Abstract
Long-term, persistent cancer-related fatigue (CRF) is the most common side effect reported by lymphoma survivors. CRF reduces quality of life, and treatments are limited. This pilot study aimed to determine feasibility of recruiting and retaining diffuse large B-cell lymphoma (DLBCL) survivors in a 12-week remote Fatigue Reduction Diet (FRD) intervention and evaluate preliminary efficacy of the intervention. Participants met remotely with a registered dietitian nutritionist for eight individual sessions. FRD goals included consuming specific fruits, vegetables, whole grains, and omega-3 fatty acid rich foods. Acceptability was assessed by session attendance, FRD goal attainment, and exit surveys. Self-reported dietary intake and fatigue were measured using the Healthy Eating Index-2015 and PROMIS Fatigue Short Form, respectively, at baseline and post-intervention. Ten DLBCL survivors enrolled; nine attended all sessions and completed the intervention. Weekly adherence to targeted food intake goals improved significantly throughout the study (all p < 0.05), with participants meeting goals over 4 day per week by week 11. Mean[SD] diet quality improved significantly from baseline (65.9[6.3]) to post-intervention (82.2[5.0], p < 0.001). Mean[SD] fatigue reduced significantly from baseline (50.41[9.18]) to post-intervention (45.79[6.97], p < 0.05). The 12-week remote FRD intervention was feasible, acceptable, and holds promise to improve diet quality and fatigue in DLBCL survivors.
Introduction
Advancements in screening and treatments for cancer have resulted in larger numbers of people living for years after diagnosis (Citation1). As survivors live longer, there is a greater need to find effective interventions to reduce side effects of cancer and its treatments, such as fatigue, in order to improve quality of life. Non-Hodgkins lymphoma (NHL), which constitutes 80% of lymphoma cases (Citation2), is the most commonly diagnosed blood cancer, with diffuse large B-cell lymphoma (DLBCL), making up approximately 25% to 30% of all NHL cases (Citation2, Citation3). Long term, persistent fatigue is the most commonly reported side effect in NHL survivors (Citation4, Citation5), and is associated with depression, anxiety, and reduced quality of life (Citation6, Citation7). In prior studies, about 20 to 30% of cancer survivors experienced long-term fatigue for 5 to 10 years after treatment (Citation8). While there are currently no standards and limited guidance for the management of cancer related fatigue (CRF) in cancer survivors, some non-pharmacological treatment options include physical activity, psychosocial interventions such as cognitive behavioral therapy, and mind-body interventions such as yoga or mindfulness-based approaches (Citation9). Barriers to these treatment options include physical and disease-related limitations to body-based interventions, costs of psychotherapy, and demands of travel and time. Despite the prevalence of CRF and implications on quality of life, treatments are limited and barriers to implementation exist.
Human studies have shown that CRF is associated with high levels of pro-inflammatory markers (Citation10–12). Associations between inflammation and fatigue have been observed during cancer treatment (Citation13), after recent completion of primary treatment (Citation14), and years after treatment completion (Citation15). Chronic disruption of inflammatory pathways resulting from cancer and cancer treatment not only exacerbate fatigue but worsen other cancer treatment-related side effects impacting quality of life (Citation8, Citation12, Citation16). Due to the association of CRF with inflammation, dietary interventions that reduce inflammatory markers are promising strategies to treat CRF (Citation16–19). The results of a pilot randomized controlled trial by Zick et al. (Citation17) in 30 breast cancer survivors suggest that a 12 week hybrid (i.e., in-person and phone) nutrition counseling intervention to increase intake of fruit, vegetables, whole grains, and omega-3 polyunsaturated fatty acid (n-3 PUFA)-rich foods, named the fatigue reduction diet (FRD) pattern, significantly improved fatigue and sleep compared to an attention control group. The FRD pattern has the potential to benefit many cancer survivor populations as the dietary goals exceed the minimum intake for a healthy diet recommended by the American Cancer Society (ACS) for cancer survivors to improve long-term survival, which emphasizes a plant-based dietary pattern that includes a variety of vegetables, fruits, whole grains, and beans/legumes, and limits red and processed meats, added sugars, and highly processed foods (Citation20). Further, adult cancer survivors in the U.S. have worse overall diet quality compared to individuals without cancer history, highlighting the need for interventions to improve diet quality in this vulnerable population (Citation21). However, the feasibility of implementing the intervention in other cancer survivor populations that are particularly vulnerable to CRF, such as lymphoma survivors, has not been examined. Further, there is a need to test a fully remote delivery method, such as telehealth, to facilitate access and scalability.
