?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Our study aimed to evaluate the effects of nano and chelated Fe fertilization on nutrient and heavy metals uptake and their translocation from soil to root, stem, leaf, and fruits of fresh market tomatoes (Solanum Lycopersicon, cv. Sunbrite). A completely randomized design in a factorial experiment, with two Fe sources (nano vs. chelated) applied at 0 (control), 10, 20, and 40 mg/L via drip-irrigated plasticulture, was conducted. Nutrient and heavy metals concentrations in soil, water, tomato roots, stems, leaves, and fruits were determined. Soil had higher K, Fe, Ca, Mg, P, and Mn but lower Ni, Cd, and Cr concentrations. The P, K, Fe, Cu, Mn, and Na concentrations in the roots were significantly affected by both nano and chelated Fe fertilization; however, without following a consistent pattern. Averaged across the Fe treatments, K, Ca, Mg, Fe, and P that translocated from the soil were dominated in the roots, stems, and leaves. The nutrient translocation order, from roots to fruits in response to Fe fertilization, was nano Fe (40%) > chelated Fe (37%) > control (23%). Based on the treatment index values calculated across treatments, it is evident that Fe fertilization plays a pivotal role in nutrient uptake and translocation. The use of chelated Fe at a concentration of 20 mg/L had a pronounced effect on improving the nutritional quality of the tomatoes and, by extension, their associated public health benefits. In conclusion, the chelated Fe (20 mg/L) stands out as the most preferable and effective Fe fertilization for tomatoes.
Introduction
Iron, as one of the essential micronutrients, plays an important role in chlorophyll and DNA synthesis, enzymatic functions, and photosynthesis and respiration processes to support plant productivity (El-Desouky et al. Citation2021a; Mimmo et al. Citation2014; Rout and Sahoo Citation2015; Samaranayake, Peiris, and Dssanayake Citation2012; Ye et al. Citation2015; Zargar et al. Citation2015; Zuo and Zhang Citation2011). While abundant in most soils, Fe is often complexed with clay minerals and other reactive components to form insoluble Fe compounds, and its availability to plants becomes limited (Bindraban et al. Citation2015; Rout and Sahoo Citation2015). It is reported that Fe deficiency is a common nutritional disorder in crops, resulting in low yields with poor nutritional quality (López-Millán et al. Citation2009; Rout and Sahoo Citation2015). In contrast, Fe toxicity acts catalytically via the Fenton reaction to generate reactive oxygen-based radicals that can damage vital cellular constituents of plants by lipid peroxidation (Rout and Sahoo Citation2015). Several studies reported that Fe toxicity is a complex nutrient disorder that causes deficiencies of P, K, Ca, Mg, and Zn and, consequently, affects plant productivity (Ottow et al. Citation1983; Rout and Sahoo Citation2015). Currently, Fe deficiency or toxicity is a yield-limiting factor with major implications for crop production in the world (Hansen et al. Citation2006; Rout and Sahoo Citation2015). Therefore, Fe nutrition of plants must respond to Fe stress in between Fe deficiency and overloading by optimizing Fe sources and rates.
Among the Fe compounds used as fertilizers, the Fe chelates are the most common for their water solubility and gradual release, nitrogen content, and absorption by plants when compared to conventional mixed Fe fertilizers (Klem-Marciniak et al. Citation2021; Schlegel, Schönherr, and Schreiber Citation2006). The effectiveness of Fe, Mn, and Zn with traditional or novel chelates, and neutral complexing agents, has been investigated for crop nutrition and production (López-Rayo, Nadal, and Lucena Citation2016; Nadal et al. Citation2012). Several studies have reported that chelated Fe is a better source when compared to conventional mixed Fe fertilizers. However, a number of studies have reported that chelated micronutrients, especially Fe, has limitations associated with its application and plant absorption (Karagiannidis et al. Citation2008). It is reported that at pH above 6, almost 50% of the chelated Fe becomes unavailable to plants.
Nanotechnology, an approach of precision nutrient management practices, is increasingly adapted in agriculture (Bárzana, Garcia-Gomez, and Carvajal Citation2022). Nano-materials with their extremely smaller size (between 1 and 199 nm in dia.), faster reactivity, and ability to interact with biological systems enhance growth, development, and stress tolerance of plants (Aqeel et al. Citation2022; El-Desouky et al. Citation2021b; Kah et al. Citation2018; Pinto et al. Citation2013). Several studies have reported that Fe nanoparticles are very effective to minimize leaching and volatilization and increase Fe availability to growing plants when compared to the commonly used traditional Fe fertilizers or chelated Fe fertilization (Bárzana, Garcia-Gomez, and Carvajal Citation2022; El-Desouky et al. Citation2021b). It has been shown that nano Fe fertilizer increased tomato yields by 11% when compared to the chelated Fe fertilization (El-Desouky et al. Citation2021). While Fe deficiency or toxicity is responsible for complex nutritional disorders in plants, substituting conventional or chelated Fe fertilizers with nano Fe fertilization is expected to improve the nutritional balance of crops. Over the last 100 years, high-input mechanized agriculture has increased crop production to meet food demand while nutritive values of the produce or yield were overlooked (Hossain et al. Citation2017).
Tomato is one of the widely consumed, high-value specialty crops in the world (FAOSTAT Citation2019). Epidemiological studies on tomatoes have reported its capacity to reduce the risk of certain diseases and balance human nutrition (Salehi et al. Citation2019). Tomatoes contain a substantial amount of both macro- and micronutrients, which depend on their availability in the soil, acquisition by roots, transportation, and storage by stem and leaf (Hossain et al. Citation2017). Along with essential nutrients, heavy metals toxic to public health are also present ubiquitously in the soil (Hossain et al. Citation2017); however, the information on nutrient concentration of tomatoes, including heavy metals, in response to the effects of nano and chelated Fe fertilization, are limited (Hossain et al. Citation2017; Litskas et al. Citation2019; Montgomery et al. Citation2022).
We hypothesize that due to its rapid and efficient interactions with biological systems, nano Fe fertilization is expected to balance nutrient uptake and improve nutritional quality of tomatoes. The specific objectives of our field research study were to: (1) determine the effect of variable rates of nano and chelated Fe fertilization on nutrients and heavy metals concentration in the soil and water and their uptake and subsequent translocations to the root, stem, leaf, and tomato fruits; (2) evaluate the economic viability of the Fe-fertilized tomato productions; and (3) relate potential benefits of essential nutrients in tomatoes via Fe fertilization with public health.
Method and materials
Study site
A replicated plasticulture field study under drip irrigation was conducted at The Ohio State University South Centers at Piketon, (lat. 39.07° N, long. 83.01° W), Ohio, USA. Average monthly air temperature of 32.2 °C was recorded highest in August and lowest of less than 15.6 °C in September during the cropping season. Mean annual rainfall is 96.2 cm, with about 55% of the precipitation falling during the crop-growing season (May to September). The highest monthly rainfall (15 cm) was recorded in July. The monthly relative humidity ranges between 79 to 93%, soil temperatures at 15 cm deep ranges between 3 and 30 °C, and solar radiation ranges between 9,980 and 43,000 KW/m2.
The soil at the site is a deep, nearly level and somewhat poorly drained Doles silt loam with a pH of 6.0 ± 0.3; electrical conductivity of 170 µS/cm; total organic carbon of 0.82 ± 0.23%; total nitrogen of 0.105 ± 0.024%; bulk density of 1.28 ± 0.04 g/cm3; and sand, silt, and clay composition of 30 ± 4, 55 ± 2, and 15 ± 2%, respectively, at 0 to 20 cm depth.
