Abstract
Inoculation of crop plants with beneficial root-associated microorganisms may be a useful strategy for sustainable intensification of agriculture. In recent years, interest has grown in using rhizobacteria from extreme environments to develop high-performing inoculants, as some strains may possess plant-growth promoting traits and increase host fitness under abiotic stress. Only two vascular plant species – Antarctic hair grass (Deschampsia antarctica) and Antarctic pearlwort (Colobanthus quitensis) – are currently found on the Antarctic continent, one of the most extreme environments on Earth. Few studies have examined the rhizosphere microorganisms associated with these two plants and their potential contribution to crop nutrition, productivity, and stress tolerance. The present study assesses the potential of a novel rhizobacterium extracted from the rhizosphere of Deschampsia antarctica, Pseudomonas sp. ATCC PTA-122608, to improve growth of soybean (Glycine max L.) and investigates potential underlying mechanisms. Soybean plants were grown for 118 days in a glasshouse study and plant growth, nutrition, and root systems were analyzed. Inoculation with both the bacterial treatment and sometimes the kaolin substrate increased root biomass, the production of medium-diameter and coarse roots, nodulation by Bradyrhizobium japonicum, total biomass production, and C/N accumulation. These results indicate that ATCC PTA-122608 inoculation with kaolin substrate can promote soybean nutrition and productivity, potentially via modification of root system architecture and enhancement of the soybean-rhizobia symbiosis. Broadly, our work demonstrates the potential for rhizosphere microorganisms from extreme environments to promote the growth of economically and nutritionally important crops by influencing plant root architectural traits and plant-microbe interactions.
Introduction
Rhizosphere bacteria and fungi influence several aspects of plant growth and development (Bakker et al. Citation2012; Panke-Buisse et al. Citation2014; Lu et al. Citation2018). Despite recent advances in our understanding of rhizosphere assembly and plant-microbe interactions (Philippot et al. Citation2013; De-la-Peña and Loyola-Vargas Citation2014; Poole Citation2017), the rhizosphere still holds a largely untapped reservoir of biodiversity from which high-performing microbial inoculants may be derived. Applications of inoculants containing beneficial microorganisms to crops could help sustain or intensify agricultural production with fewer resources and aid in cultivation of marginal lands (Bhardwaj et al. Citation2014; García-Fraile, Menéndez, and Rivas Citation2015; Ahmad et al. Citation2018).
Through millions of years of co-evolution, rhizosphere symbionts have contributed to the fitness and adaptive plasticity of plant traits (Friesen et al. Citation2011; Rao et al. Citation2016; Timmusk et al. Citation2014; Yang, Kloepper, and Ryu Citation2009), especially in harsh environments (Goh et al. Citation2013; Gopal and Gupta Citation2016; Li et al. Citation2018). Several studies have highlighted the ability of plant growth promoting rhizobacterial (PGPR) strains to promote plant growth under abiotic stresses through multiple direct and indirect mechanisms (Yang, Kloepper, and Ryu Citation2009; Shrivastava and Kumar Citation2015; Enebe and Babalola Citation2018). However, thousands of years of crop improvement in high-input agricultural systems may have altered root adaptation to resource-poor environments and recruitment of beneficial microbes to the rhizosphere (Brisson et al. Citation2019; Perez-Jaramillo, Mendes, and Raaijmakers Citation2016; Schmidt, Bowles, and Gaudin Citation2016; Schmidt et al. Citation2020; Wissuwa, Mazzola, and Picard Citation2009). Biofertilizers developed from extremophilic microorganisms could thus be a promising strategy to shape root and rhizosphere traits that increase resource use efficiency and sustainable productivity in high-input systems (Grover et al. Citation2011; Ramadoss et al. Citation2013).
With year-round snowfall, extreme cold, and limited nutrient availability, Antarctica is one of harshest environments on Earth and only two vascular plant species – Antarctic hair grass (Deschampsia antarctica) and Antarctic pearlwort (Colobanthus quitensis) – have been able to successfully survive on the continent. Co-evolved rhizosphere microorganisms have likely facilitated persistence of these hosts in such an extreme environment by conferring a fitness advantage through alteration of root traits and phytohormone metabolic pathways (Glick Citation2012). However, whether Antarctic rhizobacteria can improve productivity of agriculturally relevant non-host plants such as soybean (Glycine max L.) has not been explored.
