ABSTRACT
Introduction
Medical imaging in pregnancy (antenatal imaging) is routine. However, the effect of seeing fetal images on the parent–fetal relationship is not well understood, particularly for fathers or partners, or when using advanced imaging technologies. This review aimed to explore how parent experience and prenatal attachment is impacted by antenatal imaging.
Method
Database searches were performed between September 2020 and April 2021 Inclusion criteria were English language primary research studies published since 2000, describing or reporting measures of attachment after antenatal imaging in expectant parents. The Pillar Integration Process was used for integrative synthesis.
Findings
Twenty-three studies were included. Six pillar themes were developed: 1) the scan experience begins before the scan appointment; 2) the scan as a pregnancy ritual; 3) feeling actively involved in the scan; 4) parents’ priorities for knowledge and understanding of the scan change during pregnancy; 5) the importance of the parent–sonographer partnership during scanning; and 6) scans help to create a social identity for the unborn baby.
Conclusion
Antenatal imaging can enhance prenatal attachment. Parents value working collaboratively with sonographers to be actively involved in the experience. Sonographers can help facilitate attachment by delivering parent-centred care tailored to parents’ emotional and knowledge needs.
Introduction
Prenatal attachment is described as the emotional connection parents form to their unborn child (Condon, Citation1993). It is important for healthy infant brain development (Glover, Citation2014), parental emotional well-being (Göbel et al., Citation2018) and represents a transformation during pregnancy whereby expectant parents start to reconceptualise their identity from self to care-giver (Walsh, Citation2010). The concept arose from Bowlby’s early definitions of attachment theory (Bowlby, Citation1982); however, the term ‘prenatal bonding’ is often used interchangeably in literature to describe the parent-fetal relationship (Walsh, Citation2010). For clarity, the parent-fetal relationship is referred to as ‘attachment’ throughout this review to incorporate all constructs, although bonding is recognised as a synonym in this context, and therefore included as a key word to ensure all relevant records were captured during searches.
Routine medical ultrasound imaging during the antenatal period is generally regarded as a positive pregnancy experience which also facilitates the bonding process as it allows expectant parents to create a mental image of their unborn baby (Walsh, Citation2020). This image can be central to the developing attachment by providing a visual catalyst for parents to construct and fantasise about their ‘imagined child’ (Trombetta et al., Citation2021), to which they attribute personal characteristics to humanise the fetus and thus experience a deeper emotional tie towards the unborn baby (Condon, Citation1993). It is thought that prenatal attachment can predict the quality of the parent–child relationship after birth, thus antenatal imaging may provide a unique and early opportunity during pregnancy for parents to establish a positive emotional connection towards the fetus, or for health-care professionals to provide timely intervention and support to parents if required (Della Vedova & Burro, Citation2017). For most parents, scans are an enjoyable and reassuring experience (G.M. Thomas et al., Citation2017). However, there is evidence to suggest that the experience may also lead to increased anxiety and stress in parents, particularly those who are unable to interpret the image (National Institute for Health and Care Excellence, Citation2019), which may impact on the developing relationship.
Research in this field is warranted, especially with the increasing use of advanced fetal imaging techniques to complement routine ultrasound imaging, including three-dimensional (3D) and four-dimensional (4D) ultrasound and fetal magnetic resonance imaging (MRI). These modalities produce highly detailed images and videos of fetuses, yet the effect of seeing these images on the developing parent-fetal relationship is not well studied or reported (Van den Bergh & Simons, Citation2009), especially for pregnancies in which a fetal anomaly is suspected or has been diagnosed. For some parents, visualising the anomaly may aid their understanding of a diagnosis (Gonçalves et al., Citation2005; Leung et al., Citation2006; Sreejith et al., Citation2018), although for some it may increase distress, for example, if faced with a decision to continue the pregnancy (Mitchell, Citation2004). Furthermore, studies evaluating the effect of imaging on the paternal-fetal relationship are sparse (Walsh et al., Citation2017), even though quality prenatal attachment is associated with a positive effect on maternal emotional well-being and the maternal-foetal relationship (Borg Cunen et al., Citation2017; Lindgren, Citation2001).
This review aimed to explore the research question ‘what is the effect of medical imaging in pregnancy on prenatal attachment?’, to enhance current understanding of the impact antenatal imaging may have on the parent-fetal relationship and identify factors of the parent imaging experience that may affect attachment.
Materials and methods
The protocol for this review was registered on the PROSPERO database (CRD42020197259). No patients were involved in the development or conduct of this review. Funding was received from the College of Radiographers Doctoral Fellowship Award (DF017) and City, University of London.
Search strategy
The PRISMA statement (Page et al., Citation2021) was used to develop a search strategy from keywords identified using the PEO framework (Khan et al., Citation2003; ). This was reviewed by a university librarian specialising in literature searches and piloted for efficacy. Identified keywords and synonyms were combined with Wildcard and Boolean operators to generate search queries (e.g. TI (mother* or maternal or mum*) AND TI (magnetic resonance imaging or MRI) OR TI (ultrasound or sonography or sonogram or ultrasonography) AND TI (bonding or attachment or relationship or behaviour or experience)) (Supplementary Material S1). During September 2020-April 2021, searches of 10 electronic databases were performed (MEDLINE, CINAHL, PsychNET, Academic Search Complete (via EBSCOhost), (Embase, MIDRIS (via OVID), The Cochrane Library, PubMed, Scopus and Web of Science) in addition to searches of grey literature (HMIC, OpenGrey, NICE, and TRIP) and doctoral dissertations (ProQuest Dissertations, Theses Global).
Table 1. PEO framework to identify keywords and develop search terms.
Eligibility criteria
Studies were eligible if they were primary research studies published in English describing or reporting measures of attachment in expectant adult (≥18 years old) parents in the context of medical imaging in pregnancy. To incorporate the impact of fetal imaging advances, studies published before 2000 were excluded. Only studies where the antenatal imaging examination was the research intervention (e.g. 2D/3D/4D ultrasound or fetal MRI) were considered for inclusion. There was no restriction on gestational age or fetal anomaly diagnosis. Studies measuring prenatal attachment after birth were excluded to reduce recall bias.
Selection of papers
References were imported into review management software program, EPPI-Reviewer4 (Thomas et al., Citation2010). After removing duplicates, titles and abstracts were screened for relevance. Full texts of studies meeting the eligibility criteria were retrieved for further evaluation. Using the same keywords as the electronic databases, additional searches were performed on Google Scholar and using web-based literature searching platform, ResearchRabbit Beta3 (www.researchrabbit.ai). Reference lists of included studies were hand-searched for additional relevant studies. All references were reviewed by ES, with independent double-screening performed by RW on randomly allocated 10% of the total studies for both title/abstract and full-text screening. Discrepancies were discussed by the reviewers to reach consensus following each level of screencing.
Data extraction and quality appraisal
A database was developed for extraction of study characteristics including participant demographics, study design, and reported measure of attachment or qualitative insights. Included studies were quality appraised using Joanna Briggs Institute checklists (Lockwood et al., Citation2015; Moola et al., Citation2020; Tufanaru et al., Citation2020). These assist reviewers in evaluating the rigour and validity of published research, helping to identify flaws in reported study designs and methods through focused questions. Reviewers can respond with ‘yes’, ‘no’, ‘unclear’ or ‘not applicable’. Whilst a scoring system is not embedded in these checklists, studies with a high proportion of ‘no’ or ‘unclear’ responses are suspected to be of lower quality.
