Abstract
Objective: Chronic Chemotherapy-Induced Peripheral Neuropathy (CIPN) is highly prevalent among colorectal cancer (CRC) patients. Ergothioneine (ET) – a dietary antioxidant -protected against CIPN in experimental models, but human studies are lacking. We explored whether whole blood ET levels were associated with chronic peripheral neuropathy among CRC patients who had completed chemotherapy.
Methods: At diagnosis, median ET-concentration in whole blood of 159 CRC patients was 10.2 μg/ml (7.2–15.8). Patients completed questionnaires on peripheral neuropathy 6 months after completion of chemotherapy. We calculated prevalence ratios (PR) to assess associations of ET-concentrations and prevalence of peripheral neuropathy and used linear regression to assess associations with severity of peripheral neuropathy.
Results: Prevalence of total and sensory peripheral neuropathy were both 81%. Higher ET-concentrations tended to be associated with lower prevalence of total and sensory peripheral neuropathy, but not statistically significant (highest versus lowest tertile of ET: PR = 0.93(0.78, 1.11) for total neuropathy, and PR = 0.84(0.70, 1.02) for sensory neuropathy). ET-concentrations were not associated with severity of neuropathy.
Conclusion: Statistically significant associations were not observed, possibly because of limited sample size. Although data may putatively suggest higher levels of ET to be associated with a lower prevalence of neuropathy, analyses should be repeated in larger populations with larger variability in ET-concentrations.
Introduction
The treatment of choice for colorectal cancer (CRC) is mostly surgery, combined with (neo) adjuvant chemotherapy and/or radiotherapy depending on the age of the patient, disease stage, and tumor location (Citation1). Chemotherapy can have severe direct and continued adverse effects (Citation2). One common and severe adverse effect reported by CRC patients is Chemotherapy-Induced Peripheral Neuropathy (CIPN). During treatment, CIPN is a major reason to modify treatment intensity (Citation3). CIPN regularly continues after discontinuation of chemotherapy, and can become chronic and irreversible (Citation4). Previous studies support that about one-third of patients suffer from chronic and persistent CIPN 6 months after the end of chemotherapy (Citation5). CIPN is predominantly characterized by sensory symptoms such as numbness and tingling of hands and feet, pain, and aberrant temperature sensation (Citation3,Citation6). Platinum-derived chemotherapeutic agents used in CRC treatment, such as oxaliplatin, are frequently causing CIPN (Citation4).
Strategies to prevent or reduce chronic CIPN are needed, since CIPN strongly affects quality of life (Citation5,Citation7). Ergothioneine (ET) has been suggested as a potential dietary factor that may impact CIPN based on experimental studies (Citation8–10). ET is a sulfur-containing amino acid (Citation11), and a potent antioxidant produced only by fungi and some cyanobacteria (Citation12). Only some foods contain ET, of which mushrooms are the most predominant source (Citation12,Citation13); other foods containing ET are organ meat as kidney and liver, red and black beans, and oat bran (Citation13). ET is found in the human body with relatively high concentrations in red blood cells (Citation11). The specific role of ET for human health has yet to be fully elucidated, but it has been proposed that ET may serve as an adaptive antioxidant to protect injured tissues (Citation14).
ET has been suggested to have neuroprotective effects against toxicity of platinum-derived chemotherapeutics in several experimental models (Citation8–10), but this has not been explored in humans. Based on rodent studies, it has been suggested that ET may inhibit accumulation of oxaliplatin in dorsal root ganglion neurons (Citation10). The uptake of ET is thought to be regulated through the Organic Cation/Carnitine Transporter (OCTN1)) (Citation15); interestingly, through rodent studies it has been suggested that the uptake of oxaliplatin by dorsal root ganglion neurons may occur via this same transporter OCTN1 (Citation8,Citation16). As accumulation of platinum in dorsal root ganglia has been reported as a major determinant of neurotoxicity, inhibition of oxaliplatin accumulation by ET may partially prevent CIPN-related symptoms (Citation10,Citation17). Moreover, ET acts as a potent antioxidant (Citation11) and hence, it has been suggested that ET mediates its potential preventive effects on CIPN through reduction of oxidative stress in dorsal root ganglion neurons (Citation10).
