ABSTRACT
Tulip is one of the most important cut flowers in the world market and abscission of the tepals is this species’ most common symptom during vase life. To prevent these symptoms and extend postharvest life, passive and active modified atmosphere packaging (MAP) was applied at 0°C. Respiratory rate (CO2 Kg−1h−1), ethylene production (µL kg−1h−1) and concentration of CO2 and O2 (%) inside the packaging were evaluated during storage, while fresh weight loss (FWL) (%) and vase life (days) were assessed. Flowers stored using MAP performed significantly better than conventional packaging. A lower FWL (only up to 0.3%) was observed on MAP while FWL was as high as 18 to 21% for conventional packaging after 20 and 31 days, respectively. Vase life was 5.7 and 6.0 days for active and passive MAP, respectively compared to 3.3 days for conventional packing. Thus, MAP successfully extended postharvest life of tulip flowers.
Introduction
Postharvest life of cut flowers is a very challenging process, including the effect of both biotic and abiotic factors that trigger the senescence of the organs (Reid & Jiang Citation2012). Genotype, cultivation system and conditions during storage and transport have been described as the main factors that determine the extension of the vase life of a cut flower (van Doorn & Han Citation2011). Tulip (Tulipa spp.) is one of the most important cut flowers in the market and senescence of the tepals, usually followed by tepal abscission, is the most common symptom that limits this species’ vase life (Iwaya-Inoue & Takata Citation2001; Iwaya-Inoue & Nonami Citation2003). Premature leaf yellowing has also been described in tulips as an important symptom of senescence (Ferrante et al. Citation2003).
Various factors influence the postharvest life of cut flowers, including storage at low temperatures (Çelikel & Reid, Citation2002; Bunya-atichart et al. Citation2004) and high relative humidity (RH) (Reid & Jiang, Citation2012). Moreover, flower preservative solutions are applied to promote water uptake (van Doorn et al. Citation2002; Eason Citation2002; Asrar Citation2012), controlling microorganisms (Knee Citation2000; Macnish et al. Citation2008), reducing the effect of ethylene (Çelikel et al. Citation2002; Serek et al. Citation2006) and inducing (Han Citation2003) and improving flower opening (Eason et al. Citation2004). More recently and less investigated is the use of modified atmosphere packaging (MAP) to increase vase life of cut flowers.
MAP has been used successfully in fruits (Cantín et al. Citation2008; Costa et al. Citation2011), vegetables (Serrano et al. Citation2006; Lee & Baek Citation2008) and fresh cut vegetables (Escalona et al. Citation2007), but few studies have been conducted using cut flowers. Although some authors have reported disappointing results using MAP in cut flowers (Reid Citation2001; Reid & Jiang Citation2012), evaluation of this technique in rose (De Pascale et al. Citation2005; Bishop et al. Citation2007), lily (De Pascale et al. Citation2005; Wu et al. Citation2013) and carnation (Bishop et al. Citation2007) has shown promising results in extending postharvest life. No MAP studies have been performed on tulips, one of the most important cut flowers in the market; consequently, this research is focused on the evaluation of the postharvest life of this species after 20 and 31 days of cold storage using passive and active MAP.
MAP is a system based on the modification of the atmosphere within a package and is aimed at improving the shelf-life of the stored commodity. For fresh products such as cut flowers, decreasing the oxygen (O2) concentration and increasing the carbon dioxide (CO2) concentration to reduce the metabolic activity of the product is a common practice when using this system (Rennie & Tavoularis Citation2009). Moreover, decreasing O2 also reduces ethylene (C2H4) production and therefore retards the ripening of fresh products (Sandhya, Citation2010). The internal atmosphere in packages can be modified artificially by flushing a gas mixture before closing (active modification) or passively by sealing the package under normal air conditions (passive modification), and this depends on the respiration rate of the product and the permeability of the package (Montanez et al. Citation2010). Therefore, once equilibrium between the permeability of the packaging and the product respiration rate has been reached, the shelf-life of the product increases (Sandhya Citation2010).
Packaging films used for MAP can be made of low-density polyethylene (LDPE), polyester (PET), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC) and saran (PVDC) (Smith et al. Citation2003). Each of these materials present specific ranges of O2 and CO2 permeabilities and perforations might be required to obtain the optimal gas composition inside the package (Del-Valle et al. Citation2003).
