?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Chromium isotope and chemical compositions in runoff from two felsic rock catchments, the Lysina (LYS; Czech Republic) and the Neglinka (NEG; northwest Russia), were studied. Three types of water were identified: Na-Ca-SO4 type for the LYS, Mg-Ca-Cl-HCO3 type for the NEG downstream, and Mg-Ca-Cl to Mg-Ca-HCO3 type for the NEG upstream. The Cr (III) liberated during soil/bedrock weathering oxidized to Cr (VI), which was the main dissolved Cr species in both catchments. Variations in δ53Cr values for LYS runoff (–0.07‰ to +0.37‰) reflected a sole Cr source, seasonality of weathering and hydrologically controlled leaching/export processes. In the NEG catchment, back-reduction of Cr (VI) to Cr (III) during riverine transport resulted in a decrease in Cr concentrations (to 10–30%) and in an increase of δ53Cr values (+0.09 to +0.42‰ for the upstream to +0.72 to +1.73‰ for the downstream). Groundwater discharge could have influenced the chemical composition of the downstream NEG runoff to some extent.
Editor A. Fiori; Associate editor G. Chiogna
1 Introduction
Traditional stable isotopes of elements such as O, H, N, C, S, and Si are shown to be very useful and applicable tools for hydrological and environmental studies. On the other hand, application of non-traditional stable isotopes to study natural materials is still very limited. Presently, most information on weathering and hydrological processes is provided by non-traditional stable isotopes of elements such as Li, Mg, Ca and Fe. Application of stable isotopes of other metals such as Sr, Cu, Zn, Cr, Ba, Mo, Hg, Cd, and Ni, and exploration of their potential for studies related to weathering and hydrology, is only in the beginning stages (Bullen Citation2014 and references therein). Among these, a Cr isotope system emerges as proxy in environmental, paleoenvironmental and hydrological studies. A number of researchers have developed and employed this system in studies on detoxification/remediation of water contaminated with Cr (VI) (e.g. Ellis et al. Citation2002, Izbicki et al. Citation2008, Basu and Johnson Citation2012, Novak et al. Citation2014, Citation2017a), whereas other possibilities of the system are still little investigated.
Chromium is a redox-active transition metal that occurs in natural waters mostly in a soluble Cr (VI) form as chromate (CrO42−) and/or dichromate anions (Cr2O72−), and in rocks and soils mostly in a poorly soluble Cr (III) form bounded in Cr-bearing minerals. Chromium (VI) in ground- and surface water may originate from geogenic and/or industrial sources (e.g. Richard and Bourg Citation1991, Oze et al. Citation2007, Qin and Wang Citation2017, Wu et al. Citation2017). Geogenic Cr (VI) results from oxidative weathering of Cr (III)-bearing bedrock. Industrial Cr (VI) results from direct release of industrial chemicals into the environment (Ellis et al. Citation2002, Novak et al. Citation2017b). Because variations in Cr isotope composition can point to the origin and sources of Cr, in particular in water streams, attempts have been made in recent years to distinguish between geogenic and polluting industrial Cr (VI) by studying an abundance ratio of Cr isotopes (Izbicki et al. Citation2008, Citation2012, Citation2015, Economou-Eliopoulos et al. Citation2014, Novak et al. Citation2014, Citation2017b).
Chromium isotope fractionation at the Earth’s surface is driven mostly by oxidation and reduction reactions (D’Arcy et al. Citation2016 and references therein). Chromium (III) oxidation induces equilibrium isotopic fractionation, whereas Cr (VI) reduction induces kinetic fractionation (Schauble et al. Citation2004). Chromium isotope compositions are expressed in the per mil notation relative to the Cr isotope standard reference material:
Because rivers are known to contribute major amounts of Cr to oceans, the behaviour of Cr and its isotopic features in rivers have been studied to some extent (Bonnand et al. Citation2013, D’Arcy et al. Citation2016, Wu et al. Citation2017 and references therein). However, only three studies so far deal with Cr isotope systematics of runoff at the catchment level (Novak et al. Citation2017c, Wu et al. Citation2017, Andronikov et al. Citation2019). A year-long study of runoff from a serpentinite-based catchment in Pluhuv Bor in the Czech Republic showed that runoff δ53Cr exhibited a seasonality with lower values in winter and much higher values in summer (Kram et al. Citation2009, Novak et al. Citation2017c). Runoff samples from the Connecticut River, USA (felsic and mafic terrains), had elevated δ53Cr values of the dissolved Cr (as compared to those in weathering profiles) by up to 1‰ (Wu et al. Citation2017). A seasonal effect on dissolved Cr concentration and δ53Cr values was observed in some tributaries of the Connecticut River. A pilot study of Cr isotope composition of Neglinka River runoff (felsic terrains) showed an increase in δ53Cr values by >1‰ during the riverine transport (Andronikov et al. Citation2019).
Because almost nothing is known about the behaviour of Cr in runoff from catchments located on felsic bedrock, we chose to study two such sites: the Lysina (LYS) catchment (Czech Republic) and the Neglinka (NEG) catchment (Karelia, northwest Russia) (; ). These catchments were chosen because: (i) they are located >1500 km apart with different hydrologic and slightly different climatic conditions; (ii) both catchments are underlain by monolithologic felsic bedrocks, therefore representing almost terra incognita in terms of Cr isotope behaviour; (iii) both catchments are situated in a temperate climate where only a moderate weathering regime persists; and (iv) one of the streams (LYS) drains a forested rural area, while the other (NEG) includes both rural and urban environments. We studied processes controlling variability in Cr isotope composition in runoff, investigating factors to control Cr isotope composition during mobilization and further riverine transport.
Table 1. Characteristics of the study sites
2 Materials and methods
2.1 Study sites
The LYS catchment (0.27 km2) is located 120 km west of Prague on a mountainous plateau and is underlain by coarse-grained leucogranite. A perennial small stream (~1 km long) drains the catchment. A long-term monitoring programme (1990–2019) revealed that runoff mineralization was characterized by low concentrations of base cations (total Ca2+, Mg2+, Na+ and K+ varied from 2.9 to 7.2 mg L−1) and low pH varying from 4.0 to 5.2 (Kram et al. Citation2012, Citation2017). The water discharge ranged from 0.7 to 190 L s−1 km2 (Kram et al. Citation2012, Citation2017, Kram Citation2019), and the instantaneous discharge during our sampling was within these limits, ranging from 0.4 to 63 L s−1 km2.
The 14-km-long Neglinka River begins in a forest environment, but the downstream reaches of the river run through the city of Petrozavodsk and along the shore of Lake Onega. The NEG catchment (46.1 km2) is situated in the Petrozavodsk depression, filled with Late Proterozoic quartzite-sandstone (Mikhailov et al. Citation2014). The rocks are covered with a glacial Quaternary cover and a thin layer of mostly organic-rich soil (Mikhailov et al. Citation2014, Novikov Citation2014). The river runoff was characterized by total concentrations of base cations in the range of 6–70 mg L−1, with water total mineralization changing during the year from 35 to 400 mg L−1 (Borodulina Citation2013). Over a calendar year, the daily mean water discharge ranged from 4.5 to 22 L s−1 km2 (The State Water Cadaster of the USSR Citation1986).
2.2 Sampling
We collected samples of LYS runoff monthly from June 2017 to September 2018. Samples of mineral soil were collected from three soil pits along the length of the stream () from depth intervals of 0–10, 10–20, 20–40 and 40–80 cm. Four granite bedrock samples were collected along the stream riverbed.
Figure 1. Schematic maps of the Lysina (LYS) and Neglinka (NEG) catchments, with location map of the studied sites in Europe. See insert location of the studied areas in the Czech Republic and in northwest Russia. For the LYS catchment, the location of the soil pits is shown. For the NEG catchment, the boundary of the catchment and the urban and settlement areas (diagonal pattern) is shown. NEG sampling sites: 1 – the upstream site; 2 – the downstream site; 3 – the Bezymjanny site (see text for details); the black star in the inset indicates the location of the studied area in northwest Russia
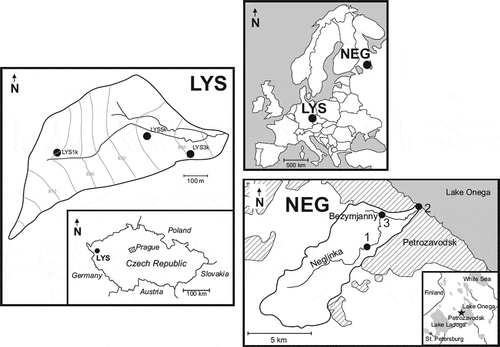
Water samples were collected at three sites along the Neglinka River (). The first site was located in the river upstream, outside the city limits; the second site was located 50 m upstream from the river mouth, within the city limits; and the third site was located 50 m upstream from the mouth of the Bezymjanny tributary. We collected samples monthly between August 2017 and July 2018. Four samples of quartzite-sandstone bedrock were collected from a quarry located in the catchment.