The aim of this study was to assess whether ET levels in blood were associated with chronic peripheral neuropathy in CRC patients who had been treated with chemotherapy.
Methods
Design and Population
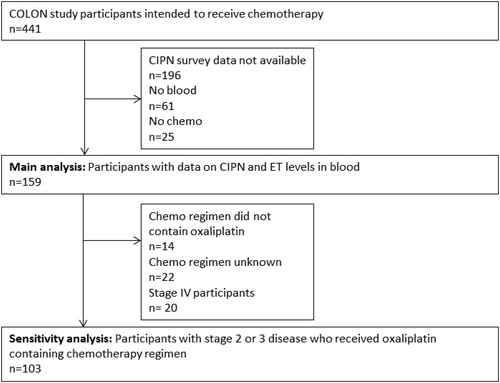
We used data of the ongoing COLON study for the current analysis (Citation18). Briefly, for the current study we included newly diagnosed CRC patients who were recruited in 11 hospitals in The Netherlands between 2010 and 2017. Participants had to be fluent in Dutch, could not have a history of CRC, could not have had a partial bowel resection, hereditary CRC, chronic inflammatory bowel disease, and should be able to fill out surveys to be eligible for the study. Eligible patients received an information leaflet from their physician or nurse practitioner during a routine visit shortly after being diagnosed with CRC. All participants provided written informed consent. The study was approved by a medical ethical review board. For the current study, we selected those participants of the COLON study who planned to receive chemotherapy (n = 441). Of this group: n = 196 participants had to be excluded because ‘Quality of Life Questionnaire to assess chronic Chemotherapy-induced Peripheral Neuropathy’ (QLQ-CIPN) data were missing (mainly because this survey was not administered in the COLON study from the start); n = 61 participants had to be excluded because blood samples were missing; n = 25 participants had to be excluded because they did not receive chemotherapy. This resulted in a total study population of n = 159 participants (see ).
Measurements
Nonfasted whole blood samples were drawn in EDTA tubes from all participants shortly after diagnosis, but before the start of any chemotherapy, and stored at −80 °C until further processing.
Twelve months after diagnosis, participants completed the QLQ-CIPN20 (Citation19). We used 16 items of this 20-item survey, as previous studies have shown that those 16 items accurately address the prevalence and severity of peripheral neuropathy and are most clinically relevant (Citation19). Of those 16 items, eight items address sensory symptoms, and eight items address motor symptoms. Validity and clinical relevance of these 16 items of the QLQ-CIPN20 have been reported previously (Citation19). In the survey, participants were asked to rate each item on a 4-point Likert scale summing up to a total score of 8–32 for the eight items on sensory neuropathy and to a total score of 16–64 for all 16 items. As recommended for this survey (Citation19), both scores were then linearly transformed to a 0–100 scale. A higher score reflects more severe symptoms. We used the data of total peripheral neuropathy based on the 16 items, and the data of the sensory subscore based on the eight items on sensory neuropathy, since CIPN is mainly characterized by sensory symptoms (Citation20–22). Cutoff points based on mean scores of the Dutch population were used to determine which participants suffered from chronic neuropathy: >3.6 for the total score and >3.2 for the sensory subscore (Citation23).
ET was analyzed in whole blood samples at the Core lab of the Pennsylvania State University, College of Medicine in Hershey, using the following method. Labeled ET (ET-d9) was spiked into whole blood samples as internal standard. Acetonitrile was added to the samples to precipitate proteins; all blood samples were diluted 100 times. Then, samples were mixed thoroughly and centrifuged at 4 °C for 10 min at 14,000 rpm. After centrifuging, the supernatant was loaded onto the ultra-performance liquid chromatography tandem mass spectrometer (UPLC/MS/MS) system. A standard curve for ET was created, which had a linear range of 5–1,000 ng/ml. An ABSciex 4000 Q Trap mass spectrometer coupled with a Waters Acquity UPLC separation system was used to analyze ET. ET was separated from impurities on a 1.7 µm Acquity UPLC ethylene bridged hybrid C18 analytical column (2.1 × 100 mm, Waters, Ireland). Mobile phase A consisted of 0.1% formic acid in water and mobile phase B consisted of acetonitrile. The ABSciex 4000 Q Trap mass spectrometer was equipped with an electrospray ionization probe operated in positive mode. The multiple reaction monitoring mode was used to analyze and quantify ET and ET-d9, with transitions of a mass over charge (m/z) of 230.3 to 127.3 for ET and m/z of 239.3 to 127.3 for ET-d9. All peaks were integrated and quantified by ABSciex Multiquan 3.0 software.