Experimental design
A 2 × 4 factorial field experiment in completely randomized design was established. Two sources of Fe (nano vs. and chelated) were applied at the rate of 0 (control), 10, 20, and 40 mg/L via drip irrigation. Both Fe treatments were replicated four times. Each replicated plot was 2 m wide x 5 m long with a 50-cm buffer between plots.
The nano Fe (Fe2O3) was acquired from the Aqua-Yield® Company (Sandy, Utah, USA) and the chelated Fe [(iron-Ethylene di-amine di(o-hydroxyphenylacetic acid, FeEDDHA)] from the Miller® Chemical and Fertilizer, LLC (Hanover, Pennsylvania, USA).
Cultural practices
Fresh market tomato (cv. Sunbrite) was seeded into 72 cell plug trays containing Metro Mix 360® soilless media and placed in a controlled plant growth chamber for germination. About 25-30 cm tall seedlings were planted in the plots during the third week of May 2021. Prior to laying plastic materials, the field was chisel plowed followed by surface application of a basal dose of NPK 19-19-19 fertilizers at a rate of 100 kg/ha. Plasticulture rows were 1.6 m apart with tomato seedlings being planted and spaced 60 cm apart within rows of raised beds using a waterwheel transplanter.
Nano and chelated Fe treatments were applied twice on tomato plants in mid-June and early July, respectively. The Fe treatments and watering of the tomato plants were applied via drip irrigation emitters that were inserted to a 10-cm soil depth. Irrigation was applied as required based on 75% of maximum allowable depletion of available soil moisture. All the irrigation valves were shut off except for the Fe treatment that was being applied. Drip lines were pressurized, and the respective Fe treatments were injected into the irrigation. Each time, the Fe treatments took almost 15 min to inject, and then were allowed to irrigate for 10 more min to purge the lines before the valve was shut off at each treatment. The header line was then uncapped to empty the header line between each treatment. Standard cultural practices were performed following recommendations from the Midwest Vegetable Production Guide for Commercial Growers (ID-56).
Sampling, processing, and analysis of tomatoes
Randomly selected fresh ripened tomatoes at peak harvesting were collected from each replication and processed to analyze for mineral nutritional quality. After the final harvest, root, stem, and leaf samples of tomato plants were collected separately. A portion of the collected fruit, root, stem, and leaf samples was oven-dried at 55 ± 2 °C, ground with a Wiley Mill® grinder, followed by sieving with a 125 µm mesh and stored in sealable plastic bags until analysis.
A 1.0 g processed sample of tomato fruit, root, stem, and leaf was placed into a 50 mL Teflon tube and digested using a mixture of 10 mL of concentrated HNO3 and 5 mL of 30% H2O2 (2: 1) at 185 ± 2 °C for 10 min using Anton Parr® Microwave Digestion System. After cooling, the digestates were diluted with distilled deionized water, followed by filtration with Whatman® filter paper to obtain clear aliquots. Nutrient concentration in the aliquot of fruit, root, stem, and leaf samples was determined in triplicates using Shimadzu® Inductively Coupled Plasma-Emission spectrometry (ICPE-9000).
Soil and water analysis
Prior to establishing the field experiment, composite soil cores (2.54 cm internal dia.) at 0-20 cm depth were collected using a JMC® stainless-steel soil environmental probe lining with plastic tube and placed in sealable plastic bags for a short-term storage at 4 °C until analyzed. A portion of the field-moist soil was air-dried for a period of 15 d under shade at room temperature, ground using an Agate mortar and pestle, and sieved through 2 mm mesh prior to analysis.
For essential nutrients and heavy metals analysis, a 1.0 g finely ground (<125 µm) soil sample was placed into a 50 mL Teflon tube and mixed with 16 mL of concentrated HNO3 and HCl (1:3 ratio) at 185 °C for 10 min using Anton Parr® Microwave Digestion System. After cooling, the digested aliquot was diluted with distilled deionized water, followed by filtration with white ribbon filter paper (Macherey–Nagel®, Germany, 640 m, Ø 125 mm, Cat No. 203 210). Nutrient and heavy metals concentration, such as B, Ca, Cu, Cr, Cd, Fe, K, Mg, Mn, Mo, Ni, P, S, and Zn, in soil aliquot were analyzed using Shimadzu® ICPE-900 spectrometry.
Soil pH was determined using a glass electrode pH meter (Model 520 A, Orion®, Boston, MA, USA). Electrical conductivity in 1:1 soil-water suspension was measured using a conductivity probe. Total organic C and total N were determined on finely ground (< 125 µm), air-dried soil using a FlashEA-1112 series CNHS-O dry combustion analyzer. Bulk density was determined by following the standard core method. Soil particle size analysis was performed using the standard Bouyoucos hydrometer method after removing the soil organic matter by 3% hydrogen peroxide oxidation followed by an overnight treatment with 10% sodium hexametaphosphate solution. Using the standard pressure plate apparatus procedure, the field-moisture capacity and permanent wilting point of soil were determined to be 0.033 and 1.5 MPa, respectively (Klute Citation1986).
Water analysis
Water used for irrigation was randomly collected in dry, sterilized 500-mL plastic bottles and filtered with Whatman® filter paper (# 42) prior to analyzing for pH, electrical conductivity, and nutrients and heavy metals (B, Ca, Cu, Cr, Cd, Fe, K, Mg, Mn, Mo, Ni, P, S, and Zn) using standard methods of analysis.
Nutrient and heavy metals transfer factors
The transfer factor (TF) was used to assess the potential of tomato plants to accumulate nutrients or heavy metals in their below-ground biomass tissues and translocate them to the above-ground biomass tissues and fruits (Eid et al. Citation2018). The transfer factors were calculated as follows:
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
Where, Csoil, Croots, Cstems, Cleaves, and Ctomato were the nutrient and heavy metals concentrations (mg/kg) in the soil, root, stem, leaf, and fruit of tomatoes, respectively.
Daily intake rate of nutrients
The daily intake rate (DIR) of nutrients through the consumption of tomato fruits was calculated using following equation (Sharma, Agrawal, and Marshall Citation2009):
(5)
(5)
Where Cnutrient is the average nutrient concentration (mg/kg) in the tomato fruits (dry-weight basis); Cfactor is the conversion factor (typically 0.085 to convert fresh vegetable weight to dry-weight); Dintake is the daily intake of tomato (200 g/person/day) for adults; and BW is the average body weight for adult (60 kg).
Quality analysis/quality control
After every 10 samples, a QC/QA sample prepared from certified standard solution was analyzed to check the analytical quality with a relative standard deviation of QA/QC (5 to 8%). The detection limits of nutrients and heavy metals such as B, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Mo, Ni, Zn, P, and S were 0.2, 0.5, 0.2, 0.3, 0.2, 0.3, 0.5, 0.5, 0.2, 0.3, 0.1, 0.2, 0.5, 0.5 µg/L, respectively. Analytical quality control was maintained by analyzing certified reference material NIST 1567b (wheat flour). Replicated analysis of the reference material showed a recovery of 94 ± 12%. Analytical precision, as determined by QA/QC procedures, reagent blanks, and internal standards, was better than ±10%.
Treatment index
To assess nutritional quality of the tomatoes under different treatments, the treatment index (TI) was calculated using the following equation:
(6)
(6)
(7)
(7)
Here, 11 essential nutrients such as Na, K, Ca, Mg, Fe, Mn, Cu, Zn, B, P, and S were used to calculate the treatment index.