Recent work has suggested extremophile rhizosphere microorganisms, including those found in Antarctica, can improve plant abiotic stress tolerance in non-host species. Work by Fardella et al. (Citation2014) demonstrated that fungal endophytes isolated from Antarctic plants improved the survival and water use efficiency of several tree and shrub species in xerophytic formations. Beneficial effects of extremophilic rhizosphere microorganisms on non-host plants were further demonstrated by Acuña-Rodríguez et al. (Citation2019), where rhizosphere consortia reduced osmotic stress in lettuce. In combination with past work showing PGPR can directly alter root systems (El Zemrany et al. Citation2007; Poitout et al. Citation2017), these results suggest that certain strains of rhizobacteria from Antarctica may be useful tools in promoting abiotic stress tolerance and productivity in important crop species. In this study, we test the effects of inoculation with Pseudomonas sp. ATCC PTA-122608, a newly discovered Antarctic rhizobacterium from the rhizosphere of D. antarctica, on root growth and productivity of soybean. Given the origin of ATCC PTA-122608 from an extreme environment and evidence that other members of the genus Pseudomonas can promote plant growth (Choudhary et al. Citation2009), we hypothesized that inoculation would increase soybean productivity by altering root and rhizosphere traits. Further, because interactions between Pseudomonas and rhizobial species have been observed (Andrade, De Leij, and Lynch Citation1998; Egamberdieva et al. Citation2010), we hypothesized that this strain would also promote rhizobial nodulation of soybean roots.
Materials and methods
Plant material and growth
Glasshouse trials were performed at the University of California, Davis Environmental Horticulture Glasshouse Facility from May 6th, 2016 to September 1st, 2016 (25 °C/17 °C day/night temperature with natural light). Soybean seeds (Peaceful Valley Farm Supply Co., Grass Valley, CA, USA) were coated with powdered rhizobium (Bradyrhizobium japonicum) and planted into trays containing 1:1:1 (v/v/v) peat:sand:redwood compost potting medium. Five weeks after germination, seedlings were transplanted into 11.3 L pots containing the same potting medium. Plants were arranged on a single glasshouse bench in a completely randomized design.
One seedling was transplanted per individual pot. Three days after transplanting, ten plants were inoculated with 200 mL of a solution containing 104 colony forming units (CFU) mL−1 of Pseudomonas sp. ATCC PTA-122608 formulated into a biofertilizer using kaolin as a substrate for a total of 2,000,000 CFU per plant (inoculated treatment). Ten plants received 200 mL of deionized water only (non-inoculated control) and ten received 200 mL of a solution containing kaolin only (substrate control). Plants were watered daily with 670 mL of deionized water until harvest.
Root traits, biomass, and nutrient analysis
Aboveground and belowground plant biomass was harvested 118 days after planting. Roots were preserved in deionized water for analysis of root architecture using WinRHIZO (Reagent Instrument, INC.) shortly after harvest. To analyze potential shifts in root system architecture (i.e., variation in root order), the entire root system was scanned and images were analyzed using color analysis with three color classes (dark root, light root, and background) and three distinct diameter classes (fine root (d < 0.5 cm), intermediate root (0.5 cm < d < 1cm) and coarse root (d > 1cm)). Specific root length (SRL, cm g−1) was calculated by dividing the total root length by dry root weight for each plant. Nodulation was also recorded by manually counting nodules on the entire root system. Root and shoot tissues including root nodules were dried at 60 °C for three days and weighed. Plant total carbon (C) and nitrogen (N) were measured on dried leaf tissue using a TruSpec CN analyzer at the University of California, Davis Analytical Laboratory (Davis, CA, USA).
Statistical analyses
Data were analyzed using R 3.5.1 (R Foundation for Statistical Computing). Analysis of variance (ANOVA) was used to test the effect of treatments on plant traits. A linear model was created to assess the effect of bacterial treatment on each response variable and a one-way ANOVA on each model was used to test for significant differences. Residuals were tested for homogeneity and normality using Levene’s test and the Shapiro-Wilk test, respectively. Data transformations were performed as necessary to meet model assumptions. Multiple pairwise comparisons of least square means were performed using the Tukey method of p-value adjustment with a significance level of alpha = 0.05.