In addition, participants completed surveys to report personal and demographic factors around time of diagnosis, including a 204-item semi-quantitative food frequency questionnaire (FFQ) developed by the Division of Human Nutrition and Health of Wageningen University & Research, The Netherlands, to assess habitual dietary intake during the previous month. This FFQ has been validated for several nutrients (Citation24,Citation25), and has been shown to capture energy intake reasonably well (Citation26).
Statistics
The association of ET-concentration in blood and peripheral neuropathy prevalence (yes/no) was assessed in two ways: (Citation1) with ET as a continuous variable, and (Citation2) with ET categorized into tertiles. In the first analysis, we assessed associations between ET-concentrations and the prevalence of total or sensory peripheral neuropathy; ET-concentrations were natural log-transformed to approach normal distributions. In the second analysis, we created tertiles of ET-concentrations in blood, and assessed whether there was an association between tertile of ET and prevalence of total or sensory peripheral neuropathy. We used Cox regression models with a constant time period to determine prevalence ratios (PRs), and used robust variance estimation in the estimation confidence intervals. PRs were used and not odds ratios, since odds ratios tend to overestimate the size of the association when the outcome is common (Citation27).
In the tertile analysis, the lowest tertile served as reference category. Based on the literature, age, gender, physical activity, energy intake, diabetes (Citation28), and smoking (Citation5) were considered as possible confounding variables. Variables were added to the model one by one. We adjusted for factors that changed PRs with at least 10%. In test for trend analyses (p for trend), we assigned the median score of each tertile of ET to every individual in this tertile. This new variable was included in the Cox model as a continuous variable.
In addition, we assessed the association between ET-concentrations in blood and the severity of total CIPN score and severity of sensory symptoms. For these analyses, we used multiple linear regression and adjusted for possible confounding variables as described above. In the linear regression analyses, we included ET and severity of total peripheral neuropathy as continuous variables: both peripheral neuropathy severity scores and ET-concentrations were natural log-transformed to approach normal distributions.
As an additional analysis, we repeated all analyses with a different cutoff point for prevalence of total peripheral neuropathy and sensory peripheral neuropathy. We included this analysis, because the prevalence of CIPN in our study based on cutoff points published in the literature, was much higher than the prevalence found in other studies (Citation5). In this additional analysis, we created tertiles based on the peripheral neuropathy scores, and defined peripheral neuropathy ‘yes’ as a score in the upper tertile.
In a sensitivity analysis, all analyses were repeated excluding participants who did not receive oxaliplatin as part of their chemotherapy regimen (n = 14) or for whom type of chemotherapy was unknown (n = 22), as oxaliplatin is particularly known to cause neuropathy (Citation4), and we excluded patients with stage IV disease (n = 20) as chemotherapy treatment for those patients may be very different than for patients with an earlier stage of disease, e.g. it could be a lower number of cycles or in combination with bevacizumab. This dataset for the sensitivity analyses consisted of n = 103 patients. All analyses were performed using StataSE 14 software (Statacorp, TX, USA).
Results
Median age of the participants was 64.3 years; most participants were diagnosed with stage III cancer (71%), with the majority of participants having a tumor in the colon (89%). Of all participants, 78% of participants were treated with CAPOX (also known as Xelox) or FOLFOX, which are both oxaliplatin-containing regimens. The median age of participants who had to be excluded of the dataset (see ) was 64.6 years, 69% had stage III disease, and 84% with a tumor in the colon. Of these excluded participants, 82% were treated with CAPOX or FOLFOX.