2.3 Chemical analyses
Water samples were acidified with HNO3 to pH < 2 and kept in a refrigerator until analysis for major cations (Na+, K+, Ca2+, and Mg2+) and trace metals. Non-acidified subsamples were filtered through a 0.45-μm membrane filter just before the analysis for anions (SO42− and Cl−) and dissolved organic carbon (DOC). Unfiltered and non-acidified samples were used to determine electrical conductivity, pH and phosphorus concentration. The <2 mm mineral soil fraction and bedrock samples were homogenized and dissolved by HF-HClO4 acid digestion followed by Na2CO3-Li2B4O7 oxidative alkaline fusion.
2.3.1 The Lysina catchment
Chemical analyses of water samples were conducted at the Central Accredited Laboratory of the Czech Geological Survey (Prague, Czech Republic). Concentrations of Cr, Fe, and Mn were measured with a quadrupole inductively coupled plasma mass spectrometer (Q-ICP-MS) X-Series 2 (Thermo) under standard analytical conditions. A concentric glass nebulizer with a spray chamber was used for sample introduction. A standard-blank solution was prepared using successive dilutions of the 5% HNO3 standard-carrier solution. Concentrations of major cations (Na+, K+, Ca2+, and Mg2+) were determined with a Perkin-Elmer AAnalyst 200 atomic absorption spectrometer. Samples were aspirated through the glass nebulizer, and the absorbance was measured with a blank as the reference. The high-performance liquid chromatography (HPLC) method was used to measure both SO42− and Cl− with a Knauer 1000 liquid chromatograph. DOC concentrations were measured on a Tekmar-Dohrmann Apollo 9000 analyser with a chemiluminescent detector (0.1 mg L−1 detection limit) by high-temperature oxidation using a non-purgeable organic carbon method. Water pH was measured using a combination of glass electrodes. Electrical conductivity was measured using a standard conductivity cell. Phosphorus concentration was determined by the photometric method with the use of a Perkin-Elmer Lambda 25 spectrophotometer.
Concentrations of Cr, Fe and Mn in solid samples and Cu in water samples were determined with the use of the Agilent Technologies 5110 ICP-optical emission spectrometer (OES) under standard operating conditions. Samples in 0.3 N HNO3 were introduced to plasma with a concentric glass nebulizer and an Agilent Technologies SPS-4 autosampler.
2.3.2 The Neglinka catchment
Chemical analyses of water samples were conducted at the Accredited Chemical Laboratory of the Northern Water Problems Institute (Petrozavodsk, Russia). Concentrations of Cr, Fe, Cu and Mn were measured on an AA-6800 Shimadzu atomic spectrophotometer. Concentrations of Fe and Mn were measured by an atomic absorption method, and concentrations of Cu and Cr were measured using an electro-thermal atomization method. Samples were aspirated through the glass nebulizer, and absorbance was measured with a blank as the reference. Determination of Na, K, Ca, and Mg concentrations was performed on an AA-6200 Shimadzu spectrophotometer. Sodium and K concentrations were measured in the atomic emission mode, while Ca and Mg concentrations were measured in the atomic absorption mode. Samples were aspirated through the quartz nebulizer and absorbance was measured using blank as a reference. A photometric method (spectrophotometer Portlab 501) with Sulfonazo III Na salt was used to analyse SO42−. Optical density was measured at wavelengths of 610 and 640 nm. A photometric method with Hg thiocyanate and Fe(III) nitrate was used to analyse Cl−. Optical density was measured at a wavelength of 460 nm. Phosphorus concentrations were determined after oxidation of P-bearing compounds to phosphates by boiling the sample in a water bath with the addition of H2SO4 and (NH4)2S2O8 as oxidants. The concentration of phosphates in the resultant solution was determined on a Portlab 501 spectrophotometer. For DOC concentrations, photo-chemical oxidation of the organic material to CO2 with the (NH4)2S2O8 in a constant stream of nitrogen was applied (Zobkov and Zobkova Citation2015). The resultant CO2 was analysed on an IR Fourier FSM-1201 spectrometer. Electrical conductivity was measured with an Agilent 3200 C conductometer. A potentiometric method was used to determine pH with a pH meter PH-410. Concentrations of Cr, Fe and Mn in bedrock samples were determined at the Czech Geological Survey with the use of an Agilent Technologies 5110 ICP-OES.
2.4 Isotopic analyses
Sample preparation and Cr isotopic analyses were conducted at the laboratories of the Czech Geological Survey. All runoff samples were adjusted to pH ≥ 9 by adding ultrapure NH4OH in order to maintain Cr (VI) stability (see Cadkova and Chrastny Citation2015). After filtering with 0.45-μm membrane filters, water samples containing ~1–2 μg Cr were loaded on 10-mL polyethylene columns filled with 2 mL of AG1-X8 anion exchange resin (100–200 mesh, Bio-Rad, USA). Elution chromatography modified from Chrastny et al. (Citation2011) and Farkas et al. (Citation2013) was applied.
Water samples were drained through the columns, and Cr (VI) in the form of CrO42− was retained on the resin (D’Arcy et al. Citation2016). Matrix cations were eluted with 0.1 M HCl, whereas Cr (VI) was eluted with 6 M HCl. The resulting Cr fraction still contained Fe, Ti and V, which had to be removed to avoid isobaric interferences on Cr isotopes. The sample in 0.1 M HCl was loaded on the column filled with 2 mL of AG1-X8 anion exchange resin, and the Cr fraction was collected by flushing the resin with 0.1 M HCl. The collected Cr sample was treated with concentrated HNO3 + H2O2 to remove chlorides and organics. Finally, H2O (Milli-Q System, <1 × 10−18 Ω cm−1) was added to dilute the solution to 2% HNO3 for the analyses. For soil and bedrock samples, an additional step was introduced (Chrastny et al. Citation2011). NH4OH was added to the sample in 0.1 M HCl to maintain a pH of ≥9, and H2O2 was added to oxidize Cr (III) to Cr (VI). Then the sample was loaded on the column and processed. The National Institute of Standards (NIST) 979 Cr standard was processed for each sample set.
Isotope measurements were performed on a multi-collector (MC)-ICP-MS Neptune (Thermo) in a high-resolution mode (M/ΔM(95%) = 10 000). The instrumental set-up was described by Farkas et al. (Citation2013). Because Cr has four stable isotopes, a 50−54Cr double spike was used for mass bias and isobaric interference corrections. A sample solution in 2% HNO3 was introduced into the plasma using an ARIDUS II (Cetac, USA) desolvating nebulizer and a ASX-112FR (Cetac) autosampler. All four Cr isotopes and elements with isobaric overlaps (48Ti for overlap on 50Cr with 50Ti, 51V for overlap on 50Cr with 50V and 56Fe for overlap on 54Cr with 54Fe) were measured simultaneously. Each measurement consisted of 40 cycles with a 4-s integration time. A blank was measured between each pair of samples/standards. The standard was analysed after every four samples.
The δ53Cr value of the NIST 979 standard doped with the double spike was monitored over the course of measurement, providing a mean value of +0.014 ± 0.020‰ (2σ). The isotope composition of all samples was corrected to this value.
The average reproducibility of δ53Cr measurements was ±0.054‰ (2σ). The total procedural blank contribution was 10–20 ng Cr, which was negligible and did not affect Cr isotope measurement beyond the external reproducibility of the NIST 979 standard. The analytical accuracy and precision for analysed samples were assessed by repeatedly processing and measuring JG-3 and BHVO-2 reference materials. The measured JG-3 δ53Cr value (–0.114 ± 0.018‰ (2σ), n = 6) was almost identical to that reported by Li et al. (Citation2017). The measured BHVO-2 δ53Cr value (–0.136 ± 0.020‰ (2σ), n = 6) was within the range of the values reported by Zhu et al. (Citation2018).
3 Results
3.1 The Lysina catchment
3.1.1 Bedrock and soil
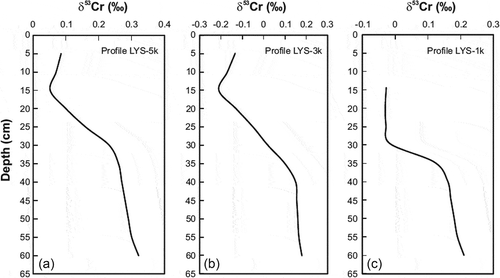
The concentration of Cr in LYS bedrock varied from 8.3 to 14.3 ppm, whereas concentrations of Fe and Mn varied from 9000 to 10 700 ppm and from 320 to 350 ppm, respectively (). The bedrock displayed variation in δ53Cr values from −0.15 to −0.06‰, consistent with known δ53Cr values for granite (Li et al. Citation2017, Zhu et al. Citation2018). The uppermost mineral soil horizons (0–20 cm) were usually depleted in Cr (14.3–48.4 ppm) as compared to deeper horizons (29.9–54.3 ppm). An exception was the soil from pit LYS-3 k (), where the 0–20 cm horizon displayed Cr concentrations of 42.3–48.4 ppm, whereas the deeper horizons were poorer in Cr (29.9–34.8 ppm). Soil samples displayed Fe and Mn concentrations of 3900–20 900 and 50–535 ppm, respectively (), with the highest concentration of these elements typical for deeper horizons. Mineral soil displayed δ53Cr values from −0.21 to +0.32‰ (). The deepest soil horizons were richer in the 53Cr isotope than the shallower horizons (). In all three soil pits, the deepest horizons had higher δ53Cr values than those of the bedrock ().
