 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study examined whether eccentric exercise increases nuclear factor erythroid 2-related factor 2 (NRF2) activity in peripheral blood mononuclear cells (PBMCs). Twenty-six recreationally active males (mean [SD]; age 25 [5] y, height 178.4 [6.9] cm, body mass 77.6 [7.4] kg) were allocated to either an exercise (n = 16) or non-exercise (resting) control (n = 10) group. Eccentric exercise involved performing 100 drop jumps from a 0.6 m box. Blood was collected pre-, immediately post- and 1 h post-exercise or rest. NRF2/antioxidant response element (ARE) binding was measured in PBMCs; glutathione reductase (GR) and peroxidase (GPX) were measured in plasma. NRF2/ARE binding was greater immediately post- and 1 h post in the exercise vs. rest group (p < 0.05 for all). NRF2/ARE binding was higher at 1 h post vs. post-exercise (fold change from baseline: [post] 1.31 ± 1.31, [1 h] 2.79 ± 3.30; p = 0.003). GR did not increase in response to exercise or rest (p = 0.377) but was higher at all time-points in the exercise vs resting group (p = 0.023). Exercise or rest had no impact on GPX activity (p > 0.05 for all). Eccentric exercise increases NRF2/ARE binding in PBMCs compared to rest.
Pre-registration number
osf.io/kz37g
Introduction
Exercise transiently elevates the production of reactive oxygen and nitrogen species (RONS) from various subcellular sources, most notably superoxide (O2•−) from NADPH oxidases (Jackson et al., Citation2016; Michaelson et al., Citation2010; Sakellariou et al., Citation2013). It is well established that exposure to exercise-induced RONS stimulates beneficial adaptive responses, such as a strengthened antioxidant defence network (Abruzzo et al., Citation2013; Steinbacher & Eckl, Citation2015). Indeed, exercise has shown to increase antioxidant enzyme content such as glutathione peroxidase (GPX), catalase and superoxide dismutase, which maintain redox balance and prevent oxidative damage to cells (Ji et al., Citation2006; Parise et al., Citation2005; Tofas et al., Citation2021).
This antioxidant defence network is chiefly regulated by the transcription factor nuclear erythroid 2-related factor 2 (NRF2) (Vomund et al., Citation2017). During unstressed conditions, NRF2 is sequestered within the cytosol by its negative regulator, kelch-like ECH-associated protein 1 (KEAP1), through continual ubiquitination (Kobayashi & Yamamoto, Citation2006). Under conditions of oxidative stress, the cysteine residues of KEAP1 are oxidised, inducing a conformational change to its structure that inhibits NRF2 ubiquitination (Dinkova-Kostova et al., Citation2017). Newly synthesized NRF2 can then translocate to the nucleus, where, following heterodimerization with small musculoaponeurotic fibrosarcoma proteins, it can activate antioxidant response element (ARE) DNA sequences, enabling transcription of cytoprotective genes involved in antioxidative, anti-inflammatory and detoxifying responses (Harvey et al., Citation2009). As these gene targets could bolster cellular resistance to pathologies associated with aberrant inflammation and oxidative distress, there is a growing interest in strategies that could activate NRF2 (Cuadrado et al., Citation2019; Dinkova-Kostova & Kazantsev, Citation2017; Robledinos-Antón et al., Citation2019).
In humans, changes in NRF2 activity have been reported to occur following aerobic exercise (Ballmann et al., Citation2014; Done et al., Citation2016, Citation2017; Ostrom & Traustadóttir, Citation2020; Scott et al., Citation2015). Such responses have predominantly been measured in peripheral blood mononuclear cells (PBMCs), an easily accessible surrogate tissue that may mimic exercise-induced changes in transcriptional and redox-specific responses in skeletal muscle (García-López et al., Citation2007; Spanidis et al., Citation2018). Indeed, Done et al. (Citation2016) reported that cycling at 70% O2max for 30 min increased NRF2 whole-cell protein content from PBMCs in younger (~23 years; peak at 30 min post) and older (~63 years; peak at 10 min post) males, with nuclear-bound NRF2 accumulation also increasing in the younger males (peak at 1 h post) but attenuated in the older group. More recently, Ostrom and Traustadóttir (Citation2020) corroborated these findings, reporting increased NRF2 nuclear localization from PBMCs in young males (peak at 30 min post) and females (peak at 10 min post) after the same acute cycling intervention. These initial human studies therefore suggest that exercise may increase whole-cell and nuclear NRF2 content in PBMCs within 1 h of completion.
Nonetheless, these studies all utilised aerobic exercise, and as such, there is a lack of research exploring the effects of less metabolically demanding exercise, such as eccentric exercise, on NRF2 activity. Eccentric muscle contractions, which typically impose low metabolic but high mechanical stress through active lengthening, are frequently used to promote muscle hypertrophy, strength and neural adaptations in healthy and vulnerable populations (Harris-Love et al., Citation2021). Although eccentric muscle contractions do not generate as much RONS as aerobic exercise, at least immediately post-exercise (Kamandulis et al., Citation2017; Nikolaidis et al., Citation2008), they do activate other signalling proteins such as extracellular signal-regulated kinases (ERK) (Franchi et al., Citation2014) and mammalian target of rapamycin complex 1 (mTORC1) (Ashida et al., Citation2018; Burry et al., Citation2007; Eliasson et al., Citation2006) in the immediate hours post-exercise (30 min for ERK; 1 h for mTORC1). These signalling proteins are reported to activate NRF2 non-canonically, independent of RONS (Ichimura et al., Citation2013; Zipper & Mulcahy, Citation2000), and thus, it may be possible that eccentric exercise activates NRF2 through these pathways.
