ABSTRACT
Microgreens are gaining interest for claimed high nutraceutical properties, but data on their chemical composition are so far limited. Although often grown hydroponically, their mineral requirements are still unknown. This study aimed to provide an insight into yield, mineral uptake, and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. With reference to data reported in literature for the same species hydroponically grown but harvested at adult stage, these microgreens yielded about half, with lower dry matter percentage, but higher shoot/root ratio. They showed high concentrations of some minerals, but their nutrient uptake was limited due to low yield. Nitrates content was lower if compared with that usually measured in baby leaf or adult vegetables of the same species, as well as the concentration of chlorophylls, carotenoids, phenols, and sugars. Therefore, microgreens seem to be interesting and innovative low-nitrate-salad crops requiring low fertiliser inputs. Nevertheless, an improvement in yield as well as in the content of nutraceutical compounds would be desirable.
Introduction
Microgreens are a category of salad crops that have gained popularity as a new culinary trend over the past few years. They are seedlings of vegetables and herbs consumed with tender cotyledons and the first pairs of leaves more or less developed. At harvest, plant height is from 2.5 to 8 cm depending on the species (Xiao et al. Citation2012). It is known that they are mainly produced hydroponically or semi-hydroponically with very short growing cycles (usually 10–20 days following plantlets emergence [Lee et al. Citation2004]), but cultural practices, included fertilisation, have not been standardised yet, and mineral requirements are even not known. A low yield is usually indicated as a limiting factor for these crops (Murphy et al. Citation2010; Kou et al. Citation2014; Sun et al. Citation2015), and no formal guidelines for produce quality exist (Murphy & Pill Citation2010).
Initially, microgreens were appreciated especially for the large variety of tastes, the range of colours, and the different texture, then also for their potential health benefits, since claimed to be nutritionally beneficial and often associated with the terms ‘nutraceutical’ and ‘functional food’ (Janovská et al. Citation2010; Samuolienė et al. Citation2013).
In fact, although data on the chemical composition of microgreens are so far limited (Di Gioia & Santamaria Citation2015), according to some studies the variety or the amount of bioactive compounds in microgreens is higher than that of the adult plants of the same species. In 25 commercially available microgreens varieties Xiao et al. (Citation2012) found considerably higher concentrations of vitamins and carotenoids than those typical of the respective species. In five Brassica species, microgreens showed more complex polyphenol profiles compared to their mature plant counterparts (Sun et al. Citation2013). On the other hand, a lower content of fibre, proteins, and microelements were detected in Brassica rapa as microgreens than at the mature stage (Di Gioia & Santamaria Citation2015).
This study aimed to provide an insight into yield, mineral uptake, and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system.
Materials and methods
Plant material and growing system
Basil (Ocimum basilicum L.), Swiss chard (Beta vulgaris L. subsp. vulgaris) and rocket (Eruca vesicaria (L.) Cav. subsp. sativa (Mill.) Thell.) were grown in a floating hydroponic system. Seeds were sown in polystyrene cell trays (27.0 × 53.5 cm2, 392 cells) filled with vermiculite (Asfaltex S.A., Sant Cugat del Vallés, Barcelona, Spain).
The sowing was carried out on 9 September 2014, with a density of 48.5, 242 and 45 g m−2 for basil, Swiss chard and rocket, respectively. Seed amount was calculated considering 1000 seed weight and germination percentage in order to achieve a crop density of approximately 21,700 plants m−2 (about eight plants per cell). Seed germination occurred in a climatic chamber in the dark at 24°C. Rocket seeds germinated after two days from sowing, while three days were necessary for basil and Swiss chard germination. After germination, trays were transferred in polyethylene (PE) tanks (30 × 60 × 6.5 cm3) containing each 5 L of half-strength Hoagland’s nutrient solution (macroelements expressed in mM: N 7.5, P 0.5, K 3.0, Ca 2.5, Mg 1.0; microelements expressed in µM: Fe 25.0, B 23.1, Mn 4.6, Zn 0.39, Cu 0.16, Mo 0.06; initial pH: 5.56; initial electric conductivity: 1.12 mS cm−1), prepared with distilled water (three tanks per species). Tanks were placed outdoor on a bench equipped with a transparent PE roof. Minimum and maximum temperatures during the growing cycle were 9.7°C and 43.1°C, respectively. During cultivation, every time the solution level turned down below 4 cm height, a known volume of fresh nutrient solution was added in the tanks until the initial volume was restored.