Table 2. Concentrations of Fe, Mn and Cr, and Cr isotope composition of the Lysina (LYS) catchment mineral soil and bedrock samples, and the Neglinka (NEG) catchment bedrock samples
3.1.2 Water
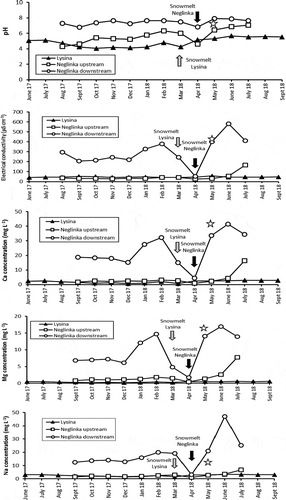
Physico-chemical parameters of the LYS runoff varied insignificantly (). Water hardness values were 0.07–0.17 mmol L−1. Electrical conductivity was 41.4–51.6 μS cm−1 (higher in winter). Water was acidic (pH = 4.1–5.6) over the whole period of observation, with the acidity increasing during higher streamwater flow ().
Table 3. Hydrological features of LYS and NEG runoff, and meteorological conditions during sampling. Sampling sites: LYS: Lysina; NEG: Neglinka; UpS: upstream; DS: downstream; B: Bezymjanny creek. EC: electrical conductivity; T: Temperature; TH: total hardness; n.d.: not determined. All sampling was conducted between 10:00 and 11:00 h local time
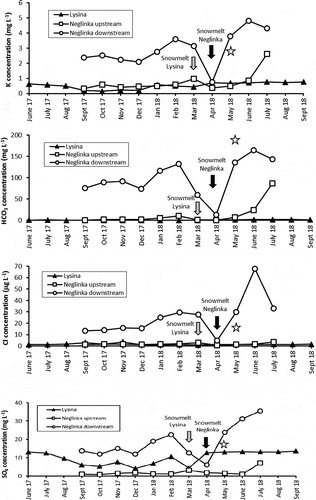
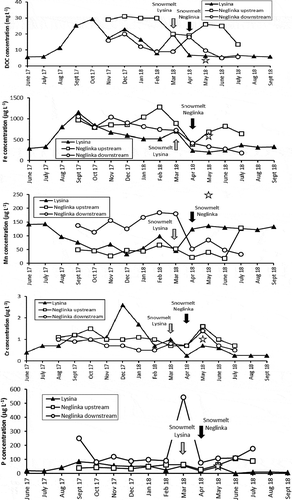
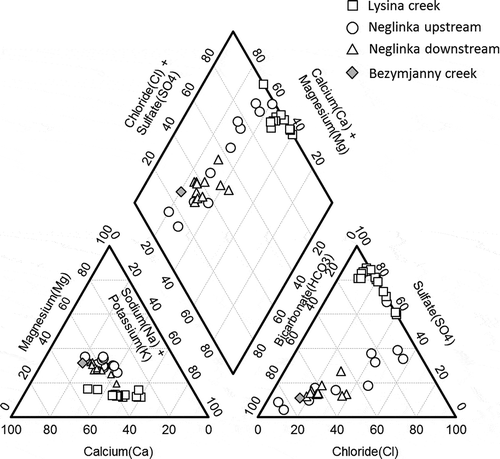
The Piper diagram () shows that LYS runoff water belongs to the Na-Ca-SO4 type. During most of the year, a Ca = Na-SO4 type prevailed, whereas it became a Na > Ca-SO4 type in winter and after snowmelt. During low discharge in summer, it was a Ca > Na-SO4 type. The total concentration of major ions (Na+, K+, Ca2+, Mg2+, SO42−, and Cl−) varied from 7.4 mg L−1 in March 2018 to 20.9 mg L−1 in June 2017 (), with overall higher concentrations of major ions observed in summer. The highest Cr concentrations (1.7–2.6 μg L−1) were found in LYS samples collected in winter, whereas the lowest concentrations (0.3–0.4 μg L−1) were measured in summer and in early autumn (; ). Concentrations of Fe and Mn in runoff changed in the ranges of 290–1150 and 48–143 μg L−1, respectively. Concentrations of P were season dependent, with the lowest concentrations typical for late summer and early autumn (). DOC concentrations varied from 5.5 to 29 mg L−1 with the highest concentrations typically found in winter (; ).
Table 4. Chemical and isotopic features of LYS and NEG runoff. Sampling sites: LYS: Lysina; NEG: Neglinka; UpS: upstream; DS: downstream; B: Bezymjanny creek. n.d.: not determined. SD: standard deviation
The LYS runoff displayed δ53Cr values from −0.07‰ to +0.37‰ (). Moderate seasonality was observed in runoff δ53Cr values, with higher values typical for summer (+0.16‰ to +0.37‰) and lower values typical for winter (–0.07‰ to +0.13‰) ().
3.2 The Neglinka catchment
3.2.1 Bedrock
The NEG bedrock () displayed Cr concentrations from 11.5 to 40.1 ppm. Concentrations of Fe and Mn varied from 6100 to 11 600 ppm and from 116 to 164 ppm, respectively. Our first determination of NEG bedrock isotope composition showed that the bedrock was only insignificantly enriched in the 53Cr isotope (δ53Cr = +0.01 to +0.05‰).
3.2.2 Water
The water type of NEG upstream changed significantly from Mg-Ca-Cl type in winter and after the snowmelt to Mg-Ca-HCO3 type during summer low discharge (). However, downstream NEG water was characterized as Mg-Ca-Cl-HCO3 type, varying from Mg-Ca-Cl≪HCO3 (this type also characterized the Bezymjanny tributary) during most of the year to Mg-Ca-Cl = HCO3 after snowmelt and during summer low discharge.
Concentrations of major ions were almost an order of magnitude higher in the river downstream (21.1–209.3 mg L−1) than upstream (5.1–14.3 mg L−1). An exception was observed in July 2018 when the total concentration of major ions for the upstream area sharply increased to 44.1 mg L−1 (). Concentrations of most metals were also higher downstream than upstream (; ). Concentrations of Cu varied from 0.6 μg L−1 upstream to 10 μg L−1 downstream. Concentrations of Cr were higher upstream (1.0–1.5 μg L−1) than downstream (0.5–1.0 μg L−1) for most of the observation period. Phosphorus was much more abundant downstream (76–544 mg L−1 vs. 26–107 mg L−1 upstream). DOC displayed slightly lower concentrations upstream (9–20 mg L−1) than downstream (19–31 mg L−1), with the highest concentrations measured in late autumn and early winter (). The behaviour of Fe in the upstream water was erratic, with concentrations varying from 390 to 1280 μg L−1, whereas the downstream samples displayed a steady decrease in Fe concentrations (except for November 2017), from 1050 μg L−1 in September 2017 to 180 μg L−1 in July 2018 ().
This study confirms observations made by Borodulina (Citation2013) and Andronikov et al. (Citation2019) regarding water hardness: it varied from 0.08 to 0.30 mmol L−1 in upstream samples, and from 0.37 to 2.84 mmol L−1 in downstream samples, depending on the season (). Electrical conductivity upstream varied from 24.8 to 41.7 μS cm−1, whereas it was almost an order of magnitude higher downstream (206–402 μS cm−1; a drop to 53.4 μS cm−1 was observed after snowmelt in early May 2018; ; ). Upstream runoff displayed a wide range of pH values varying from acidic to neutral (pH = 4.3–7.0), whereas the acidity was about neutral (pH = 6.8–7.9) in the downstream samples (; ).
In addition, we considered the Bezymjanny tributary (~750 m) of the Neglinka River, which originates in a small spring and enters the river downstream in the middle of the city (). Bezymjanny runoff was significantly richer in Fe and Mn than the NEG downstream runoff, with concentrations of other elements and compounds very close to those in the NEG downstream (). The physico-chemical properties of Bezymjanny water were very close to those for the Neglinka downstream: pH of 6.9–7.5, and EC of 199–405 μS cm−1 (Borodulina Citation2013, Andronikov et al. Citation2019) (; ).
Our study of the Cr isotope systematics of the Neglinka River runoff confirm that the downstream is much richer in 53Cr isotope than the upstream (see Andronikov et al. Citation2019) (; ). The only sample of Bezymjanny water analysed had a δ53Cr value of +0.67‰, which was between those measured at the same time in the NEG downstream and upstream (+1.28‰ and +0.18‰, respectively; ).