Results
Inoculation of soybean with ATCC PTA-122608 increases plant growth
We measured the impact of inoculation with ATCC PTA-122608 on biomass accumulation, partitioning between shoot and roots, and plant nutrition (). Inoculation increased total dry biomass production by 71% (p < 0.05) and total C accumulation by 64% (p < 0.05) compared to the non-inoculated control, while the substrate control did not differ significantly from the other treatments. In addition, inoculated plants contained 55% (p < 0.05) more N when compared to the non-inoculated controls. Inoculation also increased root dry weight by 110% (p < 0.001) and 65% (p < 0.05) compared to the non-inoculated and substrate controls. As a result, root:shoot (R:S) ratio did not vary among any bacterial treatments.
Table 1. Mean total shoot dry weight, total root dry weight, total dry weight, total plant carbon, and total plant nitrogen for each treatment with standard errors and ANOVAs.
Inoculation promotes the development of coarse roots
Inoculation altered root system architecture toward more large diameter roots (). Increases in total root length of inoculated plants were not significant, despite their greater root biomass. Medium root length of the inoculated plants was 54% (p < 0.05) greater than the substrate control plants, while coarse root length of the inoculated plants was 53% (p < 0.1) and 69% (p < 0.05) greater than the non-inoculated and substrate control plants, respectively (). Fine root length did not differ between any of the treatments. Total root surface area was 36% greater in the inoculated plants compared to the substrate controls (p < 0.05), while the non-inoculated controls did not differ between the other two treatments (). Fine root surface area did not differ between treatments, but coarse root surface area was 67% greater in inoculated plants compared to the substrate controls (p < 0.05). Medium root surface area was also 52% greater in inoculated plants compared to the substrate controls (p < 0.05). SRL was 71% (p < 0.05) and 41% (p < 0.05) greater in non-inoculated plants compared to the inoculated and substrate controls, respectively ().
Figure 1. A) Total root length and B) root surface area in soybean plants inoculated with Pseudomonas sp. ATCC PTA-122608, not inoculated, or inoculated with a substrate control, partitioned by root diameter class. C) Specific root length in each treatment. Letters indicate significant differences between treatments at the p = 0.05 level. Letters in A) and B) represent comparisons between treatments for each diameter class as well as total root length and surface area. Error bars indicate standard error.

Inoculation enhances nodulation
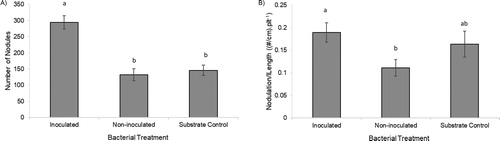
Inoculation increased nodulation by 122% (p < 0.001) and 102% (p < 0.001) compared to the non-inoculated and substrate controls, respectively (). Nodulation per unit root length was 70% higher in inoculated plants when compared to the non-inoculated controls (p < 0.05), while the substrate control did not differ between the other two treatments ().
Figure 2. A) Total nodulation and B) nodulation/length of soybean plant roots inoculated with Pseudomonas sp. ATCC PTA-122608, not inoculated with the bacteria, and inoculated with the substrate control. The letters represent significant differences between treatments at the p = 0.05 level. Error bars indicate standard error.
Discussion
The goal of this experiment was to assess the potential of a novel Antarctic rhizobacterium, Pseudomonas sp. ATCC PTA-122608, to improve nutrition and biomass production of soybean and to investigate underlying mechanisms related to root morphology and interactions with rhizobia. Inoculation promoted shoot and root growth as well as total nodulation by rhizobia. Effects of this magnitude on soybean growth parameters have been reported for a non-extremophile rhizobacterium frequently used in biofertilizers, Azospirillum brasilense (Molla et al. Citation2001), suggesting that novel rhizobacteria from extreme environments have great potential to contribute to the development of effective biofertilizers.