Materials and methods
Plant material
Tulip flowers grown under greenhouse conditions (temperature maintained at 23 ± 5°C and RH > 50%) were harvested at the stage of <50% of coloured flower bud by mid-August 2013 in the fields of ‘Araucania Flowers’ located in San José de la Mariquinia, Valdivia, Chile (39°31′S 72°58′W). Flowers were land shipped in cardboard boxes under no controlled conditions in a 9 h trip to Santiago, Chile (33°35′S70°38′W), where the evaluations were performed in the Postharvest Laboratory at the Universidad de Chile. Upon arrival to the laboratory, flowers were immediately packed and no holding solutions were used. The initial approach to the use of modified atmosphere on tulip cut flowers involved analysis of eight different cultivars, sorting them equally into each treatment performed ().
Table 1. Identification of the eight tulip cultivars evaluated during postharvest.
MAP of tulip flowers
Tulip flowers were distributed into bunches of 10 flowers each and wrapped using a cellulose film (Cellophane™) for conventional packaging. For MAP, bunches were packaged in a sealed LDPE homemade bag of 20 µm with a permeability of 2000 mL m−2 day−1 for O2 and 6000 mL m−2 day−1 for CO2 (at 23°C). Active MAP was made up of 10:0:90 O2:CO2:N2 and passive MAP was sealed under atmospheric conditions. Packed tulips were stored at 0°C under dark conditions and RH between 90 and 95%, over plastic trays for 20 and 31 days in order to simulate long maritime shipping from Chile to the Northern hemisphere. Thus, a completely randomised factorial design was set using 10 flowers for each replicate, using at least one stem of each cultivar. Three replicates were performed for each treatment and results were obtained from all the eight cultivars pooled together ().
Table 2. Details of the treatments performed using active and passive MAP to store tulip flowers at 0°C for 20 and 31 days.
Gas concentration and respiration rate characterisation
Evaluations were performed at days 0, 3, 5, 10, 13, 17, 20, 31 during the cold storage, extracting a gas sample (1 mL) from the headspace of the bags with a syringe and injecting it into a gas-metre (Checkpoint, PBI Dansensor, Ringsted, Denmark) to determine percent CO2 and O2 concentrations. Respiration rate was calculated according to Fonseca et al. (Citation2002), expressing the results as CO2 production (mL kg−1 h−1). The gas sample was injected into a flame ionisation gas chromatograph (Agilent Technologies 7820A, USA) fitted with a Porapak QN 80/100 column (1.20 m × 3.18 mm) (Norwalk, Connecticut, USA) to determine ethylene production. Injector and detector temperature was set at 200°C while column temperature was set at 50°C. Helium was employed as the carrier gas with a flow rate of 55 mL min−1. Results were expressed as µL C2H4 kg−1 h−1.
Fresh weight loss (FWL)
The weight of the samples was determined using a digital precision balance (± 0.1 g) (Belltronic ES-300HA; Korea). FWL of the flower stems at the end of postharvest storage was calculated according to the following equation:where %FWL(t) is the percentage of the FWL at time t, FWL0 is the initial sample weight and FWL(t) is the sample weight at time t.
Vase life evaluation
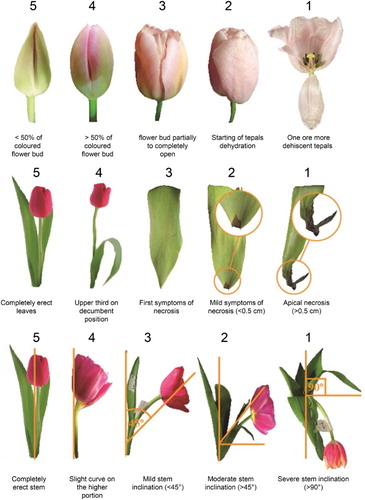
After cold storage for 20 or 31 days, tulip stems were placed in 1.5 L plastic vases filled with 250 mL of distilled water in a room with a temperature between 20 ± 2°C (diurnal temperature) and 16 ± 2°C (nocturnal temperature), 60% ± 5% RH and a light period of 10 h provided by fluorescent lamps. Evaluations on flower, leaf and stem were performed based on a rating scale created for this evaluation (; Supplementary Table 1), which ranged from 1 (worst quality) to 5 (best quality). Each organ on each of the 10 flower stems from the treatments was independently scored. Vase life was determined according to the lowest score of each organ, therefore an average score of any organ below 3 was considered as the end of the vase life. Photos were taken during vase life evaluations at days 0, 1, 5 and 10 using a Nikon D3000 10 Mpx Black (Nikon Co., Tokio, Japan). Colour assessment was also performed during vase life on each organ using a Minolta chroma metre (CR– 300, Minolta Corp., Tokyo, Japan). Evaluations were performed on the middle third section of the flower bud, the adaxial face of the leaf and the stem. Results were expressed using the CIELab system.