We recently examined the effects of eccentric exercise alone and in combination with green tea on NRF2 and found no effect of eccentric exercise on NRF2/ARE binding in PBMCs after 24 h (Thorley et al., Citation2023). However, this study did not employ a resting control group to compare to the exercise group, meaning we could not rule out if circadian oscillations in NRF2 activity, purportedly controlled by diurnal alterations in the circadian transcription factors brain and muscle ARNT-Like 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK), masked any exercise-induced changes (Pekovic-Vaughan et al., Citation2014; Sun et al., Citation2021). Thus, the purpose of this present investigation was to expand upon these findings and examine whether a bout of mechanically demanding eccentric exercise, with a low metabolic load, can augment NRF2/ARE binding in PBMCs. A secondary aim was to assess whether the exercise bout altered the activity of NRF2 target enzymes glutathione reductase (GR) and GPX. It was hypothesized that eccentric exercise would increase NRF2 and target enzyme activity compared to a non-exercise resting group.
Materials and methods
Participants
Twenty-six male participants (age: 25 ± 5 years, height: 178.4 ± 6.9 cm, body mass: 77.6 ± 7.4 kg) volunteered for this study. Participants completed a health screening survey to determine their eligibility. Individuals who reported that they had a history of cardiovascular or metabolic disease, musculoskeletal injury (currently or in the past 6 months), a food allergy that was relevant to the investigation, or were taking medication (currently or in the past 6 months), were excluded from participating. Trained individuals, defined as those completing >4 resistance training sessions per week for the previous 6 months, were excluded from the study on the premise that due to their familiarity with similar exercise stressors, they would be less responsive to the exercise protocol. Per the inclusion criteria, participants were required to be recreationally active (tier 1) as defined by a participant classification framework (McKay et al., Citation2021). This meant participants were required to be completing at least 150 to 300 min of moderate-intensity activity or 75–150 min of vigorous-intensity activity per week, plus 2 or more muscle-strengthening activities per week.
For the duration of the study, participants refrained from using any putative recovery interventions or consuming any dietary supplements. In addition, all planned exercise (i.e., resistance training, aerobic exercise, participation in sport) was restricted in the 48 h prior to the second visit. These restrictions were enforced since they may activate NRF2 independent to the exercise under investigation. Participants provided written informed consent and all procedures conformed to the guidance presented by the Declaration of Helsinki.
Experimental design
This study employed a single blind (exercise vs. non-exercise control) between-subjects design; ethical approval was granted by Loughborough University Research Committee, Human Participants Sub-Committee. Some of the data presented in this study forms part of a larger investigation examining the effects of a nutritional intervention on NRF2 activation. As detailed in our pre-registration for that research (osf.io/kz37g), the exercise vs. non-exercise control data presented here were a secondary exploratory aim. We aimed to recruit ≥ n = 11 per group, as per the sample size calculation in our pre-registration, but data collection was affected by the COVID-19 pandemic. A sensitivity power analysis (80% power, α = 0.05) performed with GPower (3.1.9.2, Dusseldorf, Germany), calculated that with our final sample size for our primary outcome (n = 24; NRF2 binding), the smallest effect size we could reliably detect for a within-between interaction was a partial eta squared (ηp2) of 0.189.
To support recruitment and conceal the main aim of the study, all participants were provided with 6 placebo capsules containing 500 mg of inulin (Blackburn Distributions, Burnley, UK). Participants were told to consume 1 capsule per day for 6 consecutive days (5 days prior to second visit and during second visit) and were informed they were taking part in a study investigating the cellular responses to nutritional supplementation at rest or following exercise. Participants were then unblinded upon study completion.
Experimental trials
All participants attended the laboratory twice. First, for a familiarization session, in which participants height (cm), body mass (kg) and maximal counter movement jump (CMJ) height (cm) were measured. The second visit was performed within 1 month of the first visit and testing started at 09:00. For this visit, participants arrived after an overnight fast lasting 8 to 10 h where a blood sample was collected following a 5 min seated rest. They then consumed a final dose of the placebo supplement, continued by a seated rest lasting 1 h. 30 min into the rest period, participants consumed a cereal bar (Nature Valley Honey and Oat cereal bar, 42 g, General Mills International, Sárl, Switzerland). Following 1 h of rest, participants exercised or rested after which blood samples were collected immediately post- and 1 h post. Water consumption was allowed ad libitum throughout the resting periods and during exercise.
Eccentric exercise protocol
Participants in the exercise group performed an eccentric exercise protocol that consisted of 100 drop jumps with 20s rest intervals between each jump and 2 min rest between every 20 jumps. The extended rest periods between each jump served to isolate the mechanical demands of eccentric contractions and minimise metabolic stress, as previously described (Kamandulis et al., Citation2021; Skurvydas et al., Citation2016). Participants dropped from a 0.6 m box with their arms constrained of movement to prevent arm swing. Upon landing with two feet on a contact jump mat (JumpMat™, FSL Scoreboards, Cookstown, Northern Ireland), participants immediately jumped vertically with a maximal rebound effort. Knee angle in the deceleration phase and general technique were visually monitored by a researcher who provided verbal guidance if form began to deteriorate. Jump height was instantaneously measured through the contact jump mat. Maximal effort was regularly assessed by comparing each recorded jump height to their maximal CMJ height recorded at familiarization. Prior to beginning exercise, participants were instructed to reach within 20% of their maximal CMJ height throughout the exercise protocol; thus, if participants began to deviate away from a 20% difference, verbal encouragement was given. Participants in the non-exercise group remained seated in the lab for 35 min, the average completion time of the exercise protocol.
Dietary restrictions
Participants recorded their dietary intake for 48 h prior to the second visit using a weighed food diary. Participants were asked to refrain from consuming food and drink high in (poly)phenols, as these foods may increase NRF2 activity independent of exercise (Nabavi et al., Citation2016); a list of restricted foodstuffs was provided to the participants during familiarization. Energy, carbohydrate, fat, protein, Omega-3, vitamin C, D and E intakes were analysed using online dietary analysis software (Nutritics Education v5.81, Nutritics, Dublin, Ireland).
