ABSTRACT
Objectives: Ischemic stroke (IS) is often associated with long-lasting physical deficits, linked to emotional symptoms (ES) and lowered quality of life (QoL). However, recent observations raised doubts regarding the traditional perspective of solely impairment-driven ES. In fact, anxiety and depression were also reported after transient ischemic attack (TIA) with a per definition absence of infarction and thus lacking physical deficits. This study follows the hypothesis that TIA patients might exhibit non-physical symptoms affecting individual QoL.
Methods: In a prospective single-center observational study, IS patients (n = 73) were compared with TIA patients (n = 24) regarding their neurological deficit, ES and QoL, whereas the latter were evaluated by the Hospital Anxiety and Depression Scale (HADS) and the Short Form 36 Heath Survey (SF-36). Assessments were conducted six times within a one-year follow-up period.
Results: Overall, anxiety and depression decreased over time, while anxiety decreased more substantially. TIA patients showed similar levels of anxiety and depression when compared to IS patients. ES were detectable very early after the event and remained throughout the follow-up period in both groups. ES were associated with an impaired QoL including non-functional dimensions, while the strongest interrelations were observed for TIA patients, emphasizing interrelations between QoL and anxiety.
Discussion: This study indicates that ES after TIA are comparable to the emotional burden after IS. ES after TIA were associated with QoL, pointing out their crucial role for individual well-being. Although confirmation in larger studies is necessary, these data underpin the need for early clinical awareness regarding non-physical symptoms in TIA patients.
Introduction
Stroke is known to range amongst the three most frequent causes of death worldwide and is a major cause for disabilities and subsequent diseases [Citation1]. Rate of deaths due to acute ischemic stroke declined in recent years, mainly as a consequence of the introduction of new treatment options and specialized care units [Citation2]. Along with a rising number of survivors, persisting stroke symptoms were increasingly recognized since they critically contribute to the perceived increase in disability-adjusted life years [Citation3].
In addition to physical stroke sequelae such as motor and sensory deficits, research confirmed the presence of non-physical symptoms among stroke survivors [Citation4]. Among those, post-stroke depression represents a common and serious condition, affecting about one-third of stroke survivors [Citation5]. In addition, about 30% of all stroke patients experience anxiety symptoms that were shown to represent a crucial predictor for depression and impaired quality of life (QoL) [Citation6]. Consequently, awareness for specific symptoms and knowledge on possible therapeutic interventions appear essential in stroke aftercare [Citation5], even more as there is an increased mortality in patients fulfilling diagnostic criteria for post-stroke depression [Citation7]. As a strong association between post-stroke depression and functional outcome was shown in earlier studies [Citation8,Citation9], research focused on mechanisms promoting this fact and found complex and multi-directional relationships between neurological impairment, determinants of acute stroke care, disability and mood disorders [Citation10,Citation11]. Further, depression and anxiety are known to critically affect the individual QoL, especially in the elderly and people suffering from severe medical conditions [Citation12]. Consequently, stroke survivors were found to exhibit an impaired QoL along with increased levels of depression, anxiety, and disability [Citation13].
Recently, studies indicated the existence of anxiety and depression-related symptoms also in patients with transient ischemic attack (TIA) [Citation14,Citation15]. These findings are remarkable since TIA patients per definition suffer from a neurological deficit disappearing spontaneously in a period of less than 24 hours and not resulting in any structural brain lesions [Citation16]. Hence, the observed emotional symptoms raised doubts concerning the traditional, impairment-driven concept for the development of these non-physical stroke sequelae.
Consequently, these observations led to the hypothesis that TIA patients may develop emotional symptoms as anxiety and depression along with a lowered QoL – perhaps in a dimension that is comparable with stroke patients. This study therefore investigates the long-term course of emotional symptoms and QoL in TIA and stroke patients. Subsequently, interrelations between different psychometric dimensions were investigated to explore potential mechanisms underlying the observed non-physical impairments after cerebral ischemia.