Data synthesis
Owing to the heterogeneity of the studies, a research synthesis (Sandelowski et al., Citation2006) was more appropriate than the separate meta-analytical and meta-ethnographic approaches proposed in the protocol (Skelton et al., Citation2020). This method is becoming popular in health research as it enables extraction of categories and themes from quantitative and qualitative data pertaining to a research question, facilitates validation or triangulation of findings (Moon, Citation2019), and creates a more complete understanding of complex phenomena of interest (Stern et al., Citation2020). The Pillar Integration Process (Johnson et al., Citation2019) was used to integrate and synthesise quantitative and qualitative studies in a convergent approach, as it is flexible for use across study designs, yet provides a well-defined methodological approach to the analysis in keeping with the nature of a systematic review (Flanagan et al., Citation2020). After initial, separate analyses of quantitative and qualitative studies had been completed, the 4-stage (listing, matching, checking, Pillar building) Pillar Integration Process was used to build central, integrated themes. In the first stage, coded categories from quantitative studies and themes for qualitative studies were listed in the respective column on the joint display matrix. The data was then matched to the opposite quantitative or qualitative column and horizontally rearranged to reflect the corresponding content. The rows of matched data were then cross-checked for quality and completeness, before the Pillars were built in the central column to represent the integrated themes from each row (Johnson et al., Citation2019).
Results
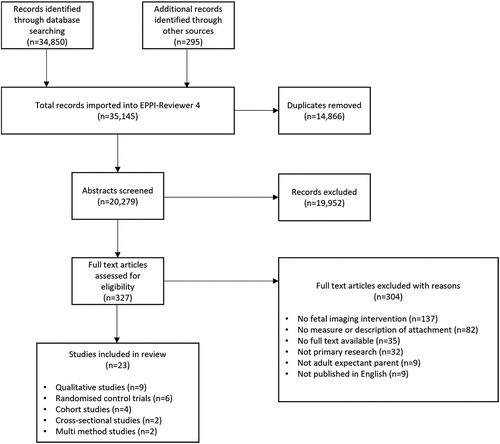
Results of the searches are given in . After removing duplicates, 20,279 references were screened by title and abstract. Of these, 19,952 did not meet the inclusion criteria and were excluded. After detailed review of 327 full-text references, 304 were excluded (see Supplementary Material S2). Twenty-three studies (13 quantitative and 10 qualitative/descriptive studies) were eligible for inclusion. Agreement between the two reviewers was 98% on title and abstract, and 91% on full-text screening.
Study characteristics
Study characteristics are presented in Supplementary Material S3 and S4. There were nine qualitative studies, (Cadogan et al., Citation2009; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Firth et al., Citation2011; Freeman, Citation2000; Øyen & Aune, Citation2016; Stephenson et al., Citation2016; Wadephul et al., Citation2015; Westerneng et al., Citation2019), 6 RCTs (Boukydis et al., Citation2006; De Jong-Pleij et al., Citation2013; Lapaire et al., Citation2007; Righetti et al., Citation2005; Rustico et al., Citation2005; Sedgmen et al., Citation2006), 4 cohort studies (Edwards et al., Citation2010; Lalor & Devane, Citation2007; Polizzi et al., Citation2017; Robak-Chołubek et al., Citation2015), 2 cross-sectional (Harpel & Barras, Citation2018; Sidi & Josheu, Citation2019), and 2 studies using a multi-method approach (Cristofalo et al., Citation2006; Murakami et al., Citation2012).
Most studies (n = 13) were conducted in European countries (Cadogan et al., Citation2009; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; De Jong-Pleij et al., Citation2013; Lalor & Devane, Citation2007; Lapaire et al., Citation2007; Øyen & Aune, Citation2016; Polizzi et al., Citation2017; Righetti et al., Citation2005; Robak-Chołubek et al., Citation2015; Rustico et al., Citation2005; Wadephul et al., Citation2015; Westerneng et al., Citation2019), followed by the USA (n = 5; Boukydis et al., Citation2006; Cristofalo et al., Citation2006; Edwards et al., Citation2010; Freeman, Citation2000; Harpel & Barras, Citation2018), Africa (n = 2; Firth et al., Citation2011; Sidi & Josheu, Citation2019), Australia (n = 2; Sedgmen et al., Citation2006; Stephenson et al., Citation2016), and Japan (n = 1; Murakami et al., Citation2012).
Seventeen studies featured maternal participants only (Boukydis et al., Citation2006; Cristofalo et al., Citation2006; Dykes & Stjernqvist, Citation2001; Edwards et al., Citation2010; Firth et al., Citation2011; De Jong-Pleij et al., Citation2013; Lalor & Devane, Citation2007; Murakami et al., Citation2012; Øyen & Aune, Citation2016; Polizzi et al., Citation2017; Robak-Chołubek et al., Citation2015; Rustico et al., Citation2005; Sedgmen et al., Citation2006; Sidi & Josheu, Citation2019; Stephenson et al., Citation2016; Wadephul et al., Citation2015; Westerneng et al., Citation2019), five included both parents (Cadogan et al., Citation2009; Ekelin et al., Citation2004; Harpel & Barras, Citation2018; Lapaire et al., Citation2007; Righetti et al., Citation2005), and one study only recruited fathers (Freeman, Citation2000). Studies with specific research foci included 3D/4D ultrasound (n = 8; Cadogan et al., Citation2009; Edwards et al., Citation2010; De Jong-Pleij et al., Citation2013; Lapaire et al., Citation2007; Righetti et al., Citation2005; Rustico et al., Citation2005; Sedgmen et al., Citation2006; Wadephul et al., Citation2015), scans in third trimester (n = 5; Edwards et al., Citation2010; De Jong-Pleij et al., Citation2013; Lapaire et al., Citation2007; Robak-Chołubek et al., Citation2015; Westerneng et al., Citation2019), known or high chance of foetal anomaly (n = 3; Cadogan et al., Citation2009; Cristofalo et al., Citation2006; Polizzi et al., Citation2017), first-time mothers (n = 2; Dykes & Stjernqvist, Citation2001; Sedgmen et al., Citation2006), and ethnic minorities (n = 2; Firth et al., Citation2011; Sidi & Josheu, Citation2019). No studies reporting fetal MRI as the imaging modality were included.
Increased prenatal attachment was measured or reported after scanning in 16 studies (Boukydis et al., Citation2006; Dykes & Stjernqvist, Citation2001; Edwards et al., Citation2010; Ekelin et al., Citation2004; Firth et al., Citation2011; Freeman, Citation2000; De Jong-Pleij et al., Citation2013; Lalor & Devane, Citation2007; Murakami et al., Citation2012; Øyen & Aune, Citation2016; Righetti et al., Citation2005; Robak-Chołubek et al., Citation2015; Rustico et al., Citation2005; Sedgmen et al., Citation2006; Sidi & Josheu, Citation2019; Stephenson et al., Citation2016), was unchanged in one study (Westerneng et al., Citation2019), and temporarily decreased after scanning in one study following diagnosis of foetal anomaly (Cristofalo et al., Citation2006). Four studies reported no difference in attachment between imaging modalities (De Jong-Pleij et al., Citation2013; Righetti et al., Citation2005; Rustico et al., Citation2005; Sedgmen et al., Citation2006); however, one study found a significant difference in prenatal attachment after 3D/4D compared to 2D ultrasound (Lapaire et al., Citation2007).
