Abstract
Schizophrenia (SCZ), is considered as one of the most debilitating mental disorders around the world. Symptom-based clinical interview and numerous tests have been used to evaluate the diagnosis and also cognitive disturbances in patients with SCZ. All these tests measure phenotype-based functions. Thus, it seems accurate diagnosis of such complex disorders must rely on more valid and reliable factors. In this study, we evaluated the association of transcription factor 4 (TCF4) gene mRNA level in peripheral blood with SCZ, and also its psychopathology, cognitive and intellectual impairments. In this study, using real-time PCR, we compared TCF4 mRNA level between the case (70 unmedicated schizophrenia patients) and healthy control (n = 72) groups. In addition, all subjects underwent Psychopathology (PANSS) and cognitive and intelligence (WAIS, WMS, Stroop, WCST) assessments, and scores were compared between the two groups. Also, to determine the effect of TCF4 expression on psychopathology, cognitive and intellectual functions, the correlation between expression level and test scores was measured. The correlation between gene expression and age of onset and duration of the disorder was evaluated as well. Our results showed that the TCF4 mRNA level, psychopathology, cognitive and intellectual functions were significantly different in all, male, and female patients compared to healthy participants. Additionally, it was found that TCF4 level is positively correlated with scores of WAIS and WMS and is negatively correlated with Stroop and WCST errors and PANSS score. Our results showed that the mRNA level of TCF4 may be associated with SCZ, its psychopathology, IQ and cognitive impairments in an Iranian group of patients with SCZ. These results may help to better understanding the TCF4 role in the psychopathogenesis of SCZ and also may shed some light on the ongoing works conducted on peripheral biomarker-based diagnosis of complicated mental disorders.
1. Introduction
Schizophrenia, with a lifetime prevalence of about 1% (Schizophrenia Working Group of the Psychiatric Genomics, C Citation2014), is considered as one of the most debilitating mental disorders around the world. Cognitive impairments, including attention, working memory and executive functions, are known as the main core deficits of SCZ (Green, Kern, Braff, & Mintz, Citation2000). A large number of studies have shown impaired working memory (Lee & Park, Citation2005; Park & Gooding, Citation2014), disrupted attention (Demeter, Guthrie, Taylor, Sarter, & Lustig, Citation2013; Ward et al., Citation2015) and inability to make decisions and solve problems appropriately in patients with SCZ. A post-morbid decline in general intellectual abilities in affected individuals has been reported by several studies as well (Kremen et al., Citation1998; Kremen, Seidman, Faraone, Toomey, & Tsuang, Citation2004).
Existing results point to alterations of mechanisms involved in brain development (Nour & Howes, Citation2015), neuroplasticity (McCullumsmith, Clinton, & Meador-Woodruff, Citation2004), various neurotransmitter systems (Grace, Citation2016; Selvaraj, Arnone, Cappai, & Howes, Citation2014), neuronal myelination (Hakak et al., Citation2001) and intercellular and intracellular signaling in SCZ (Corfas, Roy, & Buxbaum, Citation2004; Emamian, Hall, Birnbaum, Karayiorgou, & Gogos, Citation2004). Furthermore, high heritability of SCZ emphasizes influence of genetic factors on its etiology. In a quantitative meta-analysis of 12 published twin studies, Sullivan, Kendler, and Neale, (Citation2003) estimated 81% heritability for SCZ (Sullivan et al., Citation2003).
Generally, there is no actual test (physical or laboratory) for the diagnosis of SCZ and clinicians usually rely on clinical symptoms and signs. Currently, researchers are evaluating possible physical diagnostic tests (blood-based, cognitive tests, eye movements, brain imaging, ‘smell tests’, and …), nevertheless, these tests do not work well in case of complicated mental disorders like SCZ, because these tests only assess outward symptoms. In order to improve diagnosis accuracy, genetic-based surveys have recently attracted much attention.