Harvesting and measurements
Microgreens were harvested at the first true leaf stage, with green and swollen cotyledons. Shoot and root fresh weight (FW) and dry weight (DW) were measured before and after oven desiccation at 80°C for 48 h or until a constant weight. Leaf area was determined by a planimeter LI-3000 (LI-COR, Lincoln, NE, USA) on 10 plants per tank. Shoots and roots were analysed for mineral content (N, P, K, Ca, Fe, and Mn). Besides, chlorophylls, carotenoids, phenols, anthocyanins, sucrose, total and reducing sugars, and nitrates were measured in the edible part (shoots).
The volume of the nutrient solution consumed by the crops was calculated subtracting the volume residual in the tanks at harvest from the overall supplied volume.
Mineral content and uptake
Shoot and root dry matter were ground and digested with nitric acid, and P, K, Ca, Fe, and Mn were measured using inductively coupled plasma mass spectrometry following the procedures described in Nocito et al. (Citation2011). Mineral uptake by the crops was then calculated multiplying the element concentration by shoot and root dry matter production.
Nitrogen and nitrates content
Total N was determined with the Dumas method performed by using an elemental analyser (ThermoQuest NA 1500 N; Thermo Electron, Milan, Italy).
Nitrates content was measured with the salicylsulphuric acid method (Cataldo et al. Citation1975). One g fresh samples were ground in 3 mL of distilled water. The extract was centrifuged at 4000 rpm for 15 min and the supernatant was recovered and used for the colorimetric determination. Twenty microlitres of sample was added to 80 μL of 5% salicylic acid in sulphuric acid and to 3 mL of NaOH 1.5 N. The samples were cooled at room temperature and the spectrophotometer readings were performed at 410 nm. Nitrates content was calculated referring to a KNO3 standard calibration curve.
Chlorophylls, carotenoids, phenols, and anthocyanins content
Chlorophylls and carotenoids were extracted from fresh leaf tissues using methanol 99.9% as solvent. Samples were kept in a dark room at 4°C for 24 h. Quantitative determination of chlorophylls was carried out immediately after extraction. Absorbance readings were measured at 665.2 and 652.4 nm for chlorophyll pigments and 470 nm for total carotenoids. Chlorophylls and carotenoids concentrations were calculated by Lichtenthaler’s formula (Lichtenthaler Citation1987).
Phenols were spectrophotometrically determined in fresh shoot samples (about 1 g) following two different approaches: the direct measure of the methanolic extract absorbance at 320 nm (phenolic index) and the Folin–Ciocalteu method (Kang & Saltveit Citation2003). Phenolic index was expressed as ABS320nm g−1 FW, while the Folin–Ciocalteu method expresses total phenols as μg g−1 FW gallic acid equivalent (GAE).
For anthocyanins determination, samples of frozen shoot tissue (about 1 g) were ground in pre-chilled mortar and extracted into methanolic HCl (1%). Samples were then incubated overnight at 4°C in the dark. The concentration of cyanidin-3-glucoside equivalents was determined spectrophotometrically at 535 nm (Klein & Hagen Citation1961).
Sucrose, reducing sugars, and total sugars content
For the determination of sucrose, reducing sugars, and total sugars, 1–2 g of fresh samples were ground in 3 mL of distilled water. Homogenate was centrifuged at 4000 rpm for 15 min. For sucrose determination, 0.2 mL of extract was added to 0.2 mL NaOH 2N and incubated at 100°C for 10 min; then 1.5 mL of hot resorcinol solution was added and the sample was incubated at 80°C for 10 min. The resorcinol solution was prepared by adding 35 mg of resorcinol and 90 mg of thiourea in 250 mL HCl 30%, mixed with 25 mL of acetic acid and 10 mL of distilled water. Samples were cooled at room temperature and spectrophotometer readings were performed at 500 nm (Rorem et al. Citation1960). A calibration curve was built with sucrose standards at 0, 0.5, 1, 1.5, and 2 mM.