Sample processing and analysis
Blood sampling and processing
At all 3 time points (pre-, post-, 1 h post-intervention), 18 mL of blood was collected from an antecubital vein into vacutainers treated with ethylenediaminetetraacetic acid (EDTA). EDTA tubes were immediately centrifuged for plasma separation (1500 × g, 4°C, 10 min). Plasma was then pipetted into cryovials and stored at −80°C until assayed. For the collection of PBMCs, 10 mL of EDTA blood was diluted at a 1:1 ratio with 1X phosphate buffer saline (PBS) (ThermoFisher Scientific, Massachusetts, United States) then dispensed upon 15 mL Ficoll® paque PLUS (Merck, Darmstadt, Germany) and centrifuged with the brakes off (400 × g, 20°C, 35 min) to isolate PBMCs. The buffy coat interphase containing PBMCs was harvested and washed twice with PBS at 300 × g for 10 min at 20°C. Once washed, the supernatant was discarded, and the pellet was resuspended in RPMI 1640 Complete Medium (Merck, Darmstadt, Germany). PBMCs were aliquoted at 9 × 106 cells/mL in cryopreservation media containing 50% RPMI 1640, 40% FBS and 10% dimethyl sulphoxide (Merck, Darmstadt, Germany) and frozen at a rate of −1°C/min to −80°C using a Mr. Frosty™ freezing container (ThermoFisher Scientific, Massachusetts, United States).
Nuclear fractionation and NRF2/ARE binding
Nuclear proteins were fractionated from PBMCs using a commercial nuclear extraction kit (Nuclear extraction kit, Cat No. 40010, Active Motif, Waterloo, Belgium) according to the manufacturer’s instructions. Protein content of nuclear fractions was measured in duplicate at multiple dilutions using a commercial BSA assay (Prostain™ Protein Quantification Kit, Cat No. 15001, Active Motif, Waterloo, Belgium). NRF2/ARE binding was measured using a commercially available human NRF2 activity assay (Cat. No. TFEH-NRF2-1, RayBiotech, Georgia, United States) according to the manufacturer’s instructions. Nuclear-bound protein was added to a 96-well plate containing immobilized oligonucleotides possessing ARE consensus binding sites (5′-GTCACAGTACTCAGCAGAATCTG-3′) and incubated overnight at 4°C with gentle agitation. Following colorimetric development, absorbance was read at 450 nm on a Varioskan™ LUX multimode microplate reader (ThermoFisher Scientific, Loughborough, UK).
NRF2 target antioxidant activity
The enzymatic activity of NRF2 targets GR and GPX was measured in plasma using commercially available assays (GR: Cat No. 703202; GPX: Cat No. 703102, Cayman Chemical, Michigan, USA) according to the manufacturer’s instructions. One unit of GR and GPX activity is defined as the number of enzymes causing the formation of 1 nmol of NAPDH to NADP+ per min (nmol/min/ml). All samples were run in duplicate, and the intra-assay CV for GR and GPX were 6.5% and 7.5%, respectively.
Statistical analysis
Data was analysed using IBM SPSS Statistics 27 for Windows (Surrey, UK) and displayed as mean ± SD unless stated otherwise. Statistical significance was set at p < 0.05 prior to the analysis. Graphs were produced using GraphPad Prism (v9.4.1, Boston, USA). Normal distribution of data was assessed by inspecting histograms, skewness and kurtosis and the Shapiro–Wilk test. NRF2/ARE binding and GR activity data were skewed, and therefore, data were log10 transformed prior to analysis, which successfully reduced skewness. To make it easier to interpret the data and compare the results to previous studies with these measures, we have presented values prior to log transformation in figures. A mixed (2 × 3) analysis of variance (ANOVA) was used to assess group (exercise vs. rest), time (pre-, post-, 1 h post-intervention) and interaction effects to NRF2/ARE binding and NRF2 gene target activity data. NRF2/ARE binding data were analysed and presented as fold change from pre-intervention values due to minor differences in protein concentration between participants. Significant main or interaction effects were further explored using post-hoc tests with Bonferroni corrections to locate differences. Independent samples t-tests were performed to compare group differences in physical characteristics and energy intake data. Where data were missing at random for one time point (<5% of total data), expectation-maximization approach was used to calculate maximal likelihood estimates (Bennett, Citation2001). If multiple data points (≥75%) were missing for one participant, they were excluded from analysis. If sphericity was violated, as indicated using Mauchly’s test (p < 0.05), Greenhouse–Geisser corrections were used. For ANOVA analyses, effect sizes were calculated with partial eta squared (ηp2: small: 0.01, medium: 0.05, large 0.14 (Cohen, Citation1988)); for post hoc interactions, effect sizes were calculated with hedges g (g: small: 0.2, medium: 0.5, large: 0.8 (Cohen, Citation1988)).
Results
Participant’s physical characteristics and 2 d average energy intake are presented in . No significant group differences were found for any of these variables (p > 0.05). Examination of food diaries suggested that all participants avoided foodstuffs high in (poly)phenols as instructed. All participants successfully completed 100 drop jumps, with every participant achieving a mean exercise CMJ height ≤20% of their maximal height, indicating that near-maximal intensity was achieved by all. No group differences in maximal CMJ height were observed ().
Table 1. Group comparison of participants’ physical characteristics and 2 d average energy intake.
Table 2. Group comparison of jump characteristics during exercise.