According to the recent convergent functional genomics study, DISC1, TCF4, MBP, MOBP, NCAM1, NRCAM, NDUFV2 and RAB18 are among the top genes associated with SCZ (Ayalew et al., Citation2012). Meanwhile, TCF4, despite its central role in regulating expression of many other genes, has not received much attention. TCF4 encodes a basic helix-loop-helix (bHLH) transcription factor which activates transcription by binding to an Ephrussi-box (‘E-box’) site (de Pontual et al., Citation2009).
TCF4 is widely expressed in the developing central nervous system in mammals (de Pontual, et al., Citation2009). It mainly controls proliferation and differentiation of neuronal and glial progenitor cells (Ross, Greenberg, & Stiles, Citation2003). It seems that mammalian protein TCF4 integrates multiple bHLH networks controlling critical neurodevelopmental stages and is possibly involved in neural plasticity transcriptional programs (Quednow, Brzózka, and Rossner, Citation2014).
Decreased TCF4 activity has been suggested to cause drastic mental retardation (Giurgea et al., Citation2008). Additionally, Kurian et al. (Citation2011) have suggested reduced expression of TCF4 in the psychotic states (Kurian et al., Citation2011), and Mudge et al. (Citation2008) have noted its insufficient expression in the cerebellar cortex of patients with SCZ (Mudge et al., Citation2008). Moreover, the association of TCF4 with deficient verbal and visuospatial memories (Hui et al., Citation2015; Lennertz et al., Citation2011), disrupted attention and context-processing (Zhu et al., Citation2013) and inefficient sensorimotor gating (B. B. Quednow et al., Citation2011) have been affirmed. Also, the excessive expression of TCF4 in Pitt-Hopkins syndrome, which is associated with severe mental retardation, has been identified (Zweier et al., Citation2007).
The aim of this study was to further clarify TCF4 role in SCZ, its psychopathology, cognitive and intellectual impairments, through testing the hypothesis questioning if TCF4 expression is associated with SCZ, its executive functions, memory, attention, and intelligence impairments in a sample of Iranian patients with SCZ. The results of this study may help to better understand the role of TCF4 in the psychopathogenesis of SCZ and may also shed light on the ongoing studies of peripheral biomarker-based diagnosis of complex mental disorders.
2. Materials and methods
2.1. Participants
Using the available sampling approach, 70 unrelated patients with SCZ (39 males [56%]; and 31 females [44%]) diagnosed based on the fifth edition of diagnostic and statistical manual of mental disorders (DSM-V) were selected at Roozbeh hospital, Tehran University of medical sciences (TUMS), Tehran, Iran. The mean age of patients (±standard deviation; SD) was 38.87 ± 10.71 (35.85 ± 10.26 in males and 41.80 ± 9.07 in females).
Patients had not received medication for at least six months before assessments. All patients were clinically assessed by two expert psychiatrists and clinical psychologists. Patients were excluded if they were mentally retarded or had a history of a severe head injury, serious disorders (e.g. thyroid, heart and liver diseases) and also history of substance or alcohol use or addiction during one year before the study. Healthy subjects were excluded from the study if they or their first-degree relatives had a lifetime history of any psychiatric or non-psychiatric disorders interfering with brain function, or if they had history of substance or alcohol use or addiction during one year before the study. Written informed consent form was obtained from all participants. This study was approved by the ethics committee of the School of Medicine, Tehran University of Medical Sciences.
2.2. Psychopathology assessment
The positive and negative syndrome scale (PANSS) was used to measure severity of patient symptoms. PANSS captures general psychopathology (depression and anxiety) and higher order cognitive abilities (attention and abstract thinking) in addition to psychosis-related symptoms (Kay, Flszbein, & Opfer, Citation1987). This instrument does not depend on individual’s self-insight and provides reliable self-reports.
2.3. Cognitive assessment
2.3.1. Intelligence
General intelligence abilities were measured using the Wechsler Adult Intelligence Scale–Forth Edition (WAIS-IV). The WAIS is an IQ test originally published in 1955 by David Wechsler (Kaufman & Lichtenberger, Citation2005). Wechsler test includes verbal, and non-verbal (known as performance scales) items.