Therefore, the primary aims of this pilot study were to determine the feasibility of recruiting and retaining DLBCL patients to a fully remote 12-week dietary counseling intervention based on the FRD, and to evaluate adherence to the FRD goals. Exploratory analyses were performed to examine the preliminary efficacy of the FRD to improve overall diet quality and fatigue scores in DLBCL survivors.
Materials and Methods
Study Design
This small feasibility study utilized a single group, pre-post study design to test remote delivery of a dietary intervention to reduce fatigue in DLBCL survivors. This feasibility study was based on the FRD intervention completed by Zick et al. (Citation17) in breast cancer survivors.
Eligible participants in the present study were adults aged 18 years and older who were DLBCL survivors that had completed upfront chemoimmunotherapy and remained in remission for at least 2 years before being enrolled. Participants were recruited from the Wexner Medical Center at The Ohio State University from March to June of 2021. Potentially eligible patients from collaborating medical oncologists’ clinics were identified via electronic chart review, and medical oncologists confirmed patient eligibility for the study. The study coordinator contacted patients via phone to discuss the study and invite them to participate. The study protocol 2020C0143 was approved by The Ohio State University Cancer Institutional Review Board and all participants provided informed consent prior to study entry. The study was listed at ClinicalTrials.gov with identifier NCT05327725.
Intervention
The FRD intervention provided remote nutrition counseling by a Registered Dietitian Nutritionist (RDN) and/or dietetic intern aimed at altering daily food choices to include higher amounts of whole grains, specific vegetables and fruits, fatty fish, and n-3 PUFA-rich plant-based foods; this FRD pattern was previously shown to be efficacious in lowering CRF in breast cancer survivors (Citation17). All study sessions occurred via telehealth videoconference or phone calls. The 12-week intervention was divided into two parts (). Weeks 1–4 included weekly video or phone sessions of approximately 30 minutes with an RDN (four total sessions) and focused on introducing the goals of the FRD. During each of the first four sessions, the RDN and participant worked together to select one food group as a goal to add for that week. This was a step-wise process, meaning every week another goal/food group was added in order to build participant self-efficacy to achieve the study goals. Weeks 5–12 included bi-weekly video or phone sessions of 15–20 minutes with an RDN (four total sessions) and focused on overcoming barriers to achieving FRD goals, problem solving, and evaluating progress. Daily calorie goals were not prescribed: participants were encouraged to swap current foods with foods to meet the study goals and improve diet quality, with no focus on weight loss. The goal for fruit intake was to consume two servings of fruit (one high in vitamin C) daily, with a serving being 1 cup of fresh, ½ cup of canned, or ¼ cup of dried fruit. The goal for vegetable intake was to consume five servings of vegetables daily (one leafy green, one tomato, and one yellow/orange), with a serving being 1 cup raw or fresh, ½ cup canned or cooked, or two cups of raw leafy greens. Both the fruit and vegetables would replace some of the carbohydrates from non-fruit or vegetable sources. There was a goal to consume two servings of n-3 PUFAs per day, one from fatty fish and one from nuts and/or seeds and n-3 rich oil (vegetarians were to replace fatty fish with plant-based n-3 sources). A serving was defined as 3 oz fish, one tablespoon of oils or seeds, or 1-2 tablespoons nuts. The fish, nuts or seeds and oils would replace existing sources of fats and proteins in the diet. The goal for whole grain intake included consuming at least three servings daily of whole grains (serving sized varied depending on the food), aiming for at least 50% of grains to be whole by replacing servings of refined grain foods. Participants self-monitored daily FRD goal attainment using an electronic checklist tracking system with shared access with the study RDN for personalized feedback during study sessions.
Table 1. FRD intervention timeline and syllabus.