Quality appraisal
Critical appraisal indicated moderate level of quality across the studies. Quality was considered higher where studies used a pre- and post-scan comparative design (Boukydis et al., Citation2006; Cadogan et al., Citation2009; Dykes & Stjernqvist, Citation2001; De Jong-Pleij et al., Citation2013; Lalor & Devane, Citation2007; Øyen & Aune, Citation2016; Polizzi et al., Citation2017; Righetti et al., Citation2005; Sedgmen et al., Citation2006; Wadephul et al., Citation2015), chose a validated questionnaire for quantitative data collection (Boukydis et al., Citation2006; De Jong-Pleij et al., Citation2013; Polizzi et al., Citation2017; Righetti et al., Citation2005; Rustico et al., Citation2005; Sedgmen et al., Citation2006), and fully described the analytical process (Cristofalo et al., Citation2006; Freeman, Citation2000; Øyen & Aune, Citation2016; Wadephul et al., Citation2015).
Elements of qualitative studies that were considered of lower quality were: not disclosing the relationship of the researchers to participants (e.g. to determine whether this relationship could have influenced data collection; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Stephenson et al., Citation2016; Wadephul et al., Citation2015; Westerneng et al., Citation2019), not describing the data collection environment in sufficient detail (Cristofalo et al., Citation2006; Ekelin et al., Citation2004; Øyen & Aune, Citation2016; Wadephul et al., Citation2015), and not reporting triangulation methods (Cristofalo et al., Citation2006; Dykes & Stjernqvist, Citation2001).
Elements of quantitative studies that were considered of lower quality were: using non-validated questionnaires (Edwards et al., Citation2010; Lalor & Devane, Citation2007; Lapaire et al., Citation2007; Murakami et al., Citation2012; Robak-Chołubek et al., Citation2015; Sidi & Josheu, Citation2019), unclear participant recruitment, or randomisation procedures (Boukydis et al., Citation2006; De Jong-Pleij et al., Citation2013; Righetti et al., Citation2005), and not providing details for the ultrasound scan protocol (Polizzi et al., Citation2017; Righetti et al., Citation2005; Robak-Chołubek et al., Citation2015; Sidi & Josheu, Citation2019).
Fourteen studies did not report ethical approval (Boukydis et al., Citation2006; Cadogan et al., Citation2009; Cristofalo et al., Citation2006; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Freeman, Citation2000; Harpel & Barras, Citation2018; De Jong-Pleij et al., Citation2013; Lapaire et al., Citation2007; Polizzi et al., Citation2017; Righetti et al., Citation2005; Robak-Chołubek et al., Citation2015; Rustico et al., Citation2005; Sedgmen et al., Citation2006).
Excluding lower-quality studies based on these considerations, however, would severely reduce the quantity of data available for use in this review, therefore it was decided they should still be included.
Convergent integrated Pillar data synthesis
Analysis of the included studies identified 58 codes related to prenatal attachment. Six central themes were then developed from the Pillar Integration Process (): 1) the scan experience begins before the scan appointment; 2) the scan as a pregnancy ritual; 3) feeling actively involved in the scan; 4) parents’ priorities for knowledge and understanding of the scan change during pregnancy; 5) the importance of the parent–sonographer partnership during scanning; and 6) scans help to create a social identity for the unborn baby. For simplicity, the term sonographer is used throughout the paper to represent any health-care professional or ultrasound practitioner who performs pregnancy imaging. These themes are described below with illustrative quotes.
Table 2. Adapted Pillar Integration process analysis (Johnson et al., Citation2019).
The scan experience begins before the scan appointment
This theme conceptualises pregnancy scans as experiences that are not confined to the scan room. Prior to the scan, expectant parents sought information from a range of sources, including leaflets provided by scan departments, the internet, and social interactions (Cristofalo et al., Citation2006; Ekelin et al., Citation2004; Firth et al., Citation2011; Øyen & Aune, Citation2016), which they used to develop individual expectations about the scan (Cristofalo et al., Citation2006; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Firth et al., Citation2011; Lalor & Devane, Citation2007; Murakami et al., Citation2012; Øyen & Aune, Citation2016; Wadephul et al., Citation2015). Parents looked forward to the scan, but were simultaneously apprehensive of the potential to receive unexpected news about their baby (Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Firth et al., Citation2011; Øyen & Aune, Citation2016; Stephenson et al., Citation2016; Westerneng et al., Citation2019), with a ‘fear that something could be wrong’ always present (Cadogan et al., Citation2009). This emotional conflict created a paradox, whereby in the excitement of visualising their unborn baby, parents also had to consider the possibility of the scan detecting a fetal anomaly and further antenatal care decisions that may have been needed (Cristofalo et al., Citation2006; Ekelin et al., Citation2004; Firth et al., Citation2011; Stephenson et al., Citation2016).
The scan as a pregnancy ritual
Parents regarded scans as a milestone event, which they expected, and wanted (Harpel & Barras, Citation2018):
One of the first-time mothers even considered the ultrasound examination to be an initiation rite into pregnancy, making it obvious not only to herself but also to others that she really was expecting a baby (Ekelin et al., Citation2004).
Many parents had made the decision to attend the scan before receiving information about it, and were unaware that it is not obligatory in antenatal care (Ekelin et al., Citation2004). Parents viewed scans as an opportunity to see their baby (Cadogan et al., Citation2009; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Firth et al., Citation2011; Øyen & Aune, Citation2016; Stephenson et al., Citation2016; Wadephul et al., Citation2015; Westerneng et al., Citation2019) and to confirm the presence of a new life (Ekelin et al., Citation2004; Firth et al., Citation2011; Øyen & Aune, Citation2016; Stephenson et al., Citation2016). Guided by the sonographer’s commentary, they used the scan images to create mental images of their baby (Stephenson et al., Citation2016). Whilst some parents showed a clear preference for advanced imaging techniques, such as 3D/4D ultrasound for ease of recognition (Sedgmen et al., Citation2006), particularly for first-time mothers (Rustico et al., Citation2005), scan type, did not seem to significantly impact on parents’ perception of the fetus or attachment (Edwards et al., Citation2010; Lapaire et al., Citation2007; Righetti et al., Citation2005; Rustico et al., Citation2005; Sedgmen et al., Citation2006).
For some, novel modalities felt more exciting and therefore desirable; however, they also created uncertainty and feelings of disappointment in parents who have high expectations that may not be met (Cristofalo et al., Citation2006; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Firth et al., Citation2011; Øyen & Aune, Citation2016; Wadephul et al., Citation2015).
All [parents] expected clear, detailed images of the baby, particularly the face, enabling them to see ‘what the baby looks like’ (Wadephul et al., Citation2015).
Pregnancy scans also enabled parents to engage with fetal anomalies by providing a visual image (Cadogan et al., Citation2009; Cristofalo et al., Citation2006; Dykes & Stjernqvist, Citation2001; Stephenson et al., Citation2016).