Materials and methods
Study design
The study followed a prospective observational design with a follow-up period of one year. Approval by the local ethics committee was granted under reference number 198–08. Recruitment took place between July 2008 and October 2010. Patients were screened during their hospital stay at the stroke unit of the Department of Neurology, University of Leipzig. After informed consent, patients were assessed for a spectrum of emotional, QoL-related and clinical parameters (see below) at the beginning of the study (day (d)0), as well as on d7, d30, d90, d180, and d360. Staff conducting study inclusion and follow-up visits was experienced in stroke care and respective scores.
Main inclusion criteria were an acute onset of a focal neurological deficit related to the territory of the middle cerebral artery, and a possibility of study inclusion within 24 hours after symptom onset or time last seen well. Main exclusion criteria were an impaired level of consciousness, intravenous or intraarterial thrombolysis, epileptic seizures, previous stroke or intracerebral bleeding, as well as any malignant or degenerative central nervous system diseases. Further exclusion criteria were a history of depression, anxiety disorder, dementia or other relevant psychiatric disorders.
According to current standards, TIA was defined as a focal neurological deficit with a duration of less than 24 hours and lacking radiological evidence for infarction [Citation16]. In contrast, ischemic stroke was defined as a focal neurological deficit with a duration of more than 24 hours or a radiologically visible infarction.
Emotional symptoms
Emotional symptoms were assessed by the Hospital Anxiety and Depression Scale (HADS) [Citation17,Citation18]. This self-assessment questionnaire consists of 14 items, seven of which rate for anxiety or depression, respectively. The HADS was previously shown to identify depressive and anxious individuals among patients with physical illnesses with high sensitivity and specificity (0.8 each) and is widely used to assess the mental status of patients [Citation19]. A cut-off-score of at least 8 was defined as an indicator for relevant anxiety or depression. For comparison to the general population, respective values were extracted from normative values collected from a representative German sample cohort [Citation20].
Quality of life
Individual QoL was assessed by the Short Form 36 Health Survey (SF-36) [Citation21–23], reliably addressing different aspects of health-related QoL [Citation24–26]. The results of this survey allow calculation of subscales regarding physical functioning, role limitations due to physical problems, pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. Overall scores, one reporting physical and one mental health, were compared with representative data from the general population. For this purpose, data were extracted from the SF-36 manual that features figures from normative populations [Citation23].
Clinical data and medical history
Moreover, details regarding neurological deficit and functional outcome were recorded. The National Institute for Health Stroke Scale (NIHSS) [Citation27] was used to identify the neurological deficit up to day 7, while the modified Rankin scale [Citation28,Citation29] was used to evaluate the patients’ overall physical impairment in the throughout all study visits. During recruitment, individual medical history was assessed for cardiovascular risk factors such as arterial hypertension, diabetes mellitus, hyperlipoproteinemia, as well as nicotine and alcohol consumption. Furthermore, regular intake of an antidepressive medication was recorded.
Statistics
The SPSS Software package (Version 24, 2016, IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA) was used for data analysis. Descriptive statistics were performed on patient characteristics as well as HADS and SF-36 scores, while the Mann-Whitney-U-test and the Wilcoxon rank test were applied to reveal statistically significant inter-group differences. In case of multiple comparisons, the Bonferroni method was used to correct for alpha-accumulation. Further, Fisher’s exact test and McNemar test were used to compare unpaired and paired percentages in cross tables. Interrelations between the assessed emotional and QoL-related parameters were explored by bivariate correlations, while the respective Spearman coefficients indicated the strength of any interrelation. Patient characteristics in the manuscript were reported as mean values ± standard deviation (SD). For outcome variables representing discrete parameters, scores from psychometric assessments were presented as median with interquartile interval, which also prevent overestimation by extreme values. For comparison with the general population, mean scores are used as these are given in the publication of a representative sample [Citation20,Citation23]. A p-value <0.05, or adapted p-values in the setting of multiple comparisons (given in figure legends), were considered as statistically significant.
Results
A total of 99 patients were included in the study, while two patients were subsequently excluded from further analyses due to the final evaluation of an underlying stroke mimic. In conclusion, this study is based on 97 patients suffering from ischemic stroke or TIA.
