a constant dose” (aOR = 2.91; 95% CI = 1.21–6.98), “no longer taking a daily treatment” (aOR = 2.91; 95% CI = 1.21–6.98), and XR-BUP being a “more discreet form” of MOUD compared to traditional formulations (aOR = 1.75; 95% CI = 1.01–3.10). Conversely, incarcerated individuals less commonly endorsed the potential reduction of withdrawal symptoms (aOR = 0.54; 95% CI = 0.29–0.99), the risk of misuse (i.e., no longer having the option of injecting, snorting or inhaling their medications) (aOR = 0.56; 95% CI = 0.34–0.94), and the fact that “a subcutaneous injection is involved” (aOR = 0.24; 95% CI = 0.14 to 0.43) compared to non-incarcerated individuals as potential reasons for switching to XR-BUP.
Similarly, Martin et al. (Citation55) found high interest in and preferences for XR-BUP in their retrospective cohort study among n = 56 (96% male) incarcerated individuals in Rhode Island who received XR-BUP (2019–2022). More than half of the participants (54%) preferred XR-BUP to their previous oral MOUD pharmacotherapies of methadone or buprenorphine/naloxone. Over a quarter (26%) were either switched from their previous MOUD as a disciplinary measure for hoarding (17%) or requested to change to XR-BUP due to concerns of being accused of future diversion (9%) by staff.
Safety
In addition to examining preferences for XR-BUP, Martin et al. (Citation55) also found that XR-BUP was generally safe, with no evidence of tampering with the injection site and no attempts to remove, hoard, or divert the medication. However, 46.3% of individuals reported an adverse effect related to the first/initial injection, with an average of 1.9 side effects, while overall, including all injections provided, 61% reported at least one adverse effect, with a total average of 2.8 side effects. Most common adverse effects were gastrointestinal, general and administration site pain including discomfort or bruising, fatigue, sweats, and nervous system issues such as a rash or insomnia.
Dunlop et al. (Citation58) likewise reported minimal safety issues among participants engaged in their open-label non-randomized effectiveness trial comparing XR-BUP to methadone among n = 133 (n = 67 XR-BUP, n = 62 methadone) incarcerated individuals in Australia (2018–2019). The authors compared outcomes between the two groups at three time points: baseline, week 4, and week 16. Nearly all participants (97%) experienced a treatment-emergent adverse event (TEAE), with 94% related to the study drug, the majority of which were mild (88%) and resolved within one day, while the remaining 12% were moderate, and zero were severe. The most commonly reported drug-related TEAEs were injection site pain, constipation, injection-site swelling/erythema, headache, nausea, and vomiting. There were two serious adverse events, both of which required hospitalization, neither of which were related to the study drug. There were no overdoses or deaths. While 78% of the overall sample reported having ever heard of threats, coercion, or intimidation related to medication diversion, there were no reported attempts to remove/divert XR-BUP.
In a randomized control trial comparing treatment retention and related outcomes among individuals released from jail in New York in 2020 receiving either XR-BUP (n = 26) or buprenorphine/naloxone (n = 26), Lee et al. (Citation57) reported no significant difference in rates of serious adverse events between groups. There were eight adverse events reported, the most common being tenderness at the XR-BUP injection site and non-study–related emergency department visits. There were two total serious adverse events which occurred in the same XR-BUP participant, likely related to ongoing intravenous cocaine use. Seven (27%) XR-BUP participants chose to switch back to buprenorphine/naloxone prior to week eight with reported reasons including complaints of burning and pain during XR-BUP administration and general preferences for self-administration. There were no overdoses or deaths.
Cost
Three studies, Wong et al. (Citation59), Wright et al. (Citation51), and Ling et al. (Citation52), conducted cost analyses to evaluate and predict the cost of providing XR-BUP to incarcerated individuals (costs have been converted to current [2024] exchange rate USD for comparability). Wong et al. estimated the monthly (28 day) comparative costs of XR-BUP and buprenorphine/naloxone within one provincial correctional center in British Columbia, Canada, in 2021, housing approximately 130 incarcerated individuals, half of whom were on MOUD. Estimations were retrospective and based on observations and interviews with relevant staff. The authors found that XR-BUP had a higher initial acquisition cost (which included the cost of the medication, supplies, and pharmacist time for preparation; all costs presented below are per-person, per-month) compared to buprenorphine/naloxone (USD $488.77 vs. $108.78, respectively), but lower administration costs, which resulted in an overall lower total monthly per-person cost for XR-BUP (USD $579.30 vs. $727.74, respectively). Specifically, nursing costs (which included time spent on checking records, preparing, logging, and administering medication, patient monitoring, and finding community clinics for treatment continuity) were lower for XR-BUP than buprenorphine/naloxone (USD $50.22 vs. $202.33, respectively). Similarly, correctional costs (which included hourly wages for four correctional officers and one supervisor to monitor patients) were lower for XR-BUP compared to buprenorphine/naloxone (USD $10.95 vs. $416.71, respectively). The total lower per-person monthly cost for XR-BUP compared to buprenorphine/naloxone can be attributed to a substantial reduction in hourly wages for nurse and correctional officer time spent monitoring patients which occurs only once monthly with XR-BUP versus daily for 28 days with buprenorphine/naloxone.