NRF2/ARE binding in PBMCs
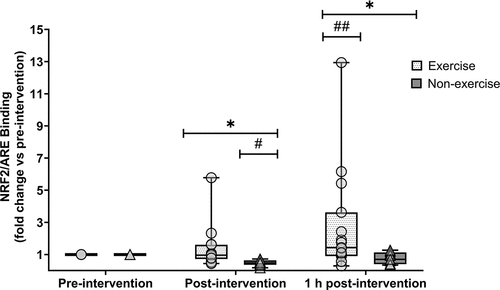
One participant’s data from the exercise group was excluded from analysis due to technical difficulties, and one participant’s data from the resting group was excluded due to issues with blood sampling; thus, n = 24 were analysed for NRF2/ARE binding. There was a group (p = 0.009, ηp2 = 0.22), time (p = 0.008, ηp2 = 0.28) and group × time interaction effect (p = 0.013, ηp2 = 0.21) for NRF2/ARE binding (). NRF2/ARE binding was higher immediately post- (1.33 ± 1.33 vs. 0.48 ± 0.18; p = 0.003) and 1 h post-exercise (2.77 ± 3.31 vs. 0.79 ± 0.36; p = 0.027) compared to the non-exercise group. In the exercise group, NRF2/ARE binding did not increase immediately post-exercise (p = 1.000) but did elevate from post-exercise to 1 h post-exercise (p = 0.008). The increase at 1 h post-exercise was not statistically significant compared to pre-exercise values (p = 0.089), but the effect size was medium (g = 0.76). In the resting group, NRF2/ARE binding was reduced immediately post rest (p = <0.001) and was no different to pre-intervention values after 1 h (p = 0.116).
Figure 1. Fold change (vs pre-intervention) of nuclear factor erythroid 2-related factor 2 (NRF2) binding to antioxidant response element (ARE) oligonucleotides at post- and 1 h post-intervention. Symbols represent individual values. Whiskers correspond to lowest and highest values. Horizontal line within-box define median values. Exercise group (circles, n = 15); non-exercise group (triangles, n = 9). * = significantly different between groups. # = significantly lower than resting values. ## = significantly higher than post-exercise values. Statistical significance set at p < 0.05.
Enzymatic activity of NRF2 gene targets
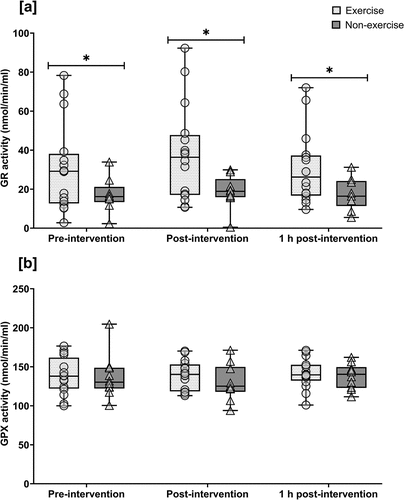
For GR activity analysis, one participant’s data from the resting group was excluded as all samples were below the assay sensitivity limit, thus n = 25 were analysed in total. GR activity displayed no time (p = 0.734, ηp2 = 0.01) or interaction effect (p = 0.542, ηp2 = 0.02); however, GR was higher across all time-points in the exercise vs resting group (group effect: p = 0.023, ηp2 = 0.20) (). GPX activity remained unchanged, displaying no time (p = 0.494, ηp2 = 0.03), group (p = 0.481, ηp2 = 0.02), or time × group (p = 0.687, ηp2 = 0.02) effect ().
Figure 2. Enzymatic activity of NRF2 target genes (a) glutathione reductase (GR) and (b) glutathione peroxidase (GPX) at pre-, post- and 1 h post-intervention. Symbols represent individual values. Whiskers correspond to lowest and highest values. Horizontal line within-box define median values. Exercise group (circles, n = 16); non-exercise group (triangles, n = 9). * = significantly different between groups. Statistical significance set at p < 0.05.
Discussion
This study tested whether mechanically demanding eccentric exercise with low metabolic stress can increase NRF2 activity and target redox enzyme activity compared to resting conditions. We found that NRF2/ARE binding in PBMCs was greater immediately post- and 1 h post-exercise compared to resting conditions, but there were no differences in GPX and GR activity. This is the first study to report that eccentric exercise increases NRF2/ARE binding in human PBMCs.
The changes in NRF2 activity are consistent with previous studies that found aerobic exercise increased nuclear accumulation of NRF2 in PBMCs ≤1 h post-exercise (Done et al., Citation2016, Citation2017; Ostrom & Traustadóttir, Citation2020). One of the main mechanisms by which aerobic exercise was thought to activate NRF2 in these studies was through a shift in cellular redox balance due to elevated RONS. Indeed, an increase in intracellular RONS by exercise has been reported to coincide with elevated NRF2 activation (Abruzzo et al., Citation2013; Muthusamy et al., Citation2012). In the present study, we did not measure RONS directly to assess whether they were associated with changes in NRF2 activity; however, there are several lines of evidence to suggest RONS may not have played a major role in augmenting NRF2 in the present study. Firstly, the lack of change in GPX and GR activity between the exercise and resting groups suggests that RONS generation was minimal, at least in circulation. Secondly, in our previous study using the same exercise protocol, 8-hydroxy-2’-deoxyguanosine, a marker for oxidative DNA damage, was not increased in the 24 h following (Thorley et al., Citation2023). Finally, using similar exercise (100 drop jumps from 0.5 m, 20 s rest), Kamandulis et al. (Citation2017) reported no change to markers of oxidative damage (protein carbonyl and malondialdehyde content in vastus lateralis muscle) after 24 h. Collectively, these data suggest that the eccentric exercise performed in the present study was not sufficiently stressful to elevate RONS production, at least ≤1 h post-exercise in the circulation; thus, the increase in NRF2 activity was possibly unrelated to exercise-induced RONS production and/or shifts in intracellular redox balance. Rather, the recently described non-canonical mechanisms of NRF2 activation may have played a larger role.