Feeling actively involved in the scan
This theme focuses on parents as active participants in the scan (Ekelin et al., Citation2004; Stephenson et al., Citation2016). Scans were generally felt to be reassuring or a relief (Cadogan et al., Citation2009; Cristofalo et al., Citation2006; Ekelin et al., Citation2004; Øyen & Aune, Citation2016; Wadephul et al., Citation2015; Westerneng et al., Citation2019), and parents reported positive feelings when the baby’s health was confirmed (Ekelin et al., Citation2004). Parents enjoyed recognising personal characteristics in the baby (Boukydis et al., Citation2006; Ekelin et al., Citation2004), and this helped validate their role as creators of life (Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Øyen & Aune, Citation2016). The presence of fathers at scans was important (Ekelin et al., Citation2004; Øyen & Aune, Citation2016; Westerneng et al., Citation2019), not only for maternal support (Murakami et al., Citation2012), but also for enhanced attachment as fathers who attended scans felt closer to their unborn baby than those who had not (Harpel & Barras, Citation2018). Partner behaviour changed after the scan to be ‘more understanding and gentle’ towards mothers (Ekelin et al., Citation2004). As with mothers, the imaging modality did not seem to significantly impact paternal attachment (Lapaire et al., Citation2007; Righetti et al., Citation2005), rather it was being present and active in the real-time, dynamic scan experience which helped them to feel closer to their unborn baby:
The women and their partners used the ultrasound examination in planning the pregnancy process leading towards birth and a new life with the baby (Cadogan et al., Citation2009).
Parents’ priorities for knowledge and understanding of the scan change during pregnancy
Interacting with, and understanding the visual images of babies during scan evoked positive pregnancy-related behaviours in parents, such as reducing alcohol/caffeine consumption and preparing the house for the baby’s arrival (Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Øyen & Aune, Citation2016; Sedgmen et al., Citation2006; Westerneng et al., Citation2019).
These behaviours represented the developing attachment, and parents’ transformation of themselves as a care-giver with ‘feelings of responsibility and concern for fetal development’ (Stephenson et al., Citation2016). The pattern to these behaviours was progressive, and as such, the need to inform parents with specific knowledge and understanding from scans changed with gestation (Cadogan et al., Citation2009; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Firth et al., Citation2011; Øyen & Aune, Citation2016; Stephenson et al., Citation2016; Wadephul et al., Citation2015; Westerneng et al., Citation2019). At earlier gestations, parents prioritised knowing that their pregnancy was viable (Murakami et al., Citation2012). At later gestations, it was important for parents to know about the presence of foetal anomalies (Murakami et al., Citation2012). Knowledge of the fetal sex was highlighted as an important insight for some parents in trying to learn more about their baby (Firth et al., Citation2011; Freeman, Citation2000; Øyen & Aune, Citation2016). However, limitations of fetal sex determination were understood by parents, with reports to suggest doubt, particularly if it did not align with their preferences (Murakami et al., Citation2012; Robak-Chołubek et al., Citation2015). This implied that fetal sex does not have a substantial impact on bonding, as parents seemed to value knowing the health of the baby as a priority (Freeman, Citation2000).
The importance of the parent–sonographer partnership during scanning
During scans, sonographers facilitated the connection between expectant parents and their babies (Firth et al., Citation2011), and contributed to parents’ knowledge and understanding of the scan procedure (Ekelin et al., Citation2004; Firth et al., Citation2011; Øyen & Aune, Citation2016; Westerneng et al., Citation2019).
Certain aspects of the sonographer’s practice-influenced parents’ perceptions of their overall scan experience (Ekelin et al., Citation2004; Øyen & Aune, Citation2016; Stephenson et al., Citation2016), which impacted on attachment (Boukydis et al., Citation2006). Parents’ confidence in their sonographer was linked with narration of the scan (Freeman, Citation2000), highlighting the importance of the role of the sonographer in partnership with parents:
The women also stressed the importance of having had the picture on the screen thoroughly explained (Dykes & Stjernqvist, Citation2001).
Two studies noted the importance of language choices for sonographers (Ekelin et al., Citation2004; Stephenson et al., Citation2016). Limiting the use of non-medical terminology humanised the fetus, implied to parents that the sonographer recognised their unborn baby as an individual, and demonstrated professional investment in their care (Stephenson et al., Citation2016). Making parents feel like they had been given sufficient time during the scan to engage with the experience and ask questions (Øyen & Aune, Citation2016) helped to reduce parental anxiety and was also important to delivering parent-centred care (Westerneng et al., Citation2019):
I wish there was more time for the ultrasound so that she could explain more and also go through what can and cannot be seen (Ekelin et al., Citation2004).
Scans help to create a social identity for the unborn baby
The final theme represents parents’ ongoing scan experience and how they continued to reflect on it to enhance attachment. Many parents centred their news about pregnancies around a scan (Sidi & Josheu, Citation2019), using it as ‘proof’ of their unborn baby to others (Ekelin et al., Citation2004). Some parents waited until their first scan to tell friends and family about their pregnancies, sharing their pictures or videos (Cadogan et al., Citation2009; Ekelin et al., Citation2004; Wadephul et al., Citation2015). Scan mementos represented the baby in a physical presence that others could interact with, and this helped create a social identity before birth (Murakami et al., Citation2012). After birth, both the parents and the parents’ social circle had a sense of ‘knowing’ the baby already (Cadogan et al., Citation2009; Cristofalo et al., Citation2006; Dykes & Stjernqvist, Citation2001; Ekelin et al., Citation2004; Firth et al., Citation2011; Freeman, Citation2000; Øyen & Aune, Citation2016; Stephenson et al., Citation2016; Westerneng et al., Citation2019).
The perception of social support was an important factor in the developing prenatal attachment (Polizzi et al., Citation2017) and some mothers chose to bring people other than their partner to scans:
I took my mother in law and my father … so there are also involved a little bit more with the pregnancy (Westerneng et al., Citation2019).
Discussion
Main findings
This review explored how prenatal attachment is impacted by antenatal imaging. The objectives were to enhance current understanding of parents’ experiences of pregnancy imaging, and to identify factors, which could impact on attachment. Six pillar themes were developed, each describing an element of the scan experience and how it impacts the developing attachment. The experience begins in advance of the scan with the creation of expectations, and continues by sharing pregnancy images and knowledge outside of the parental relationship.
Strengths and limitations
A strength of this review was in using the Pillar Integration Process, which enabled rigorous integration of results from quantitative and qualitative studies. It was chosen above other integration methods because the process is displayed clearly and therefore replicable by others (Johnson et al., Citation2019). An additional strength was the external support received from a subject expert librarian in developing the robust search strategy.
However, a few limitations need to be considered before drawing conclusions. First, the wide publication date range yielded many eligible studies but findings from earlier studies (particularly those involving 3D/4D ultrasound) may not be wholly generalisable to the current context given technological advancements and improvements in image quality (Pulliainen et al., Citation2019). Additionally, increasing availability of access to pregnancy imaging through private scan clinics may also have an impact on the parent experience, which may not be fully represented within older studies.
A meta-analysis of quantitative studies was not conducted due to heterogeneity of data. The richness of common themes across both qualitative and quantitative studies, however, highlighted the importance of using the Pillar Integration Process to ensure these key themes were strong and thematic saturation was achieved between all eligible studies while addressing the conceptual complexity of prenatal attachment and parental experiences in antenatal imaging.
Despite the high yield returned from the searches, a thorough screening process was enabled by use of the EPPI-Reviewer 4 software (Thomas et al., Citation2010). This facilitated management of the records across a shared platform and kept an electronic ‘audit trail’ of the reviewer’s judgment against the defined inclusion criteria. Although partial double screening of the records may be considered a limitation of the study, high concordance between reviewers demonstrates the clarity of the inclusion criteria. Finally, to ensure breadth of knowledge in the review, some of the included studies were of low quality. Although it may be argued that this could limit the extent to which the findings can be transferred beyond the review, use of the Pillar Integration Process to synthesise qualitative and quantitative findings helps to mitigate conflicts by highlighting the commonalities across the studies. The need for more methodologically rigorous studies in future research is also emphasised.