Plants growing in the substrate control treatment were often not statistically different from the inoculated treatments suggesting some effect of the kaolin substrate on soybean root system and growth. Kaolin is a natural clay substrate which has been shown to impact both soil and plant properties and likely influenced the growth of soybean in this experiment. Kaolin has been shown to promote soil nitrate retention (Mohsenipour et al. Citation2019) and plant salinity tolerance (Boari et al. Citation2014). Additionally, kaolin stores elements such as iron (Guo et al. Citation2010), which is an important plant nutrient. While this likely had an influence on the growth of our soybean plants, clear alteration to traits like root dry weight and nodulation by rhizobia in the inoculated treatment suggest the bacteria still showed growth promotion potential.
Our results suggest that increasing root biomass, altering root architecture, and enhancing nodulation by rhizobia may be important mechanisms by which ATCC PTA-122608 promotes nutrient accumulation and biomass production in soybean. Higher root biomass could improve plant nutrition given the essential role of roots in foraging for soil resources (McNickle and Cahill Citation2009). In the case of legumes, increased root biomass can also promote nodulation by effective strains of rhizobia (Batstone et al. Citation2017). Increased root biomass in soybean leading to greater nutrient capture and improved nodulation and subsequent atmospheric N fixation could be a primary mechanism by which ATCC PTA-122608 promotes growth under non-stressed conditions.
Shifts in root system architecture were also observed in plants inoculated with ATCC PTA-122608, with greater partitioning to the production of medium and coarse roots, resulting in decreased SRL. These changes may have been due to manipulation of phytohormone metabolic pathways by ATCC PTA-122608, as has been shown for other PGPR (Vacheron et al. Citation2013). Increasing coarse root production could be advantageous in promoting resource transport and providing structural support for lateral roots (Zhang and Wang Citation2015). Furthermore, coarse roots can determine the ability of plants to exploit compacted soil layers that fine roots lack the mechanical strength to penetrate (Comas et al. Citation2013), ultimately affecting plant rooting depth. Thus, a greater proportion of coarse roots could be even more beneficial under field conditions where structural support and penetration of compacted soils have a larger effect on yield outcomes than under glasshouse conditions.
Given the importance of rhizobia for N accumulation in legumes and the importance of N for plant growth, the improved growth observed in inoculated plants was likely due in large part to increased nodulation. The increases in nodulation could have occurred through changes to the soybean root system that promoted rhizobial colonization (Batstone et al. Citation2017; Bourion et al. Citation2010), increased resource availability to symbionts as a consequence of improved host nutrition overall, or through other interactions between ATCC PTA-122608 and B. japonicum (Korir et al. Citation2017). Synergistic effects of Pseudomonas sp. and rhizobia have been reported in a variety of legumes, perhaps due to Pseudomonas sp. outcompeting rhizobia-inhibiting microorganisms or via indirect effects on the host (Ahmad et al. Citation2013; Andrade, De Leij, and Lynch Citation1998; Egamberdieva et al. Citation2010; Sánchez et al. Citation2014). Although beyond the scope of this study, further molecular and biochemical work could illuminate the mechanisms by which this rhizobacterium promotes nodulation.
Root system architectural shifts and increased nodulation following inoculation show that ATCC PTA-122608 is capable of altering soybean root and rhizosphere traits, leading to growth promotion under greenhouse conditions. Given uncertainties of reproducibility of greenhouse results (Schmidt and Gaudin Citation2018) and previous work on other rhizobacteria from extreme environments, future studies should evaluate whether this strain increases crop productivity under ecologically relevant field conditions and alleviates abiotic stresses, particularly extremely low temperatures. The mechanisms by which extremophilic rhizobacteria may promote plant growth remain to be fully explored, but our results highlight the potential for these microorganisms to contribute to the development of high-performing biofertilizers for sustainable production of nutritionally and economically important crops.
Acknowledgments
The authors would like to thank Uxmal S.A., Santiago, Chile for the strain ATCC PTA-122608 isolated from Antarctic soil, the Chilean Antarctic Institute for logistical support (INACH 0301 Grants) and the Chilean Development Agency - CORFO.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Acuña-Rodríguez, I. S., H. Hansen, J. Gallardo-Cerda, C. Atala, and M. A. Molina-Montenegro. 2019. Antarctic extremophiles: Biotechnological alternative to crop productivity in saline soils. Frontiers in Bioengineering and Biotechnology 7 (22):1492–1507. doi: 10.3389/fbioe.2019.00022.