Several non-canonical mechanisms of NRF2 activation have been described (Silva-Islas & Maldonado, Citation2018). Of relevance to the present study, in vitro and rodent studies have shown that NRF2 is activated following phosphorylation by ERK (Chen et al., Citation2017; Yang et al., Citation2019, Citation2011). Given that eccentric exercise activates mitogen activated protein kinases such as ERK1/2 after 30 min in muscle (Franchi et al., Citation2014), activation of this signalling pathway could at least partly explain the increased NRF2 activation in the present study. Another signalling pathway associated with eccentric exercise and NRF2 activation is mTORC1. Indeed, mTORC1 signalling is greater in skeletal muscle 1–6 h following eccentric versus concentric contractions (Burry et al., Citation2007; Eliasson et al., Citation2006; Rahbek et al., Citation2014) and can phosphorylate the autophagic cargo adaptor protein p62, which, in turn, interacts with KEAP1 to stabilise NRF2 (Ichimura et al., Citation2013; Woo et al., Citation2019). Collectively, this data supports the supposition that, perhaps due to the low metabolic load and therefore limited RONS generation immediately post-exercise, changes in NRF2 activity following eccentric exercise are driven more by non-canonical mechanisms. We acknowledge that this is speculative as we did not measure these signalling pathways in the present study. Thus, further research is needed to delineate the interaction between NRF2 and these protein signalling pathways in PBMCs and other tissues following eccentric exercise. Changes in NRF2 binding reported in this study should also be interpreted with caution due to the log10 transformations performed to reduce skewness per recommendations by Field (Citation2016), and thus, we acknowledge this as a limitation to our work.
Exercise did not increase GPX or GR activity in the present study. GR levels were higher in the exercise vs. rest group at all time-points, including at baseline, but it is unclear why. All participants were randomised so the groups were well matched in their physical characteristics, so the group differences may not be related to a physiological difference but rather to random chance, which can occur in smaller trials like ours (Elkins, Citation2015). Irrespective of the precise reasons, this could have limited our ability to detect group differences. There are limitations with our analysis that could have also impeded our ability to detect exercise-induced changes in these enzymes. Firstly, we measured GR and GPX in plasma, thus only capturing concentrations that have leaked from other cell compartments (e.g., cytoplasm, endoplasmic reticulum and mitochondria) (Montero et al., Citation2013). We aimed to measure these enzymes in the cytosolic fraction of PBMCs, which would better reflect changes in NRF2 activity, but were unable to as the detergent used during the fractionation process reacted with the reagents used for enzyme assays. Nonetheless, glutathione-specific enzymes can be found in the extracellular space, albeit at relatively low concentrations (Banerjee, Citation2012), so changes in these enzymes are still widely reported and thought to have biological relevance (de Oliveira et al., Citation2012; Onur et al., Citation2011; Wiecek et al., Citation2018). Secondly, limiting our analysis to 1 h post-exercise means we may have missed any later exercise-induced changes in GR and GPX activity. Increased gene expression following transcriptional activity does not always equate to immediate protein synthesis; rather, the presence of proteins or enzyme activity may take several hours, days, or even weeks to appear after elevated mRNA expression (Perry et al., Citation2010). Thus, we cannot rule out that plasma levels of these enzymes increased ≥1 h post-exercise, following peak NRF2 activation. We also acknowledge that because markers of oxidative damage can peak several days after eccentric exercise (Nikolaidis et al., Citation2007), restricting our analyses to 1 h post-exercise means we may have missed later increases in NRF2 activity.
We and others (Done et al., Citation2016, Citation2017; Ostrom & Traustadóttir, Citation2020) opted to measure changes in PBMCs as opposed to muscle, as they offer a more accessible and less invasive alternative to muscle biopsies, enabling multiple samples to be collected in a short period of time. In addition, there is concern that muscle biopsies may trigger an acute inflammatory response (i.e., nuclear factor-κB activation) that could independently activate NRF2, thereby affecting the interpretation of transcriptional changes (Groeger et al., Citation2010; Liu et al., Citation2008). Nonetheless, studies suggest that PBMCs are valid surrogate cells for skeletal muscle in nutritional and exercise-specific time-course studies (Rudkowska et al., Citation2011; Zeibig et al., Citation2005). Their accessibility and response to cell stress means PBMCs are also increasingly recognised as a promising cell type for monitoring NRF2 and gene target activity in clinical trials (Neilson et al., Citation2020). Despite the possible advantages of measuring NRF2 changes in PBMCs, it is important that the findings from ours and other studies (Done et al., Citation2016, Citation2017; Ostrom & Traustadóttir, Citation2020) are corroborated in other tissues, including skeletal muscle.
Conclusion
In conclusion, we report that NRF2 activity is elevated <1 h following a bout of eccentric exercise compared to resting conditions, whilst gene targets GR and GPX are unaffected. These novel findings suggest that eccentric exercise could be used as a non-pharmacological strategy to increase NRF2 signalling, a beneficial adaptation which could be useful to individuals looking to strengthen their antioxidative defence systems (i.e., those with pathologies associated with dysfunctional redox responses such as cardiovascular disease and cancer) (Aggarwal et al., Citation2019).
Author Contribution
Conceptualization (T.C, J.T, S.J.B, N.M, N.C.B). Collection, analysis, or interpretation of data (T.C, C.T, J.T). Writing – original draft preparation (T.C, J.T). Writing – review and editing (T.C, J.T, S.J.B, N.M, N.C.B). All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data can be provided at a reasonable request from the corresponding author.