Using a predictive model, Wright et al. (Citation51) drew on national data sources, scientific literature, and experiences in prison healthcare settings to estimate and compare the cost of providing treatment as usual (i.e., methadone) versus XR-BUP if treatment was hypothetically provided to 30% of 150 incarcerated individuals in England (i.e., n = 105 methadone patients versus n = 45 XR-BUP patients) (the year in which the costs are in reference to is not reported). Estimations consisted of direct costs (i.e., medication and staff costs) and indirect costs (i.e., healthcare utilization and security/criminal justice costs). Direct medication costs were based on an average daily dose of 60 mg/patient and unit cost data for methadone, and the fixed cost for a 30-day supply of XR-BUP. Direct staff costs were based on time to complete the following tasks: 1) setup (e.g., preparation); 2) escorting patients to treatment; 3) dispensing (e.g., daily dispensation and supervision); 4) completion (e.g., cleaning and recording/logging files); and 5) administration (e.g., medication procurement, transport, etc.). Indirect healthcare utilization costs included diversion-related medication review, overdoses, ambulance calls for overdoses, and drug-related death (in both prison and post-release). Indirect security and criminal justice system costs included management time for investigation of and arrests for violence related to the diversion of MOUD medication. This included adjudications or sentencing and punishment (including added sentence days). Total annual methadone costs were equal to USD $782,342.75, comprised of $370,495.85 for direct medication and staff costs, $122,432.65 for indirect healthcare costs, and $289,414.25 for indirect security or criminal justice system costs. Total annual XR-BUP costs were equal to USD $771,364.20, comprised of $440,337.90 for direct medication and staff costs, $101,547.44 for indirect healthcare costs, and $229,478.86 for indirect security and criminal justice costs. Taken together, the provision of XR-BUP resulted in an overall annual cost savings of $10,978.55 and would result in a 17% reduction in indirect healthcare costs, 21% reduction in security and criminal justice system costs, and 27% reduction in staff time, allowing for an additional 3159 of staff hours.
Ling et al. (Citation52) conducted a cost estimation comparison between XR-BUP, methadone, and buprenorphine/naloxone, using data taken from a non-randomized comparative effectiveness trial across seven correctional centers in New South Wales, Australia. Costs included pharmacy, inventory management, medication administration, and supervision (all costs presented below are per-person, per-month). Analyses were done with the assumption of a steady incremental treatment transfer from methadone and buprenorphine/naloxone to XR-BUP starting with 0% of patients receiving XR-BUP. The percent of patients receiving XR-BUP increased by 5% each month to reach 60% at month 12, while buprenorphine patients were reduced until there were only two left by month 12. The authors found that in month zero, the total monthly per-patient costs for methadone were USD $245.49 and $977.36 for buprenorphine/naloxone. Total first month XR-BUP cost was $116.84. As XR-BUP was slowly introduced and assumed to comprise a growing number of patients, per-patient costs for methadone and buprenorphine/naloxone increased. At six months, methadone costs increased to USD $270.43, buprenorphine/naloxone costs increased to $1041.69, and XR-BUP decreased to $68.92. By 12 months, methadone costs increased to USD $347.89, buprenorphine/naloxone costs increased to $1419.12, and XR-BUP decreased to $60.39. XR-BUP was consistently the least costly MOUD option.
Effectiveness
Drug use
In addition to describing safety and diversion outcomes (above), both Dunlop et al. (Citation58) and Lee et al. (Citation57) reported on drug use outcomes. When examining drug use in custody, Dunlop et al. (Citation58) found a significant decrease in self-reported non-prescribed opioid use from baseline to week 4 and week 16 among XR-BUP patients. At baseline, 97% self-reported non-prescribed opioid use, which decreased to 61% at week 4 (Odds Ratio (OR) = 0.048, 95%CI = 0.010–0.221; p = .0001) and to 12% at week 16 (OR = 0.0035, CI = 0.0007–0.018; p = <0.0001). The authors also found a significant decrease in the frequency of self-reported non-prescribed opioid use between baseline and week 4 (22.51 days [Standard Deviation SD = 9.67] to 5.41 days [SD = 7.70]; OR = 0.35; 95% CI = 0.26–0.48; p = <0.0001). Additionally, they found a significant the decrease in prevalence of injection drug use from 81% at baseline to 52% at week 4 (OR = 0.22, 95%CI = 0.09–0.53; p = .0008) and 17% at week 16 (OR = 0.032, 95%CI = 0.012–0.087; p = <0.0001). Frequency of injecting also decreased significantly between baseline and week 4 (17.55 [SD = 12.5] days to 4.36 [SD = 7.14] days; OR = 0.35; 95% CI = 0.24–0.50; p = <0.0001), but the decline between weeks 4 and 16 was not statistically significant.
Using descriptive statistics, Lee et al. (Citation57) found a decrease in opioid use post-release, with 55% of those who received XR-BUP producing opioid-negative urinalysis tests at 8 weeks post-release compared to 38.4% of those who received buprenorphine/naloxone among individuals enrolled in their random effectiveness trial.
Cheng et al. (Citation50) qualitatively examined perceptions and experiences of XR-BUP pre- and post-release among n = 16 (81.3% male) participants recently released from incarceration in New York City (2019–2022). They found that although some participants indicated they had tried opioids to test if the XR-BUP blocked the euphoric effects of opioid use, nearly all reported no longer using illicit opioids. However, many participants described still using stimulants.
Treatment retention
Three studies reported on treatment retention. When comparing individuals engaged in methadone to those on XR-BUP in their non-randomized effectiveness trial, Dunlop et al. (Citation58) reported that among the n = 67 participants receiving XR-BUP, 5 participants were considered failures (4 for treatment-related reasons and one transferred to another site). Seven were censored before 113 days, six of whom were involuntarily withdrawn from treatment for reasons unrelated to the study drug (e.g., administrative transfer [n = 3], unexpected release [n = 2]), and the remaining 1 was due to an unrelated serious adverse event). The probability of XR-BUP treatment retention was 92.3%; probability of methadone retention was not reported.