2.3.2. Attention
The Stroop color and word test was used to assess cognitive flexibility, resistance to interference from outside stimuli, and sustained attention (Golden & Freshwater, Citation1978). It measures cognitive processing and provides practical diagnostic information on brain dysfunction and cognition.
2.3.3. Memory
The Wechsler Memory Scale III (WMS-III) includes visual and nonverbal memory sub-tests, which evaluates nonverbal memory without the requirement for visuographic representation (Seelye, Howieson, Wild, Moore, & Kaye, Citation2009). The WMS-III also helps to assess global cognitive functioning in people with suspected memory deficits (Hunsley & Lee, Citation2014).
2.3.4. Executive functions
The Wisconsin Card Sorting Test (WCST) measures frontal or executive functions. The computer version of the test is employed to scoring mainly perseverative and non-perseverative errors (Hodges, Citation2007). WCST includes strategic planning, organized searching and cognitive shifting to direct behavior towards the interested goal and to control impulsive responding (Kohli & Kaur, Citation2006).
2.4. RNA isolation and expression analysis
Peripheral whole blood (4 ml.) was collected from all participants into a potassium EDTA (K2) anticoagulant containing tube. Peripheral blood mononuclear cells (PBMC) were extracted and total RNA content was isolated and quantified (Thermo Scientific NanoDrop 2000 Spectrophotometer). Intended cDNA was synthesized (TAKARA kit, Japan) for quantitative real-time polymerase chain reaction (RT-PCR). The expression of TCF4 was normalized with the expression of the housekeeping gene, HPRT1, and fold changes were calculated as 2-ΔΔCT.
2.5. Statistical analysis
The number of males and females and the handedness status in both case and control groups were compared using cross-tabs analysis. The mean age was compared between the two groups using independent t-test. The fold change of TCF4 expression in patients was calculated using REST 2009 software. TCF4 expression between patients and controls was compared using ANCOVA with PANSS, intelligence and cognitive scores (each score separately) as covariates to remove the effects of scores.
In order to evaluate the correlation between TCF4 expression level and test scores, the partial correlation method was used and group (case/control) was considered as control variable. Correlation between age of onset, duration of illness and smoking status with TCF4 expression level was also evaluated using Pearson correlation coefficient.
3. Results
Three patients (4%) were left handed. In addition, 72 unrelated healthy participants (36 males [50%] and 36 [50%] females) were selected as the control group from the same area. The mean age of healthy individuals (±SD) was 35.65 ± 9.40 (39.53 ± 9.10 in males and 37.78 ± 9.75 in females). Six people (8%) were left handed. Statistical analysis showed that the two groups were matched in terms of sex, age and handedness. The demographical features of the participants are shown in .
Table 1. Demographic characteristics of participants and comparison of characteristics between case and control groups.
The results showed that there was a significant difference between the two groups in all psychopathology, cognitive and intelligence scores. This difference was apparent both in all and between males and females (). The control group was better than the case group in all tests.
Table 2. Comparison of psychopathology, cognitive and intellectual functions between case and control groups using independent t-test.
It was also found that the expression of TCF4 is significantly different between the control and case groups (). Gene expression in the case group was significantly lower than the control group.
Table 3. Comparison of TCF4 expression level between case and control groups.
Further analysis showed that, in general, decreased gene expression is correlated significantly with increasing the PANSS, Stroop and WCST total errors (p < .001), and decreasing the WAIS and WMS test scores (p < .001) ().
Figure 1. Correlation of gene expression with PANSS, cognitive and intellectual functions (p < .001). Fit lines are drawn on each graph.
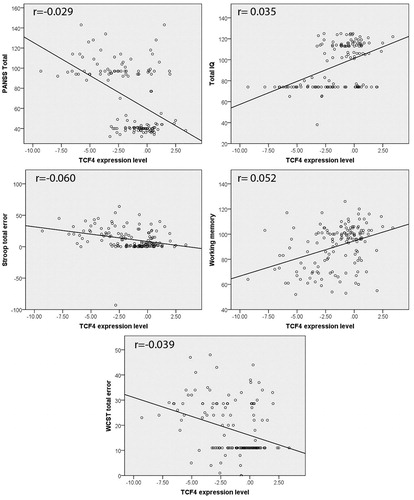
The results showed no significant correlation between TCF4 expression level and age of onset and between TCF4 expression level and duration of illness. We also found statistically significant correlation between TCF4 expression level and smoking status; however, the correlation coefficient was zero ().