Additional information
Funding
References
- Abruzzo, P. M., Esposito, F., Marchionni, C., DiTullio, S., Belia, S., Fulle, S., Veicsteinas, A., & Marini, M. (2013). Moderate exercise training induces ros-related adaptations to skeletal muscles. International Journal of Sports Medicine, 34(8), 676–687. https://doi.org/10.1055/s-0032-1323782
- Aggarwal, V., Tuli, H. S., Varol, A., Thakral, F., Yerer, M. B., Sak, K., Varol, M., Jain, A., Khan, M. A., & Sethi, G. (2019). Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules, 9(11), 735. https://doi.org/10.3390/BIOM9110735
- Ashida, Y., Himori, K., Tatebayashi, D., Yamada, R., Ogasawara, R., & Yamada, T. (2018). Effects of contraction mode and stimulation frequency on electrical stimulation-induced skeletal muscle hypertrophy. Journal of Applied Physiology, 124(2), 341–348. https://doi.org/10.1152/JAPPLPHYSIOL.00708.2017
- Ballmann, C., McGinnis, G., Peters, B., Slivka, D., Cuddy, J., Hailes, W., Dumke, C., Ruby, B., & Quindry, J. (2014). Exercise-induced oxidative stress and hypoxic exercise recovery. European Journal of Applied Physiology, 114(4), 725–733. https://doi.org/10.1007/s00421-013-2806-5
- Banerjee, R. (2012). Redox outside the box: Linking extracellular Redox remodeling with intracellular Redox metabolism. The Journal of Biological Chemistry, 287(7), 4397. https://doi.org/10.1074/JBC.R111.287995
- Bennett, D. A. (2001). How can I deal with missing data in my study? Australian and New Zealand Journal of Public Health, 25(5), 464–469. https://doi.org/10.1111/J.1467-842X.2001.TB00294.X
- Burry, M., Hawkins, D., & Spangenburg, E. E. (2007). Lengthening contractions differentially affect p70s6k phosphorylation compared to isometric contractions in rat skeletal muscle. European Journal of Applied Physiology, 100(4), 409–415. https://doi.org/10.1007/S00421-007-0444-5
- Chen, M., Dai, L. H., Fei, A., Pan, S. M., & Wang, H. R. (2017). Isoquercetin activates the ERK1/2-Nrf2 pathway and protects against cerebral ischemia-reperfusion injury in vivo and in vitro. Experimental and Therapeutic Medicine, 13(4), 1353. https://doi.org/10.3892/ETM.2017.4093
- Cohen, J. (1988). Statistical power analysis for the social Sciences (2nd ed.). Lawrence Erlbaum Associates.
- Cuadrado, A., Rojo, A. I., Wells, G., Hayes, J. D., Cousin, S. P., Rumsey, W. L., Attucks, O. C., Franklin, S., Levonen, A. L., Kensler, T. W., & Dinkova-Kostova, A. T. (2019). Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nature Reviews Drug Discovery, 18(4), 295–317. https://doi.org/10.1038/s41573-018-0008-x
- de Oliveira, V. N., Bessa, A., Jorge, M. L. M. P., da Silva Oliveira, R. J., de Mello, M. T., de Agostini, G. G., Jorge, P. T., & Espindola, F. S. (2012). The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Applied Physiology, Nutrition, and Metabolism, 37(2), 334–344. https://doi.org/10.1139/H2012-004
- Dinkova-Kostova, A. T., & Kazantsev, A. G. (2017). Activation of Nrf2 signaling as a common treatment of neurodegenerative diseases. Neurodegenerative Disease Management, 7(2), 97–100. https://doi.org/10.2217/nmt-2017-0011
- Dinkova-Kostova, A. T., Kostov, R. V., & Canning, P. (2017). Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Archives of Biochemistry and Biophysics, 617, 84–93. https://doi.org/10.1016/j.abb.2016.08.005
- Done, A. J., Gage, M. J., Nieto, N. C., & Traustadóttir, T. (2016). Exercise-induced Nrf2-signaling is impaired in aging. Free Radical Biology and Medicine, 96, 130–138. https://doi.org/10.1016/j.freeradbiomed.2016.04.024
- Done, A. J., Newell, M. J., & Traustadóttir, T. (2017). Effect of exercise intensity on Nrf2 signalling in young men. Free Radical Research, 51(6), 646–655. https://doi.org/10.1080/10715762.2017.1353689
- Eliasson, J., Elfegoun, T., Nilsson, J., Köhnke, R., Ekblom, B., & Blomstrand, E. (2006). Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. American Journal of Physiology Endocrinology and Metabolism, 291(6), E1197–E1205. https://doi.org/10.1152/AJPENDO.00141.2006
- Elkins, M. R. (2015). Assessing baseline comparability in randomised trials. Journal of Physiotherapy, 61(4), 228–230. https://doi.org/10.1016/J.JPHYS.2015.07.005
- Field, A. (2016). An adventure in statistics: The reality enigma. SAGE PublicationsSage CA.
- Franchi, M. V., Atherton, P. J., Reeves, N. D., Flück, M., Williams, J., Mitchell, W. K., Selby, A., Beltran Valls, R. M., & Narici, M. V. (2014). Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiologica, 210(3), 642–654. https://doi.org/10.1111/APHA.12225
- García-López, D., Cuevas, M. J., Almar, M., Lima, E., De Paz, J. A., & González-Gallego, J. (2007). Effects of eccentric exercise on NF-κB activation in blood mononuclear cells. Medicine and Science in Sports and Exercise, 39(4), 653–664. https://doi.org/10.1249/mss.0b013e31802f04f6
- Groeger, A. L., Cipollina, C., Cole, M. P., Woodcock, S. R., Bonacci, G., Rudolph, T. K., Rudolph, V., Freeman, B. A., & Schopfer, F. J. (2010). Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature Chemical Biology, 6(6), 433. https://doi.org/10.1038/NCHEMBIO.367
- Harris-Love, M. O., Gollie, J. M., & Keogh, J. W. L. (2021). Eccentric exercise: Adaptations and applications for Health and performance. Journal of Functional Morphology and Kinesiology, 6(4), 96. https://doi.org/10.3390/JFMK6040096
