ABSTRACT
The common use of peat as a growing medium for plant production is questioned due to the negative effects on the environment and climate. Therefore, it is of great importance to find substitutes for peat with similar positive properties that have made peat one of the most important substrates in the horticultural sector. Production of biogas from organic residues is a valuable process, producing both energy and a residue, anaerobic digestate (AD), with a high content of plant nutrients. In this study, peat was partly substituted with AD of plant material origin up to 80% in a growing substrate for basil (Ocimum basilicum). Germination, yield and plant nutrient content were measured as well as chemical and physical properties of the growing media. The results showed that with 50% substitution of peat the growth in the AD/peat mix gave the same yield as for the fertilised peat on its own. No toxic or deficiency symptoms could be seen in the 50% mix. The important physical properties of the substrate were similar in peat and AD/peat mixes. However, the water-holding capacity was slightly decreased when part of the peat was substituted.
Introduction
Throughout the year, a considerable part of commercial vegetable production is performed in protected cropping systems based on the use of non-renewable resources, such as mineral fertilisers and fossil fuels, in a rather linear system (Stewart et al. Citation2005; Stoknes Citation2020). The development of resource-efficient and recirculated fertilisers and substrates for vegetable production provides an important step towards more sustainable food production required to meet future challenges. Increased public demand for sustainable and organically produced food (Irandoust Citation2016) and new ways to close the loop between producers and consumers calls for increased circulation of food waste, crop residues and other agricultural sources of organic waste materials (Reganold and Wachter Citation2016; Tittarelli Citation2020).
Several municipal and agro-industrial residues have been investigated and used for plant nutrition, such as sewage- and agro-sludge, various types of composts, manure slurries (Alvarenga et al. Citation2015) as well as anaerobic digestates (AD) from biogas production based on residues from crop production and the food industry (Tambone et al. Citation2010). The use of AD from biogas production as a soil amendment and fertiliser has been established in agricultural field production (Tambone et al. Citation2010; Lee et al. Citation2020) and it is currently investigated for use in protected vegetable production as a liquid fertiliser (Barzee et al. Citation2019; Liu et al. Citation2011; Lind et al. Citation2020; Bergstrand et al. Citation2020a) and as a growing substrate in soilless systems (Restrepo et al. Citation2013; Stoknes et al. Citation2018). Anaerobic digestate has been shown to have good fertiliser qualities (Tambone et al. Citation2010; Lind et al. Citation2020) and its increased use could lead to a reduced need for mined or industrially produced mineral fertilisers connected with a high use of fossil fuel and increased CO2 emissions (Stewart et al. Citation2005). Furthermore, production of AD is part of an efficient waste handling process, which at the same time provides renewable energy as methane through the biogas production (Möller and Müller Citation2012).
In the EU, greenhouse production of, for example, vegetable transplants, herbs and pot plants largely rely on the use of peat as the predominant substrate (Schmilewski Citation2009). Several negative environmental and ecological issues connected with the use of peat provide a strong incentive to introduce alternative horticultural substrates (Ceglie et al. Citation2015). In the light of this, the solid residue from the dewatered fraction of AD has been suggested as a growing medium and/or fertiliser for greenhouse vegetable production (Stoknes et al. Citation2018; Bergstrand et al. Citation2020b). Similar to the liquid fraction of the AD, the dewatered (solid) fraction of the AD is also rich in plant nutrients, however, with slightly different relative proportions of nutrients as compared to the unseparated sludge (Stoknes et al. Citation2016). If peat could be partly or completely substituted by solid AD as a substrate with fertilising properties, the sustainability of pot plant production would evidently be increased. EU legislation allows for organic certification of pot production in substrates, as long as the products are intended to be sold to the end consumer together with the pot, as is typically the case for ornamentals and herbs (EC Citation2018; KRAV Citation2021). Thus, solid AD may also provide a promising candidate as a growing medium for certified organic production in protected cropping systems.