Patient characteristics
There were no significant differences between the TIA and ischemic stroke group regarding age, sex, pre-existing vascular risk factors, pre-morbid physical impairment as measured by the modified Rankin Scale (mRS), and treatment with antidepressants (). Four patients in the stroke subgroup formally reported regular intake of antidepressive medication without the diagnosis of depressive disorder but for other purposes, e.g. sleeping disorder or chronic pain syndrome. As expected, TIA and stroke patients exhibited different clinical scores at admission. In good accordance with the TIA definition, ascribed patients showed a neurological deficit at hospital admission (NIHSS > 0) which completely dissolved afterward as indicated by a missing deficit at d7 (NIHSS = 0).
Table 1. Patient characteristics
Emotional symptoms
The HADS questionnaire was conducted at six pre-defined points of time between the beginning of the study (d0) and d360. The course of achieved scores is shown in for the two dimensions anxiety ()) and depression ()) independently.
Figure 1. Anxiety and depression in TIA and ischemic stroke patients over time. Emotional symptoms were assessed in both groups using the Hospital Anxiety and Depression Scale (HADS). Data are given as median ± interquartile range as represented by boxes. Whiskers indicate for maximum and minimum score. *: p < 0.003 (adjusted for multiple comparison with Bonferroni correction), lines with asterisk (*) indicate statistically significant changes) between d0 or d7 and later time points in either ischemic stroke population (black) or TIA group (light grey). #: p < 0.05, black line with hash (#) indicate for statistically significant results in the inter-group comparison. Sample sizes for ischemic stroke and TIA varied between individual assessment sessions due to patients lost to follow-up and ranged from n = 65 to n = 73 for stroke and n = 18 to n = 23 for TIA
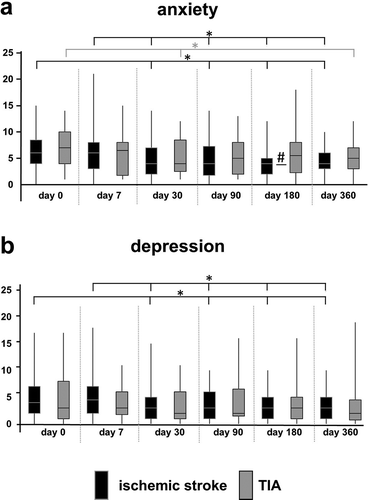
Anxiety did not differ significantly between TIA and ischemic stroke patients at the early points of time. However, at day 180, TIA patients were significantly more affected than stroke patients (TIA: median 5.50, interquartile range 5.75 vs. ischemic stroke: median 4.00, interquartile range 3.00; p = 0.042, Mann-Whitney-U-test). Depression scores did not differ substantially between the two groups at any time.
Concerning the temporal course during the one-year observation period, a statistically significant reduction of the anxiety scores were found for both ischemic stroke and TIA patients between d0 to d360 ()). From d30 on there were more stable courses showing no significant differences with the later survey results. Depression scores significantly declined in ischemic stroke over time, but not in TIA patients ()).
Taking the common cut-off of 8 points in one subscale into account, the percentage of patients affected by anxiety and depression decreased over time. At d0, a relevant score for anxiety was detected in 34.78% of patients in the ischemic stroke group and in 43.48% of patients suffering from TIA. Respective proportions decreased to 8.96% after ischemic stroke and 21.05% after TIA at d360 (ischemic stroke: p < 0.001; TIA: p = 0.031; McNemar test). A relevant score in the depression subscale occurred in 22.86% of the stroke patients and in 26.09% of TIA patients at d0, while percentages decreased to 4.62% after ischemic stroke and 5.56% after TIA at d360 (ischemic stroke: p = 0.013; TIA: p = 0.250; McNemar test).
Quality of life
The SF-36 questionnaire was conducted at six pre-defined time points between d0 and d360 as well. The eight SF-36 subscale values and their course over time are shown in , while a value of 100 indicating best achievable result. Concerning the overall course, stable conditions with a slight trend towards a better QoL in most of the subscales were noted towards d360.