- Harvey, C. J., Thimmulappa, R. K., Singh, A., Blake, D. J., Ling, G., Wakabayashi, N., Fujii, J., Myers, A., & Biswal, S. (2009). Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radical Biology & Medicine, 46(4), 443. https://doi.org/10.1016/J.FREERADBIOMED.2008.10.040
- Ichimura, Y., Waguri, S., Sou, Y. S., Kageyama, S., Hasegawa, J., Ishimura, R., Saito, T., Yang, Y., Kouno, T., Fukutomi, T., Hoshii, T., Hirao, A., Takagi, K., Mizushima, T., Motohashi, H., Lee, M. S., Yoshimori, T., Tanaka, K., Yamamoto, M., & Komatsu, M. (2013). Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Molecular Cell, 51(5), 618–631. https://doi.org/10.1016/J.MOLCEL.2013.08.003
- Jackson, M. J., Vasilaki, A., & McArdle, A. (2016). Cellular mechanisms underlying oxidative stress in human exercise. Free Radical Biology and Medicine, 98, 13–17. https://doi.org/10.1016/j.freeradbiomed.2016.02.023
- Ji, L. L., Gomez-Cabrera, M. C., & Vina, J. (2006). Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Annals of the New York Academy of Sciences, 1067(1), 425–435. https://doi.org/10.1196/annals.1354.061
- Kamandulis, S., De Souza Leite, F., Hernández, A., Katz, A., Brazaitis, M., Bruton, J. D., Venckunas, T., Masiulis, N., Mickeviciene, D., Eimantas, N., Subocius, A., Rassier Di, E., Skurvydas, A., Ivarsson, N., & Westerblad, H. (2017). Prolonged force depression after mechanically demanding contractions is largely independent of Ca2+ and reactive oxygen species. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 31(11), 4809–4820. https://doi.org/10.1096/FJ.201700019R
- Kamandulis, S., Mickevicius, M., Snieckus, A., Streckis, V., Montiel-Rojas, D., Chaillou, T., Westerblad, H., & Venckunas, T. (2021). Increasing the resting time between drop jumps lessens delayed-onset muscle soreness and limits the extent of prolonged low-frequency force depression in human knee extensor muscles. European Journal of Applied Physiology, 122(1), 255–266. https://doi.org/10.1007/S00421-021-04834-X
- Kobayashi, M., & Yamamoto, M. (2006). Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Advances in Enzyme Regulation, 46(1), 113–140. https://doi.org/10.1016/j.advenzreg.2006.01.007
- Liu, G. H., Qu, J., & Shen, X. (2008). NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochimica et Biophysica Acta, 1783(5), 713–727. https://doi.org/10.1016/J.BBAMCR.2008.01.002
- McKay, A. K. A., Stellingwerff, T., Smith, E. S., Martin, D. T., Mujika, I., Goosey-Tolfrey, V. L., Sheppard, J., & Burke, L. M. (2021). Defining training and performance caliber: A participant classification framework. International Journal of Sports Physiology and Performance, 17(2), 1–15. https://doi.org/10.1123/ijspp.2021-0451
- Michaelson, L. P., Shi, G., Ward, C. W., & Rodney, G. G. (2010). Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle and Nerve, 42(4), 522–529. https://doi.org/10.1002/mus.21724
- Montero, D., Tachibana, C., Rahr Winther, J., & Appenzeller-Herzog, C. (2013). Intracellular glutathione pools are heterogeneously concentrated. Redox biology, 1(1), 508. https://doi.org/10.1016/J.REDOX.2013.10.005
- Muthusamy, V. R., Kannan, S., Sadhaasivam, K., Gounder, S. S., Davidson, C. J., Boeheme, C., Hoidal, J. R., Wang, L., & Rajasekaran, N. S. (2012). Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radical Biology and Medicine, 52(2), 366–376. https://doi.org/10.1016/j.freeradbiomed.2011.10.440
- Nabavi, S. F., Barber, A. J., Spagnuolo, C., Russo, G. L., Daglia, M., Nabavi, S. M., & Sobarzo-Sánchez, E. (2016). Nrf2 as molecular target for polyphenols: A novel therapeutic strategy in diabetic retinopathy. Critical Reviews in Clinical Laboratory Sciences, 53(5), 293–312. https://doi.org/10.3109/10408363.2015.1129530
- Neilson, L., Quinn, J., & Gray, N. (2020). Peripheral blood NRF2 expression as a biomarker in human Health and disease. Antioxidants, 10(1), 1–12. https://doi.org/10.3390/ANTIOX10010028
- Nikolaidis, M. G., Jamurtas, A. Z., Paschalis, V., Fatouros, I. G., Koutedakis, Y., & Kouretas, D. (2008). The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: Magnitude and time-course considerations. Sports Medicine, 38(7), 579–606. https://doi.org/10.2165/00007256-200838070-00005
- Nikolaidis, M. G., Paschalis, V., Giakas, G., Fatouros, I. G., Koutedakis, Y., Kouretas, D., & Jamurtas, A. Z. (2007). Decreased blood oxidative stress after repeated muscle-damaging exercise. Medicine and Science in Sports and Exercise, 39(7), 1080–1089. https://doi.org/10.1249/MSS.0B013E31804CA10C
- Onur, E., Kabaroĝlu, C., Günay, Ö., Var, A., Yilmaz, Ö., Dündar, P., Tikiz, C., Güvenç, Y., & Yüksel, H. (2011). The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergologia et immunopathologia, 39(2), 90–95. https://doi.org/10.1016/J.ALLER.2010.04.006
- Ostrom, E. L., & Traustadóttir, T. (2020). Aerobic exercise training partially reverses the impairment of Nrf2 activation in older humans. Free Radical Biology and Medicine, 160, 418–432. https://doi.org/10.1016/j.freeradbiomed.2020.08.016
- Parise, G., Phillips, S. M., Kaczor, J. J., & Tarnopolsky, M. A. (2005). Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radical Biology and Medicine, 39(2), 289–295. https://doi.org/10.1016/j.freeradbiomed.2005.03.024
- Pekovic-Vaughan, V., Gibbs, J., Yoshitane, H., Yang, N., Pathiranage, D., Guo, B., Sagami, A., Taguchi, K., Bechtold, D., Loudon, A., Yamamoto, M., Chan, J., van der Horst, G. T. J., Fukada, Y., & Meng, Q. J. (2014). The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes & Development, 28(6), 548. https://doi.org/10.1101/GAD.237081.113