The analysis of reducing sugars was performed using 0.2 mL of crude extract that was added to 0.2 mL of a solution containing 62.6 mM dinitrosalicylic acid and 1.52 M potassium sodium tartrate. The reaction mixture was heated at 100°C for 5 min, then 1.5 mL of distilled water was added and absorbance readings were performed at 530 nm (Miller Citation1959). The reducing sugars were expressed as glucose equivalent using a glucose standard curve (0, 1, 2, 3, and 4 mM). Total sugars were calculated by anthrone method: 0.2 g of anthrone was melted in 100 mL of H2SO4 and shaken for 30–40 min. One millilitre of extract was added to 5 mL of anthrone solution, cooled in ice for 5 min and mixed thoroughly. Samples were incubated at 95°C for 5 min and then cooled on ice. Absorbance readings were performed at 620 nm and a calibration curve was built with glucose standards at 0, 1, 2, 3 and 4 mM (Yemm & Willis Citation1954).
Statistical analysis
Data were subjected to ANOVA and significant differences among means (n = 3) were determined using Bonferroni’s post-hoc test at P < .05.
Results
Consumption of nutrient solution, yield, and growth parameters
Swiss chard and rocket reached the growth stage suitable for harvest 17 days after sowing, while basil had a crop cycle of 27 days. The three crops consumed 35 L m−2 of nutrient solution on average, without significant difference among them (data not shown).
The highest yield (shoot FW) was obtained in Swiss chard and the lowest in basil ((a)). Basil produced the lowest amount of shoot also on a DW basis, while no difference in shoot DW was observed between Swiss chard and rocket ((b)). Swiss chard shoots had the lowest DW percentage and basil shoots had the highest ((c)). Basil showed the less developed roots, considering both FW and DW; between Swiss chard and rocket, root FW and DW were higher in the latter ((d–e)). Leaf area was significantly higher in Swiss chard than in the other two species, and basil plants showed a leaf area higher than rocket ((f)).
Figure 1. Yield, root FW, shoot and root DW, shoot DW percentage, and leaf area of basil, Swiss chard and rocket microgreens grown in a hydroponic system.
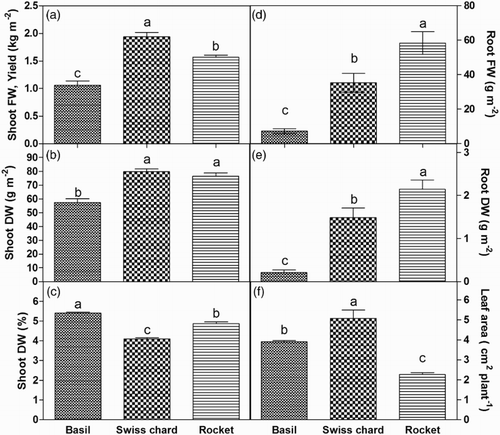
The three crops showed significant differences in the distribution of the dry matter between shoots and roots, although in all of them roots constituted less than 3% of the total biomass (data not shown). Root to shoot ratio was 0.004, 0.019, and 0.028 in basil, Swiss chard, and rocket, respectively.
Mineral content in the edible part
The analysis of the mineral content in the edible part (shoots) of microgreens revealed significant differences among the species only for some elements (). Concentration of nitrogen and phosphorus ranged, on average, from 56 to 66 g kg−1 DW and from 8.4 to 8.9 g kg−1 DW, respectively, without differences among the three crops ((a)). In Swiss chard shoots, potassium concentration was higher (92 g kg−1 DW) than in basil and rocket ((a)). The three species showed different calcium concentration, with the highest value in rocket (18 g kg−1 DW) and the lowest in Swiss chard (4.75 g kg−1 DW) ((a)). Swiss chard and rocket did not differ in iron concentration, while, in basil, accumulation of this element was significantly higher ((b)). Finally, manganese concentration showed no difference among the three species and ranged, on average, from 0.13 to 0.24 g kg−1 DW ((b)).
Figure 2. Mineral content (N, P, K, Ca, Fe, Mn) in the edible part (shoots) of basil, Swiss chard and rocket microgreens grown in a hydroponic system.