Table 4. Correlation of gene expression with age of onset, duration of illness and smoking status.
4. Discussion
Schizophrenia, with a median incidence of 15.2/100,000 (McGrath, Saha, Chant, & Welham, Citation2008), is often described by positive (delusions, hallucinations, disordered thoughts and speech), negative (flat expressions or little emotion, poverty of speech, inability to experience pleasure, lack of desire to form relationships and lack of motivation) symptoms (Crow, Citation1981), and cognitive impairments (Keefe & Harvey, Citation2012). The PANSS is commonly used for measuring symptoms severity of patients with SCZ (Kay et al., Citation1987).
We performed a case control study to evaluate the association of TCF4 mRNA with SCZ, its psychopathology (PANSS scores), IQ, and cognitive impairments in a sample of 70 unrelated patients with SCZ and 72 matched unrelated healthy people.
Similar to other studies (Edwards et al., Citation2016; Selvaraj, et al., Citation2014), our results showed that patients with SCZ demonstrate higher scores in PANSS scores compared to control individuals. In addition, we found significant decrease in terms of WAIS scores in patients with SCZ rather than the healthy participants. These results are consistent with several previous studies. For example, in a meta-analysis, Aylward, Walker, and Bettes, (Citation1984) argued that significant IQ deficits are apparent after onset of SCZ (Aylward et al., Citation1984). In another meta-analysis study conducted by Woodberry, Giuliano, and Seidman, (Citation2008), patients with SCZ demonstrated a moderate impairment in premorbid intelligence (Woodberry et al., Citation2008).
In addition, memory impairment was clearly seen in our study. WMS scores were significantly decreased in patients with SCZ in comparison with the control group, even when males and females were analyzed separately. Studies have shown that working memory impairment is a promising endophenotypic biomarker for SCZ (Park & Gooding, Citation2014). Short and long-term memory impairments have been documented as well (Green, et al., Citation2000; Lee & Park, Citation2005; Park & Gooding, Citation2014; Tamlyn et al., Citation2009).
Our study, consistent with the previous studies, revealed impaired attention of patients (decreased performance in Stroop test). We found significant decrease in terms of color errors in all, and word and color errors in female patients with SCZ rather than the healthy participants. Carter, Mintun, Nichols, and Cohen, (Citation1997) have demonstrated that patients with SCZ respond with more errors in color naming in color-incongruent condition (Carter et al., Citation1997). Furthermore, it has been suggested that patients with SCZ face difficulty when they are posed with multiple attentional demands.
The results of the present study showed that the executive functions of the patients with SCZ; WCST scores are significantly decreased rather than the healthy people. This finding has also been confirmed by numerous studies. Everett et al. (Citation2001) have reported that patients with SCZ show a perseverative deficit in WCST score (Everett, Lavoie, Gagnon, & Gosselin, Citation2001). Prentice, Gold, and Buchanan, (Citation2008) have shown that patients perform poorly in WCST and this reflects a disability in feedback-guided behaviors (Prentice et al., Citation2008). Recently, Singh, Aich, and Bhattarai, (Citation2017) have indicated that patients with SCZ demonstrate WCST measures deficits (Singh et al., Citation2017). Executive functions actually depend on the components such as attention, working memory and the speed of information processing in brain (Diamond, Citation2013; Friedman, Citation2016). Since defects in each of these components have been observed in patients with SCZ, it can be expected that executive functions will be disrupted in these patients.