Substitution of an established and thoroughly investigated substrate such as peat calls for careful investigations of both physical and chemical properties of the intended new substrate. Different combinations of solid AD and peat or other organic constituents, with or without fertigation with liquid digestate, have been tested for production of a variety of horticultural crops. Restrepo et al. (Citation2013) found improved nutritional status of seedlings of several horticultural species established in peat/AD mixes (from maize silage and cattle manure) containing 25–75% of AD, compared with a growing medium of (pure) fertilised peat. Pokhrel et al. (Citation2018) grew parsley (Petroselinum crispum) in growing media consisting of AD originating entirely from plant residues. They found that, even when mixed with different organic residues, the plant-derived AD needed supplementary nitrogen (N) to be adequate for the plants. In all mixes with the AD, they also observed increased values for NH4: NO3 ratio, pH, electric conductivity (EC) and for concentrations of potassium (K), compared with when conventional inorganic fertilisers were used. For a crop with a longer growing cycle (tomato, Solanum lycopersicum), Stoknes et al. (Citation2018) also found the need for additional fertilisation when solid AD, mixed with vermicompost, was used as growing medium and fertiliser. When AD, vermicompost and fertigation with the liquid portion of AD were combined, the result was a balanced fertiliser/substrate, and the harvested yields of the tomatoes corresponded well with that of the control with mineral fertiliser. The challenges of this system, as described by the authors, were to control the NH4+ concentrations, pH and EC, as well as high concentrations of Cl and low concentrations of NO3−.
Parallel with the possible benefits achieved by increased use of AD in plant production, its use as a fertiliser is not without challenges. The digestate is characterised by a high pH (7.5–8.5) and a high NH4+: NO3− ratio, both of which are traits unfavourable for plant production (Möller and Müller Citation2012). Chemical reactions during the biogas process may result in low concentrations of Mg, Ca, P and S by the formation of calcium and magnesium phosphates, for example, struvite, hydroxylapatite and iron sulphides (Abubaker et al. Citation2012; Möller and Müller Citation2012; Zirkler et al. Citation2014). The impact of the diverse origin of the substrate used in the biogas process further enhances the complexity, and the problems that may arise are high Cl concentrations from food waste, unfavourable nutrient ratios in animal by-products, as well as changing physical properties due to the continuous biological activities. Thus, to allow for the increased use of AD in containerised production and to ensure the stability and quality of the substrate during its use in pot plant production, further studies are needed. This study therefore investigated how chemical and physical properties in peat-based growing media changed with increased proportions of plant-derived AD and monitored the effects on the growth and mineral content of pot-produced basil.
Materials and methods
Plant material, substrates and growing conditions
Basil, Ocimun basilicum cv. Edwina, (Olssons frö AB, Sweden) were grown in different mixes of a peat-based substrate and the solid fraction of anaerobic digestate (AD). The solid AD was provided from a commercial biogas producer (Gasum Jordberga AB, Sweden). The biogas plant used a feedstock of crop residues (33.4%), plant-based residues from food industries (20.1%), iron chloride (0.6%) and water (45.8%). After the biogas process, the residue was dewatered by mechanical pressure to a dry matter content of 22%. The peat substrate used in the experiment (S-soil, Hasselfors Garden AB, Örebro, Sweden) consisted of 45% light peat (H2-4), 25% dark peat (H6-8), 25% perlite and 5% sand. It had been fertilised by the manufacturer with 0.9 kg m−3 N-P-K (14-7-15% w/w) and limed with 6 kg m−3 of Ca-Mg carbonate. This peat substrate was considered as a substrate with a low addition of fertiliser, formulated to be used as a propagation substrate.
The peat was thoroughly mixed with the AD in the following proportions: 0, 20, 50 and 80% AD (v/v, weighed to the desired proportion by use of the bulk density). Half of the volume of each mix was fertilised with 4 g L−1 of a controlled-release fertiliser (Basacote 3 M controlled release, N-P-K 16-4-10, Compo GmbH & Co KG, Münster, Germany) (treatments coded: +), while the other half received no additional fertiliser (coded: -). Ten pots (12 cm, 0.7 L) were used for each AD-mix-level and ± fertiliser giving a total of 80 pots. Trays were placed under the pots to prevent loss by runoff. Twenty-five seeds of basil were sown in each pot and thinned to 15 plants per pot at six days after emergence. Irrigation of the pots was done manually with tap water to a weight corresponding to 50% of the water-holding capacity for each mix (see ). Irrigation was performed when the weight was approximately 10% below the target value as determined using a balance.
Table 1. Physical properties of mixes of peat substrate and solid anaerobic digestate (AD, 0–100%). Different letters denote significant differences at p < 0.05, n = 2. Normal distribution and equal standard deviations were assumed.
The plants were placed in a greenhouse at The Swedish University of Agricultural Sciences campus Alnarp (55°N), Sweden. The set points for the climate were 20°C for heating and 22°C for ventilation via rooftop vents. Shading screens were set to close when the outside radiation exceeded 700 W m−2. The climate was controlled by a greenhouse computer (Priva Intégro v. 730, Priva, de Lier, the Netherlands). The trial was performed between 11 May and 16 June with a natural photoperiod of 13–15 h. No supplementary lighting was used.