Statistical analysis
Data regarding FWL and vase life were subjected to analysis of variance (ANOVA) using the MINITAB software. Means were compared using Tukey’s honestly significant difference (HSD) test for multiple pair-wise comparisons with a significance level of 0.05. Statistical analyses were conducted after arcsine conversion of % of FWL.
Results
Respiration rate and ethylene production of tulip flowers
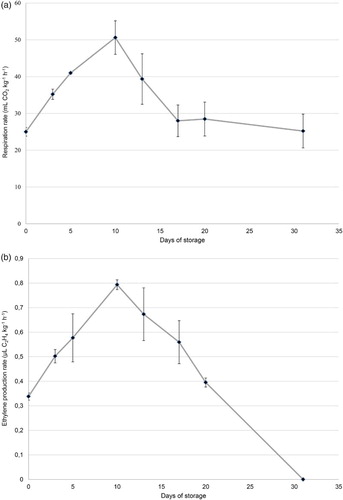
In order to characterise the metabolism of tulip flowers during postharvest storage, respiration rate and ethylene production were evaluated in the control treatment (conventional packaging). Respiration rate increased during the first days of cold storage, reaching a peak at around day 10 (50.61 mL CO2 kg−1 h−1) ((A)). Similar behaviour of the evolution of ethylene production was observed, finding a peak of production of this gas at around day 10 (0.79 µL C2H4 kg−1 h−1) ((B)). After this peak, both respiration rate and ethylene production dropped, reaching a minimum value of 25.21 mL CO2 kg−1 h−1 and a non-detectable C2H4 production rate by the end of storage.
Evolution of the concentration of CO2 and O2 inside MAP
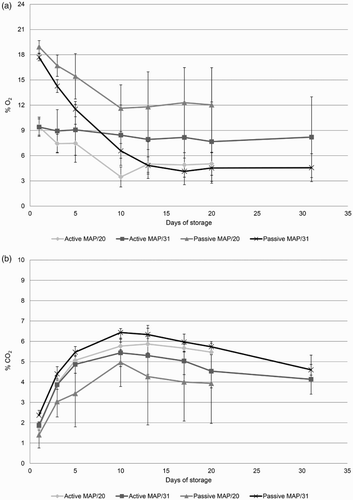
Concentrations of CO2 and O2 inside the passive and active MAP were evaluated during cold storage to analyse the evolution of these gases and their relationship with the permeability of the film and the physiology of the flowers. Concentration of O2 started at about 9% in active MAP and 18% in passive MAP. In both cases, O2 concentration dropped until day 10, when a point of stability was observed until the end of storage ((A)). Concentration of CO2 started with values close to 2% and, as expected, increased until reaching a peak at day 10 with values between 4.9% (Passive MAP/20) and 6.4% (Passive MAP/31) ((B)). However, after this peak a reduction in CO2 concentration was observed for all treatments and a point of stability was not as clear as with the O2 concentration ((b)).
Fresh weight of tulip flowers
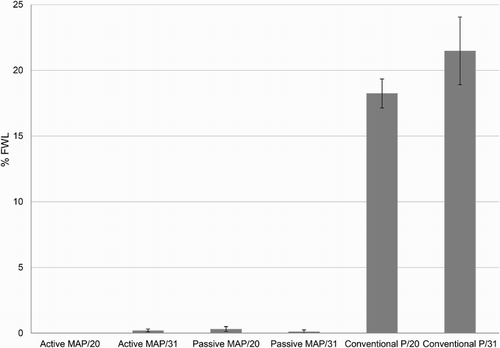
FWL evaluated at the end of storage was significantly lower in the tulip flowers packaged with both the passive and active MAP than with conventional packaging (). In fact, % FWL was very close to 0 with values between 0 and 0.32% when using MAP, while values up to 18.25 (after 20 days) and 21.48% (after 31 days) of FWL were observed when conventional packaging was applied ().