Figure 2. Different dimensions of quality of life in ischemic stroke and TIA patients over time. Quality of life was measured by the SF-36 questionnaire, which allows assessing eight dimensions of quality of live. Median scores and their shift over time are illustrated in the graphics for each subscale of SF-36. Results for the ischemic stroke group are given as continuous lines with dark grey diamonds, whereas the results for the TIA group are shown in dashed lines with light grey squares. Significant differences between the two subgroups are indicated by asterisk (*). Sample sizes varied from n = 82 to n = 95 between individual assessment sessions (n = 64 to n = 73 for ischemic stroke patients; n = 18 to n = 23 for TIA patients) due to patients lost to follow-up
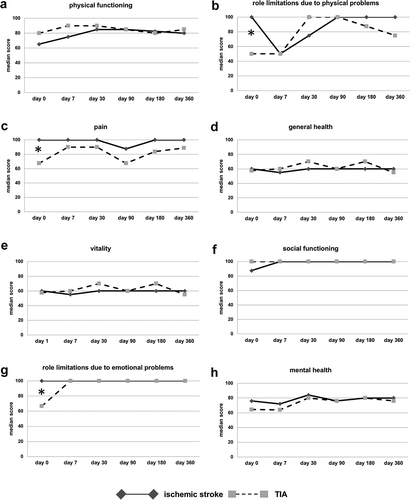
Most subscales did not differ significantly between TIA and ischemic stroke patients at any point of time. Surprisingly, subscales for role limitations due to physical problems (), median 100.00, interquartile range 50.00 for ischemic stroke vs. median 50.00, interquartile range 100 for TIA patients, p = 0.024, Mann-Whitney-U-Test), pain (), median 100.00, interquartile range 32.50 vs. median 67.50, interquartile range 57.50;p = 0.017, Mann-Whitney-U-Test), and role limitations due to emotional problems (), median 100.00, interquartile range 16.67 vs. median 66.67, interquartile range 100.00; p = 0.016, Mann-Whitney-U-Test) achieved higher scoring in ischemic stroke patients at d0. Only very small changes over time could be observed in the general health subscale ()), indicating a remarkable stability of this parameter. Furthermore, the subscales for social functioning ()) and role limitations due to emotional problems ()) showed stability over time as more than half of the patients reached the best possible score of 100 from day 7 on in both dimensions. Lowest values indicating lowest QoL were found for the role limitations due to physical problems ()), general health ()), and vitality subscales ()). During the observation period, there were numerous significant changes in individual subscales for stroke patients (data not shown), while the physical functioning subscale showing highest improvement. In contrast, no statistically significant changes over time were observed in patients suffering from TIA.
Comparison to general population
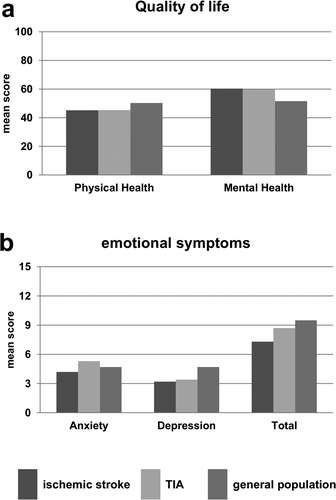
For comparison of emotional symptoms and QoL at d360 after stroke or TIA, the representative values for the general population were extracted from earlier studies for both the HADS [Citation20] and the SF-36 questionnaire [Citation23], allowing an orienting comparison (). The overall scores for QoL, i.e. physical health and mental health, in patients suffering from TIA and ischemic stroke appeared nearly similar to that of the general population ()). Emotional symptoms, i.e. anxiety and depression, provided a more inhomogeneous pattern ()): TIA and ischemic stroke patients appeared to be slightly less affected concerning emotional symptoms when compared to the general population.