Data Collection
All data collection was conducted remotely. Demographic and anthropometric data were collected at baseline only. Due to the remote nature of the intervention, height and weight were collected as self-report measures.
Adherence to the FRD pattern throughout the intervention was assessed using the FRD checklist tracker (Supplementary Table 1). The checklist tracker was a self-monitoring tool participants used daily to track attainment of each of the study goals. The checklist tracker included one row for each day, and participants could track whether or not they met each of the specific goals daily. Participants received the checklist tracker via email during Session 1, and were instructed to complete it daily until Session 8, which resulted in a total of 11 weeks of possible tracking. The participant and RDN had shared access to the checklist tracker, allowing for individualized feedback of progress during the sessions.
Dietary data were collected via the VioScreen™ web-based electronic food frequency questionnaire (eFFQ) (Citation22) at baseline and post-intervention. This remotely accessed eFFQ can address limitations inherent in paper questionnaires, by allowing very complex skip patterns and portion size estimation based on food pictures. Direct entry of data by the participant reduces potential copying errors and increases efficiency. Data produced from this eFFQ reflects the prior 3 months of eating habits, nutrient intake, and diet quality measured by the Healthy Eating Index (HEI) 2015. The HEI-2015 compares a person’s diet to the Dietary Guidelines for Americans. There are 13 subcomponents with nine reflecting adequacy (total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids) and four reflecting moderation (refined grains, sodium, added sugars, saturated fats). The maximum (i.e., best) score on the HEI-2015 is 100, with >80 reflecting good diet quality, 50-80 reflecting diet quality that needs improvement, and <50 reflecting poor diet quality.
Fatigue levels were assessed at baseline and post-intervention electronically using the Patient-Reported Outcomes Measurement Information System (PROMIS) Fatigue Short Form v1.0 - Fatigue 7a questionnaire. The PROMIS Fatigue Short Form has reliable internal consistency (Cronbach’s alpha = 0.84) (Citation23), and is considered a reliable, valid, and efficient way to measure fatigue in a variety of populations (Citation24, Citation25). The instrument includes seven questions with Likert scale response options. The short forms were scored using item-level calibrations done by the HealthMeasures Scoring Service. Each raw score was converted to a T-score, which also came with a standard error score. A score of 50 is the average for the United States general population with a standard deviation of 10. Higher PROMIS fatigue T-scores represent more fatigue. For example, a T-score of 60 is one SD worse than average. Comparatively, a fatigue T-score of 40 is one SD better than average.
A program evaluation was conducted via online questionnaire for each participant upon study completion. Participants were given an opportunity to provide open-ended feedback on their experiences with the intervention, with the primary goal being to collect insight on how the intervention might be modified in the future to meet patients’ needs. The seven questions on the instrument are included in the Supplementary Table 2.
Table 2. Baseline demographics of nine participants who completed FRD intervention.
Statistical Analysis
Descriptive statistics including means, standard deviations, and frequencies were used to summarize outcome measures at each time point. Paired t-tests assessed differences from baseline to post-intervention for dietary variables and fatigue. Repeated measures analysis of variance fixed effect tests assessed within-subject changes in FRD intervention goal attainment over the course of the intervention. For variables failing to meet assumptions of normality, alternative nonparametric tests were used. In all analyses, a two-sided p-value of ≤0.05 was considered statistically significant. Feasibility and acceptability of the intervention were assessed by study session attendance, adherence to dietary goals reported in the daily food checklists, study completion, and a qualitative program evaluation upon study end. Power analyses were not used for this pilot study as the primary goal was assessing feasibility in the DLBCL population. An enrollment goal of 10 participants was set a priori based on limitations in funding and research staff time constraints. Using data from the pilot study of the FRD intervention in breast cancer survivors by Zick, et al. (Citation17), we expected ≥90% of participants to attend all study sessions and complete the study. We also expected participants to meet their dietary goals >70% of the time during the study (Citation17). All statistical analyses were conducted using JMP (version 16, SAS Institute Inc., Cary, NC, USA).