Economics analysis
To evaluate the economic feasibility of using nano and chelated Fe fertilizers in comparison to traditional Fe sources, we determined the cost of tomato production per hectare (ha) using nano, chelated, and control (standard practice) treatments. The primary formula employed in the cost analysis is as follows:
(6)
(6)
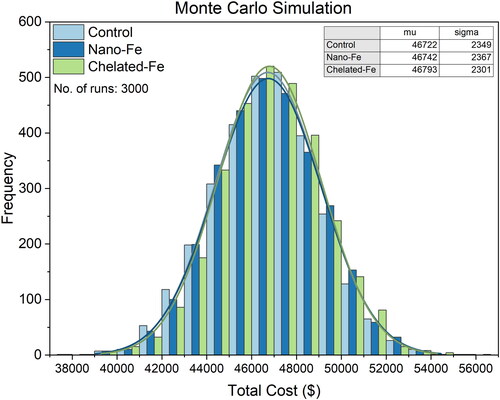
Where, TC is total cost, dollars/hectare of tomatoes production. A detailed breakdown of the estimated costs and returns for fresh market tomato production was provided in the supplementary material (Tables S1–S3). The average cost of tomato production across the three different fertilization methods was computed using Monte Carlo simulation techniques (OriginPro® 2022b).
Table 1. Total nutrient and heavy metals concentration in soil and water used for irrigation (values were in mean ± standard deviation).
Table 3. Effects of different rates of the nano and chelated iron fertilization on total nutrient and heavy metals concentrations in tomato plant stems under a drip-irrigated plasticulture system.
Statistical analysis
Data on tomato nutrients were subjected to a two-way analysis of variance (Fe source and rate) procedure using OriginPro®. However, the effects of different Fe treatment sources (chelated vs. nano fertilizer) and their rates (0, 10, 20, and 40 mg Fe/kg) on nutrient and heavy metals concentration in root, stem, leaf, and fruit of tomatoes were evaluated using three-way analysis of variance (Fe source, rate, and plant components). The Fe source, rate, and plant components were considered as fixed predictor variables. Main and interactive effects of predictor variables on dependent variables were separated by the p ≤ 0.05, unless otherwise mentioned. Principal components analyses and correlation were performed to understand the complex connection between Fe treatments and nutrient and heavy metals concentrations in tomatoes using OriginPro®. A correlation coefficient (r) higher than 0.85 and significantly different from the zero at p ≤ 0.01 was selected to draw a correlation network model.
Results and discussion
Soil and water characteristics
While the soil pH was 6.4 ± 0.2, the pH of water used for irrigation was 7.2 ± 0.3, indicating a slightly acidic soil and neutral quality water used for growing tomatoes (). Soil contained the highest concentration of K (1834 ± 351 mg/kg) while the Fe and Ca concentrations were 1755 ± 100 and 1104 ± 72 mg/kg, respectively. The nutrient and heavy metals concentration of the soil was in the following order: K > Fe > Ca > Mg > Mn > P>Na > S>Zn > Cu > Cr > Ni > B>Mo > Cd; however, soil had a substantial concentration of Cd, Cr, Cu, Mn, P, S, and Zn. In contrast, the water had the highest concentration of Mg (10.5 ± 0.9 mg/L) while the Cd, Cr, Cu, Mo, and Zn concentration was below the detection limits (0.2 to 0.5 µg/L). The nutrient and heavy metals concentration in water was in the following order: Mg > K>Ca > S>Mn > Na > Fe > P > B.
Based on the comparison of results, the concentration of the Ca, Fe, Mg, K, Mn, and P in soil were several folds higher than in water. It is expected that soil would contribute more nutrients to tomato plants when compared to the water used for irrigation. Therefore, the nutrient contribution to tomato plants by soil is expected to be higher than the water used for irrigation.
Nutrient and heavy metals concentration in tomato root, stem, leaf, and fruits
Nutrient and heavy metals concentrations in tomato roots, stems, leaves, and fruits were variably influenced by Fe fertilization (). The chelated Fe significantly increased (by 16 to 270%) but decreased in heavy metals concentration (by 70 to 250%) in roots when compared to the control. The effect of chelated Fe was more pronounced on Mg, Fe, Ca, P, and Cd concentrations (). In contrast, the nano Fe significantly increased the Mg and Mo with an associated decrease in Mn, Zn, and B concentrations in roots than that of the control. Likewise, chelated Fe increased P, S, Mg, Fe, Mn, Cu, Zn, B, Mo, Na, Cd, Cr, and Ni, but decreased Ca and K concentrations in stems compared to those of the control (). The nano Fe had only increased the Cu, Zn, and Na, but decreased K, Fe, and Mn concentrations when compared to the control. Chelated Fe, when applied at 20 mg/L, had the highest concentrations of P, S, and Fe in both roots and stems compared to other Fe treatments. The Fe source x rate did not exert any interactions on nutrient concentration in roots. While the chelated Fe significantly increased P, Fe, Zn, Na, Cr, and Ni and decreased S, Ca, K, Mn, and B in leaves, the nano Fe, in contrast, increased Fe, Na, Cd, Cr, and Ni and decreased S, Ca, K, Cu, and B concentrations when compared to the control (). The Mg, Fe, and Mo concentrations were affected by a Fe source x rate interaction. Both nano and chelated Fe fertilization affected P, S, Ca, Mg, K, Fe, Mn, Zn, B, and Na, except Cu concentrations in tomato when compared to the control, and the effect of chelated Fe was more pronounced especially at 20 and 40 mg/L on fruits than that of the nano Fe (). The P, S, Ca, K, Fe, Zn, and Na concentrations were affected by Fe source x rate interactions. The heavy metals (Cd, Cr, and Ni) concentrations were below the detection limits.
Table 2. Effects of different rates of the nano and chelated iron fertilization on total nutrient and heavy metals concentrations in tomato plant roots under drip-irrigated plasticulture system.
Table 4. Effects of different rates of the nano and chelated iron fertilization on total nutrient and heavy metals concentrations in tomato plant leaves under a drip-irrigated plasticulture system.
Table 5. Effects of different rates of the nano and chelated iron fertilization on total nutrient and heavy metals concentrations in tomato fruits under a drip-irrigated plasticulture system.
While the order of nutrient concentration in the fruits was dominated by K > P > Mg > Ca > S>Na regardless of Fe treatments, it was K > Ca > Mg > P > S > Fe in the stems (). In contrast, the order of nutrient concentration was dominated by Ca > K>Mg > P > S > Fe in the leaves, but it was significantly affected by Fe sources. In roots, the order of nutrient concentration under nano Fe was dominated by Mg > K>Ca > P > S > Zn, but under the chelated Fe, it was dominated by Ca > K>Mg > P > S > Fe when compared to the control. After summarizing the data, it showed that the nutrient concentration was higher in the leaves followed by stems, roots, and fruits, but the heavy metals concentration was higher in the roots and leaves. Overall, the heavy metals concentration was low in roots, stems, and leaves, and below detection limits in tomato fruits.
Table 6. Summary of the nutrient and heavy metals orders in roots, stem, and leaves of the tomato plants with different rates of the nano and chelated iron fertilization under a drip-irrigated plasticulture system.