Figure 4. Percentage of fresh weight loss (% FWL) (± SE, n = 3) evaluated at the end of the storage of 3 replicates of 10 tulip flowers each (composed by a mixture of 8 cultivars) submitted to active and passive modified atmosphere packaging (MAP) and cellulose film (cellophane™) packaging (Conventional P) for 20 and 31 days.
Vase life of tulip flowers
Evaluation of vase life showed better results with MAP in comparison with conventional packaging, where tulip flowers only lasted for 6 and 3 days after storage for 20 and 31 days, respectively. For all the treatments, the flower was the limiting factor and the longest vase life was observed when tulip flowers were stored for 20 days using active and passive MAP, showing results between 6.3 and 6.7 days ( and ). Analysis of the vase life observed independently in each organ revealed that flower and leaf showed the lowest vase life values, particularly when using conventional packaging, which lasted for less than 4 days. Conversely, the stem kept its quality, showing more than 10 days of vase life in all the treatments evaluated ().
Figure 5. Pictures of the evolution of the vase life of 3 replicates of 10 tulip flowers each (composed by a mixture of 8 cultivars) after storage at 0°C for 20 and 31 days using active and passive modified atmosphere packaging (MAP) and cellulose film (conventional).
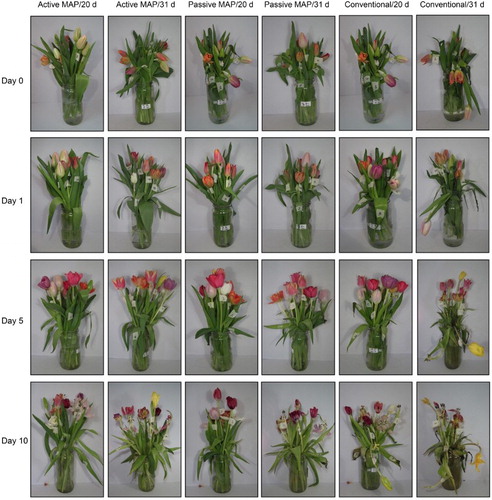
Figure 6. Vase life observed on 3 replicates of 10 tulip flowers, stems and leaves (composed by a mixture of 8 cultivars) (± SE, n = 3) after storage at 0°C using active and passive modified atmosphere packaging (MAP) and cellulose film (cellophane™) packaging (Conventional P) for 20 and 31 days.
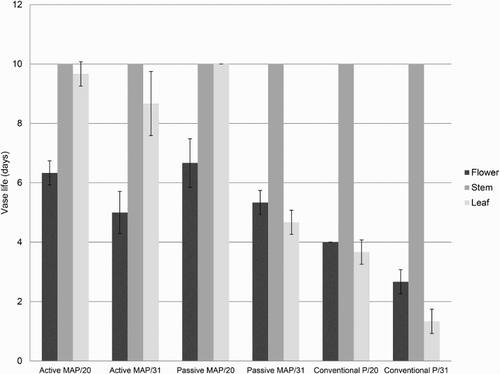
Statistical analysis performed on vase life showed no interaction between ‘cold storage time’ and ‘packaging type’; therefore, they were independently analysed using an ANOVA. Vase life was significantly longer when tulip flowers were stored using MAP compared to conventional packaging (). No differences were observed between active (5.7 days) and passive (6.0 days) MAP (). Regarding the cold storage, vase life was significantly longer when flowers were stored for 20 days (5.7 days), compared to storage for 31 days (4.3 days) ().
Table 3. Vase life mean (n = 6) of tulip flowers stored for 20 and 31 days at 0°C showing the differences between the type of packaging.
Table 4. Vase life mean (n = 9) of tulip flowers stored using conventional packaging, active and passive MAP showing the differences between 20 and 31 days of storage at 0°C.