The median concentration of ET in whole blood was 10.2 µg/ml, see for a description of baseline characteristics. Total median peripheral neuropathy score was 14.6; for the sensory domain median score was 20.8. Based on cutoff points derived from scores in the general population (Citation23), the prevalence of peripheral neuropathy in our study population was 81% (n = 129 participants), and 81% (n = 129 participants) for sensory peripheral neuropathy. Total peripheral neuropathy scores and sensory peripheral neuropathy scores were highest among participants in the lowest tertile of ET. Prevalence of total peripheral neuropathy or sensory peripheral neuropathy were highest among participants in the lowest tertiles of ET as compared with the higher tertiles.
Table 1. Baseline description of the colorectal cancer patients in a study on ergothioneine (ET) levels and peripheral neuropathy.
ET levels in blood were not associated with prevalence of total peripheral neuropathy (PR = 0.93 per ln(µg/ml) ET (0.1; 1.07)), nor with prevalence of sensory peripheral neuropathy (PR = 0.91 per ln(µg/ml) ET (0.79, 1.03)), see . In the tertile analysis, being in the highest tertile of ET tended to be associated with a lower prevalence of (total) peripheral neuropathy, but this was not statistically significant (PR for the highest vs. the lowest tertile 0.93 (0.78, 1.11). For sensory peripheral neuropathy, being in the highest tertile of ET tended to be associated with a lower prevalence of sensory peripheral neuropathy, but again this association was not statistically significant (PR for the highest vs. the lowest tertile 0.84 (0.70, 1.02).
Table 2. Association of ET-concentration in blood and prevalence of CIPN in CRC patients.
When prevalence of peripheral neuropathy was defined as scores in the upper tertile of total peripheral neuropathy scores, having a high blood concentration in the highest tertile of ET-concentrations (T3) was associated with a lower prevalence of total peripheral neuropathy, but again not statistically significant (PR for T3 vs. T1 0.63 (0.35, 1.13).
In the linear regression analysis presented in , ET levels in blood were not associated with severity of total peripheral neuropathy (β= −0.11 (−0.33; 0.11)), nor with severity of sensory peripheral neuropathy (β= −0.14 (−0.37; 0.10)).
Table 3. Association of ET-concentration in blood and severity of peripheral neuropathy in CRC patients.
The results of the sensitivity analyses, excluding participants who had not received oxaliplatin-based chemotherapy and excluding stage IV patients, did not differ substantially from the main analysis as shown in and .
Discussion
This study did not find statistically significant associations between ET levels in blood and chronic peripheral neuropathy in CRC patients who had been treated with chemotherapy. However, although none of the associations was statistically significant, it is noteworthy that the direction of the associations reported through PRs and betas in this paper were all in agreement with our hypothesis. This may putatively suggest that a higher ET-concentration in blood could be associated with lower prevalence and severity of chronic peripheral neuropathy, but that there was insufficient statistical power to detect this association.
To the best of our knowledge this is the first human study to explore possible associations of circulating ET-concentrations and peripheral neuropathy. Experimental rodent studies intervened with ET and assessed ET and oxaliplatin levels directly in dorsal root ganglions, while in our study we used blood levels of ET as a biomarker of ET status. Although we could not directly assess ET levels in neurons, we believe that our findings provide support for a possible association between ET levels and CIPN in humans. Importantly, we measured levels of ET in blood and studied a possible association with CIPN; we did not intervene with ET in this study as safety and relevance need to be established first. Observational studies like this are necessary before any potential step toward intervening with ET in cancer patients should be made.
We did not have complete data on the cumulative dose of oxaliplatin received during chemotherapy. CAPOX and FOLFOX both are combinations of chemotherapy including oxaliplatin, and specifically oxaliplatin is known to be associated with acute and with chronic CIPN (Citation3,Citation20). In an earlier observational study among 207 CRC patients who underwent chemotherapy, chronic CIPN years after treatment was associated with having received higher cumulative dose of oxaliplatin (Citation3), but patients who had received a dose reduction were still at risk of developing chronic CIPN (Citation3). In our study, a higher dose of oxaliplatin may have resulted in a higher risk of chronic CIPN, but severe symptoms of acute CIPN may have resulted in modification of treatment, resulting in a lower cumulative dose of oxaliplatin. Yet, acute CIPN is correlated with a higher risk of developing chronic CIPN (Citation3). This makes it challenging to predict how including information on the total cumulative dose of oxaliplatin would affect our results.