Interpretation
Most studies in this review measured or reported increased attachment following scanning (Boukydis et al., Citation2006; Dykes & Stjernqvist, Citation2001; Edwards et al., Citation2010; Ekelin et al., Citation2004; Firth et al., Citation2011; Freeman, Citation2000; De Jong-Pleij et al., Citation2013; Lalor & Devane, Citation2007; Murakami et al., Citation2012; Øyen & Aune, Citation2016; Righetti et al., Citation2005; Robak-Chołubek et al., Citation2015; Rustico et al., Citation2005; Sedgmen et al., Citation2006; Sidi & Josheu, Citation2019; Stephenson et al., Citation2016). Fetal imaging provided a visual image of the unborn baby that enabled expectant parents to place them in their own familiar reality (Pedreira & Leal, Citation2015), thus promoting feelings of closeness. Scans gave visual confirmation of the pregnancy, and this reassurance of viability helped to enhance attachment. This is related to parents’ pre-scan anxiety of receiving unexpected news, including that of a fetal anomaly (Brisch et al., Citation2002; Van der Zalm & Byrne, Citation2006). Rothman historically described the concept of the ‘tentative pregnancy’ (Rothman, Citation1987) where parents delay feelings towards the pregnancy until they are more confident the pregnancy is viable (Rowe et al., Citation2009). Similarly, some find it more difficult to develop an attachment to their baby when an anomaly is diagnosed (Boztepe et al., Citation2016). In this review, one study reported a temporary decrease in attachment after mothers were informed of a fetal brain anomaly (Cristofalo et al., Citation2006). Detection of a fetal anomaly may be particularly distressing beyond the first trimester, as previous unremarkable scans may create a false sense of assurance (Ekelin et al., Citation2008). Parents must address the uncertainty of the diagnosis on their emotional investment to the pregnancy, whilst simultaneously processing the loss of the baby they had initially begun to connect with (Ruschel et al., Citation2014).
Parent-centred care and implications for sonographers
Parent-centred care by sonographers was highlighted as an essential element of the scan experience to positively enhance attachment (Businelli et al., Citation2021). When parents feel actively involved, their overall perception of the scan improves (Ranji et al., Citation2012). A ‘good’ scan is determined by positive interaction with sonographers (Van der Zalm & Byrne, Citation2006), which helps to improve parents’ recognition of images and strengthen their understanding of the examination (Masroor et al., Citation2008; J. Walsh, Citation2010). Parents are then more likely to feel satisfied that their expectations for the scan have been met, even if the overall image quality during the scan is limited (Whynes, Citation2002). The review supports this finding, as whilst novel imaging techniques (e.g. 3D/4D ultrasound) may be considered desirable, any perceived impact on attachment may be attributed by parents to the explanation given to help interpret the image rather than the image itself (Ranji et al., Citation2012). This emphasises the integral role of the sonographer during the scan, who personifies the interface between technology and parental knowledge and collaborates with expectant parents to help construct an identity for the fetus (Roberts, Citation2012). Whilst the medical purpose of the examination is paramount, viewing the scan more holistically to incorporate parent-centred care practices should also be considered in the provision of sonographic education and training (Walsh, Citation2020).
Making the scan a satisfactory experience for parents (Chudleigh, Citation1999) should also be considered in providing parent-centred care, with a focus on promoting open communication (Øyen & Aune, Citation2016). However, with the extensive clinical requirements of scans placing heavy demand on sonographers (Masroor et al., Citation2008) it is not surprising parents may feel their expectations are not met (Ekelin et al., Citation2004). Emerging technologies using artificially intelligent algorithms to automate scan processes or provide clinical support for sonographers may help reduce demand (Baumgartner et al., Citation2018; Sinclair et al., Citation2018; Yaqub et al., Citation2019); however, further, large-scale prospective testing is required to evaluate the real-world clinical utility and impact on the delivery of parent-centred care (Drukker et al., Citation2020).
A cross-cutting theme was the importance of partners at scans. For fathers and non-pregnant partners, the lack of physical cues can make it difficult to accept the reality of the pregnancy, leading to distress, depression, and poor attachment (Fenwick et al., Citation2012). Scans help fathers and non-pregnant partners to engage with the pregnancy and get to know the baby through visual cues (Ekelin et al., Citation2008). The baby represents a project shared between a couple (Cristofalo et al., Citation2006; Pedreira & Leal, Citation2015) and the scan is a pregnancy-related event that both parents can experience simultaneously (Fenwick et al., Citation2012). Knowledge about the unborn baby is acquired together, and physical movements can be witnessed in real time during the scan, providing fathers and non-pregnant partners with a glimpse into the otherwise privileged access their partner has of the pregnancy (Harpel and Barras, Citation2018). The ‘thrill’ of being present cannot be fully felt through images shared afterwards (Firth et al., Citation2011), and the scan experience may further support the intrapsychic dynamics of the expectant parents by enabling an encounter with their imagined child (Walsh et al., Citation2017). When the coronavirus pandemic reached the UK in March 2020, many maternity departments placed temporary restrictions on those accompanying pregnant people to antenatal scans to minimise virus transmission. The impact of these restrictions on prenatal attachment is yet to be formally evaluated, however a recent report concludes there may be long-term implications for parents, babies and families including ‘psychosocial functioning, early parenting and child developmental outcomes’ (Lalor et al., Citation2021).
Implications for future research
This review identified the lack of published studies exploring the potential impact of fetal MRI on prenatal attachment. The searches identified two studies reporting the parent’s perspective of foetal MRI (Lie et al., Citation2019; Reed et al., Citation2016); however, they were excluded because they did not specifically describe the impact on prenatal attachment. As fetal MRI becomes popular to complement conventional ultrasound, the acceptability of this modality to parents and its potential effect on attachment requires further evaluation to facilitate successful integration into clinical pathways. In addition, the studies in this review emphasise pregnancy scans as a visual experience. Advances in 3D printing help the scan experience to be more accommodating for expectant parents who are visually impaired, contributing to attachment (Coté et al., Citation2020; Werner et al., Citation2016). These studies were excluded from this review because the 3D print was the research intervention, however they highlight the importance for sonographers performing scans to consider the needs of parents with additional sensory requirements when delivering high-quality, parent-centred care.
Conclusion
This review highlights antenatal scans as an important pregnancy experience that can enhance prenatal attachment. As well as giving reassurance regarding the health of their unborn baby, scans also provide a visual image, which parents can engage with in real-time, and utilise for attachment by attributing physical and psychological characteristics, thus transforming the baby from a medical entity (fetus) to be recognised as an individual person. The success of this transformation is dependent on sonographers interpreting images in a way that becomes accessible to parents. Sonographers can help facilitate the attachment process by providing an interactive, parent-centred scan experience that is tailored to parents’ individual emotional needs and requests of information and knowledge for the gestation of pregnancy.
Protocol registration
The protocol for this review is registered on the PROSPERO database (CRD42020197259).
Contribution to authorship
ES, CM and SA developed the review protocol. ES performed searches, reviewed papers, undertook the analysis and drafted the paper. RW reviewed references for inclusion. ES, RW, MR, CM and SA revised the review for publication.
Details of ethical approval
Not required.
