Abstract
The possibilities of using gene therapy for bone regeneration have been extensively investigated. Improvements in the design of new transfection agents, combining vectors and delivery/release systems to diminish cytotoxicity and increase transfection efficiencies have led to several successful in vitro, ex vivo and in vivo strategies. These include growth factor or short interfering ribonucleic acid (siRNA) delivery, or even enzyme replacement therapies, and have led to increased osteogenic differentiation and bone formation in vivo. These results provide optimism to consider use in humans with some of these gene-delivery strategies in the near future.
Introduction
Bone (re)generation is one of the major focus points within the field of regenerative medicine. To create bone constructs, many strategies have been developed, including different cell-based techniques, bioactive materials and various growth factors (Arthur, Zannettino, & Gronthos, Citation2009; Chimutengwende-Gordon & Khan, Citation2012). One of the possibilities is the use of genetic intervention techniques to stimulate bone formation. This review discusses the main gene therapy strategies, their possible application and some future directions in the field of bone tissue engineering.
For engineering of bone tissue several components play a key role, such as scaffold materials, bone-forming cells or their progenitors and other biological stimuli, often growth factors. Depending on its composition, a hybrid bone replacement construct will elicit a number of molecular signals at the implantation site, leading ideally to osteogenic differentiation followed by bone formation. Scaffolds mainly function to provide a three-dimensional structure, a biomechanical component and function as vehicle for biological components. Recent discussions on bone-replacement materials and scaffolds can be found elsewhere (Bose & Tarafder, Citation2012; Deng, James, Laurencin, & Kumbar, Citation2012; Chimutengwende-Gordon & Khan, Citation2012). Many studies have investigated the feasibility of addition of growth factors (Kempen et al., Citation2010), either as protein or delivered as plasmid deoxyribonucleic acid (DNA) via gene therapy. The best-known growth factors used in tissue-engineered constructs are bone morphogenetic protein-2 (BMP-2) and -7, insulin like growth factor-1 (IGF-1), stromal derived factor-1α (SDF-1α) and tumor necrosis factor α (TNF-α). The most commonly used cell type in bone-tissue-engineering constructs is the multipotent stromal cell (MSC). MSCs can be isolated from various adult tissues such as bone marrow, adipose tissue, dental pulp, muscle and umbilical cord blood and are known for their ability to differentiate into different lineages such as cartilage, adipose tissue and bone. Furthermore, they elicit immune repressing capacities, making them also suitable for allogeneic transplantation (Aggarwal & Pittenger, Citation2005; Klyushnenkova et al., Citation2005). These features make MSCs also the main targeted cell type for gene intervention techniques in the field of bone tissue engineering (Santos et al., Citation2012).
Gene therapy: history and basic concepts
Gene therapy comprises the introduction of functional genes into cells in order to enhance or enforce their expression. This most common form of additive gene therapy is now used in patients for some rare conditions. An inhibitory approach using short interfering ribonucleic acid (siRNA) or micro ribonucleic acid (miRNA) is still under development and may have potential in the field of bone tissue engineering as well. Different strategies to deliver DNA can be divided in to viral transduction and non-viral transfection, both of which can be performed in vivo and ex vivo. The main difference between the application of a viral packaging unit, which may be cell-specific, versus the use of naked oligomeric DNA or plasmid DNA, which is often complexed to a transfection agent, is the ability to obtain prolonged transgene expression by viruses that incorporate their genetic material in the host genome, whereas the chance for this happening with non-viral vectors is nil (Martin et al., Citation1999; Thomas, Ehrhardt, & Kay, Citation2003) and therefore a transient effect is achieved. The importance of this aspect only became clear in 1999, when the first gene-therapy-based clinical trial started with ADA-SCID (adenosine deaminase – severe combined immune deficiency) patients. These patients have a severely impaired adaptive immune system due to a single gene mutation coding for the enzyme adenosine deaminase (ADA). Delivery of this gene in virally transduced white blood cells can cure these patients. However 4/10 patients in this study eventually developed leukemia, because the new ADA gene got incidentally inserted into the genome close to the site of a proto-oncogene. This caused the food and drug administration (FDA) to ban all clinical trials using gene therapy for nearly a decade, which damaged the image of gene intervention techniques. With safer viral vectors and the development of non-viral delivery methods these techniques are again being investigated at the moment, in more than 1840 clinical trials for various disorders (Clinical trial database). An improved version of the ADA trial was published in 2009 and described feasibility in 50% of the included patients but is still experimental because of several vector-related side effects (Montiel-Equihua, Thrasher, & Gaspar, Citation2012). No application of gene therapy in the field of bone (re)generation has been reported so far.
Gene therapy in bone tissue engineering
Use of gene intervention techniques for generation of bone tissue does not involve the replacement of a non-functional gene as is the case in genetic disorders, but involves the delivery of transcription or growth factors. Enforced expression of growth factors has several advantages compared with the addition of protein growth factors. Many proteins have a short half-life at the site of application, resulting in the delivery of up to milligram dosages to provide a sufficient stimulus that lasts longer than a few days in order to induce osteogenic differentiation and eventual bone formation. As a result of these supraphysiological local doses, protein diffusion is more likely to happen and systemic effects may occur. Recently, the negative side effects of BMP-2 in spinal fusion procedures were reported (Shimer, Oner, & Vaccaro, Citation2009; Carragee, Hurwitz, & Weiner, Citation2011; Glassman, Gum, Crawford, Shields, & Carreon, Citation2011). Gene therapy as a means to administer growth factors might overcome the use of high protein doses, because a sustained delivery of protein can be achieved (Southwood, Frisbie, Kawcak, & McIlwraith, Citation2004). Because of their ability to induce bone formation in vivo, BMPs (in particular, BMP-2, -4 and -7) are candidates for this kind of gene intervention techniques. One of the earliest studies, however, used PTH1-34 in gene-activated collagen matrices to transfect cells both in vivo and ex vivo (Bonadio, Smiley, Patil, & Goldstein, Citation1999; Fang et al., Citation1996). Later, growth factors such as IGF-1, VEGF or TNF-α have been shown to contribute to osteogenic differentiation or bone formation (Table ; Yun et al., Citation2012).