- Ahmad, M., Z. A. Zahir, M. Khalid, F. Nazli, and M. Arshad. 2013. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer's fields. Plant Physiology and Biochemistry 63:170–6. doi: 10.1016/j.plaphy.2012.11.024.
- Ahmad, M., L. Pataczek, T. Hilger, Z. Zahir, A. Hussain, F. Rasche, R. Schafleitner, and V. Solberg. 2018. Perspectives of microbial inoculation for sustainable development and environmental management. Frontiers in Microbiology 9:2992. doi: 10.3389/fmicb.2018.02992.
- Andrade, G., F. De Leij, and J. M. Lynch. 1998. Plant mediated interactions between Pseudomonas fluorescens, Rhizobium leguminosarum and arbuscular mycorrhizae on pea. Letters in Applied Microbiology 26 (4):311–6. doi: 10.1046/j.1472-765X.1998.00337.x.
- Bakker, M., D. Manter, A. Sheflin, T. Weir, and J. Vivanco. 2012. Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant and Soil 360 (1-2):1–13. doi: 10.1007/s11104-012-1361-x.
- Batstone, R. T., E. M. Dutton, D. Wang, M. Yang, and M. E. Frederickson. 2017. The evolution of symbiont preference traits in the model legume Medicago truncatula. The New Phytologist 213 (4):1850–61. doi: 10.1111/nph.14308.
- Bhardwaj, D., M. Ansari, R. Sahoo, and N. Tuteja. 2014. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microbial Cell Factories 13 (1):66. doi: 10.1186/1475-2859-13-66.
- Boari, F., G. Cucci, A. Donadio, M. Schiattone, and V. Cantore. 2014. Kaolin influences tomato response to salinity: Physiological aspects. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 64 (7):559–71. doi: 10.1080/09064710.2014.930509.
- Bourion, V., S. M. H. Rizvi, S. Fournier, H. de Larambergue, F. Galmiche, P. Marget, G. Duc, and J. Burstin. 2010. Genetic dissection of nitrogen nutrition in pea through a QTL approach of root, nodule, and shoot variability. Theoretical and Applied Genetics 121 (1):71–86. doi: 10.1007/s00122-010-1292-y.
- Brisson, V. L., J. E. Schmidt, T. R. Northen, J. P. Vogel, and A. C. M. Gaudin. 2019. Impacts of maize domestication and breeding on rhizosphere microbial community recruitment from a nutrient depleted agricultural soil. Scientific Reports 9 (1):15611. doi: 10.1038/s41598-019-52148-y.
- Choudhary, D. K., A. Prakash, V. Wray, and B. N. Johri. 2009. Insights of the fluorescent pseudomonads in plant growth regulation. Current Science 97 (2):170–9.
- Comas, L., S. Becker, V. M. Cruz, P. F. Byrne, and D. A. Dierig. 2013. Root traits contributing to plant productivity under drought. Frontiers in Plant Science 4:442. doi: 10.3389/fpls.2013.00442.
- De-la-Peña, C., and V. Loyola-Vargas. 2014. Biotic interactions in the rhizosphere: A diverse cooperative enterprise for plant productivity. Plant Physiology 166 (2):701–19. doi: 10.1104/pp.114.241810.
- Egamberdieva, D., G. Berg, K. Lindström, and L. A. Räsänen. 2010. Co-inoculation of Pseudomonas spp. with Rhizobium improves growth and symbiotic performance of fodder galega (Galega orientalis Lam.). European Journal of Soil Biology 46 (3-4):269–72. doi: 10.1016/j.ejsobi.2010.01.005.
- El Zemrany, H., S. Czarnes, P. D. Hallett, S. Alamercery, R. Bally, and L. Jocteur Monrozier. 2007. Early Changes in root characteristics of maize (Zea mays) following seed inoculation with PGPR Azospirillium lipoferum CRT1. Plant and Soil 291 (1-2):109–18. doi: 10.1007/s11104-006-9178-0.