Likewise, comparing XR-BUP to buprenorphine/naloxone among individuals in their randomized effectiveness trial, Lee et al. (Citation57) reported a higher proportion of participants remained engaged in treatment at week eight post-release in the XR-BUP group (69.2%) versus the buprenorphine/naloxone group (34.6%).
Finally, Martin et al. (Citation55) specified that the majority (70%) of participants remained on XR-BUP during incarceration, while 11% discontinued it at some point only to then restart it later among individuals identified within their retrospective cohort study. Within those who were released into the community (61% of XR-BUP participants), 70% engaged in either methadone, naltrexone, or buprenorphine, with 30% receiving at least one XR-BUP injection post-release, 39% receiving another formulation, and another 30% were unknown.
Healthcare utilization
Two studies reported on healthcare utilization, one during incarceration and one post-release. During incarceration, Lee et al. (Citation57) noted that participants in the XR-BUP group of their random effectiveness trial had fewer (0.11 [0.03 standard deviation [SD]] vs 1.06 [0.08 SD]) mean jail medical visits compared to those on buprenorphine/naloxone.
Will et al. (Citation54) examined the likelihood of presenting to an emergency department (ED) within 24 hours and again at 28 days post-release using a retrospective cohort of participants who received MOUD (i.e., methadone (n = 105, 80% male), naltrexone (either daily oral or XR injection; n = 21, 76.2% male), or XR-BUP (n = 114, 82.5% male) during incarceration. The authors found that those not receiving any MOUD had an average of 2.9 times as many ED visits within 28 days of release than those who did receive MOUD, and that those who received at least one dose of methadone (aOR = 0.4; 95% CI = 0.2–0.9; p = .036) or XR-BUP (aOR = 0.4; 95% CI = 0.2–0.7; p = .007) during the incarceration were less likely to present to the ED within 28 days of release when compared to no treatment at all. Results for ED visits within 24 hours were non-significant, as were the number of medication administrations during the individual’s incarceration, indicating that longer duration of treatment did not impact ED utilization.
Re-incarceration
Two studies reported on re-incarceration. Using descriptive statistics, Lee et al. (Citation57) described how fewer participants (8%) in the XR-BUP arm of their random effectiveness trial were re-incarcerated during the study, compared to those in the buprenorphine/naloxone arm (15%). In their retrospective cohort, Martin et al., (Citation55 found that among the 61% of individuals who were released on XR-BUP (n = 33/54), seven were re-incarcerated during the study period; of those, n = 1 received XR-BUP, n = 1 received both XR-BUP and buprenorphine/naloxone, one two received just buprenorphine/naloxone, and three received methadone.
Barriers and facilitators
Lastly, two studies reported potential barriers and facilitators for XR-BUP among correctional populations: the qualitative study by Cheng et al. (Citation50) and the random effectiveness trial comparing XR-BUP to buprenorphine/naloxone by Lee et al. (Citation57) Key facilitators for XR-BUP engagement cited by participants in the Cheng et al. study included reductions in the risk of opioid relapse (due to the perceived blockade effects of XR-BUP), reductions in concerns regarding pressure to divert medication, reductions in the risk of engaging in criminal activities to fund opioid use, increases in the likelihood of obtaining employment post-release, a desire to avoid opioid withdrawal and overdose, and not having to visit the clinic daily. Participants elaborated that not having to visit the clinic daily rendered the treatment more discreet, which alleviated perceived stigmatization and privacy concerns. Barriers to XR-BUP treatment described by participants included side effects such as injection site pain and potential under-dosing (including post-injection withdrawal symptoms and cravings), a fear of needles, a fear of reduced efficacy compared to traditional MOUD, a preference for the daily “boost” of traditional MOUD, and a lack of information about the medication.
Participants in the Lee et al. (Citation57) study indicated the benefits of not having to attend a clinic daily and less urgency to find follow-up care immediately post-release as key facilitators for XR-BUP over buprenorphine/naloxone. However, they also indicated a fear or opposition to needles, apprehension, and a lack of knowledge about the XR-BUP formulation, potential limited community access post-release, and a preference for traditional and known buprenorphine/naloxone routines.
Discussion
This is the first systematic review to synthesize the extant literature on the feasibility and effectiveness of XR-BUP among correctional populations with OUD. Overall, our findings demonstrate that the administration of XR-BUP among correctional populations is feasible and well-tolerated. Despite the majority of participants experiencing an adverse event after receiving XR-BUP, primarily related to injection site reactions, there were no major issues reported with the provision of XR-BUP. None of the participants within any of the studies which assessed diversion attempted to divert the medication, and there were no overdoses or deaths resulting from the intervention, highlighting the relative effectiveness of XR-BUP. Moreover, incarcerated individuals engaged in MOUD expressed a high degree of interest in and preferences for XR-BUP over other MOUD formulations and cited numerous benefits. For instance, not having to interact with correctional or healthcare staff as often reinforced the discreetness of XR-BUP compared to other MOUD options, which may reduce stigmatization and increase individuals’ willingness to engage in treatment during incarceration.