Evaluation of colour organs during vase life of tulip flowers
Evaluation of colour was also performed during vase life, finding consistent results only in leaves where values of L* (lightness) and b* (yellow/blue) increased during vase life, resulting in a lighter and yellower leaf (Supplementary Figure S1), as observed using the sensorial scale. Flower and stem colour showed no consistent pattern and values were erratic.
Discussion and conclusions
According to the results observed for respiration rate and ethylene production of tulip flowers, which both peak at day 10, a climacteric pattern for tulip postharvest is suggested. The same pattern was observed by Baolian et al. (Citation1999) during postharvest evaluation of tulip flowers. Moreover, Skrzypek et al. (Citation2005) observed similar production of ethylene for this species with values between 0.72 and 1.26 µL C2H4 kg−1 h−1. Nevertheless, Jones and McConchie (Citation1995) described non-climacteric behaviour for tulip flowers with a constant and very low ethylene production (<0.1 µL L−1) during postharvest. In any case, production of ethylene by tulip flowers observed in this study remained very low compared to other fresh products (i.e. fruits or vegetables) as reported by Kader (Citation2007), who described values between 0.1 and 1 µL C2H4 kg−1 h−1 for ethylene production in cut flowers. This explains the remarkable negative effect produced by exogenous ethylene on the postharvest life of fresh flowers.
Respiration rate observed in tulip flowers coincides with results of Coller (Citation1997), who described a respiration rate between 20 and 40 mL CO2 kg−1 h−1 for this species; and it is similar to results found in other cut flowers such as rose (2.4–45.7 µmol CO2 kg−1 h−1) and iris (21.8–60.1 µg CO2kg−1 h−1). These values confirm what was reported by Kader (Citation2007), who classified cut flowers as a product with a high respiration rate. Senescence and therefore postharvest life are closely related to the flower’s metabolism and respiration rate (Teixeira Citation2003; Reid & Jiang Citation2012). It is therefore crucial to apply innovative technologies that reduce respiration rate and can maintain the quality of highly perishable products like cut flowers.
Regarding the gases concentration in the MAP treatments, CO2 did not show stability after the peak at day 10 ((B)), whereas Zeltzer et al. (Citation2001) observed stability at 13% and 7% for O2 and CO2, respectively, using MAP on several cut flowers. According to the main goal of MAP, O2 concentration is the most important as keeping this gas at a low concentration reduces the metabolism of the flowers, extending the storability (Kader, Citation2007). On the other hand, Watkins (Citation2000) referred to the importance of CO2 during MAP, showing effects on several metabolic processes such as respiration and 1-aminocyclopropane-1-carboxylic acid (ACC) synthase activity. Physiological breakdown of most fresh products is described as occurring at very low O2 (<1%) and high CO2 (>20%) concentrations. Thus, the levels of O2 and CO2 observed in this study were successfully collaborating to extend the postharvest life of tulip flowers.
Dehydration produced by transpiration is an important process during postharvest because it is closely related to the loss of quality in fresh products, particularly in cut flowers (Teixeira Citation2003), which are very susceptible due to their high area exposed and their delicate tissue (Kader Citation2007; Reid & Jiang Citation2012) without the cuticle preventing dehydration that fruits have (Lara et al. Citation2014). Furthermore, previous studies have suggested that the commercial quality of cut flowers is lost when over 20% FWL is observed (Martínez-Madrid et al. Citation1998). In this study, MAP successfully diminished FWL in comparison with conventional packaging, due to the effect of the LDPE bags with low permeability used for this type of packaging. FWL during the storage was very important for the subsequent vase life evaluation since those flowers stored using conventional packaging were not able to recover from the dehydration, resulting in a very short vase life. In contrast, flowers stored using MAP showed a quick improvement of quality during the first days of vase life ().
MAP successfully extended storage and vase life of cut tulip flowers. The effect of MAP over the FWL observed was probably more important than the actual effect on gas concentration within the packaging. Further studies considering the isolation of these factors (gas concentration and FWL) should be performed. Results of vase life obtained look very promising, considering that tulip vase life (normally limited by tepal senescence and abscission) has been observed to be between 8 and 12.1 days using different flower preservatives with the application of a previous cold storage for only two days (van Doorn et al. Citation2011). Additionally, Ali et al. (Citation2014) showed results between 6.3 and 11.7 days for vase life of tulip flowers using pulsing solutions without previous cold storage. Thus perhaps a combination of MAP with flower preservatives during postharvest could be able to extend even more the vase life of tulip flowers.