Results
Participant Characteristics and Feasibility Outcomes
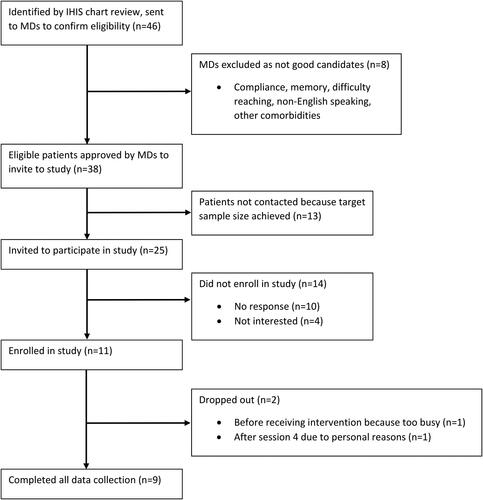
shows the CONSORT flow diagram for the study. Electronic chart review identified 46 potentially eligible patients, of which oncologists confirmed eligibility for 38. Twenty-five patients were contacted and invited to participate in the study. A total of 11 participants enrolled in the study over 4 months; nine completed the entire intervention. One female participant dropped out before the intervention began due to time constraints, and another male participant did not continue after week 4 due to personal reasons. The retention rate was 90% (9/10) for those who began the intervention. Of the nine who completed the intervention, all participants attended all eight study sessions. shows the demographics of the nine participants who finished the intervention. Most participants were male (55.6%), non-Hispanic white (77.8%), married or co-habiting with a partner (55.6%), and working at least 40 hours/week (55.6%). Most participants reported a sedentary or low physical activity level at baseline (66.7%).
A program evaluation was completed by each participant upon study end. Participants provided open-ended responses to questions pertaining to perceived benefit, suggestions for improvement, and perceptions of various elements of the intervention. All nine participants who completed the intervention also completed the program evaluation questionnaire. When asked whether the participants found the study to be beneficial to them, all participants responded positively (9/9, 100%). All participants indicated that they received enough instruction and support during the study (9/9, 100%). When asked about feelings toward the remote nature of the intervention, all participants responded either positively (8/9, 89%) or neutrally (1/9, 11%). Most participants (7/9, 78%) responded positively regarding the use of the daily checklist tool, with one responding neutrally and one negatively. When asked what could be improved with the study, three participants suggested expanding food options within each category, and two suggested changes to the daily checklist tool (simplification and/or app development).
Adherence to FRD Goals
Participants completed self-monitoring every day during the intervention using the daily checklist tool to monitor their progress. Among the nine participants who completed the intervention, daily checklist tools had a completion rate of 97.4% during the 11 weeks they were used (675 of 693 possible entries). Mean[SD] days per week that participants reported adherence to study goals are reported in . Reported adherence to all study goals changed significantly over the course of the intervention (all p < 0.05). Participants reported meeting all four vegetable goals on average 1.0[1.7] days during week 1, and 3.6[2.1] days during week 11; the whole grains goal 2.2[2.6] days during week 1, and 4.9[1.9] days during week 11; the n-3 PUFAs goal 2.4[2.6] days during week 1, and 5.6[1.7] days during week 11; and both fruit goals 5.9[1.7] days during week 1, and 5.9[1.8] days during week 11. With the exception of the fruit goals, reported adherence to study goals was higher by week 11 compared to week 1.
Table 3. Self-monitoring daily checklist: mean days per week of adherence to FRD intervention goals.
Changes in Diet Quality and Dietary Intake from Baseline to Post-Intervention
Diet quality was measured using the Healthy Eating Index 2015 from the eFFQ output at baseline and post-intervention. Changes in HEI-2015 component and total scores from baseline to post-intervention are reported in . Significant improvements were reported for Total Vegetables (p = 0.03), Greens and Beans (p = 0.03), Whole Grains (p = 0.004), Fatty Acids (p = 0.02), Added Sugars (p = 0.02), and Saturated Fats (p = 0.004). Overall diet quality, as measured by mean[SD] Total HEI-2015 Score, improved significantly from baseline (65.9[6.3]) to post-intervention (82.2[5.0]) (p = 0.0002).
Table 4. Changes in Healthy Eating Index (HEI) 2015 Component Scores from Baseline to Post-Intervention among nine Lymphoma Survivors completing a 12-week remote dietary intervention to reduce fatigue.