While the roots were the main entrance of the plants, the uptake and accumulation of nutrients and heavy metals in the fruits, via stem and leaf, depends on their form, transport, water availability, root architecture and distribution, type of plants, and soil compaction. The higher accumulation of nutrients in roots and leaves may be ascribed to complexation of metals with sulfhydryl and other reactive groups (Singh et al. Citation2004). The average nutrients accumulated in the following order: similar accumulation of nutrients in the order of leaves > roots > stems > fruits was reported by Rai et al. (Citation2019). An increased uptake and accumulation of nutrients in roots, stems, leaves, and fruits under the chelated Fe than under the nano Fe was due to the effects of greater complexation of nutrients occurring with the amine group present in the chelated Fe. A low concentration of heavy elements in roots, stems, and leaves under the chelated Fe fertilization was expected to reduce their toxicity effects on living organisms (Eid et al. Citation2018). Nutrient uptake by plants usually occurs from the soil via roots; however, several factors such as soil pH, moisture content, and compaction are expected to affect the efficiency of the nutrients acquisition by the roots (Morgan and Connolly Citation2013). It is reported that iron (Fe2+) is a cofactor for proteins that are involved in several metabolic processes in plants, including photosynthesis and respiration, to influence plant growth and development (Zia-Ur-Rehman et al. Citation2023). These processes are indirectly associated with the increased nutrient uptake from the soil via roots.
Relationship of nutrients and heavy metals among soil, root, stem, leaf, and fruits of tomato
Data presented in shows that correlation between nutrient concentration in roots and tomato fruits was observed with both nano and chelated Fe treatments (red color) and the values of the correlation coefficients (r) ranged between 0.85 to 0.87. The close 1:1 correlation (r = ∼ 1.0, blue color) was observed among tomatoes produced under the nano, chelated, and control Fe treatments, indicating that nutrient content in tomatoes follow similar mechanistic pathways depending on the availability of nutrients to roots via soil. The concentration of nutrients in tomatoes under the chelated Fe fertilization was strongly correlated with the nutrient concentration in the leaves (r = 0.90). However, the nutrient concentration in the tomato fruits under the nano Fe was relatively weakly correlated with the leaf nutrient concentration. Therefore, the chelated Fe containing ligands had more positive effects on the nutrient mobilization to the leaves than that of the nano Fe. However, a strong correlation (r = 0.94) was observed between the roots and stems under the nano Fe treatments (green color).
Figure 1. Correlation network of nutrient and heavy metals concentration in tomato (tom) root, stem, leaf, and fruits in response to different rates of iron fertilization (N, nano Fe; Ch, chelated Fe; and C, control).
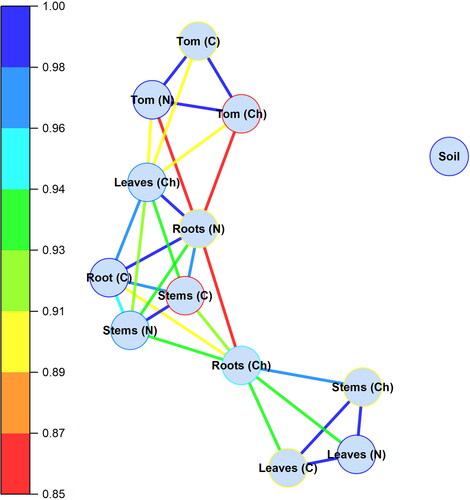
Similarly, strong and significantly higher correlations ranged between 0.96 to 0.98 and were observed between the roots and stems under the chelated Fe treatment. The higher correlation coefficients implied that the chelated Fe treatments consistently increased the nutrient mobilization from the roots to the stems and the leaves. While the roots and stems also had a strong correlation (r = 0.96) under the control, the roots, in contrast, had no correlations with the leaves under the control. The correlation analyses suggested that the nutrients concentration in tomato fruits followed a similar mobilization pattern via stems and leaves with the Fe treatments, and more pronouncedly with the chelated Fe fertilization.
Principal components analyses on nutrient and heavy metals distribution in tomato fruits
Results showed that the first principal component (PC1) and the second principal component (PC2) together accounted for 85.4% of the total variations of the nutrients and heavy metals associated with the nano Fe-treated and untreated soils (). The cluster analysis showed that the nutrient and heavy metals composition in tomatoes were influenced by the effects of 10 mg/L nano and 40 mg/L chelated Fe treatments. The variables in tomatoes under the control were compositionally distinct from the other three nano Fe treatments. In addition, the nutrient compositional variables in tomatoes with the 40 mg/L nano Fe treatment were different when compared to other Fe treatments, though the 10 and 40 mg/L nano Fe treatments were correlated to each other with higher PC1 scores. The effects of nano Fe treatments responsible for higher concentration of nutrients in tomatoes could be explained on the basis of loading values of nutrients (). By their loading values, it was evident that the higher concentration of P, S, Ca, Mg, Fe, Na, and B in tomatoes resulted from the 10 mg/L nano Fe treatment, while the higher concentration of K, Zn, and Mn resulted from the 20 and 40 mg/L nano Fe treatments. The higher concentration of Cu in tomatoes was associated with the control and 20 mg/L nano Fe treatments. Therefore, the 10 mg/L nano Fe treatment had a consistent and significant effect on the nutrient enrichment in tomatoes.
Figure 2. Principal components and cluster analyses for the nutrients and heavy metals in tomato fruits affected by different rates of the nano iron (A) and chelated Fe (B) treatments.
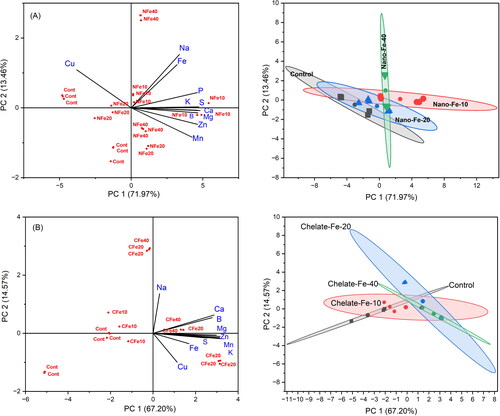
Similarly, the PCA results showed that the first and second PCAs together accounted for 81.8% of the total variations in the nutrient concentration of tomatoes as influenced by the chelated Fe fertilization (). The cluster analysis showed that the nutrient concentrations in tomatoes were related to the 40 and 20 mg/L chelated Fe treatments, while the nutrient compositions were distinct with the effect of control treatment. The effect of the 10 mg/L chelated Fe treatment was also different than the 20 and 40 mg/L chelated Fe treatments. By their loading values, it was found that the high concentration of Ca, B, Na, Zn, Mg, K, and S was associated with the 20 and 40 mg/L chelated Fe treatments. The high concentration of Fe and Cu in tomatoes was a result of the 20 mg/L chelated Fe treatment ().