No significant differences in vase life were observed between active (5.7 days) and passive (6.0 days) MAP (). Consequently, considering the easier handling of passive MAP, we can recommend this technique to store tulip flowers. Use of MAP is not frequent in the cut flower industry, although some studies have reported a longer vase life using active MAP in Gerbera (De Pascale et al. Citation2005) and using modified interactive packaging (MIP) in rose and carnation (Bishop et al. Citation2007). Active MAP has extended vase life from 11 to 18 and 20 days using N2 and 1-MCP respectively, particularly in lily (Wu et al. Citation2013).
In this study, the longer (31 days) cold storage duration the less vase life of the flowers was observed (). Long periods of cold storage have been previously identified as having a negative effect on the length of the vase life (van Doorn & Han Citation2011). Redman et al. (Citation2002) observed a significant reduction in the vase life of nine species used as cut flowers with a storage period of 3 weeks at 2°C. Han and Miller (Citation2003) also reported a shorter vase life when lilies were stored at 3.3°C for 2 weeks. In this study, storage of tulip flowers using MAP at 0°C for 20 days was significantly better than their storage for 31 days, and the vase life observed in this treatment (20 days) was in the range observed in previous studies (van Doorn et al. Citation2011; Ali et al. Citation2014), that is around 6 days.
MAP successfully extended the postharvest life of tulip flower stored for 20 days at 0°C. Further studies should be performed to clarify whether the extension of postharvest life using MAP is due to the effect on the concentration of gases or the reduction in dehydration or both. Considering now that the benefit–cost ratio should also be taken into account when applying this method, passive MAP could be the best option. Moreover, the application of this method enhanced by other techniques (i.e. flower preservatives) to extend storage up to 31 days could be an opportunity for overseas shipments of fresh cut flowers.
Acknowledgement
The authors would like to thank to ‘Araucania Flowers’ for providing the tulip flowers evaluated in this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ali A, Rehman S, Hussain R, Raza S, Sarwar M, Bashir A, Khan MA. 2014. Enhancing the vase life of tulip (Tulipa gesneriana L.) using various pulsing solutions of humic acid and NPK. International Journal of Plant, Animal and Environmental Sciences 4(2):193–200.
- Asrar AW. 2012. Effects of some preservative solutions on vase life and keeping quality of snapdragon (Antirrhinum majus L.) cut flowers. Journal of the Saudi Society of Agricultural Sciences 11:29–35. doi: 10.1016/j.jssas.2011.06.002
- Baolian D, Wei S, Yiwei T, Yimin S. 1999. Studies on senescence physiology of tulip flowers during vase life. Journal of Shanghai Agricultural College 17(4):281–289.
- Bishop CFH, Gash AJ, Mathas E, Finlayson I. 2007. Use of modified packaging with cut flowers. Acta Horticulturae 755:515–518. doi: 10.17660/ActaHortic.2007.755.71
- Bunya-atichart K, Ketsa S, van Doorn WG. 2004. Postharvest physiology of Curcuma alismatifolia flowers. Postharvest Biology and Technology 34:219–226. doi: 10.1016/j.postharvbio.2004.05.009
- Cantín CM, Crisosto CH, Day KR. 2008. Evaluation of the effect of different modified atmosphere packaging box liners on the quality and shelf life of ‘friar’ plums. HortTechnology 18(2):261–265.
- Çelikel FG, Reid MS. 2002. Storage temperature affects the quality of cut flowers from the Asteraceae. HortScience 37(1):148–150.