Figure 3. Quality of life as well as anxiety and depression in ischemic stroke and TIA patients in comparison to the general population at d360. Sum scores were calculated for SF-36 and HADS to compare individual quality of live and emotional symptoms with data from a representative general population sample cohort. To compare long-term affection as measured by both questionnaires, data from d360 was assessed. Means are given for ischemic stroke patients in dark, TIA patients in light and the general population in medium grey
Interrelations
To explore interrelations between different aspects for QoL as well as anxiety and depression at one year after the index event, bivariate correlations were performed (). Analyses were done separately for TIA and ischemic stroke patients. Interrelations were found between the eight SF-36 and two HADS subscales in both groups. Expectedly, a negative correlation was revealed between anxiety, depression and QoL, which means that increased depression or anxiety is accompanied with a decreased QoL. Interrelations between emotional symptoms and QoL were evident for most dimensions covered by the HADS and SF-36. In fact, role limitations due to physical problems was the only subscale showing only weak interrelation with depression in TIA patients, while in stroke patients only social functioning was not moderately or strongly correlated with depression. With respect to anxiety, TIA patients showed moderate to strong interrelations with most of the SF-36 subscales (Spearman’scorrelation coefficients for positive and negative correlations ranged between 0.496 and 0.603) except for pain and vitality. Remarkably, ischemic stroke patients did not show strong interrelations between anxiety and most of the SF-36 subscales except for mental health (Spearman’s correlation coefficient −0.407), while depression was correlated with all SF-36 subscales with correlation coefficients ranging from 0.250 to 0.660 (). In TIA patients, the QoL subscale with the strongest correlation to the other subscales as well as anxiety and depression was mental health (Spearman’s correlation coefficients between 0.525 and 0.784) (). The situation was similar in stroke patients, but correlation was much weaker (Spearman’s correlation coefficients ranged between 0.255 and 0.540; except for subscale pain). Further, TIA and ischemic stroke patients differed regarding the interrelation between physical functioning and role limitations due to physical problems, while a stronger correlation (Spearman’s correlation coefficient 0.640) was only found in patients with ischemic stroke ().
Table 2. A – Correlation matrix of emotional symptoms and quality of life in patients with ischemic stroke one year after the qualifying event. B – Correlation matrix of emotional symptoms and quality of life in patients with TIA one year after the qualifying event
Discussion
This study focused on non-physical stroke sequelae, i.e. emotional sysmptoms (anxiety and depression) and core aspects of individual QoL. Conditions such as depression are known to critically impact on the individual QoL [Citation30]. Other than expected, depression-related symptoms were also described in TIA patients, per definition a group of patients not suffering from persisting neurological impairment [Citation15]. Altogether, such conditions seem highly relevant when striving to optimize the post-acute management of cerebrovascular events in a comprehensive way.
Since earlier studies often explored symptoms at single points of time [Citation31,Citation32], the overall course of non-physical symptoms is still unknown and thus the determination of an optimal screening window is rather challenging. Consequently, detailed knowledge on the temporal course and the interrelations of emotional symptoms and QoL could allow a better, personalized treatment that facilitates early intervention for depression, anxiety and lowered QoL. Therefore, special emphasis in this study was given to the evaluation of respective symptoms from a long-lasting term perspective, starting very early after the initial event and covering a one-year follow-up period.
Overall, this study revealed a long-term decrease of anxiety and, in a lesser degree, depression in patients suffering from ischemic stroke and TIA, respectively. Remarkably, anxiety also tended to decrease during the acute phase, which might be explained in the context of stress caused by the acute onset of the cerebrovascular event. The concomitant neurological deficits resulting in a physical impairment could therefore cause a temporary feeling of fear and anxiety by itself. Moreover, a higher proportion of patients exhibited relevant scores for anxiety than for depression during the very early phase and also at the end of the one-year observation period, which is in excellent accordance with an earlier study that focused on respective symptoms in ischemic stroke patients between day 3 and 7 after hospital admission [Citation33].