Perhaps most importantly, in both studies that examined XR-BUP facilitators, individuals described the benefit of not having to attend a clinic daily upon release into the community as a key facilitator for engaging in XR-BUP (Citation50,Citation57). Recognizing the difficulties that many individuals face post-release, not having to find and attend an MOUD clinic/pharmacy immediately upon release can enable individuals to prioritize and refocus their energy and resources into achieving other goals such as employment, housing, and re-building their lives (Citation42). However, it must also be noted that evidence on post-release XR-BUP retention is still lacking, and individuals may also disengage from treatment and return to opioid use to alleviate withdrawal symptoms. Regardless, XR-BUP can be used as a transitional bridge to substantially reduce risks of relapse and overdose which is markedly higher within the first days and weeks of release (Citation60). Efforts to reduce barriers to treatment continuity and to ensure individuals remain engaged in XR-BUP are warranted given the prolonged risk of overdose that remains elevated for months post-release (Citation61). The potential for XR-BUP to reduce these risks underscores the importance of providing XR-BUP prerelease.
Studies that reported on the effectiveness of XR-BUP further substantiated the likelihood of increased treatment engagement and reduced risks such as substance use, emergency department visits, and re-incarceration. For instance, the studies that examined substance use outcomes reported a substantial decrease in illicit opioid use and risky drug use behaviors such as injection drug use. Qualitatively, participants also described remaining abstinent from illicit opioids while engaged in XR-BUP. Among the two studies that examined post-release treatment retention outcomes, one indicated a higher proportion of participants on XR-BUP remained in treatment post-release compared to methadone or buprenorphine/naloxone (Citation57), while the other demonstrated similar retention rates (Citation55), underscoring XR-BUP’s potential ability to increase treatment engagement during an exceptionally high-risk period.
Despite the overall benefits of XR-BUP identified in this review, several barriers were raised. Participants described a fear of needles and injection site pain as key drawbacks of XR-BUP, as well as a lack of knowledge or understanding about XR-BUP, including its effectiveness. Some participants were apprehensive about the availability of XR-BUP formulations within the community post-release and preferred traditional MOUD since those formulations and routines were considered familiar. For instance, a number of patients engaged in XR-BUP in one study did revert back to buprenorphine/naloxone once they were released citing complaints of adverse side effects of XR-BUP administration and general preferences for buprenorphine/naloxone (Citation57). These factors underscore the importance of patient preferences since preferences distinctly impact decisions to switch or cease treatment and are often further influenced by side effects, route of administration, delivery within the community, and stigma (Citation62). These dynamics should not be overlooked and could result in reduced uptake of XR-BUP. However, there are a number of strategies that could be undertaken to address these concerns, including increasing education and knowledge on the formulation’s effectiveness and side-effects as well as availability within the community. Furthermore, traditional MOUD formulations are commonly used as “currency” within the prison ecological systems, and individuals may be forced to or may purposely divert their medication for a profit (Citation63), which is difficult to do with XR-BUP and may deter individuals from engaging in this formulation. Correctional institutions may also be reluctant to implement XR-BUP due to a lack of funding and ideologies that do not support or fully recognize the benefits of providing MOUD maintenance for incarcerated individuals. However, a recent study demonstrated almost universal support from correctional and healthcare staff who participated in focus groups within an Australian prison evaluating XR-BUP formulations, and the majority of staff indicated that XR-BUP could reduce known barriers and challenges in the administration of MOUD within prisons (Citation64). The higher initial acquisition cost of XR-BUP compared to other forms of MOUD has also been cited as a barrier for implementation and correctional buy-in (Citation52). Although cost analyses can be complex and imprecise and include a variety of factors such as medication costs, administration, labor/staffing, transport, storage, as well as long-term health, social, and quality of life outcomes, all three cost analysis studies presented in this review demonstrated a net cost benefit of XR-BUP compared to traditional MOUD formulations. Specifically, XR-BUP had a total lower monthly per-person cost compared to buprenorphine/naloxone and methadone in the two studies that compared costs between formulations. The third study that predicted a hypothetical scenario in which 30% of MOUD patients switched from methadone to XR-BUP found significant savings with XR-BUP, primarily related to less staffing costs as this formulation requires less time to administer and monitor, underscoring the potential for additional staff time to be better used to strengthen treatment discharge planning or other related activities. The potential benefit is theoretically even greater when taking into consideration the possible reduction in social and healthcare costs of increased MOUD treatment engagement post-release, particularly if rates of relapse, overdose, re-incarceration, and healthcare utilization decrease. Thus, the addition of XR-BUP to the arsenal of available pharmacotherapies within correctional institutions appears to not only be economically beneficial, but may also increase uptake if correctional systems recognize the savings associated with this formulation compared to other available MOUD.
Despite the positive results identified in this review, study limitations should be noted. Due to the novelty of this specific pharmacotherapy, available data are limited and there is a lack of long-term or comparative outcomes. For instance, only two studies examined re-incarceration, and two examined healthcare utilization, only one of which examined this outcome post-release. The limited data were underscored by the vast heterogeneity in study designs, settings, and sample characteristics. An overreliance on descriptive statistics and a lack of statistical tests of association, largely due to limited sample sizes, complicated the ability to ascertain the effectiveness of XR-BUP. Most studies were non-randomized resulting in potential participant selection and other related biases. Two of the studies had overlapping samples (Citation50,Citation57), with one focusing on a subset of qualitative data from the larger study. The specific XR-BUP formulations, dosages, and pharmaceutical prices varied substantially by country and the costs outlined in this review may not be applicable to all settings, emphasizing the need for future research to take differences in XR-BUP formulations and their associated dosing schedules (e.g., weekly injection versus monthly injections, as well as dosages) into consideration. Moreover, the studies’ samples were predominately male. This minimizes any potential important observed differences between sex/gender in outcomes, which was not considered in most studies. Studies also originated from multiple countries that likely have drastically different MOUD availabilities, deliveries, processes, ideologies, and substance use-related public health problems, both in the community and within correctional settings. Studies did not incorporate analyses based on race/ethnicity so it is unclear how these results may have differed among specific populations that are overrepresented in the criminal justice system such as Indigenous populations, demonstrating the need to explore this further (Citation65,Citation66). Future research should focus on potential race/ethnicity, gender, and setting differences, as well as examining longer-term outcomes and should aim to include larger, randomized samples to be able to better and more robustly assess effectiveness. Additionally, future research should qualitatively examine the availability, accessibility, and acceptability of XR-BUP within correctional settings, as well as potential barriers and facilitators to uptake, engagement, and treatment continuity post-release to better understand this formulation’s potential as an alternative to traditional MOUD.