Measurements and analyses
After thorough mixing, the substrates were allowed to settle for 4 days before the physical and chemical analyses were performed. By the time of the mixing, the peat and AD had approximately the same water content of 20–22%. The pH and EC of the growing media were determined according to EN13038:2011 (European standards Citation2011), where 1 part of growing medium was extracted in 5 parts of distilled water. Dry bulk density was determined by the standard procedure EN13040:2007 (European standards Citation2007), where approximately 1 L of growing medium was compacted by a 634 g weight in a steel cylinder. The compressed substrate was weighed and the dry bulk density calculated.
The compact density of the material (density without pores) was determined by adding a defined amount of the growing medium (approximately 5 g) to a 50 mL volumetric flask with the addition of 25 ml ethanol, 96%. When the sample with alcohol had been shaken for 30 minutes the flask was topped up to 50 mL with a burette, the added volume of ethanol was determined, and the compact density calculated. The porosity of the growing media was calculated by the formula:
Porosity (%) = (1-(bulk density/compact density) x 100)(1)
Water filled pore-space after drainage was determined by filling a plastic cylinder (0.75 L) with the growing medium. The cylinder was perforated at the bottom allowing water to drain out and had a removable collar on the top. The cylinder and collar were filled, and the medium was compacted by a 634 g weight for three minutes. Then the medium was immersed in water for two days allowing it to become totally water saturated, and then left on a steel grid to drain for three days. The top of the cylinder was covered with plastic film to avoid evaporation. After three days of drainage, the collar was lifted and the extra substrate carefully removed to get a final volume of the medium of 0.75 L. The substrates were then weighed and dried for 4 days in 105°C and the dry weight was determined. The volume of water in each sample was compared to the total porosity and the volume of the water filled pores were calculated.
Prior to the sowing, the readily available plant nutrients in the substrates were estimated by the modified Spurway Lawton (SL) extraction procedure (Spurway and Lawton Citation1949) as this is the most commonly used extraction in the Swedish horticultural sector. Twenty-five mL of substrate was extracted for 30 min in an end-over-end shaker with 150 mL acetic acid (0.018 moles L−1) and then filtered and analysed. These extractions and analyses were made by a commercial agricultural laboratory (LMI AB, Helsingborg, Sweden).
For determination of plant available NO3− and NH4+ during the cultivation period, liquid from the substrates was sampled by installed Rhizon soil moisture samplers (Eijkelkamp Agriresearch Equipment, Giesbeek, the Netherlands). Two moisture samplers per pot in three pots per treatment were installed from the beginning of the cultivation. Samples were collected, 24 hours after irrigation, at 4 occasions, by connecting a vacuum tube (BD Vacutainer, Becton, Dickinson and Co, Franklin Lakes, NJ, USA) to the sampler. The vacuum tubes were allowed to draw samples for 24 hours before they were disconnected. Nitrate-N and ammonium-N were analysed with Hach test kits LCK303 and LCK340 using a Hach spectrophotometer (Xion 500, Hach, Loveland, CO, USA).
Six days after sowing, germination was graded from 0 to 3 according to the percentage of the 25 seeds in each pot that had developed fully expanded cotyledons: 3) 95%, 2) 50 to 94%, 1) 25 to 49% and 0 < 25%.
The height of the plants was recorded weekly using a ruler. The height was measured from the substrate surface to the top of the plants in each pot. At the end of the experiment, fresh weights (FW) were determined directly after harvest (cut 0.5 cm above the substrate surface) and the dry weights (DW) were determined after 4 days in a drying cabinet at 70°C.
Concentrations of macro- (except nitrogen) and micro-nutrients in the plant tissue were determined at harvest by a commercial analytical company (Eurofins AB Kristianstad Sweden). In short, the plants were dissolved in concentrated nitric acid and hydro peroxide by means of micro-wave technique. After dilution with distilled water the nutrients were analysed with atomic absorption spectrophotometry by flame or graphite furnace by NMKL No. 1,611,988 (NMKL Citation1998). Nitrogen was determined by the Dumas principles according to EN ISO 16634–2:2016 (European standards Citation2016) by the same company.
Statistics
The cultivation experiment was set up as a completely randomised trial with 10 pots per treatment. Data were tested for differences by analysis of variance (ANOVA) and Tukey´s multiple comparison test, p-values < 0.05 were considered significant (Minitab v. 17, Minitab inc. State College PA, USA). For the chemical analyses of plants n = 3 and for the soil parameters n = 2. In the latter case, normal distribution was assumed, and the standard deviations in this case were assumed equal.