- Çelikel FG, Dodge LL, Reid MS. 2002. Efficacy of 1-MCP (1-methylcyclopropene) and Promalin for extending the post-harvest life of Oriental lilies (Lilium x ‘Mona Lisa’ and ‘Stargazer’). Scientia Horticulturae 93(2):149–155. doi: 10.1016/S0304-4238(01)00331-4
- Collier D. 1997. Changes in respiration, protein and carbohydrates of tulip tepals and alstroemeria petals during development. Journal of Plant Physiology 150:446–451. doi: 10.1016/S0176-1617(97)80096-X
- Costa C, Lucera A, Conte A, Mastromatteo M, Speranza B, Antonacci A, Del Nobile MA. 2011. Effects of passive and active modified atmosphere packaging conditions on ready-to-eat table grape. Journal of Food Engineering 102:115–121. doi: 10.1016/j.jfoodeng.2010.08.001
- De Pascale S, Maturi T, Nicolais V. 2005. Modified atmosphere packaging (MAP) for preserving Gerbera, Lilium and Rosa cut flowers. Acta Horticulturae 682:1145–1152. doi: 10.17660/ActaHortic.2005.682.151
- Del-Valle V, Almenar E, Lagaron JM, Catala R, Gavara R. 2003. Modelling permeation through porous polymeric films for modified atmosphere packaging. Food Additives & Contaminants 20:170–179. doi: 10.1080/0265203021000042869
- van Doorn W, Abadie P, Belde P. 2002. Alkylethoxylate surfactants for rehydration of roses and Bouvardia flowers. Postharvest Biology and Technology 24:327–333.
- van Doorn W, Han SS. 2011. Postharvest quality of cut lily flowers. Postharvest Biology and Technology 62:1–6. doi: 10.1016/j.postharvbio.2011.04.013
- van Doorn W, Perik RRJ, Abadie P, Harkema H. 2011. A treatment to improve the vase life of cut tulips: effects on tepal senescence, tepal abscission, leaf yellowing and stem elongation. Postharvest Biology and Technology 61(1):56–63. doi: 10.1016/j.postharvbio.2011.02.003
- Eason JR. 2002. Sandersonia aurantiaca: an evaluation of postharvest pulsing solutions to maximise cut flower quality. New Zealand Journal of Crop and Horticultural Science, 30(4):273–279. doi: 10.1080/01140671.2002.9514224
- Eason JR, Morgan ER, Mullan AC and Burge GK. 2004. Display life of Gentiana flowers is cultivar specific and influenced by sucrose, gibberellin, fluoride, and postharvest storage. New Zealand journal of crop and horticultural science, 32(2):217–226. doi: 10.1080/01140671.2004.9514299
- Escalona VH, Aguayo E and Artés F. 2007. Modified atmosphere packaging improved quality of kohlrabi stems. LWT-Food Science and Technology 40(3):397–403. doi: 10.1016/j.lwt.2006.02.006
- Ferrante A, Tognoni F, Mensuali-Sodi A, Serra G. 2003. Treatment with thidiazuron for preventing leaf yellowing in cut tulips and chrysanthemum. Acta Horticulturae 624:357–363. doi: 10.17660/ActaHortic.2003.624.49
- Fonseca SC, Oliveira FAR, Brecht JK. 2002. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: a review. Journal of Food Engineering 52:99–119. doi: 10.1016/S0260-8774(01)00106-6
- Han SS. 2003. Role of sugar in the vase solution on postharvest flower and leaf quality of oriental lily ‘Stargazer’. HortScience 38(3):412–416.
- Han SS, Miller JA. 2003. Role of ethylene in postharvest quality of cut Oriental lily ‘Stargazer’. Plant Growth Regulation 40:213–222. doi: 10.1023/A:1025023904151
- Iwaya-Inoue M, Takata M. 2001. Trehalose plus chloramphenicol prolong the vase life of tulip flowers. HortScience 36:946–950.
- Iwaya-Inoue M, Nonami H. 2003. Effects of trehalose on flower senescence from the view point of physical states of water. Environment Control in Biology 41:3–15. doi: 10.2525/ecb1963.41.3
- Jones R, McConchie R. 1995. Characteristics of petal senescence in a non-climacteric cut flower. Acta Horticulturae 405:216–223. doi: 10.17660/ActaHortic.1995.405.28
- Kader A. 2007. Tecnología Postcosecha de Cultivos Hortofrutícolas. 3rd ed. California, CA: UCANR Publications. 571 pp.