Data sharing
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Supplemental Material
Download Zip (84.4 KB)Acknowledgments
The authors would like to thank Mrs Endang Scanlon for her assistance in developing the search strategy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02646838.2022.2088710.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Baumgartner, C., Kamnitsas, K., Matthew, J., Fletcher, T. P., Smith, S., Koch, L. M., & Rueckert, D. (2018). SonoNET: Real-time detection and localisation of fetal standard scan planes in freehand ultrasound. IEEE Transactions on Medical Imaging, 36(11), 2204–2215. https://doi.org/10.1109/TMI.2017.2712367
- Borg Cunen, N., Jomeen, J., Borg Xuereb, R., & Poat, A. (2017). A narrative review of interventions addressing the parental–fetal relationship. Women and Birth, 30(4), e141–e151. https://doi.org/10.1016/j.wombi.2016.11.005
- Boukydis, C. F. Z., Treadwell, M. C., Delaney-Black, V., Boyes, K., King, M., Robinson, T., & Sokol, R. (2006). Women’s responses to ultrasound examinations during routine screens in an obstetric clinic. Journal of Ultrasound in Medicine, 25(6), 721–728. https://doi.org/10.7863/jum.2006.25.6.721
- Bowlby, J. (1982). Attachment and loss: Retrospect and prospect. American Journal of Orthopsychiatry, 52(4), 664–678. https://doi.org/10.1111/j.1939-0025.1982.tb01456.x
- Boztepe, H., Ay, A., Kerimoğlu Yıldız, G., & Çınar, S. (2016). Does the visibility of a congenital anomaly affect maternal-infant attachment levels?. Journal for Specialists in Pediatric Nursing, 21, 200–211. https://doi.org/10.1111/jspn.12157
- Brisch, K. H., Munz, D., Bemmerer-Mayer, K., Kächele, H., Terinde, R., & Kreienberg, R. (2002). Ultrasound scanning for diagnosis of foetal abnormality and maternal anxieties in a longitudinal perspective. Journal of Reproductive and Infant Psychology, 20(4), 223–235. https://doi.org/10.1080/0264683021000033156
- Businelli, C., Bembich, S., Vecchiet, C., Cortivo, C., Norcio, A., Risso, M. F., & Stampalija, T. (2021). The psychological burden of routine prenatal ultrasound on women’s state anxiety across the three trimesters of pregnancy. European Journal of Obstetrics & Gynecololgy & Reproductive Biology, 256, 281–286. https://doi.org/10.1016/j.ejogrb.2020.11.065
- Cadogan, J., Marsh, C., & Winter, R. (2009). Parents’ views of 4D ultrasound scans following diagnosis of cleft condition. British Journal of Midwifery, 17(6), 374–380. https://doi.org/10.12968/bjom.2009.17.6.42607
- Chudleigh, T. (1999). Scanning for pleasure. Ultrasound in Obstetestrics & Gynecology, 14(6), 369–371. https://doi.org/10.1046/j.1469-0705.1999.14060369.x
- Condon, J. (1993). The assessment of antenatal emotional attachment: Development of a questionnaire instrument. British Journal of Medical Psychology, 66(2), 167–183. https://doi.org/10.1111/j.2044-8341.1993.tb01739.x
- Coté, J. J., Badura-Brack, A. S., Walters, R. W., Dubay, N. G., & Bredehoeft, M. R. (2020). Randomized controlled trial of the effects of 3D-printed models and 3D ultrasonography on maternal–fetal attachment. JOGNN – Journal of Obstetric, Gynecologic & Neonatal Nursing, 49(2), 190–199. https://doi.org/10.1016/j.jogn.2020.01.003
- Cristofalo, E. A., DiPietro, J. A., Costigan, K. A., Nelson, P., & Crino, J. (2006). Women’s response to fetal choroid plexus cysts detected by prenatal ultrasound. Journal of Perinatololgy, 26(4), 215–223. https://doi.org/10.1038/sj.jp.7211489
- de Jong-Pleij, E. A. P., Ribbert, L. S. M., Pistorius, L. R., Tromp, E., Mulder, E. J. H., & Bilardo, C. M. (2013). Three-dimensional ultrasound and maternal bonding, a third trimester study and a review. Prenatal Diagnosis, 33(1), 81–88. https://doi.org/10.1002/pd.4013
- Della Vedova, A. M., & Burro, R. (2017). Surveying prenatal attachment in fathers: The Italian adaptation of the Paternal antenatal attachment scale (PAAS-IT). Journal of Reproductive and Infant Psychology, 35(5), 493–508. https://doi.org/10.1080/02646838.2017.1371284
- Drukker, L., Noble, J. A., & Papageorghiou, A. T. (2020). Introduction to artificial intelligence in ultrasound imaging in obstetrics and gynecology. Ultrasound in Obstetrics & Gynecololgy, 56(4), 498–505. https://doi.org/10.1002/uog.22122
- Dykes, K., & Stjernqvist, K. (2001). The importance of ultrasound to first-time mothers’ thoughts about their unborn child. Journal of Reproductive and Infant Psychology, 19(2), 95–104. https://doi.org/10.1080/02646830123343
- Edwards, M. M., Wang, F., Tejura, T., Patel, A., Majewski, S., & Donnenfeld, A. E. (2010). Maternal reactions to two-dimensional compared to three-dimensional foetal ultrasonography. Journal of Psychosomatic Obstetrics & Gynecololgy, 31(2), 53–59. https://doi.org/10.3109/01674821003793038
- Ekelin, M., Crang-Svalenius, E., & Dykes, A. K. (2004). A qualitative study of mothers’ and fathers’ experiences of routine ultrasound examination in Sweden. Midwifery, 20(4), 335–344. https://doi.org/10.1016/j.midw.2004.02.001
- Ekelin, M., Crang-Svalenius, E., Nordström, B., & Dykes, A. K. (2008). Parents’ experiences, reactions and needs regarding a nonviable fetus diagnosed at a second trimester routine ultrasound. JOGNN – Journal of Obstetric, Gynecologic & Neonatal Nursing, 37(4), 446–454. https://doi.org/10.1111/j.1552-6909.2008.00258.x
- Fenwick, J., Bayes, S., & Johansson, M. (2012). A qualitative investigation into the pregnancy experiences and childbirth expectations of Australian fathers-to-be. Sexual and Reproductive Healthcare, 3(1), 3–9. https://doi.org/10.1016/j.srhc.2011.11.001
- Firth, E. R., Mlay, P., Walker, R., & Sill, P. R. (2011). Pregnant women’s beliefs, expectations and experiences of antenatal ultrasound in Northern Tanzania. African Journal of Reproductive Health, 15(2), 91–107 https://journals.co.za/doi/abs/10.10520/EJC135765.