Results
The weight to volume relationship in the peat substrate and the AD were similar, resulting in the same dry bulk density in all tested mixes (), which also proved to be the case for the total porosity. The water holding capacity in the pots, after free drainage, decreased with increased proportions of AD, and the substrate was holding approximately15% less water in the mix with the highest proportion of AD compared to the peat ().
The concentrations of readily available nutrients in the substrates before use are shown in . The analysis revealed high concentrations of most nutrient elements in the AD, which was also reflected by the increased EC. The mineralised N occurred mainly in the ammonium form (ca 85%) and the rest of the mineralised N as nitrate. All elements increased with increased AD proportions, with the exceptions for sulphur and sodium that remained constant and for nitrate and calcium, which both decreased (). Both pH and EC increased with increased AD additions. The AD had a pH one unit higher than the peat only substrate.
Table 2. Electric conductivity, EC (mS cm−1), pH and available nutrient concentrations (mg L−1 substrate), according to a modified Spurway-analysis, in mixes of peat and solid anaerobic digestate (AD, 0–100%). BD = below detection limit.
Germination recorded six days after sowing showed that the higher percentages of AD (50 or 80%) in the growing medium significantly reduced germination (). However, the seedlings in the mix with 50% AD could not be separated by visual inspection from the treatments with less AD after another 4 days (data not shown).
Table 3. Germination of basil seeds in mixtures of peat and solid anaerobic digestate (AD, 0–80%). Germination score was the average from 10 pots graded from 0 to 3 (see materials and methods). Treatments marked with (+) received mineral fertiliser and treatments marked (-) received no additional fertiliser. Different letters denotes significant differences at p < 0.05.
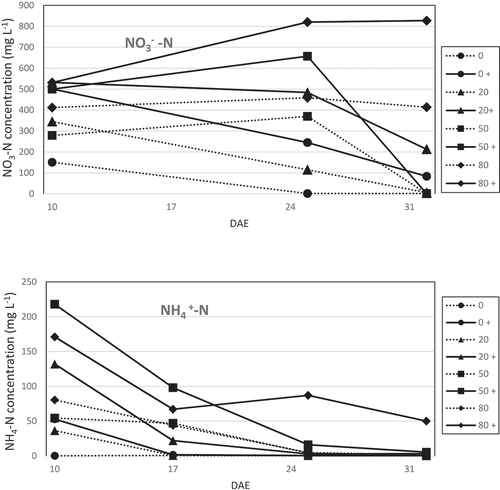
For all treatments, there was a rapid drop in the ammonium concentration in the substrate solutions during the first 15 days of the experiment (). The ammonium was derived from the AD, without any measurable amount from the peat, as can be seen in . Twenty-five days after emergence (DAE), ammonium remained only in the 50 and 80% AD treatments with additional fertiliser, and at 32 DAE only the highest AD treatment contained ammonium (). In the two highest AD-treatments, the nitrate concentration in the substrate solution increased between 10 to 25 DAE. In four of the treatments, there were neither ammonium nor nitrate left in the sampled moisture at 32 DAE.
Figure 1. Mineral nitrogen (nitrate-N and ammonium-N) concentration (mg L−1) in the substrate solution at three (nitrate) or four (ammonium) occasions (DAE, days after emergence). Treatments: 0–80% anaerobic digestate mixed with peat. Each data point represents a pooled sample of a total of six moisture samplers in three pots per treatment; the samples were pooled as only small volumes of liquid were obtained in each sampler and the data were thus not statistically analysed. The solid lines denote treatments with added mineral fertiliser (+).
The plants were harvested at 32 DAE. The plants in the two 80% AD treatments had ceased to grow in the last week and showed deficiency or toxicity symptoms in their youngest leaves with dark purple discoloration and curled leaves (data not shown). These high AD treatments had slower growth throughout the cultivation and ended up as the smallest plants together with the 50% AD with additional fertiliser (). The highest fresh weights (FW) were found in the 20%+ and 50%- mixtures (). Additional fertiliser increased the FW in the 20% AD treatment but this was not the case with 50% AD. However, there were no significant increases in DW in any of the AD treatments by the addition of the mineral fertiliser. The highest DW value was found in the 20% AD treatments, however, they were only slightly higher compared with 50% AD ().
Table 4. Plant growth parameters at harvest (32 DAE) of basil plants grown in pots with a mix of a peat substrate and solid anaerobic digestate (AD, 0–80%). – and + denotes treatments without or with additional mineral fertiliser. Different letters denote significantly different means at p < 0.05 within each pair of columns (each parameter), n= 10.