Conclusions
While the data are limited, preliminary available qualitative, quantitative, and cost estimation data indicate that providing XR-BUP to correctional populations is viable, safe, and economically beneficial, and can potentially result in reduced drug use, healthcare utilization, and re-incarceration, as well as increases in treatment retention. XR-BUP offers an alternative feasible and effective treatment option for OUD both within correctional settings and upon release into the community, and should be considered for use in order to expand treatment access for such a high-risk population.
Abbreviations
| aOR | = | Adjusted odds ratio |
| BUP/NAL | = | Buprenorphine/naloxone (Suboxone) |
| CI | = | Confidence intervals |
| ED | = | Emergency department |
| MAT | = | Medication Assisted Treatment |
| MOUD | = | Medications for Opioid Use Disorder |
| MP | = | Multi-Purpose (search strategy term indicating searches undertaken in title, original title, abstract, subject heading, name of substance and registry word fields) |
| OAT | = | Opioid agonist treatment |
| OR | = | Odds ratio |
| OUD | = | Opioid use disorder |
| US | = | United States of America |
| XR-BUP | = | Extended-release buprenorphine |
Supplemental Material
Download Zip (25.4 KB)Supplemental Material
Download MS Word (49 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00952990.2024.2360984
Additional information
Funding
References
- Proctor SL, Hoffmann NG, Raggio A. Prevalence of substance use disorders and psychiatric conditions among county jail inmates: changes and stability over time. Crim Justice Behav. 2019;46:24–41. doi:10.1177/0093854818796062.
- Raggio AL, Kopak AM, Hoffmann NG. Opioid use disorders and offending patterns among local jail inmates. Corrections. 2017;2:258–68. doi:10.1080/23774657.2017.1310003.
- Butler A, Nicholls T, Samji H, Fabian S, Lavergne MR. Prevalence of mental health needs, substance use, and co-occurring disorders among people admitted to prison. Psychiatr Serv. 2021;73:737–44. doi:10.1176/appi.ps.202000927. PubMed PMID: 34809437.
- Scott CK, Dennis ML, Grella CE, Mischel AF, Carnevale J. The impact of the opioid crisis on U.S. state prison systems. Health & Justice. 2021;9:17. doi:10.1186/s40352-021-00143-9.
- Jin H, Marshall BDL, Degenhardt L, Strang J, Hickman M, Fiellin DA, Ali R, Bruneau J, Larney S. Global opioid agonist treatment: a review of clinical practices by country. Addiction. 2020;115:2243–54. doi: 10.1111/add.15087. Epub 2020/04/15. PubMed PMID: 32289189; PubMed Central PMCID: PMCPMC7554123.
- Shulman M, Wai JM, Nunes EV. Buprenorphine treatment for opioid use disorder: an overview. CNS Drugs. 2019;33:567–80. doi:10.1007/s40263-019-00637-z
- Bruneau J, Ahamad K, Goyer M-È, Poulin G, Selby P, Fischer B, Wild TC, Wood E. Management of opioid use disorders: a national clinical practice guideline. Can Med Assoc J. 2018;190:E247–57. doi:10.1503/cmaj.170958.
- Substance Abuse and Mental Health Services Administration (SAMHSA). Treatment improvement protocol 63: medications for opioid use disorder. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration (SAMHSA); 2021.
- Moore KE, Roberts W, Reid HH, Smith KMZ, Oberleitner LMS, McKee SA. Effectiveness of medication assisted treatment for opioid use in prison and jail settings: a meta-analysis and systematic review. J Subst Abuse Treat. 2019;99:32–43. doi:10.1016/j.jsat.2018.12.003
- Santo T, Clark B, Hickman M, Grebely J, Campbell G, Sordo L, Chen A, Tran LT, Bharat C, Padmanathan P, et al. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta-analysis. JAMA Psychiarty. 2021;78:979. doi:10.1001/jamapsychiatry.2021.0976.
- Binswanger IA, Stern M, Deyo R, Heagerty P, Cheadle A, Elmore J, Koepsell TD. Release from prison — a high risk of death for former inmates. N Engl J Med. 2007;356:157–65. doi:10.1056/NEJMsa064115.
- Hartung DM, McCracken CM, Nguyen T, Kempany K, Waddell EN. Fatal and nonfatal opioid overdose risk following release from prison: A retrospective cohort study using linked administrative data. J Subst Use Addiction Treat. 2023;147:208971. doi:10.1016/j.josat.2023.208971.
- Keen C, Kinner SA, Young JT, Snow K, Zhao B, Gan W, Slaunwhite AK. Periods of altered risk for non-fatal drug overdose: a self-controlled case series. Lancet Public Health. 2021;6:e249–59. doi:10.1016/S2468-2667(21)00007-4.