- Flanagan, P., Dowling, M., & Gethin, G. (2020). Barriers and facilitators to seasonal influenza vaccination uptake among nurses: A mixed methods study. Journal of Advanced Nursing, 76(7), 1746–1764. https://doi.org/10.1111/jan.14360
- Freeman, A. (2000). The influences of ultrasound stimulated paternal fetal bonding and gender identification. Journal of Diagnostic Medical Sonography, 16(6), 237–241. https://doi.org/10.1177/875647930001600604
- Glover, V. (2014). Maternal depression, anxiety and stress during pregnancy and child outcome; What needs to be done. Best Practice & Research. Clinical Obstetrics & Gynaecology, 28(1), 25–35. https://doi.org/10.1016/j.bpobgyn.2013.08.017
- Göbel, A., Stuhrmann, L. Y., Harder, S., Schulte-Markwort, M., & Mudra, S. (2018). The association between maternal-fetal bonding and prenatal anxiety: An explanatory analysis and systematic review. Journal of Affective Disorders, 239, 313–327. https://doi.org/10.1016/j.jad.2018.07.024
- Gonçalves, L. F., Lee, W., Espinoza, J., & Romero, R. (2005). Three- and 4-dimensional ultrasound in obstetric practice does it help? Journal of Ultrasound in Medicine, 24(12), 1599–1624. https://doi.org/10.7863/jum.2005.24.12.1599
- Harpel, T. S., & Barras, K. G. (2018). The impact of ultrasound on prenatal attachment among disembodied and embodied knowers. Journal of Family Issues, 39(6), 1523–1544. https://doi.org/10.1177/0192513X17710774
- Johnson, R. E., Grove, A. L., & Clarke, A. (2019). Pillar Integration Process: A joint display technique to integrate data in mixed methods research. Journal of Mixed Methods Research, 13(3), 301–320. https://doi.org/10.1177/1558689817743108
- Khan, K. S., Kunz, R., Kleijnen, J., & Antes, G. (2003). Five steps to conducting a systematic review. Journal of the Royal Society of Medicine, 96(3), 118–121. https://doi.org/10.1258/jrsm.96.3.118
- Lalor, J. G., & Devane, D. (2007). Information, knowledge and expectations of the routine ultrasound scan. Midwifery, 23(1), 13–22. https://doi.org/10.1016/j.midw.2006.02.001
- Lalor, J., Ayers, S., Calleja Agius, J., Downe, S., Gouni, O., Hartmann, K., & Horsch, A. (2021). Balancing restrictions and access to maternity care for women and birthing partners during the COVID-19 pandemic: A commentary. https://doi.org/10.22541/au.161689510.05966705/v1
- Lapaire, O., Alder, J., Peukert, R., Holzgreve, W., & Tercanli, S. (2007). Two- versus three-dimensional ultrasound in the second and third trimester of pregnancy: Impact on recognition and maternal-fetal bonding. A prospective pilot study. Archives of Gynecology and Obstetrics, 276(5), 475–479. https://doi.org/10.1007/s00404-007-0368-7
- Leung, K. Y., Ngai, C. S. W., Lee, A., Chan, H. Y., Leung, W. C., Lee, C. P., & Tang, M. H. Y. (2006). The effects on maternal anxiety of two-dimensional versus two-plus three-/four-dimensional ultrasound in pregnancies at risk of fetal abnormalities: A randomized study. Ultrasound in Obstetrics & Gynecology, 28(3), 249–254. https://doi.org/10.1002/uog.284411
- Lie, M., Graham, R., Robson, S. C., & Griffiths, P. D. (2019). “He looks gorgeous” – IuMR images and the transforming of foetal and parental identities. Sociology of Health & Illness, 41(2), 360–377. https://doi.org/10.1111/1467-9566.12831
- Lindgren, K. (2001). Relationships among maternal-fetal attachment, prenatal depression, and health practices in pregnancy. Research in Nursing & Health, 24(3), 203–217. https://doi.org/10.1002/nur.1023
- Lockwood, C., Munn, Z., & Porritt, K. (2015). Qualitative research synthesis: Methodological guidance for systematic reviewers utilizing meta-aggregation. International Journal of Evidence Based Healthcare, 13(3), 179–187. https://doi.org/10.1097/XEB.0000000000000062
- Masroor, I., Ahmed, H., & Ajmal, F. (2008). Impact of prenatal ultrasound consultation on maternal anxiety. Journal of the Dow University of Health Sciences, 2(1), 16–20. https://mail.jduhs.com/index.php/jduhs/article/view/720.
- Mitchell, L. M. (2004). Women’s experiences of unexpected ultrasound findings. Journal of Midwifery & Women’s Health, 49(3), 228–234. https://doi.org/10.1016/j.jmwh.2003.11.004
- Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., & Mu, P. F. (2020) Explanation of analytical cross sectional studies critical appraisal. JBI Manual for Evididence Synthesis, 1–5. https://joannabriggs.org/critical_appraisal_tools (Accessed 11 August 2021)
- Moon, M. D. (2019). Triangulation: A method to increase validity, reliability, and legitimation in clinical research. Journal of Emergency Nursing, 45(1), 103–105. https://doi.org/10.1016/j.jen.2018.11.004
- Murakami, K., Tsujino, K., Sase, M., Nakata, M., Ito, M., & Kutsunugi, S. (2012). Japanese women’s attitudes towards routine ultrasound screening during pregnancy. Nursing & Health Sciences, 14(1), 95–101. https://doi.org/10.1111/j.1442-2018.2011.00670.x
- National Institute for Health and Care Excellence. (2019) Overview: Antenatal care for uncomplicated pregnancies [CG62]. https://www.nice.org.uk/guidance/cg62 (Accessed: 11 August 2021)
- Øyen, L., & Aune, I. (2016). Viewing the unborn child - pregnant women’s expectations, attitudes and experiences regarding fetal ultrasound examination. Sexual & Reproductive Healthcare, 7, 8–13. https://doi.org/10.1016/j.srhc.2015.10.003
- Page, M., McKenzie, J., Bossuyt, P., Boutron, I., Hoffmann, T., Mulrow, C. et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal, 372, 8284 . https://doi.org/10.1136/bmj.n71
- Pedreira, M., & Leal, I. (2015). What is my baby like? Representations concerning the baby in the third trimester of pregnancy. Psychology, Community & Health, 4(3), 156–170. https://doi.org/10.5964/pch.v4i3.141
- Polizzi, C., Perricone, G., Duca, V., Carollo, A., Marceca, M., & Fontana, V. (2017). A study on maternal-fetal attachment in pregnant women undergoing fetal echocardiography. Journal of Pediatric & Neonatal Individualized Medicine, 6(1), 1–10. https://doi.org/10.7363/060137
- Pulliainen, H., Niela-Vilén, H., Ekholm, E., & Ahlqvist-Björkroth, S. (2019). Experiences of interactive ultrasound examination among women at risk of preterm birth: A qualitative study. BMC Pregnancy and Childbirth, 19(1), 1–8. https://doi.org/10.1186/s12884-019-2493-2
- Ranji, A., Dykes, A. K., & Ny, P. (2012). Routine ultrasound investigations in the second trimester of pregnancy: The experiences of immigrant parents in Sweden. Journal of Reproductive and Infant Psychology, 30(3), 312–325. https://doi.org/10.1080/02646838.2012.717266
- Reed, K., Kochetkova, I., & Whitby, E. (2016). Visualising uncertainty: Examining women’s views on the role of Magnetic resonance imaging (MRI) in late pregnancy. Social Science & Medicine, 164, 19–26. https://doi.org/10.1016/j.socscimed.2016.07.012
- Righetti, P. L., Dell’Avanzo, M., Grigio, M., & Nicolini, U. (2005). Maternal/paternal antenatal attachment and fourth-dimensional ultrasound technique: A preliminary report. British Journal of Psychology, 96(1), 129–137. https://doi.org/10.1348/000712604X15518
- Robak-Chołubek, D., Chołubek, G., & Piróg, E. (2015). Determining fetal sex in pregnancy with reference to pregnant women behavior in late pregnancy. Polish Journal of Public Health, 125(2), 87–89. https://doi.org/10.1515/pjph-2015-0030
- Roberts, J. (2012). “Wakey wakey baby”: Narrating four-dimensional (4D) bonding scans. Sociology of Health & Illness, 34(2), 299–314. https://doi.org/10.1111/j.1467-9566.2011.01345.x
- Rothman, B. (1987). The Tentative pregnancy: Prenatal diagnosis and the future of motherhood. (Penguin Books, New York, USA
- Rowe, H., Fisher, J., & Quinlivan, J. (2009). Women who are well informed about prenatal genetic screening delay emotional attachment to their fetus. Journal of Psychosomatic Obstetrics & Gynecology, 30(1), 34–41. https://doi.org/10.1080/01674820802292130
- Ruschel, P., Zielinsky, P., Grings, C., Pimentel, J., Azevedo, L., Paniagua, R., & Nicoloso, L. H. (2014). Maternal-fetal attachment and prenatal diagnosis of heart disease. European Journal of Obstetrics & Gynecololgy & Reproductive Biology, 174(1), 70–75. https://doi.org/10.1016/j.ejogrb.2013.11.02961
- Rustico, M. A., Mastromatteo, C., Grigio, M., Maggioni, C., Gregori, D., & Nicolini, U. (2005). Two-dimensional vs. two- plus four-dimensional ultrasound in pregnancy and the effect on maternal emotional status: A randomized study. Ultrasound in Obstetrics & Gynecology, 25(5), 468–472. https://doi.org/10.1002/uog.1894
- Sandelowski, M., Voils, C. I., & Barroso, J. (2006). Defining and designing mixed research synthesis studies. Research in the Schools: A Nationally Refereed Journal Sponsored by the Mid-South Educational Research Association and the University of Alamaba, 13(1), 29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2809982/.