With the exception of sulphur, the concentrations of macro-nutrients in the plant tissue increased or decreased in accordance with the varying concentrations of plant nutrients in the growing media, as measured with the Spurway-Lawton extraction (compare values in and (0–80%)). The highest concentrations of macro-nutrients in the plants were typically found in the 80%- and 80%+ treatments except for Ca. Also, the decrease in plant Ca was in agreement with values presented in with decreasing values of Ca as the AD component in the substrate increased (). The correlation between plant micro-nutrient concentrations and the substrate extraction values agreed less well, but there was a general trend of increasing concentrations with increased proportions of AD (compare ).
Table 5. Macro-nutrient content, expressed as % of DW, in basil shoots grown in a mix of peat substrate and solid anaerobic digestate (AD, 0–80% digestate). Half of the plants received no additional fertiliser (-) and half were given additional mineral fertiliser (+). Values followed by different letters within each mineral (- and + columns together) are significantly different at p< 0.05 (n = 3).
Table 6. Micro-nutrient concentrations (mg kg−1 DW) in basil shoots grown in a mix of peat substrate and aerobic digestate (AD, 0–80% digestate). Half of the plants received no additional fertiliser (-) and half were given additional mineral fertiliser (+). Values followed by different letters within each mineral (- and + columns together) are significant different at p< 0.05 (n= 3). BD = below detection limit.
Discussion
Treatment of organic waste by anaerobic digestion offers several advantages compared to other waste handling practices (Ward et al. Citation2008), and this technique is increasingly used in large parts of the world. Anaerobic digestion produces a residue rich in plant nutrients, and the sustainability of the process depends greatly on how the nutrients can be recovered and reintroduced in the food production chain. The use of the digestate not only as a fertiliser but also as a growing medium constituent, for example, in container production, further increases the circularity and value of the digestate, as thoroughly summarised by Stoknes (Citation2020). This coincides well with the incentive to decrease the use of peat in the horticultural sector (Ceglie et al. Citation2015).
In the present study, the bulk density and total porosity remained almost constant within the different mixes of the peat substrate and the AD (), while the water-holding capacity decreased with increased proportions of AD in the mixture, suggesting changed ratios between micro-, meso- and macro pores (Yeager et al. Citation1997). However, the range of 66–81% water filled pores, giving a water holding capacity (WHC) ranging between 68% and 55% (with 0–80% AD) should not pose any problems for the plants, but may require different watering strategies. Yeager et al. (Citation1997) stated in a survey that the soil parameters; total porosity 50–85%, WHC 45–65% and air space 10–30% were appropriate for pot production in an organic growing medium. Furthermore, Abad et al. (Citation2005) stated that a bulk density <400 g L−1 should be ideal for an organic growing medium, strengthening the case for the peat/AD mix as a growing medium with suitable physical properties.
The additions of 20% and 50% AD to the peat substrate gave a clear fertilising effect (). The increased nutrient concentrations by the extra addition of the mineral fertiliser to the growing substrate increased the FW only when 0 or 20% AD were used in the substrate (). The addition of the mineral fertiliser to the plants was only used to establish at which levels of AD there were no fertiliser effect of the additional nutrients, which was the case at 50% AD, and this was not tested as a necessary nutrient addition for the organic production system. The choice of using a mineral fertiliser for this purpose was made to provide an easy way to find this level. The use of an organic nutrient source might have seemed more appropriate, but this would have meant having two different organic fertilisers in the system with less predictable mineralisation than the mineral source, which would have made the interpretation of the results more difficult. At 50% AD, the added mineral fertiliser decreased the growth of the basil. The fertiliser effect was not so clear regarding the DW, and in the (-) treatments the %DM decreased with increased percentage of AD. The reasons for the decrease in the growth at the higher AD proportions () may be explained by the increased pH and EC, as well as by the higher concentrations of one or more elements. One candidate being ammonium, which has been shown to become toxic at high concentrations with visual symptoms as reduced growth and lower root to shoot ratio (Britto and Kronzucker Citation2002). At neutral or higher pH, ammonia is formed from high ammonium concentrations and is released from the growing medium as a toxic gas, which was shown to impair the growth of basil after 2–3 weeks cultivation (Frerichs et al. Citation2020). The ammonia formation is elevated at high temperatures, making this a potential problem in greenhouse production. Increased turnover of ammonium to nitrate by nitrification was shown to be an efficient way to decrease the negative influence of high ammonium concentrations, as it lowered the gas emission and pH simultaneously (Frerichs et al. Citation2020). Conversion from ammonium to nitrate was clearly shown in the present study, since the highest nitrate concentrations were found at 25 DAE in the treatment that originally had the lowest concentrations of available nitrate in the unfertilised peat/AD mix (compare results shown in and ). The EC reached the highest value, 5.2 mS cm−1, in the 80% AD treatment, which should only have slightly affected the growth of a moderately sensitive plant as basil (Greenway and Munns Citation1980).