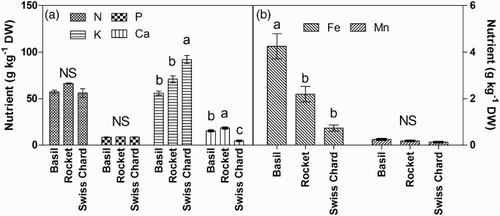
Total mineral uptake
The studied crops showed significant differences in total (shoot + root) mineral uptake for most elements (). Nitrogen uptake was lower in basil than in rocket. Basil showed also lower phosphorus and potassium requirements than the other two species that differed in potassium but not in phosphorus uptake. Potassium uptake in Swiss chard was higher than in rocket. The highest calcium uptake was observed in rocket and the lowest in Swiss chard. Swiss chard showed also a lower iron uptake than both basil and rocket. Manganese uptake was 13.1 mg m−2 as the average of the three crops without significant differences among them.
Table 1. Total mineral uptake (shoots + roots) by basil, Swiss chard and rocket microgreens grown in a hydroponic system. Values are means ± standard errors.
Nitrates, chlorophylls, carotenoids, phenols, and anthocyanins content
Significant differences in the content of nitrates were observed among the three crops. Rocket showed the highest value (2679 mg kg−1 FW) and Swiss chard the lowest (1061 mg kg−1 FW). Basil nitrates content was 1827 mg kg−1 FW.
A higher content of chlorophylls was observed in rocket than in both basil and Swiss chard, which did not show different values in total chlorophylls (). Similar concentrations of total carotenoids were found in the three species (). Phenolic index and anthocyanins content did not show differences among the species while the concentration of total phenols measured using the Folin–Ciocalteu method was lower in Swiss chard than in basil and rocket ().
Table 2. Chlorophylls (Chl a, Chl b and total), total carotenoids, total phenols, and anthocyanins in basil, Swiss chard and rocket microgreens grown in a hydroponic system.
Sucrose, reducing sugars, and total sugars
The highest contents of sugars were found in rocket shoots compared to basil and Swiss chard (). In particular, sucrose content in rocket (0.137 mg g−1 FW) was significantly higher than in basil, while Swiss chard had an intermediate content, 0.091 mg g−1 FW ((a)). Reducing sugars ranged from 0.40 to 1.42 mg g−1 FW and differed among the three species. The highest content was observed in rocket and the lowest in basil ((b)). The total sugars were similar in basil and Swiss chard, while twice the concentration was found in rocket with an average of 4.22 mg g−1 FW ((c)).
Figure 3. Sucrose, reducing sugars, and total sugars in the edible part (shoots) of basil, Swiss chard and rocket microgreens grown in a hydroponic system.
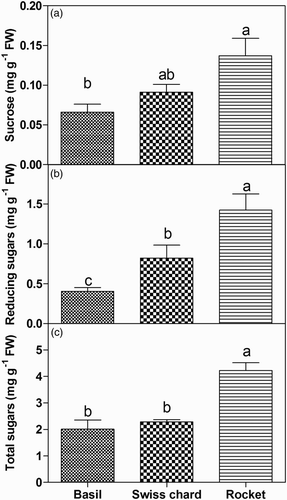
Discussion
Only few reports are available on the production of microgreens, and the achieved yield may vary somewhat among the different species and even within the same species (Murphy & Pill Citation2010; Gumble et al. Citation2015). High quantities of seed are used (approximately from 10,000 to 40,000 seeds m−2; Di Gioia & Santamaria Citation2015) to compensate the limited biomass of the single plants, which is due to the early harvesting stage. Nevertheless, a low yield remains a limiting factor for microgreen industry (Kou et al. Citation2014; Sun et al. Citation2015).
The yield we observed in basil, Swiss chard, and rocket was within the range previously reported for these microgreens (Lee et al. Citation2004; Murphy et al. Citation2010; Murphy & Pill Citation2010; Pill et al. Citation2011; Gumble et al. Citation2015), but it was only half the production obtained in a hydroponic system from the same species harvested to a full vegetative growth stage (D’Anna et al. Citation2003; Raimondi et al. Citation2006).
Our data on DW percentage were in agreement with Xiao et al. (Citation2012), who assessed this parameter in 25 commercially available microgreens demonstrating that these produce typically show high water content. According to root and shoot DW we observed, microgreens accumulated a higher portion of plant total dry matter in shoot compared to vegetables grown hydroponically to an adult stage, that usually show root to shoot ratios of about 0.15–0.20 (Fallovo et al. Citation2009; Bernstein et al. Citation2010).