- Perry, C. G. R., Lally, J., Holloway, G. P., Heigenhauser, G. J. F., Bonen, A., & Spriet, L. L. (2010). Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. Journal of Physiology, Paris, 588(Pt 23), 4795. https://doi.org/10.1113/JPHYSIOL.2010.199448
- Rahbek, S. K., Farup, J., Møller, A. B., Vendelbo, M. H., Holm, L., Jessen, N., & Vissing, K. (2014). Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids, 46(10), 2377–2392. https://doi.org/10.1007/s00726-014-1792-1
- Robledinos-Antón, N., Fernández-Ginés, R., Manda, G., & Cuadrado, A. (2019). Activators and inhibitors of NRF2: A review of their potential for clinical development. In Oxidative medicine and cellular longevity (Vol. 2019). Hindawi Limited. https://doi.org/10.1155/2019/9372182
- Rudkowska, I., Raymond, C., Ponton, A., Jacques, H., Lavigne, C., Holub, B. J., Marette, A., & Vohl, M. C. (2011). Validation of the use of peripheral blood mononuclear cells as surrogate model for skeletal muscle tissue in nutrigenomic studies. Omics: A Journal of Integrative Biology, 15(1–2), 1–7. https://doi.org/10.1089/OMI.2010.0073
- Sakellariou, G. K., Vasilaki, A., Palomero, J., Kayani, A., Zibrik, L., McArdle, A., & Jackson, M. J. (2013). Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants and Redox Signaling, 18(6), 603–621. https://doi.org/10.1089/ars.2012.4623
- Scott, H. A., Latham, J. R., Callister, R., Pretto, J. J., Baines, K., Saltos, N., Upham, J. W., & Wood, L. G. (2015). Acute exercise is associated with reduced exhaled nitric oxide in physically inactive adults with asthma. Annals of Allergy, Asthma & Immunology, 114(6), 470–479. https://doi.org/10.1016/j.anai.2015.04.002
- Silva-Islas, C. A., & Maldonado, P. D. (2018). Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacological Research, 134, 92–99. https://doi.org/10.1016/J.PHRS.2018.06.013
- Skurvydas, A., Mamkus, G., Kamandulis, S., Dudoniene, V., Valanciene, D., & Westerblad, H. (2016). Mechanisms of force depression caused by different types of physical exercise studied by direct electrical stimulation of human quadriceps muscle. European Journal of Applied Physiology, 116(11–12), 2215–2224. https://doi.org/10.1007/S00421-016-3473-0
- Spanidis, Y., Veskoukis, A. S., Papanikolaou, C., Stagos, D., Priftis, A., Deli, C. K., Jamurtas, A. Z., & Kouretas, D. (2018). Exercise-induced reductive stress is a protective mechanism against oxidative stress in peripheral blood mononuclear cells. Oxidative Medicine and Cellular Longevity, 2018. https://doi.org/10.1155/2018/3053704
- Steinbacher, P., & Eckl, P. (2015). Impact of oxidative stress on exercising skeletal muscle. Biomolecules, 5(2), 356–377. MDPI AG. https://doi.org/10.3390/biom5020356
- Sun, Q., Zeng, C., Du, L., & Dong, C. (2021). Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia-reperfusion. Experimental and Therapeutic Medicine, 21(3). https://doi.org/10.3892/ETM.2021.9622
- Thorley, J., Thomas, C., Thon, N., Nuttall, H., Martin, N. R. W., Bishop, N., Bailey, S. J., & Clifford, T. (2023). Combined effects of green tea supplementation and eccentric exercise on nuclear factor erythroid 2-related factor 2 activity. European Journal of Applied Physiology. https://doi.org/10.1007/S00421-023-05271-8
- Tofas, T., Fatouros, I. G., Draganidis, D., Deli, C. K., Chatzinikolaou, A., Tziortzis, C., Panayiotou, G., Koutedakis, Y., & Jamurtas, A. Z. (2021). Effects of cardiovascular, resistance and combined exercise training on cardiovascular, performance and blood redox parameters in coronary artery disease patients: An 8-month training-detraining randomized intervention. Antioxidants, 10(3), 1–18. https://doi.org/10.3390/antiox10030409
- Vomund, S., Schäfer, A., Parnham, M. J., Brüne, B., & Von Knethen, A. (2017). Nrf2, the master regulator of anti-oxidative responses. International Journal of Molecular Sciences, 18(12). MDPI AG. https://doi.org/10.3390/ijms18122772.
- Wiecek, M., Szymura, J., Maciejczyk, M., Kantorowicz, M., & Szygula, Z. (2018). Anaerobic Exercise-Induced Activation of Antioxidant Enzymes in the Blood of Women and men. Frontiers in Physiology, 9(JUL). https://doi.org/10.3389/FPHYS.2018.01006
- Woo, Y., Lee, H. J., Jung, Y. M., & Jung, Y. J. (2019). mTOR-Mediated antioxidant activation in solid tumor radioresistance. Journal of Oncology, 2019. https://doi.org/10.1155/2019/5956867
- Yang, Y. C., Lii, C. K., Lin, A. H., Yeh, Y. W., Yao, H. T., Li, C. C., Liu, K. L., & Chen, H. W. (2011). Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radical Biology and Medicine, 51(11), 2073–2081. https://doi.org/10.1016/J.FREERADBIOMED.2011.09.007
- Yang, S. Y., Pyo, M. C., Nam, M. H., & Lee, K. W. (2019). ERK/Nrf2 pathway activation by caffeic acid in HepG2 cells alleviates its hepatocellular damage caused by t-butylhydroperoxide-induced oxidative stress. BMC Complementary and Alternative Medicine, 19(1), 1–13. https://doi.org/10.1186/s12906-019-2551-3
- Zeibig, J., Karlic, H., Lohninger, A., Dumsgaard, R., & Smekal, G. (2005). Do blood cells mimic gene expression profile alterations known to occur in muscular adaptation to endurance training? European Journal of Applied Physiology, 95(1), 96–104. https://doi.org/10.1007/s00421-005-1334-3
- Zipper, L. M., & Mulcahy, R. T. (2000). Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochemical and Biophysical Research Communications, 278(2), 484–492. https://doi.org/10.1006/BBRC.2000.3830