4 Discussion
4.1 Hydrochemical features of runoff
We identified three different runoff water types: Na-Ca-SO4 type for the LYS catchment, Mg-Ca-Cl-HCO3 type for the NEG downstream, and a season-dependent Mg-Ca-Cl to Mg-Ca-HCO3 type for the NEG upstream (). The Pearson correlation coefficient was calculated for all measured parameters () to establish relationships between the physico-chemical characteristics of the water. Guildford’s (Citation1956) rule of thumb was applied for interpreting the correlations.
Table 5. Correlation matrix of physico-chemical parameters, major ions and trace elements of Lysina (LYS) runoff. Bold formatting indicates correlation r > 50%. T: temperature; EC: electrical conductivity; Disch.: discharge; TH: total hardness; DOC: dissolved organic carbon
Table 6. Correlation matrix of physico-chemical parameters, major ions and trace elements of Neglinka (NEG) runoff. Bold formatting indicates correlation r > 50%. T: temperature; EC: electrical conductivity; Disch.: discharge; TH: total hardness; DOC: dissolved organic carbon
Electrical conductivity had no correlation with any parameters for the LYS runoff. For the NEG upstream, EC had a significant positive correlation with almost all parameters (except for Fe and Cr) (r = 0.62–1.00 at p < 0.01), and a significant negative correlation with DOC (r = −0.69 at p < 0.05). For the NEG downstream, EC displayed a significant positive correlation with most major ions (r = 0.91–0.97 at p < 0.01) and a significant negative correlation with DOC (r = −0.82 at p < 0.01), but no correlation with trace elements. The pH displayed a consistent positive correlation with most constituents (except for trace elements in all runoff samples, and except for DOC, Cl and Fe in the LYS runoff). Discharge (measured only for the LYS runoff) has a significant (at p < 0.01) negative correlation with Na (r = −0.80), K (r = −0.62), Ca (r = −0.76), Mg (r = −0.74), SO4 (r = −0.76) and Mn (r = −0.77), pointing to the contribution of low mineralized atmospheric precipitation to the water chemistry during high flows and to the strong contribution of groundwater enriched by chemical weathering during low flows. In contrast, discharge displayed low to significantly positive correlation with DOC (r = 0.65), Fe (r = 0.34), Cr (r = 0.86) and P (r = 0.45), suggesting that during high streamwater flow, Fe and P were leached from soil horizons rich in decomposed organic matter (the relationship of DOC and Cr is considered below). Water temperature displayed a significant positive correlation with most constituents (except for discharge, DOC, Cl, Fe, Cr and P) for the LYS runoff (r = 0.56–0.83 at p < 0.01) and also for most constituents (except for DOC, Fe, Mn and Cr) for the NEG runoff (r = 0.54–0.72 at p < 0.01). A positive correlation between the water temperature and concentration of ions indicates that ion delivery to runoff was more intensive in warmer periods.
Among chemical constituents, SO4 had a significant positive correlation (at p < 0.01) with Ca (r = 0.91–0.98), Mg (r = 0.87–0.98), Na (r = 0.80–0.97) and K (r = 0.88–0.94) for all studied runoff samples. Since elevated amounts of sulphur in the LYS runoff are likely due to sulphur stored in soils from previous periods of heavy atmospheric deposition (Hruska and Kram Citation2003, Kopacek et al. Citation2016), we adopted the suggestion that SO2 from air pollution plays a dual role in weathering of silicate rocks (Schiavon Citation2007). First, it can be responsible for the sulphation of feldspar, forming gypsum (CaSO4 · 2H2O), and, second, it can cause the weathering of feldspar to kaolinite (Al2Si2O5(OH)4). A significant positive correlation between Ca and SO4 points to the contribution of gypsum. A simultaneous positive correlation among Mg, Na, K and SO4 suggests the presence of MgSO4 and (Na, K)2SO4 salts in the main source of sulphate for both catchments. For the NEG runoff, SO4 has additionally a significant positive correlation with HCO3 (r = 0.87–0.91 at p < 0.01), especially for the downstream location, possibly indicating both intensive weathering and groundwater impact (Borodulina Citation2006, Kura et al. Citation2013, Yu et al. Citation2018).
Chloride shows almost no correlation with most constituents in the LYS runoff. Only DOC, Fe and P displayed low to moderate positive correlation with Cl (r = 0.34–0.55). Thus, Cl might have originated from at least two sources different from a common source for other major ions. One source was likely related to anthropogenic pollution (Kopacek et al. Citation2016), whereas another part of the Cl along with Fe and P was leached from soil horizons rich in decomposed organic matter. In contrast, concentrations of Cl correlated positively (r = 0.71–0.99 at p < 0.01) with concentrations of all major ions for the NEG, runoff suggesting their common source. Only HCO3 displayed slightly lower but still positive correlation with Cl (r = 0.71–0.79 at p < 0.01), suggesting that part of the HCO3 might have come from a source different than that for Cl. Calcium and Mg had the highest correlation coefficient (r > 0.9 at p < 0.01) among all major ions in all studied runoffs, indicating the presence of a ubiquitous source of Ca and Mg. This could be the dissolution of low-Mg carbonate precipitates in the NEG case, and of products of granite kaolinization (Hayes et al. Citation2020) in the LYS case. These data, in combination with the geological background of the area (Mikhailov et al. Citation2014), suggest that the main anions for the Neglinka stream may have been derived from the dissolution of gypsum and carbonates.
For both catchments, runoff δ53Cr values were significantly higher than those of bedrock, but they were similar to those measured for deeper mineral soil horizons in the case of LYS (). Whereas the NEG upstream displayed moderate fractionation relative to bedrock (a fractionation factor Δ53Cr = δ53Craqueous – δ53Crbedrock was in the range +0.04 to +0.38‰), the downstream was characterized by much stronger enrichment in the 53Cr isotope (Δ53Cr varied from +0.68 to +1.69‰). Lysina runoff, similarly to the NEG upstream, displayed only slightly fractionated Cr isotopic composition (Δ53Cr was +0.04 to +0.48‰). The widest variations in δ53Cr values for the LYS runoff were observed at low discharge (<0.6 L s−1; ). This is expressed in a significant negative correlation between the discharge and δ53Cr values (r = −0.79 at p < 0.01). On the other hand, δ53Cr values varied within a narrower range (0.08–0.25‰) at higher discharge () that resulted in a low negative correlation between the discharge and δ53Cr values (r = −0.36 at p < 0.01).
4.2 Factors controlling Cr isotope composition in the LYS catchment
The LYS runoff samples were characterized by a mean δ53Cr value of +0.20 ± 0.24‰ (2σ), whereas bedrock displayed slightly negative δ53Cr values (mean: −0.11 ± 0.08‰; 2σ). The origin of higher δ53Cr values in geogenically fed water as compared to bedrock is generally due to two major processes: (i) preferential release of 53Cr during oxidation of soil/bedrock Cr (III) to Cr (VI), and (ii) back-reduction of aqueous Cr (VI) to insoluble Cr (III) (Fendorf Citation1995, Ellis et al. Citation2002, Oze et al. Citation2007, Zink et al. Citation2010, Frei and Polat Citation2013, Economou-Eliopoulos et al. Citation2014, D’Arcy et al. Citation2016).
4.2.1 Redox conditions
In the LYS catchment, mineral soil displayed δ53Cr values varying from +0.02 to +0.72‰, as compared to the mean δ53Cr value of bedrock of −0.11 ± 0.08‰ (2σ) (; ). It is known that while Cr (VI) produced by Cr (III) oxidation during bedrock weathering is still not leached from soil, the soil would be characterized by higher δ53Cr values than the bedrock because Cr (VI) soil pool is enriched in the 53Cr isotope due to fractionation (Zink et al. Citation2010, Frei et al. Citation2014, D’Arcy et al. Citation2016). However, following leaching of isotopically heavy soluble Cr (VI) affected by fractionation Cr (III)soil - Cr (VI)aqueous would leave behind soil enriched in isotopically light Cr (III) (Puzon et al. Citation2008, Paulukat et al. Citation2015, Novak et al. Citation2017c). This process could explain the isotopic inhomogeneity of LYS soil () and a slight depletion of upper soil horizons in the 53Cr isotope (mean δ53Cr values varied from −0.17 ± 0.10‰ to +0.11 ± 0.17‰ (2σ) for the horizon of 0–20 cm, as compared to mean δ53Cr values varying from +0.10 ± 0.32‰ to +0.28 ± 0.13‰ (2σ) for the >20 cm horizon). It follows from such a distribution of δ53Cr values that the uppermost soil horizons could be a main supplier of Cr (VI) to stream water.