Regarding the association of TCF4 mRNA with SCZ, we found a significant decreased mRNA level (increase in Δct) in all, male, and female patients rather than the control participants. Wirgenes et al., (Citation2012) have demonstrated an altered expression of TCF4 in psychotic groups and indicated that its expression in the peripheral blood and central nervous system is consistent with each other (Wirgenes et al., Citation2012). Moreover, in a recent study, it has been shown that peripheral expression of TCF4 is significantly correlated with grey matter thickness in the prefrontal cortex (Kochunov et al., Citation2013). Decline in volume of prefrontal grey matter in SCZ has been confirmed in numerous studies (Goghari, Macdonald, & Sponheim, Citation2014; Gur et al., Citation2000; Hirayasu et al., Citation2001; Pol et al., Citation2002). Evidence shows that the expression of TCF4 is changed in postmortem brain of patients with SCZ. TCF4 can induce pluripotent stem cells differentiation to the neurons (Ayalew, et al., Citation2012). It has been shown that subtle changes at the level of TCF4 gene expression is relevant for the psychopathology of SCZ (Quednow et al., Citation2014). In total, TCF4 may be considered as an integrator of several regulatory networks involved in various developmental stages of the CNS and alterations of its expression may affect information processing.
Our results were in line with similar available evidence which is very limited. Although, in general, the severity of negative symptoms is more in men (Ochoa, Usall, Cobo, Labad, & Kulkarni, Citation2012), when the effects of TCF4 mRNA level on cognitive functions were analyzed, results were more pronounced in females. This suggests that the expression of TCF4 may somehow be sex specific. On the other hand, this may be due to differences in basic pathology of the disorder in males and females.
Further analysis showed that TCF4 expression level is significantly correlated with smoking status. However, the correlation coefficient was zero and this shows that there is no linear relationship between the two variables and there may be a more complex relationship. Also, this relationship may be mediated by a third factor. On the other hand, in this study, only being smoker or non-smoker was evaluated, while expression rate may be directly and significantly correlated with the amount of smoking.
In summary, our study showed that healthy people and patients with SCZ may differ significantly in elementary cognitive functions. Results also show that the level of TCF4 mRNA is significantly different between the two groups and this difference is associated with significant impact on cognitive performance, especially in females. According to our study and previous findings, it seems that the level of TCF4 mRNA can be a reliable biomarker for SCZ.
Finally, each study, along with its advantages, has flaws and shortcomings as well. Perhaps the most significant shortcoming of this study, like majority of similar studies performed for the first time, is the small sample size. Another limitation is the population stratification that may be solved through a family study in future. Studies with larger non-stratified sample sizes will lead to more effective results. Evaluation of TCF4 protein expression level alongside with structural genetics and epigenetic studies can also make the available data richer.
Acknowledgements
This study has been supported by Tehran University of Medical Sciences grant No. 32178. We also thank the colleagues of the Department of Genomic Psychiatry and Behavioral Genomics (DGPBG) at the Roozbeh Hospital, school of medicine, Tehran University of Medical Science (TUMS).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ayalew, M., Le-Niculescu, H., Levey, D.F., Jain, N., Changala, B., Patel, S.D., … Niculescu, A.B. (2012). Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Molecular Psychiatry, 17, 887–905. doi:10.1038/mp.2012.37.
- Aylward, E., Walker, E., & Bettes, B. (1984). Intelligence in schizophrenia: meta-analysis of the research. Schizophrenia Bulletin, 10, 430–459. doi:10.1093/schbul/10.3.430
- Carter, C.S., Mintun, M., Nichols, T., & Cohen, J.D. (1997). Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial stroop task performance. American Journal of Psychiatry, 154, 1670–1675. doi:10.1176/ajp.154.12.1670
- Corfas, G., Roy, K., & Buxbaum, J.D. (2004). Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nature Neuroscience, 7, 575–580. doi:10.1038/nn1258
- Crow, T. (1981). Positive and negative schizophrenia symptoms and the role of dopamine. The British Journal of Psychiatry, 139, 251–254.