- Sedgmen, B., McMahon, C., Cairns, D., Benzie, R. J., & Woodfield, R. L. (2006). The impact of two-dimensional versus three-dimensional ultrasound exposure on maternal-fetal attachment and maternal health behavior in pregnancy. Ultrasound in Obstetrics & Gynecology, 27(3), 245–251. https://doi.org/10.1002/uog.2703
- Sidi, M., & Josheu, N. (2019). The role of ultrasound in enhancing maternal–fetal bonding in Kaduna Metropolis, Nigeria. Dutse Journal of Pure & Applied Sciences, 5(2), 169–179. https://fud.edu.ng/journals/dujopas/2019_DEC_Vol_5_No_2b/Page%20169-179%2041.pdf.
- Sinclair, M., Baumgartner, C., Matthew, J., Bai, W., Martinez, J. C., Li, Y., & King, A. P. (2018) Human-level performance on automatic head biometrics in fetal ultrasound using fully convolutional neural networks. International conference IEEE Engineering in medicine & biology society, 714–717. https://doi.org/10.1109/EMBC.2018.8512278
- Skelton, E., Malamateniou, C., Rutherford, M., & Ayers, S. (2020) The impact of antenatal imaging on parent-fetal bonding: A systematic review. PROSPERO 2020 CRD42020197259. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020197259 (Accessed 29 April 2022)
- Sreejith, V. P., Arun, V., Devarajan, A. P., Gopinath, A., & Sunil, M. (2018). Psychological effect of prenatal diagnosis of cleft lip and palate: A systematic review. Contemporary Clinical Dentistry, 9(2), 304–308. https://doi.org/10.4103/ccd.ccd_673_17
- Stephenson, N., McLeod, K., & Mills, C. (2016). Ambiguous encounters, uncertain foetuses: Women’s experiences of obstetric ultrasound. Feminist Review, 113(1), 17–33. https://doi.org/10.1057/fr.2016.6
- Stern, C., Lizarondo, L., Carrier, J., Godfrey, C., Rieger, K., Salmond, S., & Loveday, H. (2020). Methodological guidance for the conduct of mixed methods systematic reviews. JBI Manual for Evidence Synthesis, 18(10), 2108–2118. https://doi.org/10.11124/JBISRIR-D-19-00169
- Thomas, J., Brunton, J., & Graziosi, S. (2010) EPPI-Reviewer 4: software for research synthesis. EPPI-Centre Software. Social Science Research Unit, UCL Institute of Education.
- Thomas, G. M., Roberts, J., & Griffiths, F. E. (2017). Ultrasound as a technology of reassurance? How pregnant women and health care professionals articulate ultrasound reassurance and its limitations. Sociolology of Health & Illness, 39(6), 893–907. https://doi.org/10.1111/1467-9566.12554
- Trombetta, T., Giordana, M., Santoniccolo, F., Vismara, L., Della Vedova, A. M., & Rollè, L. (2021). Pre-natal attachment and parent-to-infant attachment: A systematic review. Frontiers in Psychology, 12, 1–17. https://doi.org/10.3389/fpsyg.2021.620942
- Tufanaru, C., Munn, Z., Aromataris, E., Campbell, J., & Hopp, L. (2020). In Aromataris E. & Munn Z. (Eds.), 3. Systematic reviews of effectiveness JBI Manual for Evidence Synthesis. https://doi.org/10.46658/JBIMES-20-04.
- van den Bergh, B., & Simons, A. (2009). A review of scales to measure the mother-foetus relationship. Journal of Reproductive and Infant Psychology, 27(2), 114–126. https://doi.org/10.1080/02646830802007480
- Van der Zalm, J. E., & Byrne, P. J. (2006). Seeing baby: Women’s experience of prenatal ultrasound examination and unexpected fetal diagnosis. Journal of Perinatololgy, 26(7), 403–408. https://doi.org/10.1038/sj.jp.7211540
- Wadephul, F., Jomeen, J., & Glover, L. (2015). Women’s experiences of commercial three -dimensional ultrasound scans. MIDIRS Midwifery Digest, 25(4), 433–438 https://core.ac.uk/download/pdf/151157181.pdf.
- Walsh, J. (2010). Definitions matter: If maternal-fetal relationships are not attachment, what are they? Archive of Women’s Mental Health, 13(5), 449–451. https://doi.org/10.1007/s00737-010-0152-8
- Walsh, T. B., Tolman, R. M., Singh, V., Davis, M. M., & Davis, R. N. (2017). Expectant fathers’ presence at prenatal ultrasounds: An opportunity for engagement. Social Work Research, 41(3), 181–185. https://doi.org/10.1093/swr/svx014
- Walsh, T. B. (2020). Your baby is so happy, active, uncooperative: How prenatal care providers contribute to parents’ mental representations of the baby. Midwifery, 83, 1–8. https://doi.org/10.1016/j.midw.2020.102630
- Werner, H., Lopes, J., Tonni, G., & Araujo Júnior, E. (2016). Maternal-fetal attachment in blind women using physical model from three-dimensional ultrasound and magnetic resonance scan data: Six serious cases. Journal of Maternal & Neonatal Medicine, 29(14), 2229–2232. https://doi.org/10.3109/14767058.2015.1085015
- Westerneng, M., Diepeveen, M., Witteveen, A. B., Westerman, M. J., Van Der Horst, H. E., Van Baar, A. L., & De Jonge, A. (2019). Experiences of pregnant women with a third trimester routine ultrasound - A qualitative study. BMC Pregnancy and Childbirth, 19(1), 1–10. https://doi.org/10.1186/s12884-019-2470-9
- Whynes, D. K. (2002). Receipt of information and women’s attitudes towards ultrasound scanning during pregnancy. Ultrasound in Obstetrics & Gynecology, 19(1), 7–12. https://doi.org/10.1046/j.0960-7692.2001.00517.x
- Yaqub, M., Cook, K., Cocks, K., Chen, Z., Chikkanna, B., Sleep, N., & Papageorghiou, A. T. (2019). Auditing the quality of ultrasound images using an AI solution: ScanNav® for fetal second trimester ultrasound scans. Ultrasound in Obstetetrics & Gynecololgy, 54(S1), 87. https://doi.org/10.1002/uog.20656