In order to model Cr fractionation during oxidation of Cr (III) to Cr (VI), the Rayleigh relation was applied (see Ellis et al. Citation2002, Johnson Citation2011):
where δ53Cr and δ53Cr0 represent the isotope compositions of Cr (III) in soil/bedrock at the sampling time and at the stage just before the oxidation started, respectively. The parameter f is the fraction of the unoxidized Cr (III) remaining in soil/bedrock, and α is an isotope fractionation factor:
where Rprod and Rreact are the 53Cr/52Cr ratios of the reaction product [Cr (VI)] and the reactant [Cr (III)], respectively. In the case of Cr (III) oxidation, α > 1, reflecting higher 53Cr/52Cr ratio in the Cr (VI) produced by oxidation (Fendorf Citation1995, Zink et al. Citation2010, Frei and Polat Citation2013).
We chose the soil pit LYS-1 k () for Cr fractionation modelling because a Cr concentration decrease from the lower to the upper horizons is clearly seen here (). Since we do not know either the exact isotopic composition of Cr (III) in the source or that of Cr (VI) produced by oxidation, the following simplifications and assumptions were made: (a) the Cr (III)soil - Cr (VI)aqueous is a closed system where all Cr in the source exists as Cr (III), and all aqueous Cr exists as Cr (VI); (b) oxidation is the only process affecting a Cr (III) pool; (c) the mean δ53Cr value of mineral horizon 20–80 cm of 0.10 ± 0.32‰ (2σ) reflects the Cr isotope composition of the Cr pool before oxidation and leaching began; and (d) the mean δ53Cr value of 0.20 ± 0.24‰ (2σ) reflects the isotope composition of the produced aquatic Cr (VI). The fractionation factor α can be calculated as follows (Zink et al. Citation2010, Economou-Eliopoulos et al. Citation2014):
where Δ53Cr(Cr (VI) − Cr (III)) is the difference between δ53Cr (VI) in runoff and δ53Cr (III) in the source pool. For the case under consideration, α ≈ 1.0001.
The results of the numerical modelling () suggest that if a mean δ53Cr value of 0.02 ± 0.14‰ (2σ) reflects the Cr isotope composition of the source after Cr (VI) leaching, then ~55% of the original Cr (III) pool was oxidized to Cr (VI) and removed to the aquatic system. However, if we apply a δ53Cr value of −0.03‰ (10–20 cm horizon of the soil pit LYS-1 k; ) as resulting from Cr (VI) leaching, then ~73% of the original Cr (III) pool was oxidized to Cr (VI). The calculated decrease in Cr concentration is similar to the decrease actually observed (54 ppm in the 20–80 cm horizon vs. 17 ppm in the 10–20 cm horizon; ) and is consistent with the dominant control of the Cr isotope composition by the generation of Cr (VI) via oxidative weathering.
Although the modelling we conducted explains general isotopic features of the LYS catchment system, the seasonality observed in the isotopic composition of the LYS runoff () suggests more complex Cr behaviour. The significant positive correlation (r = 0.83 at p < 0.01) observed between the δ53Cr values and water temperature () suggests that leaching of isotopically heavy Cr (VI) occurred at higher rates at higher temperatures (and also at low water discharge). The time pattern for the LYS runoff characterized by the decrease in δ53Cr values from +0.29‰ in June 2017 to −0.07‰ in March 2018, and then by a rise to +0.37‰ in July 2018, is consistent with the temperature influence (; ). Therefore, seasonality in Cr isotope composition could be partly explained by preferential mobilization of 53Cr isotope-enriched Cr from soil during warmer periods.
Figure 3. Fluctuation of the major ions and trace solute element concentration, pH and water hardness in runoff from the Lysina (LYS) and Neglinka (NEG) catchments. A star marks data on the Bezymjanny tributary. Sampling was conducted at intervals of about 1 month at either the very beginning or the very end of each consecutive month. The collecting months on the diagram are those immediately preceding the collecting date: for example, if the sampling date was 1 March, the sampling month is considered the previous February on the diagram; if the sampling date was 29 June, the sampling month is considered June on the diagram. Snowmelt periods are marked as the months when actual snowmelt occurred
4.2.2 The role of DOC
The DOC (and particularly dissolved organic acids) not only can be a carrier of hydrologically mobilized Cr (VI), but can also form complexes with metal cations such as Cr (III) which enhances metal solubility (James and Bartlett Citation1983, D’Arcy et al. Citation2016, McClain and Maher Citation2016). The presence of such complexes would simultaneously increase concentrations of dissolved Cr and DOC in water, and shift the δ53Cr of water towards lower values because a soluble part of Cr (III) leached from bedrock would have signatures similar to those of bedrock δ53Cr. A significant positive correlation between the DOC and Cr concentrations was observed for the LYS runoff (r = 0.72 at p < 0.01; ). The correlation was at its strongest (r = 0.83 at p < 0.01) during the warmer months, whereas in winter, during higher streamwater flow, the correlation was less significant (r = 0.71 at p < 0.05). An overall non-significant negative correlation between the DOC concentrations and δ53Cr values (r = −0.34 at p < 0.05; ) was due to the combination of features of winter samples displaying a significant positive correlation (r = 0.93 at p < 0.01) and features of samples collected during warmer periods (no correlation). All this combined suggests that if even the Cr (III)-organic complexes formed in water, their amount was totally overwhelmed by DOC carrying hydrologically mobilized Cr (VI).
4.3 Factors controlling Cr isotope composition in the NEG catchment
Two hydrochemically different water streams are present within the single Neglinka River system: the upstream running through the swamp and forested lowlands, and the downstream running through the environment of the Lake Onega shore (Andronikov et al. Citation2019). Chromium isotope characteristics of the NEG runoff were different in the two parts of the river: δ53Cr values were much higher in the downstream part (+0.72 to +1.73‰) than in the upstream part (+0.16 to +0.42‰) (; ; see Andronikov et al. Citation2019).
Figure 5. Runoff time series of δ53Cr values for the Lysina (LYS) and Neglinka (NEG) catchments. See explanation of sampling periods in (caption)
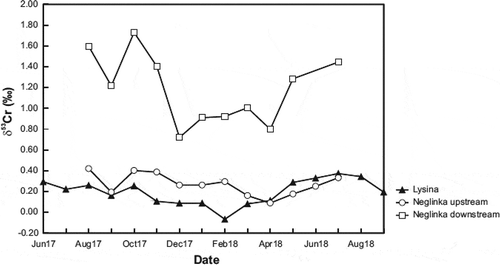
Figure 6. Decrease in δ53Cr plotted against fraction of remaining Cr (III) after oxidation of Cr (III) to Cr (VI) in the Lysina (LYS) soil. See text for details
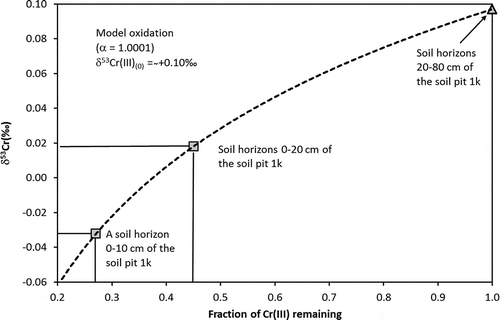
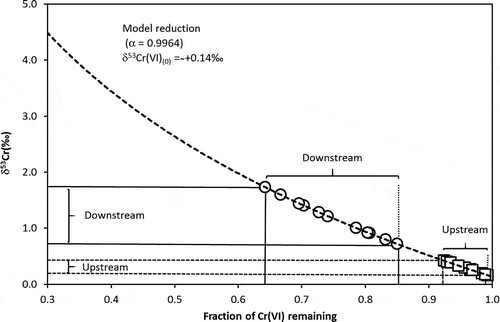
Figure 7. Concomitant increase in δ53Cr plotted against the remaining fraction of Cr (VI) during reduction in the Neglinka (NEG) runoff. The range of δ53Cr values for upstream runoff is shown as empty squares; that for downstream runoff is shown as empty circles. See text for details
4.3.1 Oxidative weathering
The Δ53Cr value can be used to distinguish between Cr isotope composition that resulted from oxidation and that resulting from reduction (D’Arcy et al. Citation2016 and references therein). Generation of Cr (VI) from oxidative weathering of Cr (III) in soil/bedrock results in a small isotope fractionation (Δ53Cr of a few tenths per mil; see our modelling), whereas back-reduction shifts δ53Cr of the residual reactant (Cr (VI)) to higher values by 2.2–5.4‰ (D’Arcy et al. Citation2016 and references therein). Lower δ53Cr values for the NEG upstream with only a moderate fractionation relative to bedrock (Δ53Cr averaging around +0.25 ± 0.20‰; 2σ) are consistent with dominant control of the Cr isotope composition by generation of Cr (VI) from oxidative weathering (Ellis et al. Citation2002, Zink et al. Citation2010, D’Arcy et al. Citation2016).