- de Pontual, L., Mathieu, Y., Golzio, C., Rio, M., Malan, V., Boddaert, N., … Amiel, J. (2009). Mutational, functional, and expression studies of the TCF4 gene in Pitt-Hopkins syndrome. Human Mutation, 30, 669–676. doi:10.1002/humu.20935
- Demeter, E., Guthrie, S.K., Taylor, S.F., Sarter, M., & Lustig, C. (2013). Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: evidence from a translational sustained attention task. Schizophrenia Research, 144, 136–141. doi:10.1016/j.schres.2013.01.003
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. doi:10.1146/annurev-psych-113011-143750
- Edwards, A.C., Bigdeli, T.B., Docherty, A.R., Bacanu, S., Lee, D., De Candia, T.R., … Wormley, B.K. (2016). Meta-analysis of positive and negative symptoms reveals schizophrenia modifier genes. Schizophrenia Bulletin, 42, 279–287. doi: 10.1093/schbul/sbv119
- Emamian, E.S., Hall, D., Birnbaum, M.J., Karayiorgou, M., & Gogos, J.A. (2004). Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nature Genetics, 36, 131–137.
- Everett, J., Lavoie, K., Gagnon, J.F., & Gosselin, N. (2001). Performance of patients with schizophrenia on the Wisconsin Card Sorting Test (WCST). Journal of Psychiatry and Neuroscience, 26, 123–130. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1407748/
- Friedman, N.P. (2016). Research on individual differences in executive functions. Linguistic Approaches to Bilingualism, 6, 535–548. doi:10.1075/lab.15041.fri
- Giurgea, I., Missirian, C., Cacciagli, P., Whalen, S., Fredriksen, T., Gaillon, T., … Moncla, A. (2008). TCF4 deletions in Pitt-Hopkins Syndrome. Human Mutation, 29, E242–E251. doi:10.1002/humu.20859
- Goghari, V.M., Macdonald, A.W., & Sponheim, S.R. (2014). Relationship between prefrontal gray matter volumes and working memory performance in schizophrenia: a family study. Schizophrenia Research, 153, 113–121. doi:10.1016/j.schres.2014.01.032
- Golden, C.J., & Freshwater, S.M. (1978). Stroop color and word test. Age, 15, 90.
- Grace, A.A. (2016). Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature Reviews Neuroscience, 17, 524–532. doi:10.1038/nrn.2016.57
- Green, M.F., Kern, R.S., Braff, D.L., & Mintz, J. (2000). Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”. Schizophr Bull, 26, 119–136.
- Gur, R.E., Cowell, P.E., Latshaw, A., Turetsky, B.I., Grossman, R.I., Arnold, S.E., …, Gur, R.C. (2000). Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Archives of General Psychiatry, 57, 761–768. doi:10.1001/archpsyc.57.8.761
- Hakak, Y., Walker, J.R., Li, C., Wong, W.H., Davis, K.L., Buxbaum, J.D., … Fienberg, A.A. (2001). Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences, 98, 4746–4751.
- Hirayasu, Y., Tanaka, S., Shenton, M.E., Salisbury, D.F., DeSantis, M.A., Levitt, J.J., … McCarley, R.W. (2001). Prefrontal gray matter volume reduction in first episode schizophrenia. Cerebral Cortex, 11, 374–381.
- Hodges, J.R. (2007). Cognitive Assessment for Clinicians (2 ed.). Oxford, UK: Oxford University Press.
- http://www.nature.com/nature/journal/v511/n7510/abs/nature13595.html#supplementary-information. Retrieved from http://dx.doi.org/10.1038/nature13595
- Hui, L., Rao, W.W., Yu, Q., Kou, C., Wu, J.Q., He, J.C., … Zhang, X.Y. (2015). TCF4 gene polymorphism is associated with cognition in patients with schizophrenia and healthy controls. Journal of Psychiatric Research, 69, 95–101. doi:10.1016/j.jpsychires.2015.07.022
- Hunsley, J., & Lee, C.M. (2014). Introduction to Clinical Psychology (2 ed.). Canada: Wiley.
- Kaufman, A.S., & Lichtenberger, E.O. (2005). Assessing Adolescent and Adult Intelligence, (3 ed.). Allyn and Bacon.
- Kay, S.R., Flszbein, A., & Opfer, L.A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia. Bulletin, 13, 261. doi:10.1093/schbul/13.2.261
- Keefe, R.S., & Harvey, P.D. (2012). Cognitive impairment in schizophrenia. In: Novel antischizophrenia treatments (pp. 11–37). Berlin, Heidelberg: Springer.