- Kinner SA, Milloy MJ, Wood E, Qi J, Zhang R, Kerr T. Incidence and risk factors for non-fatal overdose among a cohort of recently incarcerated illicit drug users. Addict Behav. 2012;37:691–96. doi:10.1016/j.addbeh.2012.01.019.
- Ray B, Victor G, Cason R, Hamameh N, Kubiak S, Zettner C, Dunnigan M, Comartin E, Costello M. Developing a cascade of care for opioid use disorder among individuals in jail. J Subst Abuse Treat. 2022;138:108751. doi:10.1016/j.jsat.2022.108751.
- Sander G, Shirley-Beavan S, Stone K. The global state of harm reduction in prisons. J Correct Health Care. 2019;25:105–20. doi: 10.1177/1078345819837909. Epub 2019/05/16. PubMed PMID: 31084277.
- von Bernuth K, Seidel P, Krebs J, Lehmann M, Neumann B, Konrad N, Opitz-Welke A. Prevalence of opioid dependence and opioid agonist treatment in the Berlin custodial setting: a cross-sectional study. Front Psychiatry. 2020;11:794. doi:10.3389/fpsyt.2020.00794.
- Khatri UG, Howell BA, Winkelman TN. Medicaid expansion increased medications for opioid use disorder among adults referred by criminal justice agencies: study examines receipt of medications for opioid use disorder among individuals people referred by criminal justice agencies and other sources before and after medicaid expansion. Health Aff (Millwood). 2021;40:562–70. doi:10.1377/hlthaff.2020.01251.
- Sufrin C, Kramer C, Terplan M, Fiscella K, Olson S, Voegtline K, Latkin C. Availability of medications for opioid use disorder in U.S. Jails. Journal Of General Internal Medicine. 2023;38:1573–75. doi:10.1007/s11606-022-07812-x.
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL. Opioid use disorder. Nat Rev Dis Primers. 2020;6:3. doi:10.1038/s41572-019-0137-5.
- Lim S, Cherian T, Katyal M, Goldfeld KS, McDonald R, Wiewel E, Khan M, Krawczyk N, Braunstein S, Murphy SM, et al. Association between jail-based methadone or buprenorphine treatment for opioid use disorder and overdose mortality after release from New York City jails 2011–17. Addiction. 2023;118:459–67. doi:10.1111/add.16071.
- Klemperer EM, Wreschnig L, Crocker A, King-Mohr J, Ramniceanu A, Brooklyn JR, Peck KR, Rawson RA, Evans EA. The impact of the implementation of medication for opioid use disorder and COVID-19 in a statewide correctional system on treatment engagement, postrelease continuation of care, and overdose. J Subst Use Addiction Treat. 2023;152:209103. doi:10.1016/j.josat.2023.209103.
- Simon R, Rich JD, Wakeman SE. Treating opioid use disorder in correctional settings. In: Wakeman SE, Rich JD, editors. Treating opioid use disorder in general medical settings. Springer, Cham; 2021. p 77–90. doi:10.1007/978-3-030-80818-1_6.
- Martin RA, Alexander-Scott N, Berk J, Carpenter RW, Kang A, Hoadley A, Kaplowitz E, Hurley L, Rich JD, Clarke JG, et al. Post-incarceration outcomes of a comprehensive statewide correctional MOUD program: a retrospective cohort study. Lancet Reg Health–Am. 2023;18:18. doi:10.1016/j.lana.2022.100419.
- Komalasari R, Wilson S, Haw S. A systematic review of qualitative evidence on barriers to and facilitators of the implementation of opioid agonist treatment (OAT) programmes in Prisons. Int J Drug Policy. 2021;87:102978. doi:10.1016/j.drugpo.2020.102978.
- Grella CE, Ostile E, Scott CK, Dennis M, Carnavale J. A scoping review of barriers and facilitators to implementation of medications for treatment of opioid use disorder within the criminal justice system. Int J Drug Policy. 2020;81:102768. doi:10.1016/j.drugpo.2020.102768.
- Russell C, Pang M, Nafeh F, Farrell Macdonald S, Derkzen D, Rehm J, Fischer B. Barriers and facilitators to opioid agonist treatment (OAT) engagement among individuals released from federal incarceration into the community in Ontario, Canada. Int J Qual Stud Health Well-Being. 2022;17:2094111. doi:10.1080/17482631.2022.2094111.
- Matsumoto A, Santelices C, Evans EA, Pivovarova E, Stopka TJ, Ferguson WJ, Friedmann PD. Jail-based reentry programming to support continued treatment with medications for opioid use disorder: Qualitative perspectives and experiences among jail staff in Massachusetts. Int J Drug Policy. 2022;109:103823. doi: 10.1016/j.drugpo.2022.103823. Epub 2022/08/23. PubMed PMID: 35994938; PubMed Central PMCID: PMCPMC10206716.
- Stopka TJ, Rottapel RE, Ferguson WJ, Pivovarova E, Toro-Mejias LD, Friedmann PD, Evans EA. Medication for opioid use disorder treatment continuity post-release from jail: a qualitative study with community-based treatment providers. Int J Drug Policy. 2022;110:103803. doi: 10.1016/j.drugpo.2022.103803. Epub 2022/08/15. PubMed PMID: 35965159; PubMed Central PMCID: PMCPMC10117037.