Nutrients and heavy metals translocation from soil within tomato plants
The transfer or translocation factors assessing the translocation of nutrients and heavy metals to tomatoes from soil via roots, stems, and leaves were variably affected by Fe fertilization (). The chelated Fe significantly increased the translocation factors of P, S, Ca, Mg, B, and Cd with an associated decrease in Zn and Ni from the soil to the roots, when compared to the control (). In contrast, the nano Fe increased the translocation factors of Mo and Cd with an associated decrease in S, Ca, and B than that of the control. Average total transfer factors of nutrients and heavy metals from soil to roots were significantly higher under the chelated Fe when compared to the nano Fe (by 26%) and control (39%) treatments. The transfer factors from the roots to the stems indicated that Ca, Cd, Cu, Mn, and Mo (3.52, 2.17, 1.50, 2.97, 2.30) accumulated in the stems, exceeding the one with the 20 mg/L chelated Fe treatment, while the 20 mg/L nano Fe showed lower values of transfer factors than the chelated Fe (). The average total transfer factors of nutrients under the chelated Fe treatment were 26.6 when compared to the nano Fe (11.7) and the control (11.4) treatments, which indicated that the translocation of nutrients from roots to stems was 2.3 folds higher under the chelated Fe than under the nano Fe treatments. The transfer factors of B, Ca, Cd, Cu, and Mn while translocating from the stems to the leaves were 3.27, 4.30, 5.14, 1.34, and 2.63, respectively, associated with the 20 mg/L nano Fe treatment, while Fe, Na, and Zn were translocated from the stems to the leaves with transfer factors of 6.43, 1.37, and 1.76, respectively, related to the 40 mg/L chelated Fe treatment (). The average total transfer factors of nutrients and heavy metals from the stems to the leaves were higher under both nano and chelated Fe treatments but did not vary when compared to the control. This indicated that the Fe treatments had synergistic effects on the translocation of nutrients from the stems to the leaves. In contrast, the transfer factors of P, S, Mg, and B, when translocated from the roots to the fruits, were significantly higher under the nano Fe than under the chelated Fe and control treatments (). However, the chelated Fe had higher values of transfer factors for Zn and B compared to the chelated Fe and control treatments. Notably, the transfer factors of all the nutrients were lower under the control than all the Fe treatments. Overall, the total transfer factor for all the nutrients did follow an order of nano Fe (7.44) > chelated Fe (6.87) > control (4.32).
Table 7. Translocation factor of total nutrient and heavy metals concentration from soil to tomato roots in response to different rates (mg/L) of the nano and chelated iron fertilization under drip-irrigated plasticulture.
Table 8. Translocation factor of total nutrient and heavy metals concentration from tomato roots to stem in response to different rates (mg/L) of the nano and chelated iron fertilization under drip-irrigated plasticulture.
Table 9. Translocation factor of total nutrient and heavy metals concentration from tomato stem to leaf in response to different rates (mg/L) of nano and chelated iron fertilization under drip-irrigated plasticulture.
Table 10. Translocation factor of total nutrient and heavy metals concentration from tomato leaf to fruits in response to different rates (mg/L) of the nano and chelated iron fertilization under drip-irrigated plasticulture.
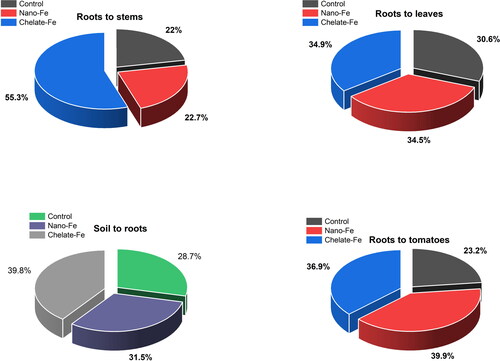
The overall Fe treatment effects on the concentration of nutrients and their partition in different parts of the plants such as roots, stems, leaves, and fruits were evaluated (). It was observed that control soil transferred only 28.7% of the nutrients to roots based on their availability in soil. In contrast, Fe-fertilized soils transferred 31.5 and 39.8% of the nutrients by nano and chelated Fe, respectively. The order of nutrient transfer factors from soil to roots had a sequence of chelated Fe > nano Fe > control. After accumulation of nutrients in the roots, those nutrients were transferred to stems, leaves, and fruits. The translocations from the roots to the stems were 22, 22.7, and 55.3% with control, nano Fe, and chelated Fe treatments, respectively. However, the translocation of nutrients from the roots to the stems was 2.4 folds higher for chelated Fe treatment compared to the nano Fe. Likewise, the transfer of nutrients from the roots to the leaves followed an order: chelated-Fe (34.9%) > nano-Fe (34.5%) > control (30.6%). The translocation was close to each other treatment, but higher than the control.
Figure 3. Overall nutrient translocation from soil to roots, stems, leaves, and fruits of tomatoes in response to different rates of nano- and chelated iron fertilization.
It is expected that nutrients in their ionic forms that have entered the plant roots from the soil with mass flow of water via stems involves the xylem and phloem transport system, which varied by type, physical and chemical properties, and reactivity of the elements (Mclaughlin et al. Citation2011; Valérie Page and Feller Citation2015; Rai et al. Citation2019). As a result, tomato plants might have accumulated nutrients in variable concentration in roots, stems, leaves, and fruits (Cobb et al. Citation2000). The higher transfer of nutrients observed in the roots under the chelated Fe treatment was due to the effects of chelated Fe containing EDDHA to enhance complexation and solubilization of soil minerals and nutrients, and subsequently translocated to the young growing roots (Elgala and Maier Citation1971). The available Fe2+ from the chelated Fe-treated soil, along with other nutrients, might have bound with the esterified pectin in the cell wall of tomato roots and further translocated the nutrients toward the stems (Krzesłowska Citation2010). The bindings were assisted by the presence of -COOH, -OH, and -SH groups in the polysaccharides of the root cells. Therefore, transfer factor values of B, Ca, Mg, Mn, S, and Zn were higher in the tomato plant roots in response to the chelated Fe treatment (Elgala and Maier Citation1971). However, a relatively lower translocation of nutrients in response to the nano Fe fertilization was due to less mobility of those nutrients, as the nano Fe had no chelating or complexing agents to bind, solubilize, and mobilize them to the roots (Küpper and Leitenmaier Citation2013).
Increased translocation of nutrients from roots to leaves compared to roots to stems indicated that the nutrients in the root zone transported easily to the leaves when they are available in the stems. Similar observations were reported in plants with heavy metals translocation form the roots to leaves to stems (Sulaiman and Hamzah Citation2018). The higher values of transfer factors in tomato plants (stems to leaves) might be due to the higher translocation of free ions or chelated ion with the transpiration stream xylem from roots to the upwards transpiring and photosynthesizing leaves through stems (Page, Weisskopf, and Feller Citation2006; Valerie Page and Feller Citation2005). Iron is one of the essential micronutrients involved in the synthesis of diverse organic compounds such as proteins and chlorophyll in plants, which leads to increased absorption and transportation of nutrients to the leaves (Zuo and Zhang Citation2011). The higher transfer of nutrients from the roots to the tomato fruits was observed with the nano Fe treatment, and the translocation order followed the sequence as: nano Fe (39.9%) > chelated Fe (36.9%) > control (23.2%). Based on the percent translocation of nutrients, the nano Fe treatment increased (16.7 and 3%) more nutrient availability in tomatoes compared to the control and chelated Fe treatments. This might be due to the indirect effects of increased leaf chlorophyll a and b synthesis, resulting in higher translocation of nutrients in tomatoes (Sheykhbaglou, Sedghi, and Fathi-Achachlouie Citation2018).