4.3.2 Back-reduction
Chromium isotope composition of the NEG runoff could have been significantly influenced by back-reduction during riverine transport. Although we cannot exclude simple mixing between the runoff and groundwater (the latter has more reduced characteristics than the stream water) as being responsible for the increase of δ53Cr values of the Neglinka runoff (see Section 4.3.4), we cannot verify this hypothesis at the present stage, and it should be a matter of future study. Hence, the most plausible current explanation for a significant increase of δ53Cr runoff values over the length of the Neglinka River is the Cr (VI) to Cr (III) back-reduction.
Spontaneous partial reduction of aqueous Cr (VI) results in insoluble Cr (III) existing as octahedral complexes such as CrOH2+, Cr(OH)3 and Cr(OH)4− (Goring-Hartford Citation2017). The process would induce fractionation and a significant increase of δ53Cr values of water accompanied by precipitation of insoluble Cr (III), lowering water Cr concentration (D’Arcy et al. Citation2016). Reduction of Cr (VI) to Cr (III) in an aquatic environment is a complex process related to many factors, and not only to the Eh-pH state of water. The presence of reducing agents, such as dissolved ferrous iron, organic substances and biological reducers, is one such factor (Fendorf and Zasoski Citation1992, Fendorf et al. Citation2000, Wielinga et al. Citation2001, Ndung´u et al. Citation2010, Døsing et al. Citation2011). Ferrous iron is an effective reductant in various settings, and may reduce aqueous Cr (VI) even under oxygenated conditions (Fendorf and Li Citation1996, Buerge and Hug Citation1997, Goring-Hartford Citation2017).
In order to numerically evaluate the extent of the suggested natural reduction of the dissolved Cr (VI) in the NEG runoff, we computed expected changes in isotope composition of dissolved Cr (VI) as a function of the progressive reduction to Cr (III). We assumed that the δ53Cr characteristics of Cr (VI) that remains in water (the reactant pool) at any given time during back-reduction can be described by the Rayleigh relation (EquationEquation 2(2)
(2) ). For the back-reduction, δ53Cr and δ53Cr0 represent the compositions of the unreacted dissolved Cr (VI) in runoff at the sampling time and at the stage when the reduction has just started, respectively. The parameter f is the fraction of still-unreacted Cr (VI). The fractionation factor α (EquationEquation 3
(3)
(3) ) is defined by Rprod and Rreact, which are the 53Cr/52Cr ratios of the reaction product (Cr (III)) and the reactant (Cr (VI)), respectively. Since we do not know the isotope composition of the reaction product, we used an α of 0.9964, which is associated with abiotic Cr (VI) reduction by aqueous Fe(II) (Døsing et al. Citation2011). Iron is abundant in the runoff studied (), and its involvement in Cr (VI) reduction is strongly plausible. The α value of <1 reflects a lower 53Cr/52Cr ratio in the Cr (III) produced compared to that in the residual Cr (VI) pool.
Because of some uncertainty in our knowledge of the isotope composition of all reacting agents, we accepted a simplistic scenario in which: (i) the Cr (VI)aqueous - Cr (III)solid was a closed system; (ii) the δ53Cr values of runoff reflected those in a residual Cr (VI) pool; (iii) the dissolved Cr (VI) in the runoff represented the total dissolved Cr (VI) pool in terms of isotopic composition and the extent of the reduction; and (iv) the reduction of Cr (VI) by aqueous Fe(II) was the predominant reduction mechanism. This scenario assumes that biotic reduction did not occur nor was an aquifer with multiple reductant present (under the pH conditions of the NEG runoff, the reduction of Cr (VI) by organic reductants is minimal; Economou-Eliopoulos et al. Citation2014). We also assumed that the geogenic background had an average δ53Cr value of +0.04 ± 0.03‰ (2σ) defined by the composition of bedrock in the catchment (). We additionally assumed that the Δ53Cr (Cr (VI)-Cr (III)) for the NEG catchment was similar to that for the LYS catchment (+0.10‰) providing δ53Cr of the initial unreacted Cr (VI) pool of +0.14‰. The results of our modelling suggest that about 1.0–7.5% of the original Cr (VI) pool was reduced to Cr (III) in the NEG upstream, whereas about 15–35% of the original Cr (VI) pool was reduced in the NEG downstream (). Such numbers are consistent with an overall 10–30% decrease in Cr concentrations from the upstream to the downstream ().
Since the measured flow rate at the Neglinka River mouth was about 1 km h−1 (and obviously lower at the upstream), it would take water at least 15–20 h to travel from the source to the river mouth. Therefore, with mean runoff δ53Cr values of +0.04 ± 0.03‰ (2σ) at the river source and +1.18 ± 0.68‰ (2σ) () at the river mouth, the rate of Cr (VI) reduction would be 0.04–0.05‰ per hour. Such data are very approximate, but are nevertheless comparable with observations by Cadkova and Chrastny (Citation2015). These authors demonstrated that in solutions containing various reducing agents, the δ53Cr values increased by up to 1.0–1.8‰ over a period of 72 h. A significant positive correlation (r = 0.66 at p < 0.05) was observed between the δ53Cr values and water temperature, suggesting that at higher temperatures processes of natural attenuation of the dissolved Cr (VI) to insoluble Cr (III) are faster.
It is also noteworthy that the study by Slukovsky and Bubnova (Citation2013) of concentrations of some trace metals in bottom sediments of the Neglinka River could support our suggestion about aqueous Cr (VI) back-reduction. These authors showed that concentrations of Cr are higher in the Neglinka downstream sediments than in the upstream sediments. Since it was shown that Cr is not the result of industrial pollution in the region (Andronikov et al. Citation2019), we suggest that the elevated Cr amounts in downstream sediments could be due to Cr precipitation from water because of Cr (VI) back-reduction.
4.3.3 The role of DOC
The DOC concentrations in the NEG upstream were twice as high as in the downstream (), likely due to the organic-rich forested environment upstream. A not-significant positive correlation between the DOC and Cr concentrations was observed for the upstream area (r = 0.57 at p < 0.05; ), which was more significant during warm periods (r = 0.77 at p < 0.05). Therefore, warmer seasons were more favourable for interaction between the DOC and Cr. Since no correlation between DOC concentrations and δ53Cr values was observed for the upstream area (), we suggest that either the DOC did not play a significant role in controlling Cr isotope composition in runoff, or the soluble Cr (III)-organic complexes with low δ53Cr signatures and the DOC-carrying hydrologically mobilized Cr (VI) with high δ53Cr signatures existed in the NEG runoff in comparable amounts. A different picture was observed for the NEG downstream. On the one hand, no correlation between the DOC and Cr concentrations was seen, but on the other hand, a not-significant negative correlation between the DOC and δ53Cr values was observed for the downstream (r = −0.54 at p < 0.05; ). This may indicate that although the back-reduction of aqueous Cr (VI) to insoluble Cr (III) shifted δ53Cr towards higher values, the dissolved Cr (III)-organic complexes with low δ53Cr signatures could have slightly reduced otherwise very high δ53Cr values in the downstream.
4.3.4 Possible influence of groundwater discharge
Although the wide range of δ53Cr values for the NEG runoff appears to be mostly due to variable redox conditions in the catchment, groundwater discharge and simple mixing could also influence runoff composition. Since there are no data on Cr isotope composition of various types of groundwater in the catchment, the only way to assess the influence of groundwater discharge would be to consider water collected from the spring-fed Bezymjanny tributary (). The spring water (i.e. groundwater) has similar values to Bezymjanny for EC (220–255 μS cm−1), pH (6.9–7.3), Mg (9.4–16.8 mg L−1), HCO3 (124–148 mg L−1), P (103–106 μg L−1), Cr (0.25–2.80 μg L−1) and Cu (0.25–7.90 μg L−1), and slightly lower concentrations of Na (5.0–6.6 mg L−1), K (1.3–2.2 mg L−1), Ca (19–25 mg L−1) and Fe (5–120 μg L−1). The only significant difference is the lower concentrations of Cl (0.7–1.3 mg L−1) and SO4 (8–20 mg L−1) in the spring water (Borodulina Citation2006, Citation2013). We sampled water from the Bezymjanny tributary only once (early June 2018), and a long-term comprehensive observation of Bezymjanny water composition has not been carried out. However, some scarce information is available for the 1993–2012 period, and it can be compared with our data. In particular, the physico-chemical characteristics of Bezymjanny water (Borodulina Citation2006, Citation2013) are consistent with our data (). Therefore, we suggest that the composition of Bezymjanny water sampled in early June 2018 was not far removed from the average Bezymjanny water compositon and can be used as a proxy for such composition. Although the Bezymjanny runoff displays a similar major ion-based water type to the NEG downstream (), trace element and isotopic compositions of Bezymjanny and NEG downstream waters are different (). The Bezymjanny δ53Cr value (+0.67‰) fell between that for the NEG upstream (+0.18‰) and the NEG downstream (+1.28‰) at the same sampling time. If we assume that the observed characteristics of Bezymjanny water are not outliers, then the addition of Bezymjanny-like water could have influenced the composition of the NEG runoff, shifting its δ53Cr from the lower values typical of the upstream towards higher values. However, no matter how much groundwater with the described isotopic characteristics was added to upstream-like runoff, it would not be enough to shift the runoff δ53Cr values to higher numbers for >1‰. Therefore, the main factor for the increase in δ53Cr values in the NEG downstream runoff would still be back-reduction of Cr (VI) to Cr (III).