Due to the early harvest, it is obvious that plants at microgreen stage show a limited leaf area. In our study, basil microgreens showed a leaf area higher than rocket but lower than Swiss chard. Similar results were obtained by Brazaitytė et al. (Citation2015).
Data on the chemical composition of microgreens are so far limited, and the mineral requirements of these produce are still unknown. Conversely, it would be useful to know the nutrient uptake by microgreens in order to manage their fertilisation. Besides, since mineral elements are important for human nutrition, the mineral composition of microgreens provides information on the contribution of these innovative produce to the human diet. After having observed in preliminary experiments that the use of nutrient solutions accelerates microgreens growing rate compared to water, in this paper we report data on the element uptake by the studied species as well as on the mineral concentration in the edible part. Comparing the latter with the elemental composition of the corresponding conventional vegetables reported in USDA (United States Department of Agriculture) Nutrition National Database (http://ndb.nal.usda.gov/ndb/search) as well as in published literature (Zheljazkov & Warman Citation2003; Cavarianni et al. Citation2008), our results seem to indicate that, on a DW basis, microgreens are slightly poorer in calcium, but richer in phosphorus, potassium, and especially iron. On the contrary, Di Gioia and Santamaria (Citation2015) found a lower iron content in Brassica rapa microgreens than in conventional vegetable.
The chlorophyll content of vegetables is important not only for reasons of health benefits, but also for the visual appearance of the produce. Considering that microgreens are mainly composed by cotyledons and only partially developed true leaves, it is not surprising that we found lower concentrations of chlorophylls, carotenoids, phenols, and anthocyanins as compared with those observed in baby leaf or adult vegetables of the same species (Ferrante et al. Citation2004, Citation2008; Spinardi & Ferrante Citation2012). However, carotenoids content was comparable to that observed by Xiao et al. (Citation2012) in microgreens.
Among the quality components of vegetables, nitrates content is very important due to possible negative effects on human health (Santamaria Citation2006). According to EU Regulation n. 1258/2011, some vegetables, to be commercialised in EU countries, must not exceed maximum acceptable nitrates levels. For rocket, these levels are 6000 mg kg−1 FW in the spring-summer season and 7000 mg kg−1 FW in autumn-winter. In this species, leaf nitrates usually ranges from 4000 to 7000 mg kg−1 FW (Santamaria et al. Citation2002). In Swiss chard and basil, nitrates contents ranging from 3800 to 4500 mg kg−1 FW and from 2000 to 4000 mg kg−1 FW have been observed, respectively (Santamaria et al. Citation1999; Pardossi et al. Citation2015). We found lower nitrates concentration in microgreens than that normally detected in baby leaf or adult vegetables, especially for Swiss chard and rocket. These species, at the microgreen stage, showed concentrations reduced to one third or even less. Similar results were obtained also in lettuce (Pinto et al. Citation2015).
Sugars are an important source of energy for maintaining the cell metabolism. After harvest they are essential for keeping cells alive and ensure a long shelf life of the produce. In basil, Swiss chard, and rocket microgreens, we measured sugar contents about 2–10 fold lower compared with baby leaf and adult vegetable leaves (Cavaiuolo et al. Citation2015). Such low sugar concentrations, observed also by Xiao et al. (Citation2015), can in part explain the short shelf life of microgreens that is limited to 1–2 days (Chandra et al. Citation2012; Xiao et al. Citation2014).