Table 1. Overview of gene therapy results in bone (re)generation.
One of the possibilities of gene therapy is the expression of heterodimeric proteins. During this process, multiple genes are expressed in the same cell, ensuring that translation and protein dimerization occur simultaneously for both genes. For instance in the case of combining different BMPs, it is known that the BMP-2/BMP-7 heterodimer is more potent in inducing bone formation than any of the homodimers (Kawai et al., Citation2009). Secretion of the dimer will then target BMP-receptor-positive cells.
Apart from growth factors, bone regeneration via gene therapy can also work on constitutive expression of proteins that are rapidly degraded, such as the transcription factors Runx2/Cbfal, Sox9 and Osterix, or on underlying genetic diseases such as hypophosphatasis (HPP), with enzyme-replacement therapies (Lai et al., Citation2011; McCanless, Jennings, Cole, Bumgardner, & Haggard, Citation2011; McKee et al., Citation2011; Park & Na, Citation2012; Yun et al., Citation2012).
Viral versus non-viral gene delivery
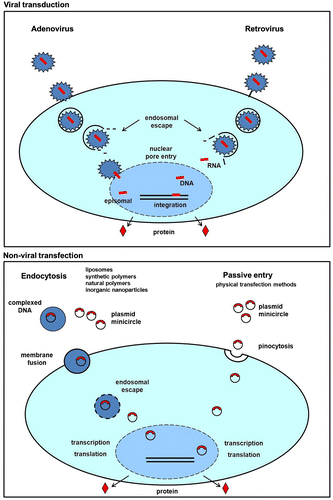
As we have already stated, genes can be delivered both virally and non-virally. Both delivery routes have their own advantages and disadvantages and the choice of suitability depends highly on the target cell type and desired expression period. Viral gene delivery is considered the most efficient way of gene transfer for many cell types. It relies on viral envelope proteins to transfer the gene of interest into the cytoplasm, where it is transported to the nucleus and subsequently expressed. The viral vector can either integrate into the host genome, leading to a stable expression of the introduced gene, or remain present as an episomal vector, which is gradually lost after cell division. The commonly used viral vectors are retroviruses, adenoviruses, adeno-associated viruses (AAVs) and lenti- and herpes viruses (Lim, Citation2012; Mingozzi & High, Citation2012; Sakuma, Barry, & Ikeda, Citation2012). The main concern of using viral transduction is triggering an immune response to the viral proteins. To avoid this, the vectors are modified to contain only the minimally necessary viral genes. In the case of retroviruses and AAVs, there still remains a risk that normal gene function can be disturbed based on insertional mutagenesis (Warnock, Daigre, & Al-Rubeai, Citation2011; Figure ).
Non-viral gene delivery is mostly used in the form of plasmid DNA, which can easily be isolated from bacteria. One of the main advantages of using bacterial DNA is that this does not stimulate any pre-existing adapted immune reaction. Cytotoxicity is only seen when unmethylated cytosine guanine dinucleotide motifs (so-called CpG motifs) are combined with liposomes (Hyde et al., Citation2008). Furthermore, localized expression of plasmid DNA is very feasible, because it shows a high turnover rate in the blood stream, which minimizes unwanted diffusion to off-target tissues. There is no limit to the amount of DNA that can be delivered, and gene transfer is efficient in a large number of cell types (Humbert & Halary, Citation2012; Lustig & Akil, Citation2012; Su, Wu, Wang, & Yeh, Citation2012). Plasmid DNA vectors do contain viral promoter sequences but do not integrate in the host genome, leading to transient expression patterns (Figure ). Since bone regeneration usually does not require a permanent expression of the desired gene, non-viral tissue engineering is very suitable for this application. The disadvantage, however, is that transfection efficiencies are often very low, particularly in vivo. Therefore, a considerable effort in the field is dedicated to optimizing the different transfection methods, which are described in detail below. To extend the transgene expression period and prevent loss of transgene mRNA, minicircle DNA can be used instead of regular plasmid DNA. This new method of producing vectors increases transgene expression up to a 1000-fold and can be considered as a candidate to replace regular plasmid DNA in the future (Kay, Citation2011).
Non-viral gene delivery methods
Non-viral gene transfer is usually performed using plasmid DNA. These small, circular, double-stranded DNAs show a stable chemistry, are easily produced in bacteria and may contain a variety of promoters and therapeutic copy deoxyribonucleic acids (cDNAs) (Gill, Pringle, & Hyde, Citation2009). The plasmid DNA has to reach the nucleus of the cell to be transcribed and therefore several barriers have to be overcome. First, degradation and body clearance (in vivo) must be prevented. Then it has to cross the cell and nuclear membranes to enter the nucleus and the DNA has to be released from any possible transfection complexes (Dang & Leong, Citation2006; Smith, Citation2008). The efficiency of non-viral gene delivery is dependent on the preparation, purification and composition of the DNA and also on the chosen transfection method, the cell type and its cell cycle phase (Table ).
In the following sections, the main non-viral methods for gene delivery are described, together with their possible applications in bone-tissue-engineering models.
Liposome-based transfection
Cellular uptake of plasmid DNA via endocytosis is enabled by formation of lipoplexes, cationic lipids that self-assemble into liposomes due to their polar heads and non-polar tails, combined with plasmid DNA. The DNA is either entrapped in the internal aqueous core or bound to the surface (Düzgüneş et al., Citation2003; Dang & Leong, Citation2006). The interaction of liposomes with the cell membrane induces endocytosis. After endocytosis, it is thought that the endosomal membrane destabilizes, causing the release of DNA into the cytoplasm (Hoekstra, Rejman, Wasungu, Shi, & Zuhorn, Citation2007; Figure ).