- Kaplowitz E, Truong A, Macmadu A, Berk J, Martin H, Burke C, Rich JD, Brinkley-Rubinstein L. Anticipated barriers to sustained engagement in treatment with medications for opioid use disorder after release from incarceration. J Addict Med. 2023;17:54–59. doi: 10.1097/adm.0000000000001029. Epub 2022/08/03. PubMed PMID: 35916404; PubMed Central PMCID: PMCPMC9892350.
- Russell C, Pang M, Nafeh F, MacDonald SF, Derkzen D, Rehm J, Fischer B. Applying the socio-ecological model to understand community reintegration experiences among individuals on opioid agonist treatment (OAT) released from federal incarceration in Ontario, Canada. SSM-Qual Res Health. 2022;2:100083. doi:10.1016/j.ssmqr.2022.100083.
- Hoffman KA, Thompson E, Gaeta Gazzola M, Oberleitner LMS, Eller A, Madden LM, Marcus R, Oberleitner DE, Beitel M, Barry DT, et al. “Just fighting for my life to stay alive”: a qualitative investigation of barriers and facilitators to community re-entry among people with opioid use disorder and incarceration histories. Addict Sci Clin Pract. 2023;18:16. doi:10.1186/s13722-023-00377-y.
- Eibl JK, Morin K, Leinonen E, Marsh DC. The state of opioid agonist therapy in Canada 20 years after federal oversight. Can J Psychiatry. 2017;62:444–50. doi:10.1177/0706743717711167.
- Vail W, Faro E, Watnick D, Giftos J, Fox AD. Does incarceration influence patients’ goals for opioid use disorder treatment? A qualitative study of buprenorphine treatment in jail. Drug Alcohol Depend. 2021;222:108529. doi:10.1016/j.drugalcdep.2021.108529
- Russell C, Lange S, Kouyoumdjian F, Butler A, Ali F. Opioid agonist treatment take-home doses (‘carries’): are current guidelines resulting in low treatment coverage among high-risk populations in Canada and the USA? Harm Reduct J. 2022;19:1–6. doi:10.1186/s12954-022-00671-z
- Frank D, Mateu-Gelabert P, Perlman DC, Walters SM, Curran L, Guarino H. “It’s like ‘liquid handcuffs”: The effects of take-home dosing policies on Methadone Maintenance Treatment (MMT) patients’ lives. Harm Reduct J. 2021;18:88. doi:10.1186/s12954-021-00535-y.
- Sharma A, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, Schwartz RP. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19:35. doi:10.1007/s11920-017-0783-9.
- Berk J, Del Pozo B, Rich JD, Lee JD. Injecting opioid use disorder treatment in jails and prisons: the potential of extended-release buprenorphine in the carceral setting. J Addict Med. 2022;16:396–98. doi:10.1097/ADM.0000000000000942
- Maremmani I, Dematteis M, Gorzelanczyk EJ, Mugelli A, Walcher S, Torrens M. Long-acting buprenorphine formulations as a new strategy for the treatment of opioid use disorder. J Clin Med. 2023;12:5575. doi:10.3390/jcm12175575
- Compton WM, Volkow ND. Extended-release buprenorphine and its evaluation with patient-reported outcomes. JAMA Netw Open. 2021;4:e219708–e. doi:10.1001/jamanetworkopen.2021.9708.
- Chappuy M, Trojak B, Nubukpo P, Bachellier J, Bendimerad P, Brousse G, Rolland B. Prolonged-release buprenorphine formulations: Perspectives for clinical practice. Therapies. 2020;75:397–406. doi:10.1016/j.therap.2020.05.007.
- Scott R, Aboud A, O’Gorman T. Long-acting injectable buprenorphine – ‘best practice’ opioid agonist therapy for Australian prisoners. Australas Psychiatry. 2021;30:498–502. doi:10.1177/10398562211059086.
- British Columbia Centre on Substance Use (BCCSU). Opioid use disorder: practice update. Vancouver (BC): British Columbia Centre on Substance Use (BCCSU); 2022.
- Soyka M, Franke AG. Recent advances in the treatment of opioid use disorders-focus on long-acting buprenorphine formulations. World J Psychiatry. 2021;11:543–52. doi: 10.5498/wjp.v11.i9.543. Epub 2021/10/12. PubMed PMID: 34631459; PubMed Central PMCID: PMCPMC8474991.
- Vorspan F, Hjelmström P, Simon N, Benyamina A, Dervaux A, Brousse G, Jamain T, Kosim M, Rolland B. What place for prolonged-release buprenorphine depot-formulation Buvidal® in the treatment arsenal of opioid dependence? Insights from the French experience on buprenorphine. Expert Opin Drug Deliv. 2019;16:907–14. doi:10.1080/17425247.2019.1649252.
- Hard B. Increased treatment engagement and adherence: flexible management with prolonged-release buprenorphine in treatment of opioid dependence. Case Rep Psychiatry. 2021;2021:6657350. doi:10.1155/2021/6657350.
- Cuperfain AB, Katznelson G, Costa T, Wong P, Beyraghi N, George TP, Lofwall MR, Chopra N. Factors to guide the use of extended-release buprenorphine formulations for specific patient populations. J Subst Use1-7. 2024. doi:10.1080/14659891.2023.2174908.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. doi:10.1186/s13643-016-0384-4
- The Joanna Briggs Institute (JBI). Critical appraisal tools for use in JBI systematic reviews: checklist for cohort studies. The Joanna Briggs Institute; 2017.