Earlier studies reported that about one-third of patients suffer from chronic CIPN 6 months after chemotherapy (Citation5), whereas peripheral neuropathy was 81% in the current study. This difference in prevalence compared with other studies likely resulted from differences in methodology to determine chronic CIPN (Citation29). Our study used a patient-reported questionnaire, which is prone to overestimate the outcome (Citation22). Nevertheless, this questionnaire is shown to be valid and reliable (Citation19). Moreover, the total median CIPN score we observed in our population (14.6) is similar to the mean score of 13.0 as observed in survivors of CRC who had received oxaliplatin as part of their chemotherapy treatment (Citation30). In addition, it is important to realize that there is no clinically defined cutoff for CIPN for this instrument. We therefore used the reported mean score of a sample of respondents from the general Dutch population, as the cutoff for CIPN. Thus, given that we used the mean in that standard population as cutoff for CIPN, the expectation was that at least 50% of our study population would have neuropathy. To mitigate this issue, we decided to also report on an analysis of tertiles with the upper tertile defined as ‘having peripheral neuropathy’. Some patients may have had neuropathy at baseline, as a result of diabetes or other comorbidities, but we did not assess neuropathy at baseline and could therefore not adjust for that.
Although the associations found in our data were not statistically significant, the directions of the association were in agreement with our hypothesis. Thus, further study into these associations using more comprehensive methods to assess CIPN throughout and after the period of cancer treatment (Citation29) is warranted. It is important to acknowledge that our study is observational, and that there could be residual confounding by other clinical/personal factors. Given the paucity of data on lifestyle factors that may affect the risk of CIPN (Citation7), it is challenging to judge whether we may have missed specific confounding variables. As stated earlier, we may not have had sufficient statistical power to detect associations. We calculated that if we assume that the prevalence of neuropathy in the upper tertile of ET is 25%, and 40% in the lowest tertile (PR∼0.63), we would have needed a total sample size of 465 participants to detect a statistically significant association, assuming a power of 80% and an alpha of 0.05. Therefore, our results should be interpreted as hypothesis-generating and future studies require a larger sample size.
Mushrooms are the most important source of ET in the human diet (Citation13). In comparison with other countries, especially Asian countries (Citation31), mushroom intake in The Netherlands is relatively low (Citation32). Additionally, in The Netherlands white buttons are the most eaten variety of mushrooms, which are low in ET compared with other mushroom varieties (Citation12). As comparison, in healthy volunteers in a study from Japan, ET blood levels ranged from 20 to 70 µg/ml, compared with a range of 4.5–23.0 µg/ml in our study. The relatively low levels of ET, and lack of wide variability in ET levels in our study, may have further reduced our ability to detect significant associations. Thus, it would be interesting to repeat these analyses in Japan or other countries where mushroom intake is traditionally higher than in The Netherlands (Citation33).
ET is a potent antioxidant. The effects of antioxidants in general on CIPN have been studied, but results are fairly mixed (Citation7,Citation34–36). For example, some studies suggest that there could be a role for the antioxidants vitamin E and acetyl-l-carnitine in protecting against CIPN (Citation37–40), but other studies could not confirm this (Citation41,Citation42), or even suggested detrimental effects on CIPN-related outcomes (Citation35,Citation41). As also proposed by others (Citation7), there could be a role for antioxidants or for dietary strategies in general in the prevention of CIPN, but further research is warranted to draw firm conclusions.
To conclude, ET-concentration in blood was not associated with prevalence or severity of peripheral neuropathy among CRC patients treated with chemotherapy. Nevertheless, data may putatively suggest that a higher ET-concentration in blood may be associated with a lower prevalence of chronic peripheral neuropathy, especially for sensory peripheral neuropathy. Future studies should consider different methodologies to assess chronic CIPN at various time points before, during and after cancer treatment, and should be performed in populations where intake of mushrooms, and therefore variability in concentrations of ET in blood is larger.