Even though liposome-based DNA delivery was one of the first methods to introduce exogenous DNA into eukaryotic cells, this method is hardly used in the field of bone tissue engineering. This is possibly due to the fact that lipoplexes are cytotoxic at higher concentrations because they are able to interact with and destabilize the cell membrane (Tachibana et al., Citation2002; Madeira et al., Citation2010). High concentrations are needed, however, to induce high transfection efficiencies. This limits their suitability for DNA-based gene therapy (Franceschi, Wang, Krebsbach, & Rutherford, Citation2000; Blum, Barry, Mikos, & Jansen, Citation2003; Wiethoff & Middaugh, Citation2003). Lipoplexes, however, are also used for RNA interference (RNAi) delivery, which seems to be more successful at inducing bone formation. In case of gene silencing using RNA interference, a temporary block of the negative regulatory genes is achieved. In this way, often multiple genes are targeted at relatively low concentrations. Zhang et al. (Citation2012) were able to target siRNA towards bone-forming areas based on liposome surface properties. The targeted RNA stimulated osteoblast activity without interfering with bone resorption, so less bone remodeling and breakdown took place, leading to higher quantities of bone. These results indicate that the use of targeted delivery of lipoplexes is a promising future strategy for bone tissue engineering.
Synthetic polymer-based transfections
Polymers can be designed to act as gene carriers. They are very versatile molecules that can vary in chemical composition, weight, size, 3D architecture, side-chain length and branching, density, etc. (T.G. Park, Jeong, & Kim, Citation2006). Most polymers investigated for gene therapy are cationic and contain a high density of positively charged groups, mainly amines (Santos et al., Citation2012). These positive groups interact with the negatively charged phosphate groups of DNA to form condensed structures called polyplexes. The structures are similar to lipoplexes and can be endocytosed by cells (Figure ).
The first cationic polymer-based vector used was poly-L-lysine (PLL). At first it appeared to be a poor transfection agent, owing to a low transfection efficiency and a tendency to aggregate and precipitate, causing cell death (Choi, Liu, Choi, Kim, & Park, Citation1998). Therefore in later studies PLL is combined with other chemicals such as palmic acid (PA) or other transfection agents such as liposomes. After conjugation of PLL to PA, the polyplexes showed improved interactions with the cell membrane thereby increasing internalization of the polyplexes. The DNA uptake and thus transfection efficiencies were enhanced by 10-fold, up to 22%, making it comparable to liposome-based transfection efficiencies, which were used as a control (Incani et al., Citation2007). The combination of both increased transfection efficiencies even more (Clements et al., Citation2007).
Other examples of synthetic polymer-based delivery agents are the biodegradable polylactic acid (PLA), poly(lactide-co-glycolide) (PLG) and poly(lactic-co-glycolic acid) (PLGA; Dang & Leong, Citation2006; Mintzer & Simanek, Citation2009). These are interesting systems because they are degraded into non-toxic waste products after delivery of the plasmid DNA. PLG was first described as gene-activated matrix from which DNA could be released slowly in vivo leading to higher transfection efficiencies, longer exposure of cells to the DNA and eventually more bone in-growth (Shea, Smiley, Bonadio, & Mooney, Citation1999). To tailor degradation rate and release profile, PLGA is often used in the form of microspheres. The advantages of the microspheres are low cytotoxicity and long-term transgene expression (Hedley, Curley, & Urban, Citation1998; Yang et al., Citation2009) probably as a result of the gradual release of DNA. Other advantages are high cell viability of different types of stem cell such as embryonic, adult adipose and cord-blood-derived MSCs (80–90%). Transfection efficiencies up to 60% (embryonic stem cells) can be achieved with an ideal poly-end modified derivate (Gwak & Kim, Citation2008).
One of the most popular polymer-based vectors is based on polyethylenimines (PEI). The widely used commercial form often serves as a control in gene-delivery studies, and is pursued as one of the most successful transfection agents (Reiser et al., Citation2005). After endocytosis, PEI increases the pumping of protons and influx of anions into the internal cell organelles, leading to osmotic rupture. PEI/DNA complexes then enter the nucleus, and it is thought that PEI-complexation prevents premature breakdown of the DNA. It has been studied as a gene carrier over a wide range of molecular weights (0.4–800 kDa), but 12–70 kDa seems to be most effective (Fischer, Bieber, Li, Elsässer, & Kissel, Citation1999; Godbey, Barry, Saggau, Wu, & Mikos, Citation2000). In MSCs the maximum transfection efficiency for tissue-engineering applications has been 50% (Saraf et al., Citation2008). Besides the molecular weight range, N/P ratio (N = molar number of primary amines in the polymer; P = molar number of phosphate groups in the pDNA backbone) also influences transfection efficiencies. A lower N/P ratio induces higher transfection rates, but the cytotoxicity is also increased (Ahn et al., Citation2008). In order to increase the transfection efficiency and decrease the cytotoxicity, several studies focus on combining PEI with other biologicals, with varying success. When PEI is covalently bound to hyaluronic acid (HA), polyplexes are formed which indeed increase transfection efficiencies comparable to lipofectamine (up to 34%). This can be explained by the fact that HA improves cell targeting by binding to CD44 expressed by many cell types including human MSCs at their surface. It also reduces the toxicity of PEI because the cationic amine groups from PEI are combined with the carboxylic groups present in HA resulting in higher cell viability levels (Lei et al., Citation2010).
The perfect synthetic polymer-based vector has yet to be found. It depends on the delivery method, target cells, host, etc. as to which polymer or combination of polymers induces the best biological effect. In the future polymers might become very efficient gene carriers. They are relatively efficient, easy to handle, can be combined with other carriers, their degradation can be tailored for optimal release profiles and the delivery route can be manipulated. For example, it is possible to design delivery agents that release the DNA as a result of external stimuli through chemical bond cleavage. This makes DNA targeting into specific cell types in vivo more efficient (Wolff & Rozema, Citation2008).
Despite all these promising features, hardly any of these strategies has been translated to the clinical practice. Similar to what is seen with liposomes, dosage determines the optimal transfection efficiency, but coincides with cell damage due to cell membrane manipulation.
Natural polymer-based transfection
Natural non-viral polymer-based vectors are often used in gene-therapy applications because of their biodegradability, biocompatibility, low/non-toxicity and because they can be modified to increase the functionality of these vectors (Dang & Leong, Citation2006).