The reduced germination rates in the treatments with high proportion of AD was probably attributable to the elevated EC. Presence of NH4+ at concentrations within the range found in this study was shown to promote rather than inhibit germination (Hendricks and Taylorson Citation1974), whereas elevated salinity already at 1 mS cm−1 was shown to reduce the germination of green basil, as compared to germination at 0 mS cm−1 (Ramin Citation2006).
The low level of mineral fertiliser in the peat substrate could most likely be omitted and substituted by a higher percent of AD, considering the proper balance of nutrient ions in the AD. This would make the system truly organic. In later experiments with the same system, the mineral fertiliser was exchanged without any significant effect on growth (data not shown).
The concentrations of the majority of the macro-nutrients in the plants correlated well with the estimated availability of nutrients in the substrates, as determined by the Spurway extraction. The remaining organic matter after the digestion process have been shown to be recalcitrant with a low mineralisation rate over several months in field production (Moorhead et al. Citation1987). Thus, for a short crop-like basil, the extra nutrient contribution from the mineralisation of the solid AD does not need to be accounted for, meaning that the weak extraction methods like Spurway, CAT+ etc. may give sufficient information for a fertiliser strategy.
As pointed out in the introduction, the digestion process might lead to the formation of less soluble compounds resulting in low nutrient availability. However, when compared to a Hoagland solution (Hoagland and Arnon Citation1950), which is balanced according to the nutrient demand for most plants, the mix of the peat substrate and AD showed higher element to Nmin ratios for all macro-nutrients as extracted by the Spurway method (data not shown). This would implicate that as long as the Nmin is sufficient this would also be the case for the rest of the macro-nutrients, however excessive concentrations may occur instead. Among the micro-nutrients, Fe stands out in the peat/AD mix by showing a low Fe: Nmin ratio as compared to the Hoagland solution. This might be surprising since iron chloride is added to the digestion process. The iron is added to decrease the amount of corrosive H2S to be formed by precipitation of the S to ferric or ferrous sulphides (Moestedt et al. Citation2013). Since the formed iron-sulphides are insoluble the availability of iron will decrease with increased sulphur concentration in the biogas feed stock. Except for Fe, which was in the low range, the other analysed micro-nutrients were in the range between deficiency and toxicity when compared to general values of deficiency and toxicity stated in the prominent textbook ‘Marschner’s Mineral Nutrition of Higher Plants’ (Marchner Citation2012).
A concern sometimes raised in the context of the use of organic waste-derived fertilisers, is the possible contamination of heavy metals and thus the threat for human health and plant growth. However, the concentrations of heavy metals were all substantially lower in the AD used (data not shown) compared to the maximum accepted levels in organic waste used as fertilisers, according to the certification authority (Avfall Sverige Citation2020), and were thus not investigated in the present study.
Conclusions
From a physical and chemical perspective, the use of AD based on plant material, as a partial peat substitute and fertiliser appeared to be promising. A mix with 50% AD with the peat resulted in yields comparable with the fertilised peat substrate. At this level of AD there was no fertilising effect by the additional application of nutrients showing that the amendment with AD can be useful for organic production since the same yield can be reached with the use of the organic fertiliser alone as with the mineral fertiliser. No toxic or deficiency symptoms were seen at the 50% substitution with AD. When the proportion of AD was increased to 80% the growth of the plants was hampered. The reason for this was not clear, but the high ammonium concentration and pH were probable reasons. Production of basil with AD provides a promising tool to increase the organic production of basil, and possibly also other herbs, in pot production. In future research it would be interesting to investigate if the ammonium concentration can be reduced in the AD, e.g. by nitrification. This may increase the levels by which the AD can be mixed in with peat or perhaps with other growing media ingredients in organic production systems.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abad M, Fornes F, Carrion C, Noguera P, Maguieira A, Puchades R, Puchades R. 2005. Physical properties of various coconut coir dusts compared to peat. Hortscience. 40(7):2138–2144. doi:10.21273/HORTSCI.40.7.2138.