Tomato nutrients and public health
The recommended daily intake rate (RDIR) and its contribution to the recommended daily allowances (RDA) and adequate intake (AI) values of the essential nutrients is presented in . While the recommended dose of Ca ranges between 1,000 and 1,300 mg/day (Yates, Schlicker, and Suitor Citation1998), it was found that the RDIR of Ca ranged between 2.76 and 3.75 mg by daily consumption of 400 g of tomatoes that were produced with Fe-treated soils. For Cu, the recommended intake values ranged between 1.0 and 1.6 mg/day (Trumbo et al. Citation2001) and the RDIR of Cu ranged between 0.01 to 0.03 mg, while the Cu contribution was 1.02 to 3.03% to the RDA. The recommended daily intake of Fe ranges between 8 and 18 mg/day (Trumbo et al. Citation2001) and the RDIR of Fe ranged between 0.15 and 0.22 mg with Fe-treated soil. These results indicated that Fe contribution to the RDA ranged between 1.83 and 2.70% with treated soil, while the contribution was only 1.26% in the control. The recommended values for Mg ranges between 320 and 420 mg/day (Yates, Schlicker, and Suitor Citation1998) and the calculated RDIR of Mg ranged between 4.22 to 6.60 mg with Fe-treated soil. The contribution by the control Fe-treated tomatoes ranged between 1.0 to 1.57%, while the contribution was 0.79% with the control tomato. The contribution of Mg by the Fe-treated tomatoes to the RDA was twice as high as the control tomato.
Table 11. Estimated daily intake rates of essential nutrients from tomato by humans.
An adequate intake of Mn is recommended to be between 1.8 to 2.3 mg/day (Trumbo et al. Citation2001). The contribution of Fe-treated tomatoes to the RDA ranged from 2.19 to 3.21%, while it was only 1.50% with the control. The Fe-treated tomatoes contributed Mn to the RDA 2.14 times more than control. The RDIR of Na ranged between 0.19 to 0.74 mg with the Fe-treated tomato, while it was 0.14 mg with control. The contribution of Na by the treated tomatoes to the RDA was five times higher than control. While the RDA value of P was 700 mg/day (Institute of Medicine Citation1997), the RDIR of P ranged between 6.4 to 10.6 mg in the Fe-treated tomatoes, while the RDIR was 5.15 mg with control. The contribution of P with treated tomatoes to the RDA was 2.1 times higher compared to control. For Zn, the recommended value ranged between 9 and 14 mg/day (Trumbo et al. Citation2001). The DIR of Zn with the Fe-treated tomatoes ranged between 0.05 to 0.08 mg, while the RDIR was 0.04 with the control. The Zn contribution by the Fe-treated tomatoes was 1.95 times higher compared to the control. The RDIR of B ranged between 0.03 to 0.04 mg with the Fe-treated tomatoes, while it was 0.02 mg with control. The RDIR with Fe-treated tomatoes ranged between 1.30 to 1.83 mg, while it was 0.98 mg with control.
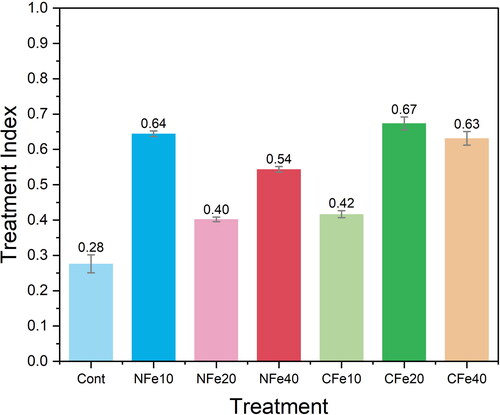
Our results indicated that a 400 g serving size of the Fe-treated tomatoes would provide a higher percentage of nutrients to the RDA and AI than the control tomatoes. Therefore, regular consumption of Fe-treated tomatoes may provide considerable public health benefits due to higher nutrient concentration in tomatoes. Several studies have reported the public health benefits of tomatoes associated with its nutrient contents (Ali et al. Citation2020; Guil-Guerrero and Rebolloso-Fuentes Citation2009). The public health benefits of nutrient-rich tomato fruits are directly influenced by the translocation of nutrients from the soil. The effectiveness of this translocation can vary based on the type and concentration of Fe treatments applied, especially when considering nano and chelated Fe. To identify the most efficient Fe treatment method, we calculated a treatment index, which quantitatively represents the efficacy of nutrient translocation from soil to tomato fruits. Our results indicated a range of treatment index values between 0.28 to 0.67 (as depicted in ). Notably, the treatment using chelated Fe at a concentration of 20 mg/L exhibited the highest index value of 0.67. In comparison, the treatment with nano Fe at 10 mg/L resulted in a slightly lower index value of 0.64. Based on these findings, we can conclude that the chelated Fe treatment at 20 mg/L is slightly more effective in promoting nutrient translocation than the nano Fe treatment at 10 mg/L. This suggests that for optimal nutrient translocation and subsequent public health benefits, chelated Fe when applied at 20 mg/L, is the preferable treatment among the methods we investigated.
Overall, the application of nano and chelated Fe fertilization techniques in tomato production has demonstrated increased efficacy in delivering Fe to plant roots, leading to improvements in both tomato yield and nutritional content. Additionally, nano Fe particles are inherently nontoxic; thus, the use of minimal amounts (20 mg/L) of the nano and chelated Fe is unlikely to pose environmental challenges. A significant concern, however, pertains to nano particles’ interactions with beneficial soil microorganisms. Notably, Fe nanoparticles may stimulate certain bacterial growth and alter the soil bacterial community structure, even though bacterial abundance remains unchanged (He et al. Citation2011; Cullen et al. Citation2011). Consequently, they might exert a positive influence on microbial communities, which in turn could improve soil health and related agroecosystem services. It remains essential to prioritize further research and monitoring to ensure the sustainability and environmental computability of the nano fertilization.
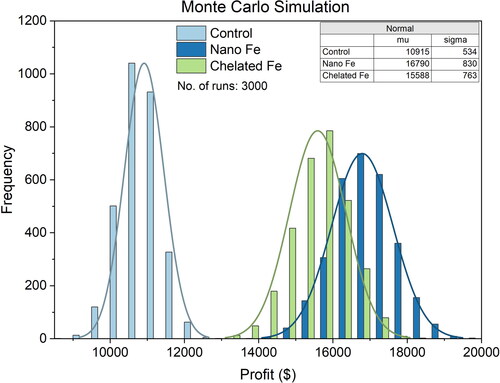
and depict the average estimations of total cost and total profits, respectively, for fresh market tomatoes produced with the control, nano, and chelated Fe fertilizations. The estimated costs per hectare (ha) were $46,722 for the control, $46,742 for the nano Fe, and $46,793 for the chelated Fe fertilization. The production cost was $20 higher with the nano Fe and $71 higher with the chelated Fe fertilization, when compared to the conventional fertilization, using the plasticulture drip irrigation. Additionally, the estimated profit per hectare (ha) stood at $10,915 for the control, $16,790 for the nano Fe, and $15,588 for the chelated Fe-fertilized tomatoes. This indicates that tomatoes fertilized with the nano Fe could yield higher profits ($5,875) than the control, while the chelated Fe-fertilized tomatoes could also yield higher profits ($15,588) than the control. The increased profits margin correlate with the 10% and 8% higher tomato yields achieved (Tables S1–S3) with the nano and chelated Fe fertilizations, respectively, when compared to control. Thus, in terms of profitability, nano Fe fertilization emerges as the most advantageous.