One of the most studied natural polymers is chitosan, a polysaccharide that is formed by deacetylating chitin. It is studied as a gel and as micro/nanoparticles (Moreira et al., Citation2009; Garcia-Fuentes & Alonso, Citation2012). Chitosan forms complexes with the DNA, but the exact mechanism behind the transfection is still unknown. When compared with liposomes, it is found that the transfection efficiency of chitosan is always a little bit lower, comparable to naked DNA, but the compound is significantly less toxic than liposomes and easy to work with (Corsi, Chellat, Yahia, & Fernandes, Citation2003). To improve the transfection efficiencies, chitosan is often combined with other materials. For orthopedic applications it can be incorporated into titanium films with BMP-2 plasmid DNA, or incorporated in alginate hydrogel as nanoparticles (Park, Choi, Leong, Kwon, & Eun, Citation2007).
Alginate as such is also used for gene delivery. This anionic linear copolymer of β-D-mannuronic acid and α-L-glucuronic acid residues gelates with bivalent cations like Ca2+ (Kong, Kim, Huang, & Mooney, Citation2008). It is considered non-toxic and biocompatible, and can be used in the form of nanoparticles or combined with other hydrogels (Krebs, Salter, Chen, Sutter, & Alsberg, Citation2010; Park et al., Citation2007). High transfection efficiencies, a slow release of biologically active BMP-2 and osteogenic differentiation in vitro and bone formation in vivo were recently found as a result of alginate-mediated transfections with plasmid DNA (Wegman et al., Citation2011, Citation2012).
Another well-known natural polymer is gelatin. Gelatin is formed after hydrolysis of collagen and easy to work with. It is very biocompatible and is mostly used as microparticles formed by emulsion techniques. After gelatin cross-linking by glutaraldehyde (GA), the stronger cross-links prevent fast degradation in vitro and in vivo. It is a widely used release system for growth factors, but can also be used to release DNA (Kasper, Kushibiki, Kimura, Mikos, & Tabata, Citation2005; Kasper et al., Citation2006; Chew et al., Citation2011).
Natural polymers in general have in common that they are often easy to work with, hardly trigger immune responses and are easily available. However, the notion that these natural polymers can be used as gene-delivery systems is only slowly being appreciated. In most cases the hydrogels that they form are used as delivery vehicles for other DNA complexes. Besides lipo- or polyplexes, these gels are also often combined with other materials such as synthetic polymers or ceramics for biomechanical and osteopromotive reasons. Although batch variation in some hydrogels might greatly influence transfection efficiencies, the addition of ceramic particles does not (Wegman et al., Citation2012).
Inorganic nanoparticles
A novel, non-viral gene-delivery method is based on the use of inorganic nanoparticles (Bourgeat-Lami, Citation2002; Chowdhury & Akaike, Citation2005). Plasmid DNA is coupled to small material particles such as gold, silica, iron oxide or calcium phosphate. These particles are endocytosed, thereby delivering the plasmid DNA intracellularly. Transfection efficiencies for these methods are very moderate, but as a result of several advantages, such as low toxicity, good shape control and easy storage ability, more research is being conducted on these types of delivery agents (Parveen, Misra, & Sahoo, Citation2012). When combined with a different kind of carrier, it was found that gold particles covered with DNA/JetPEI complexes can increase transfection efficiencies 2.5-fold (Uchimura et al., Citation2007). A similar result was found for silica nanoparticles combined with PEI used to transfect human MSCs. This combination of plasmid DNA delivery agents is more successful at inducing transfection than silica or PEI alone (Park et al., Citation2010).
One of the disadvantages of these kinds of transfection enhancements is that it is still unclear whether all particles are cleared from the body, and if eventually tissue damage will occur if these particles remain in place. Therefore, additional research should focus more on safety issues.
Physical transfection methods
With physical transfection methods, cell membranes are temporarily permeabilized to allow plasmid DNA to enter the cells. To permeabilize the cell membrane carefully, different methods are used, such as electroporation, which uses a high-intensity electric pulse. As a result, DNA can enter the cell and induce transfection before the pores of the cell close again (Mehier-Humbert & Guy, Citation2005). Unfortunately this is not a very cell-friendly method; therefore, several groups are trying to optimize the ideal voltage and duration of the procedure to increase transfection rate and reduce cell death (Mehier-Humbert & Guy, Citation2005; Ferreira et al., Citation2008; Lee et al., Citation2010). Similar to electroporation is sonoporation, which uses ultrasound to disrupt the cell membrane and induce transfection. It is still a highly experimental procedure and not very successful yet, since the majority of the cells do not survive the procedure and a very limited number of cells can be reached in tissues because only the cells at the surface receive enough ultrasound to be transfected.
In an effort to find a non-invasive transfection method without irreparable damage of the cells, electric field-induced molecular vibration is being tested. Hereby, cells are brought into suspension together with plasmid DNA in an in vitro setting. The vibration induces the cells and DNA to colloid allowing the cells to become transfected. Different cell types including MSCs were efficiently transfected without disturbance of differentiation capability (Song et al., Citation2004). In case of the ex vivo microinjection method using nano-needles, which are manipulated using an atomic force microscope, transfection efficiencies were even higher (up to 70%), while no irreparable membrane damage occurred (Han et al., Citation2008). To be able to microinject multiple cells at a time, hollow carbon nanotubes are lined up vertically and inject multiple cells simultaneously. The carbon tubes act as a nano-syringe for plasmid DNA to enter the cells (Park, Kim, Kim, & Jon, Citation2009).
All physical methods, however, destabilize the plasma membrane temporarily, which in many cases results in low cell survival. The difficulty of these techniques is to find the optimal conditions. Another difficulty is to reach deep into the tissue. Most of these techniques are only capable of penetrating the skin and maybe reach the muscle and adipose tissue just below the skin, but bone cannot be reached non-invasively, making it less optimal for orthopedic applications.