5 Conclusions
A study of two catchments underlain by different but monolithologic felsic bedrock revealed the presence of three water types, two of which were identified for the single Neglinka River system. The upstream NEG water changed from Mg-Ca-Cl type in winter and after snowmelt to Mg-Ca-HCO3 type during summer low discharge, while the downstream NEG water was characterized by a general Mg-Ca-Cl-HCO3 type varying from Mg-Ca-Cl≪HCO3 during most of the year to Mg-Ca-Cl = HCO3 after snowmelt and during summer low discharge. The main ions in the NEG runoff may have been derived from dissolution of gypsum, anhydrite, calcite and dolomite. The difference between the upstream and downstream water types is probably due to infiltration of mineralized groundwater for the latter. The third water type was identified for the LYS runoff. LYS water was of the Na-Ca(Ca-Na)-SO4 type, changing from Ca = Na-SO4 type for most of the year to Na > Ca-SO4 type in winter and after snowmelt, and to Ca > Na-SO4 type during summer low discharge. These differences might be due to preferable formation of gypsum along with the weathering of feldspar to kaolinite at higher ambient temperatures. Ion delivery to runoff was more intensive at higher temperatures for both catchments.
Low concentrations of Cr in runoff from both catchments were due to low Cr concentrations in the bedrock and the overall poor solubility of Cr during weathering. Both LYS and NEG runoff contained Cr (VI) as the main dissolved Cr species. Cr (III) oxidation and Cr (VI) back-reduction can be considered the main control for Cr isotopic composition of the runoffs. Numerical modelling suggests that between ~55% and ~73% of the original Cr (III) pool was oxidized to soluble Cr (VI) and removed to the aquatic systems. That resulted in moderately positive δ53Cr values for the LYS runoff (mean: +0.20 ± 0.24‰; 2σ) and for the NEG upstream (mean: +0.27 ± 0.21‰; 2σ). Back-reduction during riverine transport resulted in a unilateral change of Cr isotope characteristics from the NEG upstream to its downstream (δ53Cr = +0.09 to +0.42‰ for the upstream vs. δ53Cr = +0.72 to +1.73‰ for the downstream). Numerical modelling suggests that between ~1.0% and ~7.5% of the original Cr (VI) pool was reduced to Cr (III) in the NEG upstream, whereas in the downstream, this was ~15% to ~35%. Variations in δ53Cr values for LYS and NEG runoff may additionally reflect changes in water sources during the year, influence of the reducing agents and hydrological conditions. Although infiltration of mineralized groundwater could influence the isotopic composition of the NEG downstream runoff, that is a matter for a separate study.
Acknowledgements
The authors are grateful to M. Houskova, P. Janotova, H. Vitkova and V. Zoulkova for help with the chemical analyses. V. Chrastny, F. Oulehle and O. Myska helped with data interpretation. An anonymous reviewer is thanked for critical reading of an earlier version of the manuscript, and for invaluable comments and advice. This study is a contribution to the Reasearch Projects 331600 and 338100, which are a part of the Strategic Research Plan of the Czech Geological Survey (DKRVO/CGS 2018-2022).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andronikov, A.V., et al., 2019. One river, two streams: chemical and Cr isotopic features of the Neglinka River (Karelia, northwest Russia). Hydrological Sciences Journal, 64 (8), 974–982. doi:10.1080/02626667.2019.1617418
- Basu, A. and Johnson, T.M., 2012. Determination of hexavalent chromium reduction using Cr stable isotopes: isotopic fractionation factors for permeable reactive barrier materials. Environmental Science & Technology, 46 (10), 5353–5360. doi:10.1021/es204086y
- Bonnand, P., et al., 2013. The chromium isotopic composition of seawater and marine carbonates. Earth and Planetary Science Letters, 382, 10–20. doi:10.1016/j.epsl.2013.09.001
- Borodulina, G.S., 2006. Groundwater quality. In: N.N. Filatov and T.I. Regerand, ed. Water resources of Republic of Karelia and their use for drinking water supply. Petrozavodsk: Karelian Science Center, Russian Academy of Science, 127–144. (in Russian).
- Borodulina, G.S., 2013. Groundwaters. In: A.V. Litvinenko and T.I. Regerand, eds. Water objects of the city of Petrozavodsk. Petrozavodsk: Karelian Science Center, Russian Academy of Science, 31–42. (in Russian).
- Buerge, I.J. and Hug, S.J., 1997. Kinetics and pH dependence of chromium (VI) reduction by iron(II). Environmental Science & Technology, 32 (14), 2092–2099. doi:10.1021/es970932b
- Bullen, T.D., 2014. Metal stable isotopes in weathering and hydrology. Treatise in Geochemistry, 7, 329–359. Second Edition.
- Cadkova, E. and Chrastny, V., 2015. Isotope evidence of hexavalent chromium stability in ground water samples. Chemosphere, 138, 74–80.
- Chrastny, V., et al., 2011. Method of chromium (Cr) separation and establishing of measurement of its isotopic composition with the multicollector mass-spectrometer MC ICP MS. Czech Geological Sutvey open file report #TACR 010210055, 19. (in Czech).
- D’Arcy, J., et al., 2016. Processes controlling the chromium isotopic composition of river water: constrains from basaltic river catchments. Geochimica et Cosmochimica Acta, 186, 296–315. doi:10.1016/j.gca.2016.04.027
- Døsing, L.N., et al., 2011. Reduction of hexavalent chromium by ferrous iron: a process of chromium isotope fractionation and its relevance to the natural environment. Chemical Geology, 285 (1–4), 157–166. doi:10.1016/j.chemgeo.2011.04.005
- Economou-Eliopoulos, M., Frei, R., and Atsarou, C., 2014. Application of chromium stable isotopes to the evaluation of Cr (VI) contamination in groundwater and rock leachates from central Euboea and the Assopos basin (Greece). Catena, 122, 216–228. doi:10.1016/j.catena.2014.06.013
- Ellis, A.S., Johnson, T.M., and Bullen, T.D., 2002. Cr isotopes and the fate of hexavalent chromium in the environment. Science, 295 (5562), 2060–2062. doi:10.1126/science.1068368
- Farkas, J., et al., 2013. Chromium isotope variations (δ53Cr) in mantle-derived sources and their weathering products: implications for environmental studies and the evolution of δ53Cr in the Earth´s mantle over geologic time. Geochimica et Cosmochimica Acta, 123, 74–92. doi:10.1016/j.gca.2013.08.016
- Fendorf, S., Wielinga, B.W., and Hansel, C.M., 2000. Chromium transformation in natural environments: the role of biological and abiological processes in chromium (VI) reduction. International Geology Review, 42 (8), 691–701. doi:10.1080/00206810009465107
- Fendorf, S.E., 1995. Surface reactions of chromium in soils and waters. Geoderma, 67 (1–2), 55–71. doi:10.1016/0016-7061(94)00062-F
- Fendorf, S.E. and Li, G., 1996. Kinetics of chromate reduction by ferrous iron. Environmental Science & Technology, 30 (5), 1614–1617. doi:10.1021/es950618m
- Fendorf, S.E. and Zasoski, R.J., 1992. Chromium (III) oxidation by delta MnO2. 1. Characterization. Environmental Science & Technology, 26 (1), 79–85. doi:10.1021/es00025a006
- Frei, R., Poire´, D., and Frei, K.M., 2014. Weathering on land and transport of chromium to the ocean in a subtropical region (Misiones, NM Argentina): a chromium stable isotope perspective. Chemical Geology, 381, 110–124. doi:10.1016/j.chemgeo.2014.05.015
- Frei, R. and Polat, A., 2013. Chromium isotope fractionation during oxidation weathering – implications from the study of a palaeoproterozoic (ca. 1.9 Ga) paleosol, Schreiber Beach, Ontario, Canada. Precambrian Research, 224, 434–453. doi:10.1016/j.precamres.2012 October 008
- Goring-Hartford, H.J., 2017. Chromium isotope behavior in natural waters. Unpublished Ph.D thesis. University of Southampton, 187.
- Guildford, J.P., 1956. Fundamental statistics in psychology and education. New York, NY USA: McGrow-Hill, 565.