Changes in dietary variables of interest from baseline to post-intervention are reported in . No significant differences were reported for total calories or for percent of energy from carbohydrates, protein, or fat (all p > 0.05). Mean[SD] total n-3 PUFA intake increased significantly from baseline (2.09[1.42]g/day) to post-intervention (2.46[1.55]g/day) (p = 0.039). Mean[SD] intakes of eicosapentaenoic acid (EPA) (0.05[0.05]g/day, 0.11[0.12]g/day) and docosahexaenoic acid (DHA) (0.11[0.10]g/day, 0.24[0.25]g/day) more than doubled from baseline to post-intervention, however increases were not statistically significant (p = 0.09, p = 0.07, respectively). Mean[SD] intakes of whole foods targeted in the intervention increased significantly (all p < 0.05): fruit (total and those high in vitamin C), vegetables (total, dark green, orange, and tomatoes), fish, and whole grains. Micronutrients found in high concentration in the targeted foods increased significantly from baseline to post-intervention (p < 0.05), including lycopene, a carotenoid present in tomatoes, vitamin C, and beta carotene, a carotenoid found in orange and yellow vegetables.
Table 5. Change in self-reported dietary variables of interest from baseline to post-intervention among nine Lymphoma Survivors completing a 12-week remote dietary intervention to reduce fatigue.
Changes in Fatigue from Baseline to Post-Intervention
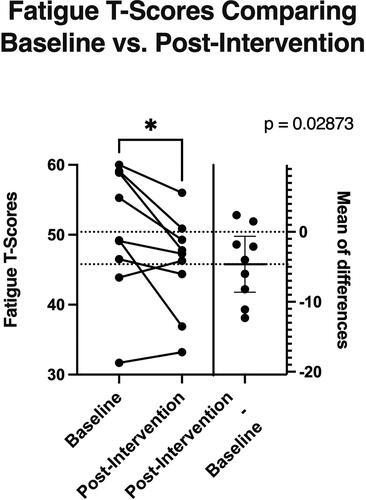
Changes in self-reported fatigue are shown in . Mean[SD] fatigue T-scores improved significantly from baseline (50.41[9.18]) to post-intervention (45.79[6.97]) (p = 0.029), with an overall mean reduction of −4.62(5.21).
Discussion
This pilot study demonstrated the feasibility of implementing a remotely delivered nutrition counseling intervention to improve diet quality and reduce fatigue in DLBCL survivors. Ten participants were recruited and enrolled over four months, which exceeded expectations of enrolling ten participants within a 6-month time frame using only recruitment from clinics of collaborating oncologists. Retention of 90% of those who started the intervention and high adherence to study goals suggests that this remote telehealth approach to changing diet quality and addressing CRF, the most commonly reported long-term symptom in NHL survivors (Citation4, Citation5), was highly acceptable to this cohort of DLBCL survivors. Indeed, participants reported on exit interviews that they appreciated the remote nature of the intervention, particularly during the COVID-19 pandemic, highlighting the value of offering accessible, convenient options for dietary intervention administration.
As this was a fully remote intervention taking place during the COVID-19 pandemic, recruitment methods were similarly conducted fully remotely. An initial review of electronic medical records from patients who attended clinic visits for the collaborating medical oncologists provided 38 oncologist-approved, eligible patients. The limited approaches to recruitment used in this study were more than sufficient to recruit and enroll 10 patients to this study within a 6 month, goal, underscoring recruitment feasibility. However, future studies with larger sample sizes would benefit from making use of other recruitment resources, such as tumor registries, healthcare databases, or research volunteer registries such as ResearchMatch.org, rather than relying solely on clinic visits. Retention of 90% of enrolled participants is consistent with expectations, as 93% (14/15) of BC survivors in the FRD pilot by Zick et al., completed the intervention. The RDN interventionist with this study worked within the healthcare system’s structure for the telehealth sessions with study participants. An unexpected challenge arose when the telehealth system generated automatic billing notices for some patients, despite no charges occurring for study visits. Conducting remote study sessions outside of the established telehealth system used by the medical system might be beneficial for future studies to avoid concerning notices for patients.