Ex vivo transfections
For non-viral gene therapy to be applied in bone (re)generation, many hurdles need to be overcome. Most techniques are based on controlled cell membrane damage or particle uptake. The main disadvantages for in vivo application are high levels of cell death/tissue damage, low penetration depth, the risk of particle migration and the risk of off-target effects. One way to overcome this problem is by doing ex vivo transfections. Hereby the DNA is not transferred into the body to transfect targeted host cells, but the desired host cells are isolated from the body, transfected in vitro, often selected afterwards, and reinfused to act as bone-forming cells or as protein factories. The two major advantages over classic in vivo transfections are preselection of the target cells and post-selection of the transfected cells. Prior quality control of the transfected cells becomes possible, which might increase safety. As safety is the primary concern, especially in the orthopedic field, ex vivo transfection models seem to be more popular at the moment (Sheyn et al., Citation2008; Citation2011; Lee et al., Citation2010; Lai et al., Citation2011; Long et al., Citation2011). A disadvantage arises with the harvesting of autologous cells, which might be time-consuming and warrants additional surgery (Aggarwal, Pompili, & Das, Citation2010). Knowledge on the use of allogeneic MSCs is increasing and several institutes are initiating safety studies for MSCs which might open up new possibilities in orthopedic gene interventions.
Safety and future outlook
Despite all the promising small and large animal studies done, it is still a huge challenge to translate these studies to human clinical practice. Safety will be the first and foremost aspect of this road, but no safety study has been reported so far for orthopedic applications. This is due to several possible reasons including the fact that gene therapy is a relatively new field of medicine, which suffered from an enormous image problem after severe side effects occurred in one of the first clinical gene therapy based trials. Second, in a field in which non-lethal diseases or injuries are treated, the possible health risks are considered too high to justify human experimentation. As soon as several gene therapy studies in other areas have proven to be effective and safe, this will add to the attempts in the field of bone tissue engineering. Since vectors are more innovative and safe, it is possible that over the next few years non-viral gene therapy trials will be set up for orthopedic applications. However, in order to design safety studies, the following aspects have to be taken into account: (i) insertion of the DNA in the host genome must be minimized to avoid possible mutagenesis; (ii) cytotoxicity of the vector must be prevented; (iii) delivery method/route needs to be optimized to prevent degradation of the DNA in the bloodstream or tissue; (iv) good manufacturing practice (GMP) protocols need to be designed to warrant the quality of the construct. These safety studies are laborious and time-consuming.
The area that most likely will be the first to proceed towards clinical gene intervention technique will possibly be the craniofacial field. Not only can the jaw be considered a relatively isolated tissue, but also it is also feasible to take biopsies of the newly formed bone from a patient, since this is part of the standard procedure to place a dental implant. Histological and genetic analysis of those biopsies would generate a sufficient amount of safety and efficacy knowledge, necessary for future developments in other applications such as spinal fusions or large bone defects.
Concluding remarks
Although the use of gene therapy for bone tissue engineering is an upcoming field, no ideal delivery agent has yet been found. There is a thin optimum between inducing high transfection efficiencies and cytotoxicity. For the orthopedic field it is hard to find an ideal technique for efficient and safe gene transfer, which is also biocompatible and mechanically suitable for bone tissue engineering. In an optimal construct for induction of bone formation, several components will need to be combined. This leads to constructs, which contain transfection agents in different forms, coated on scaffolds or mixed to hydrogels with or without the addition of ceramic or other carriers. This approach has led to some promising results in vitro in small and large animal studies. To proceed towards clinical application the next step will be designing and performing safety studies to establish gene therapy finally as a possible new therapy to induce bone (re)generation.
Acknowledgements
The authors gratefully acknowledge the support of the Smart Mix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science.
References
- Aggarwal , R. , Pompili , V. J. and Das , H. 2010 . Genetic modification of ex-vivo expanded stem cells forclinical application . Frontiers in Bioscience , 15 : 854 – 871 .
- Aggarwal , S. and Pittenger , M. F. 2005 . Human mesenchymal stem cells modulate allogeneic immune cell responses . Blood , 105 : 1815 – 1822 .
- Ahn , H. H. , Lee , J. H. , Kim , K. S. , Lee , J. Y. , Kim , M. S. , Khang , G. , … and Lee , H. B. 2008 . Polyethyleneimine-mediated gene delivery into human adipose derived stem cells . Biomaterials , 29 : 2415 – 2422 .
- Arthur , A. , Zannettino , A. and Gronthos , S. 2009 . The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair . Journal of Cell Physiology , 218 : 237 – 245 .
- Blum , J. S. , Barry , M. A. , Mikos , A. G. and Jansen , J. A. 2003 . In vivo evaluation of gene therapy vectors in ex vivo-derived marrow stromal cells for bone regeneration in a rat critical-size calvarial defect model . Human Gene Therapy , 14 : 1689 – 1701 .
- Bonadio , J. , Smiley , E. , Patil , P. and Goldstein , S. 1999 . Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration . Nature Medicine , 5 : 753 – 759 .
- Bose , S. and Tarafder , S. 2012 . Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review . Acta Biomaterialia , 8 : 1401 – 1421 .
- Bourgeat-Lami , E. 2002 . Organic-inorganic nanostructured colloids . Journal of Nanoscience and Nanotechnology , 2 : 1 – 24 .
- Carragee , E. J. , Hurwitz , E. L. and Weiner , B. K. 2011 . A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned . Spine Journal , 11 : 471 – 491 .
- Chew , S. A. , Kretlow , J. D. , Spicer , P. P. , Edwards , A. W. , Baggett , L. S. , Tabata , Y. , … and Mikos , A. G. 2011 . Delivery of plasmid DNA encoding bone morphogenetic protein-2 with a biodegradable branched polycationic polymer in a critical-size rat cranial defect model . Tissue Engineering Part A , 17 : 751 – 763 .
- Chimutengwende-Gordon , M. and Khan , W. S. 2012 . Advances in the use of stem cells and tissue engineering applications in bone repair . Current Stem Cell Research and Therapy , 7 : 122 – 126 .
- Choi , Y. H. , Liu , F. , Choi , J. S. , Kim , S. W. and Park , J. S. 1998 . Polyethylene glycol-grafted poly-L lysine as polymeric gene carrier . Journal of Controlled Release , 54 : 39 – 48 .
- Chowdhury , E. H. and Akaike , T. 2005 . Bio-functional inorganic materials: An attractive branch of gene-based nano-medicine delivery for 21st century . Current Gene Therapy , 5 : 669 – 676 .
- Clements , B. A. , Incani , V. , Kucharski , C. , Lavasanifar , A. , Ritchie , B. and Uludağ , H. 2007 . A comparative evaluation of poly-L-lysine-palmitic acid and Lipofectamine 2000 for plasmid delivery to bone marrow stromal cells . Biomaterials , 28 : 4693 – 4704 .