- Abubaker J, Risberg K, Pell M. 2012. Biogas residues as fertilisers – effects on wheat growth and soil microbial activities. Appl Energy. 99:126–134. doi:10.1016/j.apenergy.2012.04.050
- Alvarenga P, Mourinha C, Farto M, Santos T, Palma P, Sengo J, Morais M-C, Cunha-Queda C. 2015. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: benefits versus limiting factors. Waste Management. 40:44–52. doi:10.1016/j.wasman.2015.01.027
- Barzee TJ, Edalati A, El-Mashad H, Wang D, Scow K, Zhang R. 2019. Digestate biofertilizers support similar or higher tomato yields and quality than mineral fertilizer in a subsurface drip fertigation system. Front Sustain Food Syst. 3. doi:10.3389/fsufs.2019.00058
- Bergstrand K-J, Asp H, Hultberg M. 2020a. Utilizing anaerobic digestates as nutrient solutions in hydroponic production systems. Sustainability. 12(23):10076. doi:10.3390/su122310076.
- Bergstrand K-J, Löfkvist K, Asp H. 2020b. Dynamics of nutrient availability in tomato production with organic fertilisers. Biol Agric & Hort. 36(3):200–212. doi:10.1080/01448765.2020.1779816.
- Britto DT, Kronzucker HJ. 2002. NH4+ toxicity in higher plants. A critical review. J Plant Physiol. 159(6):567–584. doi:10.1078/0176-1617-0774.
- Ceglie FG, Bustamante MA, Amara MB, Tittarelli F, Singer AC. 2015. The challenge of peat substitution in organic seedling production: optimization of growing media formulation through mixture design and response surface analysis. PLOS ONE. 10(6):e0128600. doi:10.1371/journal.pone.0128600.
- EC. 2018. Regulation (EU) No 2018/848, of May 30 2018 on organic production and labelling of organic products. Off J Eur. 61:5.
- European standards. 2007. EN 13040:2007 Soil improvers and growing media. Sample preparation for chemical and physical tests, determination of dry matter content, moisture content and laboratory compacted bulk density. 978 0 580 54504 7.
- European standards. 2011. EN 13038:2011. Soil improvers and growing media. Determination of Electrical Conductivity. 1-14. 978 0 580 68880 5.
- European standards. 2016. EN ISO 16634-2:2016. Food products- determination of the total nitrogen content by combustion according to the Dumas principle and calculation of the crude protein content. 1-24. 978 0 580 54276 3.
- Frerichs C, Daum D, Pacholski AS. 2020. Ammonia and ammonium exposure of basil (Ocimum basilicum L.) growing in an organically fertilized peat substrate and strategies to mitigate related harmful impacts on plant growth. Front Plant Sci. 10:1696. doi:10.3389/fpls.2019.01696.
- Greenway H, Munns R. 1980. Mechanisms of salt tolerance in non-halophytes. Annu Rev Plant Physiol. 31(1):149–190. doi:10.1146/annurev.pp.31.060180.001053.
- Hendricks SB, Taylorson RB. 1974. Promotion of seed germination by nitrate, nitrite, hydroxylamine, and ammonium salts. Plant Physiol. 54(3):304–309. doi:10.1104/pp.54.3.304.
- Hoagland DR, Arnon DI. 1950. The culture method for growing plants without soil. In: Circ. Vol. 347. 1-32. Berkley (USA): California Agr. Exp. Stn. Univ. of California.
- Irandoust M. 2016. Modelling consumers’ demand for organic food product: the Swedish experience. Int J of Food and Agric Economics. 4:77–89.
- KRAV. 2021. Regler för KRAV-certifierad produktion 2021. (Regulations for KRAV (organic) – certified production 2021). KRAV Uppsala (Sweden. Swedish): 108–109, Krav ekonomisk förening.
- Lee ME, Steinman MW, St. Angelo SK. 2020. Biogas digestate as a renewable fertilizer: effects of digestate application on crop growth and nutrient composition. Renewable Agric and Food Systems. 1–9. doi:10.1017/S1742170520000186
- Lind PO, Hultberg M, Bergstrand K-J, Larsson-Jönsson H, Caspersen S, Asp H. 2020. Biogas digestate in vegetable hydroponic production: pH dynamics and ph management by controlled nitrification. Waste Biomass Valor. doi:10.1007/s12649-020-00965-y
- Liu WK, Yang QC, Du LF, Cheng RF, Zhou WL. 2011. Nutrient supplementation increased growth and nitrate concentration of lettuce cultivated hydroponically with biogas slurry. Acta Agriculturae Scandinavica, Section B - Soil & Plant Science. 61(5):391–394. doi:10.1080/09064710.2010.482539.
- Marchner P, editor. 2012. Mineral nutrition of higher plants. Third. London (UK): Academic press. 9780. 9780081014318. 081014318. 191–243.