The DLBCL survivors in this pilot FRD intervention reported adherence to the study goals for targeted food intake. All targeted food groups in the present study, except for nuts and seeds, increased significantly from baseline to post-intervention (all p < 0.05). These findings are similar to those by Zick et al. (Citation17) in the FRD pilot study among breast cancer survivors in which intake of all targeted foods increased significantly except for non-citrus fruits (all p < 0.05). Based on the daily checklists, which had a completion rate of 97.4%, participants were meeting targeted food goals 4.8-6.1 days/week by week 11 (study end). High adherence to the dietary goals underscores the acceptability of the FRD among this sample of DLBCL survivors. This acceptability data is consistent with findings by Zick et al. (Citation17), where BC survivors met targeted food goals 73-94% of the time. Most participants noted how important the daily checklists were for meal planning, goal setting, and overall success with adopting and maintaining dietary changes. These results suggest that the dietary goals in this intervention were feasible, acceptable, and attainable for this sample of DLBCL survivors.
Changes in diet quality were assessed using the Healthy Eating Index 2015, generated from the FFQ data. The mean HEI-2015 score of participants in this study at baseline was 65.9. This is higher than the average HEI-2015 score of American adults, which is 58 for those aged 31-70 years and 64 for those aged 71 years and older (Citation26). Following the 12-week remote dietary intervention, the DLBCL survivors in this study reported a significant mean increase in HEI-2015 score of 16.3 points to an average post-intervention score of 82.2. Despite having above-average diet quality at baseline, participants in this study reported significantly improved diet quality after following the 12-week FRD intervention. These results could be due to actual improvements in diet quality or due to misreporting of certain foods based on perceived healthfulness or in response to intervention messaging, resulting in systematic measurement error (Citation27). The HEI-2015 adequacy subcomponents that reflected significant improvements were consistent with the FRD intervention goals: total vegetables and greens and beans, whole grains, and fatty acids. Further, the moderation subcomponents with significant post-intervention improvements were added sugars and saturated fats, reflective of participants’ replacement of food choices with options better aligned with the FRD.
In this small pilot study, it was not possible to evaluate relationships of individual nutrients with CRF or underlying mechanisms; however, the beneficial changes reported in this pilot study are consistent with the available literature about the potential for specific dietary patterns and individual nutrients to reduce fatigue in cancer survivors (Citation18, Citation19). Initial evidence from a systematic review examining effects of nutrition therapy on CRF and quality of life suggests that anti-inflammatory dietary interventions, such as a whole food plant-based dietary patterns and inclusion of foods rich in n-3 PUFAs, may be promising strategies to address CRF (Citation19).
Participants reported significant reductions in fatigue scores pre- to post-intervention. The fatigue T-scores at baseline among the nine participants who completed the intervention ranged from 31.7 to 60, with a mean T-score of 50.41. A T-score of 50 represents the mean for the U.S. population, so these participants scored similar to U.S. averages but may not represent cancer survivors most likely to benefit from an intervention, namely those who are severely fatigued. Despite that, participants reported significantly less fatigue post-intervention (mean 45.79). The mean change in reported fatigue in this study (-4.62) meets the established minimal important difference of 3.0 to 5.0 points for the 7-item PROMIS fatigue instrument in a study of cancer patients (Citation28), which suggests this change may be clinically meaningful. However, due to lack of a control group, these changes cannot be definitively attributed to the FRD pattern. Future research, using a randomized controlled trial design, is needed to determine efficacy of the dietary intervention to improve diet quality and reduce fatigue.