A major finding of this study is that the existence of anxiety and depression after TIA is comparable to ischemic stroke patients. This means that at least similar non-physical impairment was detectable in the absence of physical symptoms such as sensory or motor deficits or a radiological evidence of infarction. Remarkably, at d180 an increased level of anxiety was found in TIA patients when compared to ischemic stroke patients. Contrasting the traditional view of TIA as an only temporary cerebral pathology without sequelae, these findings are of particular interest in clinical practice. The occurrence of emotional symptoms in TIA patients may support the concept of ‘vascular depression’, which has not found its way into practice because of so far lacking diagnostic criteria [Citation34]. The hypothesis here is that vascular depression forms a major etiology of late-life-depression and is caused by different kinds of cerebral damage in a structural or biochemical way as seen in cerebrovascular events. However, TIA patients only suffer from transitory cerebral ischemia what may cause biochemical alterations, but not an ischemic infarction. Thus, future studies on this group of patients might also help to explore the nature of a potential vascular-driven depression.
Surprisingly, we detected a statistically significant difference between TIA and ischemic stroke patients regarding the SF-36 subscale role limitations due to physical problems at d0 with ischemic stroke patients being less affected although experiencing more physical impairment at this point as reflected by the NIHSS score at hospital admission. However, the mean NIHSS score of 1.92 is rather low, so the formal statistically significant difference to TIA patients might be less relevant in clinical practice. Apart from the subscale role limitations due to physical problems, the subscales role limitations due to emotional problems and pain significantly differed between the two groups at d0 with TIA patients being more affected. This finding further indicates for a relevant emotional burden in TIA patients that is also reflected by the interrelations between the HADS and SF-36 scales. In particular, anxiety seemed to critically affect individual QoL in TIA patients as indicated by the revealed bivariate correlation analysis. A comparable relationship on the relevance of anxiety for health-related QoL in ischemic stroke patients was reported in earlier investigations, while this effect was previously found to be either independent from depression [Citation35] or related to depression [Citation36]. Another remarkable result of the present study is the relatively low scores for general health and vitality throughout the one-year follow-up period that might be partly explained by unhealthy lifestyle and poor overall health being common risk factors for cerebrovascular events.
Comparison of TIA and stroke patients to the general population at d360 after the initial event revealed lower global HADS scores and a slightly higher score for mental health in patients. This can be interpreted in different ways. One may assume that patients’ increased awareness of the risk of psychiatric disorders after stroke (commonly explained to patients during rehabilitation), resulted in a more differentiated perspective and thus less negative affection within the ischemic stroke group and possibly also in the TIA group. Alternatively, better scores might be explained in the context of social desirability when answering the questionnaires together with medical staff in form of study personnel. Generally, it seems crucial to see emotional symptoms after stroke not only in the context of the patients’ medical history but also in a multidimensional way. This seems especially important, as the genesis of depression and anxiety disorders as well as the occurrence of lowered quality of life is dependent on biological, psychological and social factors. In the present sample, patients showed high scores in social functioning indicating for one major resilience factor, which might further clarify the relatively low non-physical burden in comparison to the general population.
The intra- and inter-test correlations indicated strong interrelations within and between the dimensions of emotional symptoms and QoL in both groups. Almost all parameters showed moderate to strong correlations with the mental health subscale in SF-36, underlining the importance of an individual well-being for self-reported QoL. While a decrease in QoL is mainly a problem, which is affecting the individual patient, there are social and socioeconomic implications arising from the close relationship to depression- and anxiety-related symptoms that lower the potential for recovery during rehabilitation and everyday life.
This study has some limitations: First, the relatively small sample size prevents a generalization of the findings. This should stimulate further studies on this topic involving larger sample sizes and multiple centers. Second, patients with ischemic stroke showed a remarkable low neurological impairment as indicated by the NIHSS scores at admission and d7. Third, study design defined that the neurological deficit had to be related to the territory of the middle cerebral artery, potentially inducing a selection bias. Thus, this study failed to capture the full spectrum of stroke patients, which is naturally ranging from small to huge structural lesions in different supply territories and thus varying quality and quantity of neurological impairment. On the other hand, patients suffering from stronger neurological deficits are often unable to complete psychometric assessments such as the HADS and SF-36 due to speech disorders, deficits in concentration and executive functions or other neuropsychological symptoms. Fourth, this study did not include records on psychotherapeutic intervention during the observation period, although the use of antidepressants at the time of recruitment was taken into consideration. Any potential treatments during the observation period may have affected the individual emotional situation reflected by individual psychometric results, respectively.