Conclusions
Nutrients concentration and distribution in different parts of the tomato plants did not follow a similar order in response to Fe sources and their rates of application. The PCA explored that 10 mg/L nano Fe treatment resulted in higher concentrations of Fe, Na, P, S, Ca, Mg, and B in tomato fruits, while 20 and 40 mg/L nano Fe treatments increased Zn, Mn, and K concentration. In contrast, the increased concentrations of Ca, B, Na, Zn, Mg, K, and S were associated with the 40 mg/L chelated Fe treatment. Chelated Fe-treated tomato plant roots, stems, and leaves had higher concentrations of nutrients compared to those in the nano Fe, which was supported by higher correlation coefficients among the nutrients in roots, stems, and leaves (r = 0.96 to ≤1.0). The translocation factors implied that the nano Fe-fertilized tomatoes had 17% more nutrients than the control tomatoes. Therefore, 10 mg/L nano and 40 mg/L chelated Fe treatments were better with respect to the nutrient enrichment in tomatoes. Daily consumption of 200 g of Fe-fertilized tomatoes would provide a range of 0.02 to 3.0% nutrients to the RDA and AI, which was two times more than the tomatoes produced under the control. In terms of profitability, tomatoes fertilized with the nano Fe are projected to yield higher profits compared to those fertilized with chelated Fe and conventionally. However, when evaluating the index values concerning the nutritional levels in tomatoes, the 20 mg/L chelated Fe emerged as the superior fertilization method compared to all other methods examined.
Supplemental Material
Download MS Word (45.5 KB)Acknowledgements
We acknowledge Professor Gary Gao and Research Assistant Ryan Slaughter for allowing us to use their laboratory and equipment. Thanks to Bradford Sherman for his contribution to editing and reviewing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ali, M. Y., A. A. I. Sina, S. S. Khandker, L. Neesa, E. M. Tanvir, A. Kabir, M. I. Khalil, and S. H. Gan. 2020. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods (Basel, Switzerland) 10 (1):45. doi:10.3390/FOODS10010045.
- Aqeel, U., T. Aftab, M. M. A. Khan, M. Naeem, and M. N. Khan. 2022. A comprehensive review of impacts of diverse nanoparticles on growth, development and physiological adjustments in plants under changing environment. Chemosphere 291 (Pt 1):132672. doi:10.1016/J.CHEMOSPHERE.2021.132672.
- Bárzana, G., P. Garcia-Gomez, and M. Carvajal. 2022. Nanomaterials in plant systems: Smart advances related to water uptake and transport involving aquaporins. Plant Nano Biology 1:100005. doi:10.1016/j.plana.2022.100005.
- Bindraban, P. S., C. Dimkpa, L. Nagarajan, A. Roy, and R. Rabbinge. 2015. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biology and Fertility of Soils 51 (8):897–911. doi:10.1007/S00374-015-1039-7/TABLES/1.
- Cobb, G. P., K. Sands, M. Waters, B. G. Wixson, and E. Dorward-King. 2000. Accumulation of heavy metals by vegetables grown in mine wastes. Environmental Toxicology and Chemistry 19 (3):600–7. doi:10.1002/etc.5620190311.
- Cullen, L. G., E. L. Tilston, G. R. Mitchell, C. Collins, and L. J. Shaw. 2011. Assessing the impact of nano- and micro-scale zerovalent iron particles on soil microbial activities: Particle reactivity interferes with assay conditions and interpretation of genuine microbial effects. Chemosphere 82 (11):1675–82. doi:10.1016/j.chemosphere.2010.11.009.
- Eid, E. M., S. A. Alrumman, E. A. Farahat, and A. F. El-Bebany. 2018. Prediction models for evaluating the uptake of heavy metals by cucumbers (Cucumis sativus L.) grown in agricultural soils amended with sewage sludge. Environmental Monitoring and Assessment 190 (9):501. doi:10.1007/S10661-018-6885-Y/TABLES/5.
- El-Desouky, H. S., K. R. Islam, B. Bergefurd, G. Gao, T. Harker, H. Abd-El-Dayem, F. Ismail, M. Mady, and R. M. Y. Zewail. 2021. Nano iron fertilization significantly increases tomato yield by increasing plants’ vegetable growth and photosynthetic efficiency. Journal of Plant Nutrition 44 (11):1–15. doi:10.1080/01904167.2021.1871749.
- Elgala, A. M., and R. H. Maier. 1971. Effect of ethylenediamine di (o-hydroxyphenylacetic acid) application to soil columns on the distribution of certain nutrient elements in the water-soluble, acid-soluble and exchangeable forms. Plant and Soil 34 (1):607–17. doi:10.1007/BF01372816.
- FAOSTAT. 2019. Food and Agriculture Organization of the United Nations. http://fao.org/faostat/en.
- Guil-Guerrero, J. L., and M. M. Rebolloso-Fuentes. 2009. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. Journal of Food Composition and Analysis 22 (2):123–9. doi:10.1016/j.jfca.2008.10.012.
- Hansen, N. C., Hopkins, B. G., Ellsworth, J. W., and Jolley, V. D. 2006. Iron nutrition in field crops. In: Iron Nutrition in Plants and Rhizospheric Microorganisms, edited by Barton, L. L. & Abadia, B. J., 23–59. doi:10.1007/1-4020-4743-6.
- He, S., Y. Feng, H. Ren, Y. Zhang, N. Gu, and X. Lin. 2011. The impact of iron oxide magnetic nanoparticles on the soil bacterial community. Journal of Soils and Sediments 11 (8):1408–17. doi:10.1007/s11368-011-0415-7.
- Hossain, K. G., N. Islam, F. Ghavami, C. Durant, C. Durant, and M. Johnson. 2017. Effect of increased amounts of Fe, Zn, and Cd on uptake, translocation, and accumulation of human health related micronutrients in wheat. Asian Journal of Agriculture and Food Science 5 (1):19–29.
- Institute of Medicine. 1997. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press. doi:10.17226/5776.
- Kah, M., R. S. Kookana, A. Gogos, and T. D. Bucheli. 2018. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nature Nanotechnology 13 (8):677–84. doi:10.1038/s41565-018-0131-1.
- Karagiannidis, N., T. Thomidis, G. Zakinthinos, and C. Tsipouridis. 2008. Prognosis and correction of iron chlorosis in peach trees and relationship between iron concentration and Brown Rot. Scientia Horticulturae 118 (3):212–7. doi:10.1016/j.scienta.2008.06.005.
- Klem-Marciniak, E., M. Huculak-Mączka, K. Marecka, K. Hoffmann, and J. Hoffmann. 2021. Chemical stability of the fertilizer chelates Fe-EDDHA and Fe-EDDHSA over time. Molecules (Basel, Switzerland) 26 (7):1933. doi:10.3390/MOLECULES26071933.
- Klute, A. 1986. Water Retention: Laboratory Methods, Part 1: Physical and Mineralogical Methods. In: Methods of Soil Analysis, edited by Klute, A., 635–662. No. 9, Madison, WI. USA: American Society of Agronomy, Inc./Soil Science Society of America, Inc. Publisher.
- Krzesłowska, M. 2010. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiologiae Plantarum 33 (1):35–51. doi:10.1007/s11738-010-0581-z.
- Küpper, H., and B. Leitenmaier. 2013. Cadmium-accumulating plants. Metal Ions in Life Sciences 11:373–93. doi:10.1007/978-94-007-5179-8_12/FIGURES/1.
- Litskas, V. D., A. Migeon, M. Navajas, M. S. Tixier, and M. C. Stavrinides. 2019. Impacts of climate change on tomato, a notorious pest and its natural enemy: Small scale agriculture at higher risk. Environmental Research Letters 14 (8):084041. doi:10.1088/1748-9326/ab3313.