- Clinical trial database. Retrieved from http://www.wiley.com/legacy/wileychi/genmed/clinical/
- Corsi , K. , Chellat , F. , Yahia , L. and Fernandes , J. C. 2003 . Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles . Biomaterials , 24 : 1255 – 1264 .
- Dang , J. M. and Leong , K. W. 2006 . Natural polymers for gene delivery and tissue engineering . Advanced Drug Delivery Reviews , 58 : 487 – 499 .
- Deng , M. , James , R. , Laurencin , C. T. and Kumbar , S. G. 2012 . Nanostructured polymeric scaffolds for orthopaedic regenerative engineering . IEEE Transactions in Nanobioscience , 11 : 3 – 14 .
- Düzgüneş , N. , De Ilarduya , C. T. , Simões , S. , Zhdanov , R. I. , Konopka , K. and Pedroso de Lima , M. C. 2003 . Cationic liposomes for gene delivery: Novel cationic lipids and enhancement by proteins and peptides . Current Medicinal Chemistry , 10 : 1213 – 1220 .
- Fang , J. , Zhu , Y. Y. , Smiley , E. , Bonadio , J. , Rouleau , J. P. , Goldstein , S. A. , … and Roessler , B. J. 1996 . Stimulation of new bone formation by direct transfer of osteogenic plasmid genes . Proceedings of the National Academy of Science of the United States of America , 93 : 5753 – 5758 .
- Ferreira , E. , Potier , E. , Logeart-Avramoglou , D. , Salomskaite-Davalgiene , S. , Mir , L. M. and Petite , H. 2008 . Optimization of a gene electrotransfer method for mesenchymal stem cell transfection . Gene Therapy , 15 : 537 – 544 .
- Fischer , D. , Bieber , T. , Li , Y. , Elsässer , H. P. and Kissel , T. 1999 . A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity . Pharmaceutical Research , 16 : 1273 – 1279 .
- Franceschi , R. T. , Wang , D. , Krebsbach , P. H. and Rutherford , R. B. 2000 . Gene therapy for bone formation: In vitro and in vivo osteogenic activity of an adenovirus expressing BMP7 . Journal of Cell Biochemistry , 78 : 476 – 486 .
- Garcia-Fuentes , M. and Alonso , M. J. 2012 . Chitosan-based drug nanocarriers: Where do we stand? . Journal of Controlled Release , 161 : 496 – 504 .
- Gill , D. R. , Pringle , I. A. and Hyde , S. C. 2009 . Progress and prospects: The design and production of plasmid vectors . Gene Therapy , 16 : 165 – 171 .
- Glassman , S. D. , Gum , J. L. , Crawford , C. H. 3rd , Shields , C. B. and Carreon , L. Y. 2011 . Complications with recombinant human bone morphogenetic protein-2 in posterolateral spine fusion associated with a dural tear . Spine Journal , 11 : 522 – 526 .
- Godbey , W. T. , Barry , M. A. , Saggau , P. , Wu , K. K. and Mikos , A. G. 2000 . Poly(ethylenimine)-mediated transfection: A new paradigm for gene delivery . Journal of Biomedical Materials Research , 51 : 321 – 328 .
- Gwak , S. J. and Kim , B. S. 2008 . Poly(lactic-co-glycolic acid) nanosphere as a vehicle for gene delivery to human cord blood-derived mesenchymal stem cells: comparison with polyethylenimine . Biotechnology Letters , 30 : 1177 – 1182 .
- Han , S. W. , Nakamura , C. , Kotobuki , N. , Obataya , I. , Ohgushi , H. , Nagamune , T. and Miyake , J. 2008 . High-efficiency DNA injection into a single human mesenchymal stem cell using a nanoneedle and atomic force microscopy . Nanomedicine , 4 : 215 – 225 .
- He , X. , Dziak , R. , Mao , K. , Genco , R. , Swithart , M. , Li , C. and Yang , S. 2013 . Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2-gene modified MSCs for bone regeneration . Tissue Engineering Part A , 19 : 508 – 518 .
- Hedley , M. L. , Curley , J. and Urban , R. 1998 . Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses . Nature Medicine , 4 : 365 – 368 .
- Hoekstra , D. , Rejman , J. , Wasungu , L. , Shi , F. and Zuhorn , I. 2007 . Gene delivery by cationic lipids: In and out of an endosome . Biochemical Society Transactions , 35 : 68 – 71 .
- Humbert , J. M. and Halary , F. 2012 . Viral and non-viral methods to genetically modify dendritic cells . Current Gene Therapy , 12 : 127 – 136 .
- Hyde , S. C. , Pringle , I. A. , Abdullah , S. , Lawton , A. E. , Davies , L. A. , Varathalingam , A. , … and Gill , D. R. 2008 . CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression . Nature Biotechnology , 26 : 549 – 551 .
- Incani , V. , Tunis , E. , Clements , B. A. , Olson , C. , Kucharski , C. , Lavasanifar , A. and Uludag , H. 2007 . Palmitic acid substitution on cationic polymers for effective delivery of plasmid DNA to bone marrow stromal cells . Journal of Biomedical Material Research A , 81 : 493 – 504 .
- Kasper , F. K. , Kushibiki , T. , Kimura , Y. , Mikos , A. G. and Tabata , Y. 2005 . In vivo release of plasmid DNA from composites of oligo(poly(ethylene glycol)fumarate) and cationized gelatin microspheres . Journal of Controlled Release , 107 : 547 – 561 .
- Kasper , F. K. , Young , S. , Tanahashi , K. , Barry , M. A. , Tabata , Y. , Jansen , J. A. and Mikos , A. G. 2006 . Evaluation of bone regeneration by DNA release from composites of oligo(poly(ethylene glycol) fumarate) and cationized gelatin microspheres in a critical-sized calvarial defect . Journal of Biomedical Material Research A , 78 : 335 – 342 .
- Kawai , M. , Maruyama , H. , Bessho , K. , Yamamoto , H. , Miyazaki , J. and Yamamoto , T. 2009 . Simple strategy for bone regeneration with a BMP-2/7 gene expression cassette vector . Biochemical Biophysiology Research Community , 390 : 1012 – 1017 .