- Kochunov, P., Charlesworth, J., Winkler, A., Hong, L.E., Nichols, T.E., Curran, J.E., … Johnson, M. (2013). Transcriptomics of cortical gray matter thickness decline during normal aging. NeuroImage, 82, 273–283. doi: 10.1016/j.neuroimage.2013.05.066
- Kohli, A., & Kaur, M. (2006). Wisconsin Card Sorting Test: Normative data and experience. Indian Journal of Psychiatry, 48, 181–184. doi:10.4103/0019-5545.31582
- Kremen, W.S., Buka, S.L., Seidman, L.J., Goldstein, J.M., Koren, D., & Tsuang, M.T. (1998). IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. American Journal of Psychiatry, 155, 672–677. doi:10.1176/ajp.155.5.672
- Kremen, W.S., Seidman, L.J., Faraone, S.V., Toomey, R., & Tsuang, M.T. (2004). Heterogeneity of schizophrenia: a study of individual neuropsychological profiles. Schizophr Res, 71, 307–321. doi:10.1016/j.schres.2004.02.022
- Kurian, S., Le-Niculescu, H., Patel, S., Bertram, D., Davis, J., Dike, C., … Caligiuri, M. (2011). Identification of blood biomarkers for psychosis using convergent functional genomics. Molecular Psychiatry, 16, 37–58. doi: 10.1038/mp.2009.117
- Lee, J., & Park, S. (2005). Working memory impairments in schizophrenia: a meta-analysis. Journal of Abnormal Psychology, 114, 599. doi:10.1037/0021-843X.114.4.599
- Lennertz, L., Rujescu, D., Wagner, M., Frommann, I., Schulze-Rauschenbach, S., Schuhmacher, A., Mössner, R. (2011). Novel schizophrenia risk gene TCF4 influences verbal learning and memory functioning in schizophrenia patients. Neuropsychobiology, 63, 131–136. Retrieved from http://www.karger.com/DOI/10.1159/000317844
- McCullumsmith, R.E., Clinton, S.M., & Meador-Woodruff, J.H. (2004). Schizophrenia as a disorder of neuroplasticity. International Review of Neurobiology, 59, 19–45. doi:10.1016/s0074-7742(04)59002-5
- McGrath, J., Saha, S., Chant, D., & Welham, J. (2008). Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiologic Reviews, 30, 67–76. doi:10.1093/epirev/mxn001
- Mudge, J., Miller, N.A., Khrebtukova, I., Lindquist, I.E., May, G.D., Huntley, J.J., … Farmer, A.D. (2008). Genomic convergence analysis of schizophrenia: mRNA sequencing reveals altered synaptic vesicular transport in post-mortem cerebellum. PloS One, 3, e3625. doi: 10.1371/journal.pone.0003625
- Nour, M.M., & Howes, O.D. (2015). Interpreting the neurodevelopmental hypothesis of schizophrenia in the context of normal brain development and ageing. Proceedings of the National Academy of Sciences, 112, E2745–E2745. doi:10.1073/pnas.1502170112
- Ochoa, S., Usall, J., Cobo, J., Labad, X., & Kulkarni, J. (2012). Gender Differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophrenia Research and Treatment, 2012, 9. doi:10.1155/2012/916198
- Park, S., & Gooding, D.C. (2014). Working memory impairment as an endophenotypic marker of a schizophrenia diathesis. Schizophrenia Research: Cognition, 1, 127–136. doi:10.1016/j.scog.2014.09.005
- Pol, H.E.H., Schnack, H.G., Bertens, M.G., van Haren, N.E., van der Tweel, I., Staal, W.G., … Kahn, R.S. (2002). Volume changes in gray matter in patients with schizophrenia. American Journal of Psychiatry, 159, 244–250. doi:10.1176/appi.ajp.159.2.244
- Prentice, K.J., Gold, J.M., & Buchanan, R.W. (2008). The Wisconsin Card Sorting impairment in schizophrenia is evident in the first four trials. Schizophrenia Research, 106, 81–87. doi:10.1016/j.schres.2007.07.015