Acknowledgments
The authors would like to thank the coworkers from the following hospitals for their involvement in recruitment for the COLON study: Hospital Gelderse Vallei, Ede; RadboudUMC, Nijmegen; Slingeland Hospital, Doetinchem,; Canisius Wilhelmina Hospital, Nijmegen; Rijnstate Hospital, Arnhem; Gelre Hospitals, Apeldoorn/Zutphen; Hospital Bernhoven, Uden; Isala, Zwolle; ZGT, Almelo; Martini Hospital, Groningen; Admiraal de Ruyter Hospital, Goes/Vlissingen, all in The Netherlands.
Disclosure Statement
The authors declare to have no conflicts of interest. The funding agencies had no role in the design, data collection, analysis or interpretation of the data, or in the writing of the manuscript, or in the decision to submit for publication.
Additional information
Funding
Notes on contributors
Renate M. Winkels
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Lieve van Brakel
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Harm van Baar
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Robert B. Beelman
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Fränzel J. B. van Duijnhoven
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Anne Geijsen
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Henk K. van Halteren
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Bibi M. E. Hansson
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
John P. Richie
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Dongxiao Sun
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Evertine Wesselink
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Moniek van Zutphen
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Ellen Kampman
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
Dieuwertje E. Kok
Conceptualization, Renate Winkels, Lieve van Brakel, Robert Beelman, John Richie, Ellen Kampman and Dieuwertje Kok; Formal analysis, Renate Winkels and Lieve van Brakel; Investigation, Harm van Baar, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen and Dieuwertje Kok; Supervision, Renate Winkels; Writing – original draft, Renate Winkels and Lieve van Brakel; Writing – review & editing, Renate Winkels, Lieve van Brakel, Harm van Baar, Robert Beelman, Franzel van Duijnhoven, Anne Geijsen, Henk van Halteren, Bibi Hansson, John Richie, Dongxiao Sun, Evertine Wesselink, Moniek van Zutphen, Ellen Kampman and Dieuwertje Kok. All authors approved the final version of the manuscript
References
- American Cancer Society: Colorectal Cancer: Facts and Figures 2017–2019. Atlanta: American Cancer Society, 2017.
- Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, and Kalofonos HP: A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev 34, 368–377, 2008.
- Beijers AJ, Mols F, Tjan-Heijnen VC, Faber CG, van de Poll-Franse LV, et al.: Peripheral neuropathy in colorectal cancer survivors: the influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol 54, 463–469, 2015.
- Ocean AJ, and Vahdat LT: Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer 12, 619–625, 2004.
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, et al.: Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. PAIN® 155, 2461–2470, 2014.
- von Hehn CA, Baron R, and Woolf CJ: Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652, 2012.
- Derksen TM, Bours MJ, Mols F, and Weijenberg MP: Lifestyle-related factors in the self-management of chemotherapy-induced peripheral neuropathy in colorectal cancer: a systematic review. Evid Based Complem Alternat Med 2017, 7916031, 2017.
- Jong NN, Nakanishi T, Liu JJ, Tamai I, and McKeage MJ: Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J Pharmacol Exp Ther 338, 537–547, 2011.
- Song T-Y, Chen C-L, Liao J-W, Ou H-C, and Tsai M-S: Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem Toxicol 48, 3492–3499, 2010.
- Nishida K, Takeuchi K, Hosoda A, Sugano S, Morisaki E, et al. Ergothioneine ameliorates oxaliplatin-induced peripheral neuropathy in rats. Life Sci 207, 516–524, 2018.
- Cheah IK, and Halliwell B: Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta 1822, 784–793, 2012.
- Kalaras MD, Richie JP, Calcagnotto A, and Beelman RB: Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chem 233, 429–433, 2017.
- Ey J, Schomig E, and Taubert D: Dietary sources and antioxidant effects of ergothioneine. J Agric Food Chem 55, 6466–6474, 2007.
- Halliwell B, Cheah IK, and Drum CL: Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem Biophys Res Commun 470, 245–250, 2016.
- Grundemann D: The ergothioneine transporter controls and indicates ergothioneine activity – a review. Prev Med 54, S71–S74, 2012.
- Fujita S, Hirota T, Sakiyama R, Baba M, and Ieiri I: Identification of drug transporters contributing to oxaliplatin-induced peripheral neuropathy. J Neurochem 148, 373–385, 2019.