Conclusions
Based on the results obtained in basil, Swiss chard and rocket, microgreens prove to be interesting and innovative salad crops requiring low fertiliser inputs, nevertheless their yield and quality should be improved. In fact, although they provide vegetable food with low nitrates content, they also contain low amounts of many important nutraceutical components, as well as of sugars, that are important for produce shelf life. Therefore, further studies are necessary to understand the effect of growing conditions on the achievable yield and the accumulation of bioactive compounds in microgreens in order to optimise their cultivation technique.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Antonio Ferrante http://orcid.org/0000-0001-7781-9784
Anna Lenzi http://orcid.org/0000-0003-4947-7013
Additional information
Funding
References
- Bernstein N, Kravchik M, Dudai N. 2010. Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Annals of Applied Biology. 156:167–177. doi: 10.1111/j.1744-7348.2009.00376.x
- Brazaitytė A, Viršilė A, Jankauskienė J, Sakalauskienė S, Samuolienė G, Sirtautas R, Novičkovas A, Dabašinskas L, Miliauskienė J, Vaštakaitė V, et al. 2015. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. International Agrophysics. 29:13–22. doi: 10.1515/intag-2015-0004
- Cataldo DA, Maroon M, Schrader LE, Youngs VL. 1975. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Communications in Soil Science and Plant Analysis. 6:71–80. doi: 10.1080/00103627509366547
- Cavaiuolo M, Cocetta G, Bulgari R, Spinardi A, Ferrante A. 2015. Identification of innovative potential quality markers in rocket and melon fresh-cut produce. Food Chemistry. 188:225–233. doi: 10.1016/j.foodchem.2015.04.143
- Cavarianni RL, Cecílio Filho AB, Jairo Osvaldo Cazetta JO, André May A, Marotti Corradi M. 2008. Nutrient contents and production of rocket as affected by nitrogen concentrations in the nutritive solution. Scientia Agricola (Piracicaba, Brazil). 6:652–658. doi: 10.1590/S0103-90162008000600013
- Chandra D, Kim JG, Kim YP. 2012. Changes in microbial population and quality of microgreens treated with different sanitizers and packaging films. Horticulture, Environment, and Biotechnology. 53:32–40. doi: 10.1007/s13580-012-0075-6
- D’Anna F, Miceli A, Vetrano F. 2003. First results of floating system cultivation of Eruca sativa L. Acta Horticulturae. 609:361–364. doi: 10.17660/ActaHortic.2003.609.54
- Di Gioia F, Santamaria P. 2015. Microgreens. Bari: Eco-logica editore.
- Fallovo C, Rouphael Y, Rea E, Battistelli A, Colla G. 2009. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. Journal of the Science of Food and Agriculture. 89:1682–1689. doi: 10.1002/jsfa.3641
- Ferrante A, Incrocci L, Maggini R, Serra G, Tognoni F. 2004. Colour changes of fresh-cut leafy vegetables during storage. Journal of Food, Agriculture and Environment. 2(3&4):40–44.
- Ferrante A, Incrocci L, Serra G. 2008. Quality changes during storage of fresh-cut or intact Swiss chard leafy vegetables. Journal of Food, Agriculture and Environment. 6:60–62.
- Gumble J, Berghage R, Stearns D. 2015. Production and financial analyses of the rotating living wall, an urban agricultural system. Journal of Environmental Protection. 6:1029–1041. doi: 10.4236/jep.2015.69091
- Janovská D, Štočková L, Stehno Z. 2010. Evaluation of buckwheat sprouts as microgreens. Acta Agriculturae Slovenica. 95:157–162. doi: 10.2478/v10014-010-0012-2
- Kang HM, Saltveit ME. 2003. Wound-induced increases in phenolic content of fresh-cut lettuce is reduced by a short immersion in aqueous hypertonic solutions. Postharvest Biology and Technology. 29:271–277. doi: 10.1016/S0925-5214(03)00043-7
- Klein AO, Hagen CW Jr. 1961. Anthocyanin production in detached petals of Impatiens balsamina L. Plant Physiology. 36:1–9. doi: 10.1104/pp.36.1.1
- Kou LP, Yang TB, Luo YG, Liu XJ, Huang LH, Codling E. 2014. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biology and Technology. 87:70–78. doi: 10.1016/j.postharvbio.2013.08.004
- Lee JS, Pill WG, Cobb BB, Olszewski M. 2004. Seed treatments to advance greenhouse establishment of beet and chard microgreens. The Journal of Horticultural Science and Biotechnology. 79:565–570. doi: 10.1080/14620316.2004.11511806
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods in Enzymology. 148:350–382. doi: 10.1016/0076-6879(87)48036-1
- Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 31:426–428. doi: 10.1021/ac60147a030
- Murphy CJ, Llort KF, Pill WG. 2010. Factors affecting the growth of microgreen table beet. International Journal of Vegetable Science. 16:253–266. doi: 10.1080/19315261003648241
- Murphy CJ, Pill WG. 2010. Cultural practices to speed the growth of microgreen arugula (roquette; Eruca vesicaria subsp. sativa). The Journal of Horticultural Science and Biotechnology. 85:171–176. doi: 10.1080/14620316.2010.11512650
- Nocito FF, Lancilli C, Dendena B, Lucchini G, Sacchi GA. 2011. Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant, Cell & Environment. 34:994–1008. doi: 10.1111/j.1365-3040.2011.02299.x
- Pardossi A, Romani M, Carmassi G, Guidi L, Landi M, Incrocci L, Maggini R, Puccinelli M, Vacca W, Ziliani M. 2015. Boron accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves. Plant and Soil. 395:375–389. doi: 10.1007/s11104-015-2571-9
- Pill WG, Collins CM, Gregory N, Evans TA. 2011. Application method and rate of Trichoderma species as a biological control against Pythium aphanidermatum (Edson) Fitzp. in the production of microgreen table beets (Beta vulgaris L.). Scientia Horticulturae. 129:914–918. doi: 10.1016/j.scienta.2011.05.018
- Pinto E, Almeida AA, Aguiar AA, Ferreira IM. 2015. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. Journal of Food Composition and Analysis. 37:38–43. doi: 10.1016/j.jfca.2014.06.018
- Raimondi G, Orsini F, Maggio A, De Pascale S, Barbieri G. 2006. Yield and quality of hydroponically grown sweet basil cultivars. Acta Horticulturae. 723:357–360. doi: 10.17660/ActaHortic.2006.723.48
- Rorem ES, Walker HG Jr, McCready RM. 1960. Biosynthesis of sucrose and sucrose-phosphate by sugar beet leaf extracts. Plant Physiology. 35:269–272. doi: 10.1104/pp.35.2.269
- Samuolienė G, Brazaitytė A, Jankauskienė J, Viršilė A, Sirtautas R, Novičkovas A, Sakalauskienė S, Sakalauskaitė J, Duchovskis P. 2013. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Central European Journal of Biology. 8:1241–1249.
- Santamaria P. 2006. Nitrate in vegetables: toxicity, content, intake and EC regulation. Journal of the Science of Food and Agriculture. 86:10–17. doi: 10.1002/jsfa.2351
- Santamaria P, Elia A, Serio F. 2002. Effect of solution nitrogen concentration on yield, leaf element content, and water and nitrogen use efficiency of three hydroponically-grown rocket salad genotypes. Journal of Plant Nutrition. 25:245–258. doi: 10.1081/PLN-100108833
- Santamaria P, Elia A, Serio F, Gonnella M, Parente A. 1999. Comparison between nitrate and ammonium nutrition in fennel, celery, and Swiss chard. Journal of Plant Nutrition. 22:1091–1106. doi: 10.1080/01904169909365698
- Spinardi A, Ferrante A. 2012. Effect of storage temperature on quality changes of minimally processed of baby lettuce. Journal of Food, Agriculture and Environment. 10(1):38–42.
- Sun J, Kou L, Geng P, Huang H, Yang T, Luo Y, Chen P. 2015. Metabolomic assessment reveals an elevated level of glucosinolate content in CaCl2 treated broccoli microgreens. Journal of Agricultural and Food Chemistry. 63:1863–1868. doi: 10.1021/jf504710r
- Sun J, Xiao Z, Lin LZ, Lester GE, Wang Q, Harnly JM, Chen P. 2013. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS. Journal of Agricultural and Food Chemistry. 61:10960–10970. doi: 10.1021/jf401802n
- Xiao Z, Lester GE, Luo Y, Wang Q. 2012. Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. Journal of Agricultural and Food Chemistry. 60:7644–7651. doi: 10.1021/jf300459b
- Xiao Z, Lester GE, Park E, Saftner RA, Luo Y, Wang Q. 2015. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biology and Technology. 110:140–148. doi: 10.1016/j.postharvbio.2015.07.021
- Xiao Z, Luo Y, Lester GE, Kou L, Yang T, Wang Q. 2014. Postharvest quality and shelf life of radish microgreens as impacted by storage temperature, packaging film, and chlorine wash treatment. LWT – Food Science and Technology. 55:551–558. doi: 10.1016/j.lwt.2013.09.009
- Yemm EW, Willis AJ. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 57:508–514. doi: 10.1042/bj0570508
- Zheljazkov VD, Warman PR. 2003. Application of high Cu compost to Swiss chard and basil. The Science of the Total Environment. 302:13–26. doi: 10.1016/S0048-9697(02)00390-X