- Knee M. 2000. Selection of biocides for use in floral preservatives. Postharvest Biology and Technology 18:227–234. doi: 10.1016/S0925-5214(99)00074-5
- Lara I, Belge B, Goulao L. 2014. The fruit cuticle as a modulator of postharvest quality. Postharvest Biology and Technology 87:103–112. doi: 10.1016/j.postharvbio.2013.08.012
- Lee SY, Baek SY. 2008. Effect of chemical sanitizer combined with modified atmosphere packaging on inhibiting Escherichia coli O157:H7 in commercial spinach. Food Microbiology 25:582–587. doi: 10.1016/j.fm.2008.02.003
- Macnish A, Leonard R, Nell T. 2008. Treatment with chlorine dioxide extends the vase life of selected cut flowers. Postharvest Biology and Technology 50:197–207. doi: 10.1016/j.postharvbio.2008.04.008
- Martínez-Madrid M, Fernández P, Serrano M, Romojaro F. 1998. Factores que determinan la vida útil comercial del clavel. Plantflor 3:25–78.
- Montanez JC, Rodríguez FA, Mahajan PV, Frías JM. 2010. Modelling the effect of gas composition on the gas exchange rate in perforation-mediated modified atmosphere packaging. Journal of Food Engineering 96:348–355. doi: 10.1016/j.jfoodeng.2009.08.007
- Redman P, Dole JM, Maness N, Anderson A. 2002. Postharvest handling of nine specialty cut flower species. Scientia Horticulturae 92:293–303. doi: 10.1016/S0304-4238(01)00294-1
- Reid M. 2001. Advances in shipping and handling of ornamentals. Acta Horticulturae 543:277–284. doi: 10.17660/ActaHortic.2001.543.33
- Reid M, Jiang C. 2012. Postharvest biology and technology of cut flowers and potted plants. 1st ed. California, CA: University of California. Horticultural Reviews, Volume 40.
- Rennie TJ, Tavoularis S. 2009. Perforation-mediated modified atmosphere packaging. Postharvest Biology and Technology 51:1–9. doi: 10.1016/j.postharvbio.2008.06.007
- Sandhya S. 2010. Modified atmosphere packaging of fresh produce: current status and future needs. LWT – Food Science and Technology 43:381–392. doi: 10.1016/j.lwt.2009.05.018
- Serek M, Woltering EJ, Sisler EC, Frello S, Sriskandarajah S. 2006. Controlling ethylene responses in flowers at the receptor level. Biotechnology Advances 24(4):368–381. doi: 10.1016/j.biotechadv.2006.01.007
- Serrano M, Martinez-Romero D, Guillén F, Castillo S, Valero D. 2006. Maintenance of broccoli quality and functional properties during cold storage as affected by modified atmosphere packaging. Postharvest Biology and Technology 39(1):61–68. doi: 10.1016/j.postharvbio.2005.08.004
- Skrzypek E, Miyamoto K, Saniewski M, Ueda J. 2005. Jasmonates are essential factors inducing gummosis in tulips: mode of action of jasmonates focusing on sugar metabolism. Journal of plant physiology 162:495–505. doi: 10.1016/j.jplph.2004.09.007
- Smith JP, Ramaswamy HS, Ranganna B, Raghavan GSV. 2003. Packaging of fruits and vegetables. In: Chakraverty A, Mujumdar AS, Raghavan GSV, Ramaswamy HS, editors. Handbook of postharvest technology: cereals, fruits, vegetables, tea, and spices. New York (NY): Marcel Dekker, Inc; p. 539–554.
- Teixeira J. 2003. The cut flower: postharvest considerations. Journal of Biological Sciences 4:406–442.
- Watkins CB. 2000. Responses of horticultural commodities to high carbon dioxide as related to modified atmosphere packaging. HortTechnology 10(3):501–506.
- Wu XW, Suh JK, Wang LH. 2013. Effects of modified atmosphere packaging and storage conditions on freshness maintenance of cut flowers of oriental lily ‘Activa’. Acta Horticulturae. 1002:381–387. doi: 10.17660/ActaHortic.2013.1002.51
- Zeltzer S, Meir S, Mayak S. 2001. Modified atmosphere packaging (MAP) for long-term shipment of cut flowers. Acta Horticulturae 553:631–634. doi: 10.17660/ActaHortic.2001.553.152