Summary and outlook
Despite the given limitations, this study provides novel data on long-lasting emotional symptoms and their interrelations with QoL after ischemic stroke and TIA. Covering the one-year observation period, anxiety and, in a lesser degree, depression decreased over time. Remarkably, patients suffering from TIA were similarly affected by anxiety and depression as stroke patients. The observed emotional symptoms were associated with individual QoL.
In conclusion, this study indicates that TIA patients may experience a relevant burden of non-physical symptoms – such as anxiety and depression – and impaired QoL that is comparable to patients suffering from ischemic stroke with persistent functional impairment. Although this observation needs to be confirmed in larger studies, these data support the usefulness of an early clinical awareness regarding emotional symptoms in TIA patients. Standardized aftercare concepts – as already tested in stroke patients [Citation37,Citation38] – including for instance rehabilitative aspects, caregiver education, and psychometric assessment could in this context also represent useful tools for TIA patients [Citation39]. An early identification of patients at risk would allow interventions such as supporting information, pharmacological and non-pharmacological treatments with the intention to prevent long-term non-physical TIA sequelae.
Authors’ contributions
DM and CH conceived and designed the study. DM, CH, JP and KK collected the data. AH contributed to data analysis. AP and DM performed data analyses and wrote the paper. All authors provided critical revisions to the manuscript.
Disclosure of interest
The authors report no conflicts of interest.
Acknowledgments
The authors would like to thank Ms. Daniela Urban and Ms. Rita Lachmund, Department of Neurology, University of Leipzig, Germany, for excellent technical assistance during the recruitment phase of this study. Prof. Johannes Boltze, University of Warwick, United Kingdom, is acknowledged for critical revisions to an earlier manuscript version.
Additional information
Funding
Notes on contributors
Alexander Prost
Alexander Prost, Physician at the stroke unit and neurological intensive care unit, Department of Neurology, University of Leipzig, Leipzig, Germany.
Katharina Kubitz
Katharina Kubitz, Compiled her dissertation at the Department of Neurology, University of Leipzig, Leipzig, Germany, and is currently working as a neurologist.
Johann Pelz
Johann Pelz, Senior physician at the stroke unit and neurological intensive care unit, Department of Neurology, University of Leipzig, Leipzig, Germany.
Carsten Hobohm
Carsten Hobohm, Head of the Department of Neurology, Carl-von-Basedow-Klinikum Saalekreis, Merseburg, Germany.
Andreas Hinz
Andreas Hinz, Senior researcher at the Department of Medical Psychology, University of Leipzig, Leipzig, Germany.
Dominik Michalski
Dominik Michalski, Senior physician at the stroke unit and neurological intensive care unit, Department of Neurology, University of Leipzig.
References
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. .
- Hankey GJ. Stroke. Lancet. 2017;389:641–654.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254.
- Ferro JM, Caeiro L, Figueira ML. Neuropsychiatric sequelae of stroke. Nat Rev Neurol. 2016;12:269–280.
- Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017–1025.
- Rafsten L, Danielsson A, Sunnerhagen KS. Anxiety after stroke: A systematic review and meta-analysis. J Rehabil Med. 2018;50(9):769–778.
- House A, Knapp P, Bamford J, et al. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2011;32:696–701.
- Parikh RM, Robinson RG, Lipsey JR, et al. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. ArchNeurol. 1990;47:785–789.
- Bilge C, Koçer E, Koçer A, et al. Depression and functional outcome after stroke: the effect of antidepressant therapy on functional recovery. Eur J Phys Rehabil Med. 2008;44:13–18.
- Ayerbe L, Ayis S, Wolfe CDA, et al. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14–21.