- Kay , M. A. 2011 . State-of-the-art gene-based therapies: the road ahead . Nature Review Genetics , 12 : 316 – 328 .
- Kempen , D. H. , Creemers , L. B. , Alblas , J. , Lu , L. , Verbout , A. J. , Yaszemski , M. J. and Dhert , W. J. 2010 . Growth factor interactions in bone regeneration . Tissue Engineering Part B Reviews , 16 : 551 – 566 .
- Klyushnenkova , E. , Mosca , J. D. , Zernetkina , V. , Majumdar , M. K. , Beggs , K. J. , Simonetti , D. W. , … and McIntosh , K. R. 2005 . T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression . Journal Biomedical Science , 12 : 47 – 57 .
- Kong , H. J. , Kim , E. S. , Huang , Y. C. and Mooney , D. J. 2008 . Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA . Pharmaceutical Research , 25 : 1230 – 1238 .
- Krebs , M. D. , Salter , E. , Chen , E. , Sutter , K. A. and Alsberg , E. 2010 . Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis . Journal of Biomedical Material Research A , 92 : 1131 – 1138 .
- Lai , Q. G. , Yuan , K. F. , Xu , X. , Li , D. R. , Li , G. J. , Wei , F. L. , … and Li , S. 2011 . Transcription factor osterix modified bone marrow mesenchymal stem cells enhance callus formation during distraction osteogenesis . Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology , 111 : 412 – 419 .
- Lee , S. J. , Kang , S. W. , Do , H. J. , Han , I. , Shin , D. A. , Kim , J. H. and Lee , S. H. 2010 . Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells . Biomaterials , 31 : 5652 – 5659 .
- Lei , Y. , Huang , S. , Sharif-Kashani , P. , Chen , Y. , Kavehpour , P. and Segura , T. 2010 . Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds . Biomaterials , 31 : 9106 – 9116 .
- Lim , K. I. 2012 . Retroviral integration profiles: Their determinants and implications for gene therapy . BMB Reports , 45 : 207 – 212 .
- Long , J. , Li , P. , Du , H. M. , Liu , L. , Zheng , X. H. , Lin , Y. F. , … and Tian , W. D. 2011 . Effects of bone morphogenetic protein 2 gene therapy on new bone formation during mandibular distraction osteogenesis at rapid rate in rabbits . Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology , 112 : 50 – 57 .
- Lustig , L. R. and Akil , O. 2012 . Cochlear gene therapy . Current Opinion in Neurology , 25 : 57 – 60 .
- Madeira , C. , Mendes , R. D. , Ribeiro , S. C. , Boura , J. S. , Aires-Barros , M. R. , da Silva , C. L. and Cabral , J. M. 2010 . Nonviral gene delivery to mesenchymal stem cells using cationic liposomes for gene and cell therapy . Journal of Biomedicine and Biotechnology , 2010 : 735349 doi: 10.1155/2010/735349
- Martin , T. , Parker , S. E. , Hedstrom , R. , Le , T. , Hoffman , S. L. , Norman , J. , … and Lew , D. 1999 . Plasmid DNA malaria vaccine: the potential for genomic integration after intramuscular injection . Human Gene Therapy , 10 : 759 – 768 .
- McCanless , J. D. , Jennings , L. K. , Cole , J. A. , Bumgardner , J. D. and Haggard , W. O. 2011 . In vitro differentiation and biocompatibility of mesenchymal stem cells on a novel platelet releasate-containing injectable composite . Journal Biomedical Material Research A , 100 : 220 – 229 .
- McKee , M. D. , Nakano , Y. , Masica , D. L. , Gray , J. J. , Lemire , I. , Heft , R. , … and Millán , J. L. 2011 . Enzyme replacement therapy prevents dental defects in a model of hypophosphatasia . Journal of Dental Research , 90 : 470 – 476 .
- Mehier-Humbert , S. and Guy , R. H. 2005 . Physical methods for gene transfer: improving the kinetics of gene delivery into cells . Advanced Drug Delivery Reviews , 57 : 733 – 753 .
- Mingozzi , F. and High , K. A. 2012 . Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges . Nature Reviews Genetics , 12 : 341 – 355 .
- Mintzer , M. A. and Simanek , E. E. 2009 . Nonviral vectors for gene delivery . Chemistry Reviews , 109 : 259 – 302 .
- Montiel-Equihua , C. A. , Thrasher , A. J. and Gaspar , H. B. 2012 . Gene therapy for severe combined immunodeficiency due to adenosine deaminase deficiency . Current Gene Therapy , 12 : 57 – 65 .
- Moreira , C. , Oliveira , H. , Pires , L. R. , Simões , S. , Barbosa , M. A. and Pêgo , A. P. 2009 . Improving chitosan-mediated gene transfer by the introduction of intracellular buffering moieties into the chitosan backbone . Acta Biomaterials , 5 : 2995 – 3006 .
- Park , D. J. , Choi , J. H. , Leong , K. W. , Kwon , J. W. and Eun , H. S. 2007 . Tissue-engineered bone formation with gene transfer and mesenchymal stem cells in a minimally invasive technique . Laryngoscope , 117 : 1267 – 1271 .
- Park , J. S. , Na , K. , Woo , D. G. , Yang , H. N. , Kim , J. M. , Kim , J. H. , Chung , H. M. and Park , K. H. 2010 . Non-viral gene delivery of DNA polyplexed with nanoparticles transfected into human mesenchymal stem cells . Biomaterials , 31 : 124 – 132 .
- Park , S. , Kim , Y. S. , Kim , W. B. and Jon , S. 2009 . Carbon nanosyringe array as a platform for intracellular delivery . Nanotechnology Letters , 9 : 1325 – 1329 .
- Park , S. J. and Na , K. 2012 . The transfection efficiency of photosensitizer-induced gene delivery to human MSCs and internalization rates of EGFP and Runx2 genes . Biomaterials , 33 : 6485 – 6494 .
- Park , T. G. , Jeong , J. H. and Kim , S. W. 2006 . Current status of polymeric gene delivery systems . Advanced Drug Delivery Reviews , 58 : 467 – 486 .