- Enebe, M. C., and O. O. Babalola. 2018. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Applied Microbiology and Biotechnology 102 (18):7821–35. doi: 10.1007/s00253-018-9214-z.
- Fardella, C., R. Oses, C. Torres-Díaz, and M. A. Molina-Montenegro. 2014. Antarctic fungal endophytes as tool for the reintroduction of native plant species in arid zones. Bosque 35 (2):235–9. doi: 10.4067/S0717-92002014000200011.
- Friesen, M. L., S. S. Porter, S. C. Stark, E. J. von Wettberg, J. L. Sachs, and E. Martinez-Romero. 2011. Microbially mediated plant functional traits. Annual Review of Ecology, Evolution, and Systematics 42:23–46. doi: 10.1146/annurev-ecolsys-102710-145039.
- García-Fraile, P., E. Menéndez, and R. Rivas. 2015. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioengineering 2 (3):183–205. doi: 10.3934/bioeng.2015.3.183.
- Glick, B. R. 2012. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012:1–15. doi: 10.6064/2012/963401.
- Goh, C. H., D. F. Veliz Vallejos, A. B. Nicotra, and U. Mathesius. 2013. The impact of beneficial plant-associated microbes on plant phenotypic plasticity. Journal of Chemical Ecology 39 (7):826–39. doi: 10.1007/s10886-013-0326-8.
- Gopal, M., and A. Gupta. 2016. Microbiome selection could spur next-generation plant breeding strategies. Frontiers in Microbiology 7:1971. doi: 10.3389/fmicb.2016.01971.
- Grover, M., S. Z. Ali, V. Sandhya, A. Rasul, and B. Venkateswarlu. 2011. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World Journal of Microbiology and Biotechnology 27 (5):1231–40. doi: 10.1007/s11274-010-0572-7.
- Guo, M., Q. He, Y. Li, X. Lu, and Z. Chen. 2010. Removal of Fe from kaolin using dissimilatory Fe(III)-reducing bacteria. Clays and Clay Minerals 58 (4):515–21. doi: 10.1346/CCMN.2010.0580406.
- Korir, H., N. W. Mungai, M. Thuita, Y. Hamba, and C. Masso. 2017. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Frontiers in Plant Science 8:141. doi: 10.3389/fpls.2017.00141.
- Li, F., X. Zhang, J. Gong, L. Liu, and Y. Yi. 2018. Specialized core bacteria associate with plants adapted to adverse environment with high calcium contents. PLoS One 13 (3):e0194080. doi: 10.1371/journal.pone.0194080.
- Lu, T., M. Ke, M. Lavoie, Y. Jin, X. Fan, Z. Zhang, Z. Fu, L. Sun, M. Gillings, J. Penuelas, et al. 2018. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 6 (1):231. doi: 10.1186/s40168-018-0615-0.
- McNickle, G. G., and J. F. Cahill. 2009. Plant root growth and the marginal value theorem. Proceedings of the National Academy of Sciences of the United States of America 106 (12):4747–51. doi: 10.1073/pnas.0807971106.
- Mohsenipour, M., S. Shahid, K. Ebrahimi, T. Ismail, and X. Wang. 2019. Simulation of nitrate transport and fate in groundwater in presence of kaolin. Journal of Soil and Water Conservation 74 (1):67–76. doi: 10.2489/jswc.74.1.67.
- Molla, A. H., Z. H. Shamsuddin, M. S. Halimi, M. Morziah, and A. B. Puteh. 2001. Potential for enhancement of root growth and nodulation of soybean co-inoculated with Azospirillum and Bradyrhizobium in laboratory systems. Soil Biology and Biochemistry 33 (4-5):457–63. doi: 10.1016/S0038-0717(00)00186-3.
- Panke-Buisse, K., A. Poole, J. Goodrich, R. Ley, and J. Kao-Kniffin. 2014. Selection on soil microbiomes reveals reproducible impacts on plant function. The International Society for Microbial Ecology Journal 9:980.
- Perez-Jaramillo, J. E., R. Mendes, and J. M. Raaijmakers. 2016. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Molecular Biology 90 (6):635–44. doi: 10.1007/s11103-015-0337-7.