- López-Millán, A. F., F. Morales, Y. Gogorcena, A. Abadía, and J. Abadía. 2009. Metabolic responses in iron deficient tomato plants. Journal of Plant Physiology 166 (4):375–84. doi:10.1016/J.JPLPH.2008.06.011.
- López-Rayo, S., P. Nadal, and J. J. Lucena. 2016. Novel chelating agents for iron, manganese, zinc, and copper mixed fertilisation in high pH soil-less cultures. Journal of the Science of Food and Agriculture 96 (4):1111–20. doi:10.1002/JSFA.7183.
- Mclaughlin, M. J., Smolders, E., and Degryse, F., Rietra, R. 2011. Uptake of Metals from Soil into Vegetables. In: Dealing with Contaminated Sites: From Theory towards Practical Application, edited by F. Swartjes, 325–367. Dordrecht: Springer. doi:10.1007/978-90-481-9757-6_8.
- Mimmo, T., D. Del Buono, R. Terzano, N. Tomasi, G. Vigani, C. Crecchio, R. Pinton, G. Zocchi, and S. Cesco. 2014. Rhizospheric organic compounds in the soil–microorganism–plant system: Their role in iron availability. European Journal of Soil Science 65 (5):629–42. doi:10.1111/ejss.12158.
- Montgomery, D. R., A. Biklé, R. Archuleta, P. Brown, and J. Jordan. 2022. Soil health and nutrient density: Preliminary comparison of regenerative and conventional farming. PeerJ. 10:e12848. doi:10.7717/PEERJ.12848.
- Morgan, J. B., and E. L. Connolly. 2013. Plant-soil interactions: Nutrient uptake. Nature Education Knowledge 4 (8):2.
- Nadal, P., C. García-Delgado, D. Hernández, S. López-Rayo, and J. J. Lucena. 2012. Evaluation of Fe-N,N′-Bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetate (HBED/Fe 3+) as Fe carrier for soybean (Glycine max) plants grown in calcareous soil. Plant and Soil 360 (1–2):349–62. doi:10.1007/s11104-012-1246-z.
- Ottow, J. C. G., G. Benckiser, I. Watanabe, and S. Santiago. 1983. Multiple nutritional soil stress as the prerequisite for iron toxicity of wetland rice (Oryza sativa L.). Tropical Agriculture 60 (2): 102–106. https://journals.sta.uwi.edu/ojs/index.php/ta/article/view/2386
- Page, V., and U. Feller. 2005. Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Annals of Botany 96 (3):425–34. doi:10.1093/AOB/MCI189.
- Page, V., and U. Feller. 2015. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 5 (3):447–63. doi:10.3390/agronomy5030447.
- Page, V., L. Weisskopf, and U. Feller. 2006. Heavy metals in white lupin: Uptake, root-to-shoot transfer and redistribution within the plant. The New Phytologist 171 (2):329–41. doi:10.1111/J.1469-8137.2006.01756.X.
- Pinto, F. A., E. D. de Souza, H. B. Paulino, N. Curi, and M. A. C. Carneiro. 2013. Sorção e dessorção de fósforo em solos do cerrado Brasileiro como suporte para recomendação da adubação fosfatada. Ciência e Agrotecnologia 37 (6):521–30. doi:10.1590/S1413-70542013000600005.
- Rai, P. K., S. S. Lee, M. Zhang, Y. F. Tsang, and K. H. Kim. 2019. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environment International 125:365–85. doi:10.1016/J.ENVINT.2019.01.067.
- Rout, G. R., and S. Sahoo. 2015. Role of iron in plant growth and metabolism. Reviews in Agricultural Science 3:1–24. doi:10.7831/ras.3.1.
- Salehi, B., R. Sharifi-Rad, F. Sharopov, J. Namiesnik, A. Roointan, M. Kamle, P. Kumar, N. Martins, and J. Sharifi-Rad. 2019. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition (Burbank, Los Angeles County, CA) 62:201–8. doi:10.1016/J.NUT.2019.01.012.
- Samaranayake, P., B. Peiris, and S. Dssanayake. 2012. Effect of excessive ferrous (Fe2+) on growth and iron content in rice (Oryza sativa). International Journal of Agriculture and Biology 14: 296-298. http://www.fspublishers.org
- Schlegel, T. K., J. Schönherr, and L. Schreiber. 2006. Rates of foliar penetration of chelated Fe(III): Role of light, stomata, species, and leaf age. Journal of Agricultural and Food Chemistry 54 (18):6809–13. doi:10.1021/JF061149I/ASSET/IMAGES/LARGE/JF061149IF00007.JPEG.
- Sharma, R. K., M. Agrawal, and F. M. Marshall. 2009. Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 47 (3):583–91. doi:10.1016/J.FCT.2008.12.016.
- Sheykhbaglou, R., M. Sedghi, and B. Fathi-Achachlouie. 2018. The effect of ferrous nano-oxide particles on physiological traits and nutritional compounds of soybean (Glycine max L.) seed. Anais da Academia Brasileira de Ciencias 90 (1):485–94. doi:10.1590/0001-3765201820160251.
- Singh, S., R. Saxena, K. Pandey, K. Bhatt, and S. Sinha. 2004. Response of antioxidants in sunflower (Helianthus annuus L.) grown on different amendments of tannery sludge: Its metal accumulation potential. Chemosphere 57 (11):1663–73. doi:10.1016/J.CHEMOSPHERE.2004.07.049.
- Sulaiman, F. R., and H. A. Hamzah. 2018. Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecological Processes 7 (1):1–11. doi:10.1186/S13717-018-0139-3/FIGURES/4.
- Trumbo, P., A. A. Yates, S. Schlicker, and M. Poos. 2001. Dietary reference intakes. Journal of the American Dietetic Association 101 (3):294–301. https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=00028223&v=2.1&it=r&id=GALE%7CA72764304&sid=googleScholar&linkaccess=fulltext. doi:10.1016/S0002-8223(01)00078-5.
- Yates, A. A., S. A. Schlicker, and C. W. Suitor. 1998. Dietary reference intakes: The new basis for recommendations for calcium and related nutrients, B vitamins, and choline. Journal of the American Dietetic Association 98 (6):699–706. doi:10.1016/S0002-8223(98)00160-6.
- Ye, L., L. Li, L. Wang, S. Wang, S. Li, J. Du, S. Zhang, and H. Shou. 2015. MPK3/MPK6 are involved in iron deficiency-induced ethylene production in Arabidopsis. Frontiers in Plant Science 6 (NOVEMBER):953. doi:10.3389/FPLS.2015.00953/BIBTEX.
- Zargar, S. M., G. K. Agrawal, R. Rakwal, and Y. Fukao. 2015. Quantitative proteomics reveals role of sugar in decreasing photosynthetic activity due to Fe deficiency. Frontiers in Plant Science 6 (AUG):592. doi:10.3389/FPLS.2015.00592/FULL.
- Zia-Ur-Rehman, M., M. F. B. Mfarrej, M. Usman, S. Anayatullah, M. Rizwan, H. F. Alharby, I. M. Abu Zeid, N. M. Alabdallah, and S. Ali. 2023. Effect of iron nanoparticles and conventional sources of Fe on growth, physiology and nutrient accumulation in wheat plants grown on normal and salt-affected soils. Journal of Hazardous Materials 458:131861. doi:10.1016/J.JHAZMAT.2023.131861.
- Zuo, Y., and F. Zhang. 2011. Soil and crop management strategies to prevent iron deficiency in crops. Plant and Soil 339 (1–2):83–95. doi:10.1007/s11104-010-0566-0.