- Hayes, H.R., et al., 2020. Controls on granitic weathering fronts in contrasting climates. Chemical Geology, 535 (119450), 1–19. doi:10.1016/j.chemgeo.2019.119450
- Hruska, J. and Kram, P., 2003. Modelling long-term changes in stream water and soil chemistry in catchments with contrasting vulnerability to acidification (Lysina and Pluhuv Bor, Czech Republic). Hydrology and Earth System Science, 7, 525–539. doi:10.5194/hess-7-525-2003
- Izbicki, J.A., et al., 2008. Chromium, chromium isotopes and selected trace elements, western Mojave Desert, USA. Applied Geochemistry, 23 (5), 1325–1352. doi:10.1016/j.apgeochem2007.11.015
- Izbicki, J.A., et al., 2012. δ53Cr isotopic composition of native and contaminated groundwater, Mojave Desert, USA. Applied Geochemistry, 27 (4), 541–853. doi:10.1016/j.apgeochem.2011.12.019
- Izbicki, J.A., et al., 2015. Cr (VI) occurrence and geochemistry in water from public-supply wells in California. Applied Geochemistry, 63, 203–217. doi:10.1016/j.apgeochem.2015.08.007
- James, B.R. and Bartlett, R.J., 1983. Behavior of chromium in soils. VI. Interactions between oxidation-reduction and organic complexation. Journal of Environmental Quality, 12 (2), 173–176. doi:10.2134/jeq1983.00472425001200020004x
- Johnson, T.M., 2011. Stable isotopes of Cr and Se as tracers of redox processes in earth surface environments. In: M. Baskaran, ed. Handbook of environmental isotope geochemistry. Berlin: Springer-Verlag, 155–175.
- Kopacek, J., et al., 2016. Effect of industrial dust on precipitation chemistry in the Czech Republic (Central Europe) from 1850 to 2013. Water Resources, 103, 30–37.
- Kram, P., 2019. Water balance and hydrologic patterns of the Lysina catchment, Slavkov Forest, 1990–2018. Geoscience Research Reports, 52, 45–52. (in Czech with English abstract)
- Kram, P., et al., 2017. Hydrochemical fluxes and bedrock chemistry in three contrasting catchments underlain by felsic, mafic and ultramafic rocks. Procedia Earth and Planetary Science, 17, 538–541. doi:10.1016/j.proeps.2016.12.136
- Kram, P., Hruska, J., and Shanley, J.B., 2012. Streamwater chemistry in three contrasting monolithologic Czech catchments. Applied Geochemistry, 27 (9), 1854–1863. doi:10.1016/j.apgeochem.2012.02.020
- Kram, P., et al., 2009. Geoecology of a forest watershed underlain by serpentine in central Europe. Northeastern Naturalist, 16 (sp5), 309–328. doi:10.1656/045.016.0523
- Kura, N.U., et al., 2013. Evaluation of factors influencing the groundwater chemistry in a small tropical island of Malaysia. International Journal of Environmental Research and Public Health, 10 (5), 1861–1881. doi:10.3390/ijerph10051861
- Li, C.-F., et al., 2017. A low-blank two-column chromatography separation strategy based on a KMnO4 oxidizing reagent for Cr isotope determination in micro-silicate samples by thermal ionization mass spectrometry. Journal of Analytical Atomic Spectrometry, 32 (10), 1938–1945. doi:10.1039/C7JA00225D
- McClain, C.N. and Maher, K., 2016. Chromium fluxes and speciation in ultramafic catchments and global rivers. Chemical Geology, 426, 135–157. doi:10.1016/j.chemgeo.2016.01.021
- Mikhailov, V.A., Lodygin, A.N., and Kushnerenko, V.K., 2014. Features of geological structure and metal content of Shuja-Petrozavodsk area (Republic of Karelia). Regional Geology and Metallogeny, 59, 61–69. (in Russian)
- Ndung´u, K., et al., 2010. Chromium oxidation by manganese (hydr)oxides in a Califorina aquifer. Applied Geochemistry, 25 (3), 377–381. doi:10.1016/j.apgeochem.2009.12.004
- Novak, M., et al., 2014. Common occurrence of a positive δ53 Cr shift in central European waters contaminated by geogenic/industrial chromium relative to source values. Environmental Science & Technology, 48 (11), 6089–6096. doi:10.1021/es405615h
- Novak, M., et al., 2017a. The fate of Cr (VI) in contaminated aquifers 65 years after the first spillage of plating solutions: A δ53Cr study at four central European sites. Catena, 158, 371–380. doi:10.1016/j.catena.2017.07.004
- Novak, M., et al., 2017b. Chromium isotope fractionations resulting from electroplating, chromating and anodizing: implication for groundwater pollution studies. Applied Geochemistry, 80, 134–142. doi:10.1016/j.apgeochem.2017.03.009
- Novak, M., et al., 2017c. Temporal changes in Cr fluxes and δ53Cr values in runoff from a small serpentinite catchment (Slavkov Forest, Czech Republic). Chemical Geology, 472, 22–30. doi:10.1016/j.chemgeo.2017.09.023
- Novikov, S.G., 2014. Ecologic estimate of pollution by heavy metals of soils in urban territories according to categories of land management (on the example of the city of Petrozavodsk). A PhD thesis. Petrozavodsk State University, Petrozavodsk, 228. (in Russian).
- Oze, C., Bird, D.K., and Fendorf, S., 2007. Genesis of hexavalent chromium from natural sources in soil and groundwater. Proceedings of the National Academy of Science, 104 (16), 6544–6549. doi:10.1073/pnas.0701085104
- Paulukat, C., et al., 2015. Oxidative release of chromium from Archean ultramafic rocks, its transport and environmental impact: a Cr isotope perspective on the Sukinda valley ore district (Orissa, India). Applied Geochemistry, 59, 125–138. doi:10.1016/j.apgeochem.2015.04.016
- Puzon, G.J., et al., 2008. Mobility and recalcitrance of organo-chromium (III) complexes. Chemosphere, 70 (11), 2054–2059. doi:10.1016/j.chemosphere.2007.09.010
- Qin, L. and Wang, X., 2017. Chromium isotope geochemistry. Reviews in Mineralogy and Geochemistry, 82 (1), 379–414. doi:10.2138/rmg.2017.82.10
- Richard, F.C. and Bourg, A.C.M., 1991. Aqueous geochemistry of chromium: a review. Water Resources, 25, 807–816.
- Schauble, E., Rossman, G.R., and Taylor, H.P., 2004. Theoretical estimates of equilibrium chromium isotope fractionations. Chemical Geology, 205 (1–2), 99–114. doi:10.1016/j.chemgeo.2003.12.015
- Schiavon, N., 2007. Kaolinisation of granite in an urban environment. Environmental Geology, 52 (2), 399–407. doi:10.1007/s00254-006-0473-0
- Slukovsky, Z.I. and Bubnova, T.P. 2013. Fraction <0.1 mm chemical composition of Neglinka River sediments – contamination indicator of urban stream. Scientific Records of Petrozavodsk State University 4, 50–56 (in Russian with English Summary).
- The State Water Cadaster of the USSR. 1986. Perennial data on regime and resources of surface waters from the landmass. V. 1. RSFSR. Issue 5. Basins of rivers of the Baltic Sea, and Lakes Ladoga and Onega (Gosudarstvenny vodny cadaster. Mnogoletnie dannye o rezhime i resursakh poverkhnostnykh vod sushi. RSFSR. Basseiny rek Baltiiskogo morja, Onezhskogo i Ladozhskogo ozer). Leningrad: Hydrometeoizdat, 100. (in Russian).
- Water objects of the city of Petrozavodsk, 2013. A.V. Litvinenko and T.I. Regerand, eds. Petrozavodsk: Karelian Scientific Center RAS, 109.
- Wielinga, B., et al., 2001. Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environmental Science & Technology, 35 (3), 522–527. doi:10.1021/es001457b
- Wu, W., et al., 2017. Chromium isotope systematics in the Connecticut River. Chemical Geology, 456, 98–111. doi:10.1016/j.chemgeo.2017.03.009
- Yu, L., et al., 2018. Groundwater impacts on surface water quality and nutrient loads in lowland polder catchments: monitoring the greater Amsterdam area. Hydrology and Earth System Sciences, 22 (1), 487–508. doi:10.5194/hess-22-487-2018
- Zhu, J.-M., et al., 2018. An improved method of Cr purification for high precision measurement of Cr isotopes by double spike MC-ICP-MS. Journal of Analytical Atomic Spectrometry, 33 (5), 809–821. doi:10.1039/C8JA00033F
- Zink, S., Schoenberg, R., and Staubwasser, M., 2010. Isotopic fractionation and reaction kinetics between Cr (III) and Cr (VI) in aqueous media. Geochimica et Cosmochimica Acta, 74 (20), 5729–5745. doi:10.1016/j.gca.2010.07.015
- Zobkov, M.B. and Zobkova, M.V., 2015. A device for determination of organic carbon in water with photochemical persulfate oxidation in the system of continuous gas flow and FTIR spectrometric detection. Factory Laboratory. Diagnostics of Materials, 81, 10–15. (in Russian)