- Philippot, L., J. M. Raaijmakers, P. Lemanceau, and W. H. van der Putten. 2013. Going back to the roots: The microbial ecology of the rhizosphere. Nature Reviews. Microbiology 11 (11):789–99. doi: 10.1038/nrmicro3109.
- Poitout, A., A. Martinière, B. Kucharczyk, N. Queruel, J. Silva-Andia, S. Mashkoor, L. Gamet, F. Varoquaux, N. Paris, H. Sentenac, et al. 2017. Local signalling pathways regulate the Arabidopsis root developmental response to Mesorhizobium loti inoculation. Journal of Experimental Botany 68 (5):1199–211. doi: 10.1093/jxb/erw502.
- Poole, P. 2017. Shining a light on the dark world of plant root-microbe interactions. Proceedings of the National Academy of Sciences of the United States of America 114 (17):4281–3. doi: 10.1073/pnas.1703800114.
- Ramadoss, D., V. K. Lakkineni, P. Bose, S. Ali, and K. Annapurna. 2013. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2 (1):6. doi: 10.1186/2193-1801-2-6.
- Rao, I. M., J. W. Miles, S. E. Beebe, and W. J. Horst. 2016. Root adaptations to soils with low fertility and aluminium toxicity. Annals of Botany 118 (4):593–605. doi: 10.1093/aob/mcw073.
- Sánchez, A. C., R. T. Gutiérrez, R. C. Santana, A. R. Urrutia, M. Fauvart, J. Michiels, and J. Vanderleyden. 2014. Effects of co-inoculation of native Rhizobium and Pseudomonas strains on growth parameters and yield of two contrasting Phaseolus vulgaris L. genotypes under Cuban soil conditions. European Journal of Soil Biology 62:105–12. doi: 10.1016/j.ejsobi.2014.03.004.
- Schmidt, J. E., T. M. Bowles, and A. C. M. Gaudin. 2016. Using ancient traits to convert soil health into crop yield: Impact of selection on maize root and rhizosphere function. Frontiers in Plant Science 7:373. doi: 10.3389/fpls.2016.00373.
- Schmidt, J. E., and A. C. M. Gaudin. 2018. What is the agronomic potential of biofertilizers for maize? A meta-analysis. Federation of European Microbiological Societies Microbial Ecology 94 (7): fiy094. doi: 10.1093/femsec/fiy094.
- Schmidt, J. E., J. L. Mazza Rodrigues, V. L. Brisson, A. Kent, and A. C. M. Gaudin. 2020. Impacts of directed evolution and soil management legacy on the maize rhizobiome. Soil Biology and Biochemistry 145:107794. doi: 10.1016/j.soilbio.2020.107794.
- Shrivastava, P., and R. Kumar. 2015. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22 (2):123–31. doi: 10.1016/j.sjbs.2014.12.001.
- Timmusk, S., I. A. Abd El-Daim, L. Copolovici, T. Tanilas, A. Kännaste, L. Behers, E. Nevo, G. Seisenbaeva, E. Stenström, and Ü. Niinemets. 2014. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9 (5):e96086. doi: 10.1371/journal.pone.0096086.
- Vacheron, J., G. Desbrosses, M.-L. Bouffaud, B. Touraine, Y. Moënne-Loccoz, D. Muller, L. Legendre, F. Wisniewski-Dyé, and C. Prigent-Combaret. 2013. Plant growth-promoting rhizobacteria and root system functioning. Frontiers in Plant Science 4:356. doi: 10.3389/fpls.2013.00356.
- Wissuwa, M., M. Mazzola, and C. Picard. 2009. Novel approaches in plant breeding for rhizosphere-related traits. Plant and Soil 321 (1-2):409–30. doi: 10.1007/s11104-008-9693-2.
- Yang, J., J. W. Kloepper, and C.-M. Ryu. 2009. Rhizosphere bacteria help plants tolerate abiotic stress. Trends in Plant Science 14 (1):1–4. doi: 10.1016/j.tplants.2008.10.004.
- Zhang, X. Y., and W. Wang. 2015. The decomposition of fine and coarse roots: Their global patterns and controlling factors. Scientific Reports 5 (1):9940. doi: 10.1038/srep09940.