- Quednow, B.B., Brzózka, M.M., & Rossner, M.J. (2014). Transcription factor 4 (TCF4) and schizophrenia: integrating the animal and the human perspective. Cellular and Molecular Life Sciences, 71, 2815–2835. doi:10.1007/s00018-013-1553-4
- Quednow, B.B., Ettinger, U., Mossner, R., Rujescu, D., Giegling, I., Collier, D.A., … Kumari, V. (2011). The schizophrenia risk allele C of the TCF4 rs9960767 polymorphism disrupts sensorimotor gating in schizophrenia spectrum and healthy volunteers. Journal of Neuroscience, 31, 6684–6691. doi:10.1523/jneurosci.0526-11.2011
- Ross, S.E., Greenberg, M.E., & Stiles, C.D. (2003). Basic helix-loop-helix factors in cortical development. Neuron, 39, 13–25. doi:10.1016/S0896-6273(03)00365-9
- Schizophrenia Working Group of the Psychiatric Genomics, C. (2014). Biological insights from 108 schizophrenia-associated genetic loci. [Article]. Nature, 511, 421–427. doi:10.1038/nature13595
- Seelye, A.M., Howieson, D.B., Wild, K.V., Moore, M.M., & Kaye, J.A. (2009). Wechsler Memory Scale–III Faces test performance in patients with mild cognitive impairment and mild Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 31, 682–688. doi:10.1080/13803390802484763
- Selvaraj, S., Arnone, D., Cappai, A., & Howes, O. (2014). Alterations in the serotonin system in schizophrenia: A systematic review and meta-analysis of postmortem and molecular imaging studies. Neuroscience and Biobehavioral Reviews, 45, 233–245. doi:10.1016/j.neubiorev.2014.06.005
- Singh, S., Aich, T.K., & Bhattarai, R. (2017). Wisconsin Card Sorting Test performance impairment in schizophrenia: An Indian study report. Indian Journal of Psychiatry, 59, 88–93. doi:10.4103/0019-5545.204440
- Sullivan, P.F., Kendler, K.S., & Neale, M.C. (2003). Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Archives of General Psychiatry, 60, 1187–1192. doi:10.1001/archpsyc.60.12.1187
- Tamlyn, D., McKenna, P.J., Mortimer, A.M., Lund, C.E., Hammond, S., & Baddeley, A.D. (2009). Memory impairment in schizophrenia: its extent, affiliations and neuropsychological character. Psychological Medicine, 22, 101–115. doi:10.1017/S0033291700032773
- Ward, R.D., Winiger, V., Higa, K.K., Kahn, J.B., Kandel, E.R., Balsam, P.D., & Simpson, E.H. (2015). “The impact of motivation on cognitive performance in an animal model of the negative and cognitive symptoms of schizophrenia”: Correction to Ward et al. (2015).
- Wirgenes, K.V., Sonderby, I.E., Haukvik, U.K., Mattingsdal, M., Tesli, M., Athanasiu, L., … Andreassen, O.A. (2012). TCF4 sequence variants and mRNA levels are associated with neurodevelopmental characteristics in psychotic disorders. [Original Article]. Translational Psychiatry, 2, e112. doi:10.1038/tp.2012.39
- Woodberry, K.A., Giuliano, A.J., & Seidman, L.J. (2008). Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry, 165, 579–587. doi:10.1176/appi.ajp.2008.07081242.
- Zhu, X., Gu, H., Liu, Z., Xu, Z., Chen, X., Sun, X., … Chen, C. (2013). Associations between TCF4 gene polymorphism and cognitive functions in schizophrenia patients and healthy controls. Neuropsychopharmacology, 38, 683–689. http://www.nature.com/npp/journal/v38/n4/suppinfo/npp2012234s1.html
- Zweier, C., Peippo, M.M., Hoyer, J., Sousa, S., Bottani, A., Clayton-Smith, J., … Rauch, A. (2007). Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins Syndrome). The American Journal of Human Genetics, 80, 994–1001. doi:http://10.1086/515583