- Moestedt J, Nilsson Påledal S, Schnürer A. 2013. The effect of substrate and operational parameters on the abundance of sulphate-reducing bacteria in industrial anaerobic biogas digesters. Bioresour Technol. 132:327–332. doi:10.1016/j.biortech.2013.01.043
- Möller K, Müller T. 2012. Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng Life Sci. 12(3):242–257. doi:10.1002/elsc.201100085.
- Moorhead KK, Graetz DA, Reddy KR. 1987. Decomposition of fresh and anaerobically digested plant biomass in soil. J Environ Qual. 16(1):25–28. doi:10.2134/jeq1987.00472425001600010031x.
- NMKL. 1998. Determination by atomic absorption spectrophotometry after wet digestion in a microwave oven. ( NMKL 161, 1998). accessed 2022 Apr 5. https://nmkl.org/index.php/nb/.
- Pokhrel B, Sorensen JN, Moller HB, Petersen KK. 2018. Processing methods of organic liquid fertilizers affect nutrient availability and yield of greenhouse grown parsley. Renewable Agriculture and Food Systems. 1–9. doi:10.1017/S1742170517000771
- Ramin AA. 2006. Effects of salinity and temperature on germination and seedling establishment of sweet basil (Ocimum basilicum L.). J Herbs Spices Med Plants. 11(4):81–90. doi:10.1300/J044v11n04_09.
- Reganold JP, Wachter JM. 2016. Organic agriculture in the twenty-first century. NaT Plants. 2(2):15221. doi:10.1038/NPLANTS.2015.221.
- Restrepo AP, Medina E, Pérez-Espinosa A, Agulló E, Bustamante MA, Mininni C, Bernal MP, Moral R. 2013. Substitution of peat in horticultural seedling: suitability of digestate-derived compost from cattle manure and maize silage codigestions. Com Soil Sci Pl Analysis. 44(1–4):668–677. doi:10.1080/00103624.2013.748004.
- Schmilewski G. 2009. Growing medium constituents used in the EU. Acta Hortic. 819(819):33–46. doi:10.17660/ActaHortic.2009.819.3.
- Spurway CH, Lawton K. 1949. Soil testing: a practical system of soil fertility diagnosis. East Lansing: Agriculture Experiment Station, Michigan state University, USA.
- Stewart WM, Dibb DB, Johnston AE, Smyth TJ. 2005. The contribution of commercial fertilizer nutrients to food production. Agron J. 97(1):1–6. doi:10.2134/agronj2005.0001.
- Stoknes K 2020. Circular food; crops from digested waste in a controlled environment. Dissertation No. 2263, Faculty of Mathematics and Natural Sciences, Univ. of Oslo Norway. 1501-7710.
- Stoknes K, Scholwin F, Krzeinski W, Wojciechowska E, Jasinska A. 2016. Efficiency of a novel “food to waste to food” system including anaerobic digestion of food waste and cultivation of vegetables on digestate in a bubble-insulated greenhouse. Waste Management. dx, 56:466–476. doi:10.1016/j.wasman.2016.06.027.
- Stoknes K, Wojciechowska E, Jasińska A, Gulliksen A, Tesfamichael AA. 2018. Growing vegetables in the circular economy; cultivation of tomatoes on green waste compost and food waste digestate. Acta Hortic. (1215):389–396. doi:10.17660/ActaHortic.2018.1215.71.
- Sverige A. 2020. Certification rules for digestate from biodegradable waste by the quality assurance system of avfall sverige – the Swedish waste management association. Accessed 2022 Apr 5. www.avfallsverige.se/fileadmin/user_upload/4_kunskapsbank/SPCR_120_2020_Slutversion.pdf
- Tambone F, Scaglia B, D’Imporzano G, Schievano A, Orzi V, Salati S, Adani F. 2010. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere. 81(5):577–583. doi:10.1016/j.chemosphere.2010.08.034.
- Tittarelli F. 2020. Organic greenhouse production: towards an agroecological approach in the framework of the new European regulation – a review. Agronomy. 10(1):72. doi:10.3390/agronomy10010072.
- Ward AJ, Hobbs PJ, Holliman PJ, Jones DL. 2008. Optimisation of the anaerobic digestion of agricultural resources. Bioresour Technol. 99(17):7928–7940. doi:10.1016/j.biotech.2008.02.044.
- Yeager T, Gilliam C, Bilderback TE, Fare D, Niemiera A, Tilt K. 1997. Best management practice, guide for producing container-grown plants. Atlanta: Southern Nursery Association. COMAR15.20.08.07B.
- Zirkler D, Peters A, Kaupenjohann M. 2014. Elemental composition of biogas residues: variability and alteration during anaerobic digestion. Biomass Bioenergy. 67:89–98. doi:10.1016/j.biombioe.2014.04.021