- Parveen , S. , Misra , R. and Sahoo , S. K. 2012 . Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging . Nanomedicine , 8 : 147 – 166 .
- Reckhenrich , A. K. , Koch , C. , Egaña , J. T. and Plank , C. 2012 . The use of non-viral gene vectors for bioactive poly-(D, L-lactide) implant surfaces in bone tissue engineering . European Cells and Materials , 23 : 441 – 448 .
- Reiser , J. , Zhang , X. Y. , Hemenway , C. S. , Mondal , D. , Pradhan , L. and La Russa , V. F. 2005 . Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases . Expert Opinion Biological Therapy , 5 : 1571 – 1584 .
- Sakuma , T. , Barry , M. A. and Ikeda , Y. 2012 . Lentiviral vectors: Basic to translational . Biochemical Journal , 443 : 603 – 618 .
- Santos , J. L. , Pandita , D. , Rodrigues , J. , Pêgo , A. P. , Granja , P. L. and Tomás , H. 2012 . Non-viral gene delivery to mesenchymal stem cells: Methods, strategies and application in bone tissue engineering and regeneration . Current Gene Therapy , 11 : 46 – 57 .
- Saraf , A. , Hacker , M. C. , Sitharaman , B. , Grande-Allen , K. J. , Barry , M. A. and Mikos , A. G. 2008 . Synthesis and conformational evaluation of a novel gene delivery vector for human mesenchymal stem cells . Biomacromolecules , 9 : 818 – 827 .
- Shea , L. D. , Smiley , E. , Bonadio , J. and Mooney , D. J. 1999 . DNA delivery from polymer matrices for tissue engineering . Nature Biotechnology , 17 : 551 – 554 .
- Sheyn , D. , Kallai , I. , Tawackoli , W. , CohnYakubovich , D. , Oh , A. , Su , S. , … and Gazit , D. 2011 . Gene modified adult stem cells regenerate vertebral bone defect in a rat model . Molecular Pharmaceutics , 8 : 1592 – 1601 .
- Sheyn , D. , Pelled , G. , Zilberman , Y. , Talasazan , F. , Frank , J. M. , Gazit , D. and Gazit , Z. 2008 . Nonvirally engineered porcine adipose tissue-derived stem cells: Use in posterior spinal fusion . Stem cells , 26 : 1056 – 1064 .
- Shimer , A. L. , Oner , F. C. and Vaccaro , A. R. 2009 . Spinal reconstruction and bone morphogenetic proteins: Open questions . Injury , 40 ( Suppl 3 ) : S32 – 38 .
- Smith , D. K. 2008 . Dendrimers and the double helix – From DNA binding towards gene therapy . Current Topics in Medicinal Chemistry , 8 : 1187 – 1203 .
- Song , L. , Chau , L. , Sakamoto , Y. , Nakashima , J. , Koide , M. and Tuan , R. S. 2004 . Electric field-induced molecular vibration for noninvasive, high-efficiency DNA transfection . Molecular Therapy , 9 : 607 – 616 .
- Southwood , L. L. , Frisbie , D. D. , Kawcak , C. E. and McIlwraith , C. W. 2004 . Delivery of growth factors using gene therapy to enhance bone healing . Veterinary Surgery , 33 : 565 – 578 .
- Su , C. H. , Wu , Y. J. , Wang , H. H. and Yeh , H. I. 2012 . Nonviral gene therapy targeting cardiovascular system . American Journal of Physiological Heart and Circulation Physiology , 303 : H629 – 638 .
- Tachibana , R. , Harashima , H. , Ide , N. , Ukitsu , S. , Ohta , Y. , Suzuki , N. , … and Kiwada , H. 2002 . Quantitative analysis of correlation between number of nuclear plasmids and gene expression activity after transfection with cationic liposomes . Pharmaceutical Research , 19 : 377 – 381 .
- Thomas , C. E. , Ehrhardt , A. and Kay , M. A. 2003 . Progress and problems with the use of viral vectors for gene therapy . Nature Review Genetics , 4 : 346 – 358 .
- Uchimura , E. , Yamada , S. , Uebersax , L. , Fujita , S. , Miyake , M. and Miyake , J. 2007 . Method for reverse transfection using gold colloid as a nano-scaffold . Journal Bioscience and Bioengineering , 103 : 101 – 103 .
- Warnock , J. N. , Daigre , C. and Al-Rubeai , M. 2011 . Introduction to viral vectors . Methods in Molecular Biology , 737 : 1 – 25 .
- Wegman , F. , Bijenhof , A. , Schuijff , L. , Oner , F. C. , Dhert , W. J. and Alblas , J. 2011 . Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo . European Cells and Materials , 21 : 230 – 242 .
- Wegman, F., Geuze, R. E., van der Helm, Y. J., Öner, F. C., Dhert, W. J., & Alblas, J. (2012). Gene delivery of bone morphogenetic protein-2 plasmid DNA promotes bone formation in a large animal model. Journal of Tissue Engineering and Regenerative Medicine. doi:10.1002/term.1571
- Wiethoff , C. M. and Middaugh , C. R. 2003 . Barriers to nonviral gene delivery . Journal of Pharmaceutical Science , 92 : 203 – 217 .
- Wolff , J. A. and Rozema , D. B. 2008 . Breaking the bonds: Non-viral vectors become chemically dynamic . Molecular Therapy , 16 : 8 – 15 .
- Yang , F. , Green , J. J. , Dinio , T. , Keung , L. , Cho , S. W. , Park , H. , Langer , R. and Anderson , D. G. 2009 . Gene delivery to human adult and embryonic cell-derived stem cells using biodegradable nanoparticulate polymeric vectors . Gene Therapy , 16 : 533 – 546 .
- Yun , Y. R. , Jang , J. H. , Jeon , E. , Kang , W. , Lee , S. , Won , J. E. , … and Wall , I. 2012 . Administration of growth factors for bone regeneration . Regenerative Medicine , 7 : 369 – 385 .
- Zhang , G. , Guo , B. , Wu , H. , Tang , T. , Zhang , B. T. , Zheng , L. , … and Qin , L. 2012 . A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy . Nature Medicine , 18 : 307 – 314 .