- Cheng A, Badolato R, Segoshi A, McDonald R, Malone M, Vasudevan K, Badiei B, Sugarman A, Macdonald R, Mangat J, et al. Perceptions and experiences toward extended-release buprenorphine among persons leaving jail with opioid use disorders before and during COVID-19: an in-depth qualitative study. Addict Sci Clin Pract. 2022;17:4. doi:10.1186/s13722-022-00288-4.
- Wright N, Hard J, Fearns C, Gilman M, Littlewood R, Clegg R, Parimelalagan L, Alam F. OUD care service improvement with prolonged-release buprenorphine in prisons: cost estimation analysis. Clinicoecon Outcomes Res. 2020;12:499–504. doi: 10.2147/CEOR.S256714.
- Ling R, White B, Roberts J, Cretikos M, Howard M, Haber P, Lintzeris N, Reeves P, Dunlop AJ, Searles A, et al. Depot buprenorphine as an opioid agonist therapy in New South Wales correctional centres: a costing model. BMC Health Serv Res. 2022;22:1–13. doi:10.1186/s12913-022-08687-8.
- Chappuy M, Meroueh F, Trojak B, Bachellier J, Bendimerad P, Kosim M, Hjelmström P, Nubukpo P, Brousse G, Rolland B, et al. Factors of interest in extended-release buprenorphine: Comparisons between incarcerated and non-incarcerated patients with opioid use disorder. Patient Prefer Adher. 2021;15:1259–67. doi:10.2147/PPA.S311674.
- Will J, Abare M, Olson M, Chyorny A, Wilhelm-Leen E. Emergency department utilization by individuals with opioid use disorder who were recently incarcerated. J Subst Abuse Treat. 2022;141:108838. doi:10.1016/j.jsat.2022.108838
- Martin RA, Berk J, Rich JD, Kang A, Fritsche J, Clarke JG. Use of long-acting injectable buprenorphine in the correctional setting. J Subst Abuse Treat. 2022;142:108851. doi:10.1016/j.jsat.2022.108851
- Soyka M, Gross G. Transition from methadone to subcutaneous buprenorphine depot in patients with opioid use disorder in custodial setting-a case series. Am J Drug Alcohol Abuse. 2021;47:599–604. doi:10.1080/00952990.2021.1963757.
- Lee JD, Malone M, McDonald R, Cheng A, Vasudevan K, Tofighi B, Garment A, Porter B, Goldfeld KS, Matteo M, et al. Comparison oF treatment retention of adults with opioid addiction managed with extended-release buprenorphine vs daily sublingual buprenorphine-naloxone at time of release from jail. JAMA Netw Open. 2021;4:e2123032. doi:10.1001/jamanetworkopen.2021.23032.
- Dunlop AJ, White B, Roberts J, Cretikos M, Attalla D, Ling R, Searles A, Mackson J, Doyle MF, McEntyre E, et al. Treatment of opioid dependence with depot buprenorphine (CAM2038) in custodial settings. Addict (Abingdon, England). 2022;117:382–91. doi:10.1111/add.15627.
- Wong JS, Masson S, Huang A, Romm D, Fong M, Porter T, Sharifi N, Azar P, Mathew N. Cost analysis of buprenorphine extended-release injection versus sublingual buprenorphine/naloxone tablets in a correctional setting. J Correct Health Care. 2022;28:368–71. doi:10.1089/jchc.21.07.0063.
- Merrall EL, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, Bird SM. Meta‐analysis of drug‐related deaths soon after release from prison. Addiction. 2010;105:1545–54. doi:10.1111/j.1360-0443.2010.02990.x.
- Degenhardt L, Larney S, Kimber J, Gisev N, Farrell M, Dobbins T, Weatherburn DJ, Gibson A, Mattick R, Butler T, et al. The impact of opioid substitution therapy on mortality post-release from prison: a retrospective data linkage study. Drug And Alcohol Dependence. 2014;23:346. doi:10.1016/j.drugalcdep.2014.09.173.
- Kaplowitz E, Truong AQ, Berk J, Martin RA, Clarke JG, Wieck M, Rich J, Brinkley-Rubinstein, L. Treatment preference for opioid use disorder among people who are incarcerated. J Subst Abuse Treat. 2022;137:108690. doi:10.1016/j.jsat.2021.108690.
- Farrell MacDonald S, Russell C, Beauchamp T, Derkzen D, Fischer B. Comparing characteristics and outcomes of different opioid agonist treatment modalities among opioid-dependent federal men correctional populations in Canada. Int J Drug Policy. 2022;100:103480. doi:10.1016/j.drugpo.2021.103480
- Little SC, White B, Moensted M, Butler K, Howard M, Roberts J, Dunlop A. Health and correctional staff acceptability of depot buprenorphine in NSW prisons. Int J Drug Policy. 2023;114:103978. doi:10.1016/j.drugpo.2023.103978.
- Curtis M, Dietze P, Wilkinson AL, Agius PA, Stewart AC, Cossar RD, Butler T, Walker S, Kirwan A, Winter RJ, et al. Discontinuation of opioid agonist treatment following release from prison in a cohort of men who injected drugs prior to imprisonment in Victoria, Australia: a discrete-time survival analysis. Drug Alcohol Depend. 2023;242:109730.
- Gisev N, Gibson A, Larney S, Kimber J, Williams M, Clifford A, Doyle M, Burns L, Butler T, Weatherburn DJ, et al. Offending, custody and opioid substitution therapy treatment utilisation among opioid-dependent people in contact with the criminal justice system: comparison of Indigenous and non-Indigenous Australians. BMC Public Health. 2014;14:920. doi:10.1186/1471-2458-14-920.