- Ta LE, Espeset L, Podratz J, and Windebank AJ: Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology 27, 992–1002, 2006.
- Winkels RM, Heine-Bröring RC, Van Zutphen M, van Harten-Gerritsen S, Kok DE, et al.: The COLON study: Co lorectal cancer: L ongitudinal, O bservational study on N utritional and lifestyle factors that may influence colorectal tumour recurrence, survival and quality of life. BMC Cancer 14, 374, 2014.
- Smith EML, Barton DL, Qin R, Steen PD, Aaronson NK, et al.: Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res 22, 2787–2799, 2013.
- Lehky T, Leonard G, Wilson R, Grem J, and Floeter M: Oxaliplatin‐induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve 29, 387–392, 2004.
- Saif MW, Syrigos K, Kaley K, and Isufi I: Role of pregabalin in treatment of oxaliplatin-induced sensory neuropathy. Anticancer Res 30, 2927–2933, 2010.
- Park SB, Goldstein D, Krishnan AV, Lin CSY, Friedlander ML, et al.: Chemotherapy‐induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63, 419–437, 2013.
- Mols F, van de Poll-Franse LV, Vreugdenhil G, Beijers AJ, Kieffer JM, et al.: Reference data of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CIPN20 Questionnaire in the general Dutch population. Eur J Cancer 69, 28–38, 2016.
- Feunekes IJ, Van Staveren WA, Graveland F, De Vos J, and Burema J: Reproducibility of a semiquantitative food frequency questionnaire to assess the intake of fats and cholesterol in The Netherlands. Int J Food Sci Nutr. 46, 117–123, 1995.
- Verkleij-Hagoort AC, de Vries JH, Stegers MP, Lindemans J, Ursem NT, et al.: Validation of the assessment of folate and vitamin B12 intake in women of reproductive age: the method of triads. Eur J Clin Nutr 61, 610–615, 2007.
- Siebelink E, Geelen A, and de Vries JH: Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br J Nutr 106, 274–281, 2011.
- Tamhane AR, Westfall AO, Burkholder GA, and Cutter GR: Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med 35, 5730–5735, 2016.
- Zeng L, Alongkronrusmee D, and van Rijn RM: An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 10, 219, 2017.
- Cavaletti G, Frigeni B, Lanzani F, Mattavelli L, Susani E, et al. Chemotherapy-Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer 46, 479–494, 2010.
- Kieffer JM, Postma TJ, van de Poll-Franse L, Mols F, Heimans JJ, et al. Evaluation of the psychometric properties of the EORTC chemotherapy-induced peripheral neuropathy questionnaire (QLQ-CIPN20). Qual Life Res 26, 2999–3010, 2017.
- Zhang S, Tomata Y, Sugiyama K, Sugawara Y, and Tsuji I: Mushroom consumption and incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. J Am Geriatr Soc 65, 1462–1469, 2017.
- Baars JJP, and Sonnenberg ASM: Voedingswaarden Champignons en Andere Paddenstoelen. Wageningen: Plant Research International B.V., 2008.
- Royse DJ, (ed.): A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products. India: ICAR-Directorate of Mushroom Research, 2014.
- Ma J, Kavelaars A, Dougherty PM, and Heijnen CJ: Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 124, 2289–2298, 2018.
- Brami C, Bao T, and Deng G: Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit Rev Oncol Hematol 98, 325–334, 2016.
- Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, and Vitetta L: Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review. Clin Nutr 32, 888–893, 2013.
- Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, et al.: A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori 91, 135–138, 2005.
- Bianchi G, Vitali G, Caraceni A, Ravaglia S, Capri G, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-l-carnitine. Eur J Cancer 41, 1746–1750, 2005.
- Pace A, Giannarelli D, Galie E, Savarese A, Carpano S, et al. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology 74, 762–766, 2010.
- Argyriou AA, Chroni E, Koutras A, Iconomou G, Papapetropoulos S, et al. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: final results. Support Care Cancer 14, 1134–1140, 2006.
- Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 31, 2627–2633, 2013.
- Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy BP, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Support Care Cancer 19, 1769–1777, 2011.