This study had several limitations, most notably the small sample size and single arm design. However, the preliminary data on feasibility and acceptability of this fully remote dietary intervention addressed the primary aim of this pilot study and provided the foundation for future randomized controlled trials using this approach. Strengths of the study included the personalized nutrition counseling provided by a RDN, tailoring of the FRD pattern to each participant’s usual food intake using a baseline FFQ, and inclusion of behavioral change strategies such as self-monitoring and goal setting to facilitate adherence. Another strength of this remotely delivered intervention was increased accessibility and elimination of transportation burdens for enrolled participants. Indeed, this contributed to study enrollment and retention because some participants were able to continue intervention visits while traveling across the country for work and/or pleasure due to the flexibility allowed with telehealth style visits. However, forced isolation during the COVID-19 pandemic may have contributed to higher participation, adherence and enthusiasm among this patient population. Further, the remote nature of the intervention also hindered objective assessment of anthropometric data, such as body weight and height, which were collected via self-report at baseline only. Weight change was not a targeted outcome of the intervention, so post-intervention anthropometric data were not collected, and self-reported physical activity level was obtained at baseline only. In addition, blood markers of inflammation and nutritional biomarkers were not collected in this small, fully remote pilot study. Another limitation of this study was that the intervention was not strictly isocaloric. Rather, participants were counseled to replace some food choices with options that aligned with the FRD plan. Results indicated that on average, participants reduced daily caloric intake by about 300 kcal/day post-intervention compared to baseline, although this was not statistically significant. Weight reduction paired with physical activity can contribute to improved energy levels in cancer survivors (Citation29), so it is possible that potential weight change could have contributed to the preliminary evidence for reduction in fatigue reported by participants in this study. However, Zick et al. (Citation17) found similar reductions in fatigue in BC survivors using an isocaloric FRD approach in a RCT. Research in a larger sample of lymphoma survivors is needed to confirm these results.
Conclusion
A fully remote pilot dietary intervention to reduce fatigue was feasible and acceptable for this small sample of DLBCL survivors in remission. Self-reported fatigue and diet quality improved significantly post-intervention. As fatigue is the most commonly reported side effect of cancer treatment in non-Hodgkins lymphoma survivors, further research is warranted to test this dietary intervention in a larger sample of lymphoma survivors using a more rigorous randomized controlled study design.
Authors’ Contributions
The authors’ contributions were as follows – TSO and SZ designed research; KRW and AMB conducted research; KRW analyzed data; KRW, TSO, and SL wrote this article. KRW had primary responsibility for final content. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (62.7 KB)Acknowledgments
The authors would like to thank graduate students in the Ohio State University Masters of Dietetics and Nutrition program for their contributions to recruitment, intervention implementation, literature review, and data analysis (Miriam Knopp, Emily Botello, Jacob Heinl, Mitchell Coale, Amanda Johnson, and Cameryn Cohen), as well as Zihan Zhang who contributed to figure development.
Disclosure Statement
The authors report no conflict of interest.
Additional information
Funding
References
- American Cancer Society. Cancer treatment & survivorship facts & figures 2022–2024. Atlanta: American Cancer Society; 2022.
- Padala SA, Kallam A. Diffuse large B cell lymphoma. Treasure Island (FL): StatPearls Publishing; 2021.
- Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74–87. doi: 10.1016/j.pathol.2017.09.006.
- Oerlemans S, Mols F, Issa DE, Pruijt JHFM, Peters WG, Lybeert M, Zijlstra W, Coebergh JWW, van de Poll-Franse LV. A high level of fatigue among long-term survivors of non-Hodgkin’s lymphoma: results from the longitudinal population-based PROFILES registry in the south of the Netherlands. Haematologica. 2013;98(3):479–86. doi: 10.3324/haematol.2012.064907.
- Busson R, van der Kaaij M, Mounier N, Aleman BMP, Thiéblemont C, Stamatoullas A, Ribrag V, Tilly H, Haioun C, Casasnovas R-O, et al. Fatigue level changes with time in long-term Hodgkin and non-Hodgkin lymphoma survivors: a joint EORTC-LYSA cross-sectional study. Health Qual Life Outcomes. 2019;17(1):115–20190702. doi: 10.1186/s12955-019-1186-x.
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(0):S48–S57. doi: 10.1016/j.bbi.2012.06.011.
- Daniëls LA, Oerlemans S, Krol AD, van de Poll-Franse LV, Creutzberg CL. Persisting fatigue in Hodgkin lymphoma survivors: a systematic review. Ann Hematol. 2013;92(8):1023–32. doi: 10.1007/s00277-013-1793-2.
- Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127.
- Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GH, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–50. doi: 10.1200/jco.2013.53.4495.
- Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21(7):863–71. doi: 10.1016/j.bbi.2007.03.013.
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12 Suppl 1:22–34. doi: 10.1634/theoncologist.12-S1-22.
- Wang XS. Pathophysiology of cancer-related fatigue. Clin J Oncol Nurs. 2008;12(5 Suppl):11–20. doi: 10.1188/08.cjon.s2.11-20. PubMed PMID: 18842520; PubMed Central PMCID: PMC3281752.
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–40. 10.1158/1078-0432.CCR-08-2584
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–22. doi: 10.1200/jco.2011.36.1154.
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–11. doi: 10.1097/00006842-200207000-00010.
- Inglis JE, Lin P-J, Kerns SL, Kleckner IR, Kleckner AS, Castillo DA, Mustian KM, Peppone LJ. Nutritional interventions for treating cancer-related fatigue: a qualitative review. Nutr Cancer. 2019;71(1):21–40. doi: 10.1080/01635581.2018.1513046.
- Zick SM, Colacino J, Cornellier M, Khabir T, Surnow K, Djuric Z. Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res Treat. 2017;161(2):299–310. doi: 10.1007/s10549-016-4070-y. PubMed PMID: 27913933; PubMed Central PMCID: PMC5480210.
- Baguley BJ, Skinner TL, Jenkins DG, Wright ORL. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: a pilot randomised control trial. Clin Nutr. 2021;40(1):245–54. 10.1016/j.clnu.2020.05.016
- Baguley BJ, Skinner TL, Wright ORL. Nutrition therapy for the management of cancer-related fatigue and quality of life: a systematic review and meta-analysis. Br J Nutr. 2019;122(5):527–41. 10.1017/S000711451800363X
- Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, Neuhouser ML, Bandera EV, Wang Y, Robien K, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230–62. doi: 10.3322/caac.21719.
- Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: results from a national survey. Cancer. 2015;121(23):4212–21. doi: 10.1002/cncr.29488.
- Kristal AR, Kolar AS, Fisher JL, Plascak JJ, Stumbo PJ, Weiss R, Paskett ED. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J Acad Nutr Diet. 2014;114(4):613–21. doi: 10.1016/j.jand.2013.11.017.
- Yang M, Keller S, Lin JS. Psychometric properties of the PROMIS(®) Fatigue Short Form 7a among adults with myalgic encephalomyelitis/chronic fatigue syndrome. Qual Life Res. 2019;28(12):3375–84. doi: 10.1007/s11136-019-02289-4.
- Bingham Iii CO, Gutierrez AK, Butanis A, Bykerk VP, Curtis JR, Leong A, Lyddiatt A, Nowell WB, Orbai AM, Bartlett SJ, et al. PROMIS fatigue short forms are reliable and valid in adults with rheumatoid arthritis. J Patient Rep Outcomes. 2019;3(1):14. doi: 10.1186/s41687-019-0105-6.
- Ameringer S, Elswick RK, Menzies V, Robins JL, Starkweather A, Walter J, Gentry AE, Jallo N. Psychometric evaluation of the patient-reported outcomes measurement information system fatigue-short form across diverse populations. Nurs Res. 2016;65(4):279–89. doi: 10.1097/nnr.0000000000000162.
- U.S.D.A United States Department of Agriculture Food and Nutrition Service. HEI scores for Americans 2022 [cited 2022 06/23/2022]. Available from: https://www.fns.usda.gov/hei-scores-americans.
- Kirkpatrick SI, Baranowski T, Subar AF, Tooze JA, Frongillo EA. Best practices for conducting and interpreting studies to validate self-report dietary assessment methods. J Acad Nutr Diet. 2019;119(11):1801–16. doi: 10.1016/j.jand.2019.06.010.
- Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–16. doi: 10.1016/j.jclinepi.2010.11.018. PubMed PMID: 21447427; PubMed Central PMCID: PMC3076200.
- Kenzik KM, Demark-Wahnefried W, Ganz PA, Colditz G, Rock CL, Rogers LQ. Changes in body mass index and physical activity predict changes in vitality during a weight loss trial in breast cancer survivors. Ann Behav Med. 2018;52(12):999–1009. doi: 10.1093/abm/kay004.