- Paolucci S, Iosa M, Coiro P, et al. Post-stroke Depression Increases Disability More Than 15% in Ischemic Stroke Survivors: A Case-Control Study. Front Neurol. 2019;10:926.
- Sivertsen H, Bjørkløf GH, Engedal K, et al. Depression and quality of life in older persons: a review. Dement Geriatr Cogn Disord. 2015;40:311–339.
- De Wit L, Theuns P, Dejaeger E, et al. Long-term impact of stroke on patients’ health-related quality of life. DisabilRehabil. 2017;39:1435–1440.
- Broomfield NM, Quinn TJ, Abdul-Rahim AH, et al. Depression and anxiety symptoms post-stroke/TIA: prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurol. 2014;14:198.
- Kahlon CK, Nasrallah HA. Bidirectional relationship between transient ischemic attacks and depression: A review. Ann Clin Psychiatry. 2019;31:e10–e16.
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370.
- Herrmann C. International experiences with the Hospital Anxiety and Depression Scale-a review of validation data and clinical results. J Psychosom Res. 1997;42:17–41.
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77.
- Hinz A, Brähler E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J Psychosom Res. 2011;71:74–78.
- Ware JE Jr, Sherbourne CD, The MOS. 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483.
- Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. SocSci Med. 1995;41:1359–1366.
- Bullinger M, Kirchberger I. SF-36 Fragebogen zum Gesundheitszustand. Zeitschrift für Klinische Psychol Psychothera. 1999;28:143–145.
- Tarlov AR, Ware JE Jr, Greenfield S, et al. The Medical Outcomes Study. An application of methods for monitoring the results of medical care. JAMA. 1989;262:925–930.
- Tsai C, Bayliss MS, Ware JE. SF‐36 Health Survey Annotated Bibliography: second edition (1988/1996). Boston, MA: Health Assessment Lab, New England Medical Center; 1997.
- Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25:3130–3139.
- Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870.
- Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2:200–215.
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607.
- Unsworth DJ, Mathias JL, Dorstyn DS. Preliminary Screening Recommendations for Patients at Risk of Depression and/or Anxiety more than 1 year poststroke. J Stroke Cerebrovasc Dis. 2019;28:1519–1528.
- Limampai P, Wongsrithep W, Kuptniratsaikul V. Depression after stroke at 12-month follow-up: a multicenter study. Int J Neurosci. 2017;127:887–892.
- Kielbergerová L, Mayer O Jr, Vaněk J, et al. Quality of life predictors in chronic stable post-stroke patients and prognostic value of SF-36 score as a mortality surrogate. Transl Stroke Res. 2015;6:375–383.
- Fure B, Wyller TB, Engedal K, et al. Emotional symptoms in acute ischemic stroke. Int J Geriatr Psychiatry. 2006;21:382–387.
- Aizenstein HJ, Baskys A, Boldrini M, et al. Vascular depression consensus report - a critical update. BMC Med. 2016;14:161.
- Tang WK, Lau CG, Mok V, et al. Impact of anxiety on health-related quality of life after stroke: a cross-sectional study. Arch Phys Med Rehabil. 2013;94:2535–2541.
- Donnellan C, Hickey A, Hevey D, et al. Effect of mood symptoms on recovery one year after stroke. Int J Geriat Psychiatry. 2010;25:1288–1295.
- Yu HL, Cao DX, Liu J. Effect of a novel designed intensive patient care program on cognitive impairment, anxiety, depression as well as relapse free survival in acute ischemic stroke patients: a randomized controlled study. Neurol Res. 2019;41:857–866.
- Zhang L, Zhang T, Sun Y. A newly designed intensive caregiver education program reduces cognitive impairment, anxiety, and depression in patients with acute ischemic stroke. Braz J MedBiol Res. 2019;52:e8533.
- Ahmadi M, Laumeier I, Ihl T, et al. A support programme for secondary prevention in patients with transient ischaemic attack and minor stroke (INSPiRE-TMS): an open-label, randomised controlled trial. Lancet Neurol. 2020;19:49–60.
