Abstract
Impressive advances have been made in the treatment and management of HIV-1 infected individuals. Combination antiretroviral therapy (cART) has turned HIV-1 infection from an almost invariable deadly infectious disease, to a lifelong manageable infectious disease. However, a cure or vaccine has not been forthcoming. A major problem in HIV-1 infection is the persistent and latently infected cellular and tissue reservoirs. One of these reservoirs is the Gut Associated Lymphoid tissue (GALT), which has been the research focus of our group. Our group and others have shown that HIV-1 evolves differently in different parts of the gastro intestinal tract, which also appears to affect the development of antiretroviral drug resistance. The GALT is not the only reservoir. HIV-1 continues to persist and evolve in various other cell and tissue reservoirs despite intense and apparent successful antiretroviral therapy. Moreover, drug resistance mutations remain prevalent under therapy and successful viral suppression. In addition to finding a vaccine, the research on combating and eradicating the HIV-1 viral reservoirs has also been an important focus of HIV-1 cure strategies. We will discuss some of the research findings on reservoirs in the context of some of the HIV-1 cure approaches.
Introduction
The human immunodeficiency virus (HIV) acquired immunodeficiency syndrome (HIV/AIDS) epidemic has changed compared to its start in the 1980s. Although we are still faced with 35–40 million infected individuals worldwide (WHO, Citation2016b), the more readily available combination antiretroviral therapies (cART) have significantly increased life expectancy of HIV infected individuals in both the developed and developing world (WHO, Citation2016a). Despite significant efforts there is no fully protective vaccine. Recently, a cure involving a bone marrow transplant consisting of cells resistant to HIV infection, has been an exciting proof of principle (Hutter et al., Citation2009). However, this approach will not be readily available for a global cure approach. Despite setbacks, the HIV cure efforts have not diminished and a multi-prong approach based on vaccination, antiviral therapy, gene therapy, and modulating the immune system has been proposed (Chun, Moir, & Fauci, Citation2015). In the context of the HIV cure efforts, the HIV reservoirs are often mentioned as an obstacle. HIV infects and persists in many different cells and tissues over the course of infection, which are referred to as reservoirs. The reservoirs are considered important as it enables the virus to evade immune system, antiviral therapies, but also evade and complicate other HIV cure approaches (Chun et al., Citation2015). In this review, we will discuss and summarize the current knowledge on the HIV reservoirs, and how this impacts some of the HIV cure strategies.
HIV: its life cycle, pathogenesis, and origin
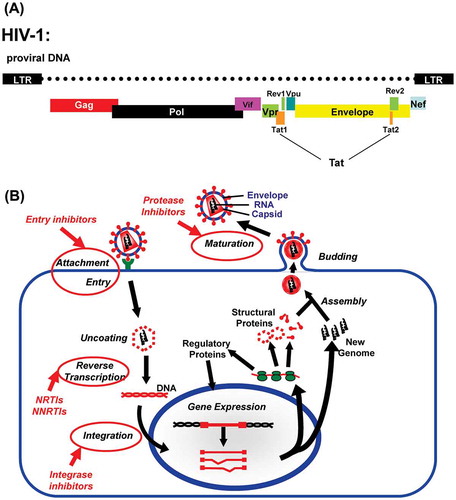
Human immunodeficiency virus (HIV) is a retrovirus belonging to the lentivirus family (reviewed in (Levy, Citation2007)), and follows the typical retroviral life cycle (Figure ). It infects cells of the immune system and in particular CD4+ cells. The main receptor for HIV entry is CD4, but the virus also needs a co-receptor (Nisole & Saib, Citation2004). The chemokine receptors CCR5 or CXCR4 are the most commonly used co-receptors, but other chemokine receptors have also been reported. Retroviruses are enveloped viruses, and upon attachment the viral envelope fuses with the cell membrane which allows the viral capsid to enter the cell. After entry of the viral capsid into the cell, the viral RNA is reverse transcribed by the viral reverse transcriptase (RT). The resulting cDNA (proviral DNA) is translocated to the nucleus and integrated into the cell’s genomic DNA by the viral integrase (IN).The RT and IN are encoded by the pol gene, and are part of the HIV virus particle (virion) (Nisole & Saib, Citation2004). Subsequently, the proviral DNA is transcribed by the host transcription machinery to produce various mRNAs encoding different viral proteins as well as full length viral genomic transcripts. In contrast to simple retroviruses, HIV encodes several other genes in addition to the gag (encoding the various capsid proteins) pol and env (encoding the envelope protein). These accessory genes (vpr, vpu, nef, rev, and tat), play important roles in viral replication and pathogenesis (Chiozzini & Toschi, Citation2016; Collins & Collins, Citation2014; Suhasini & Reddy, Citation2009). Virions assemble and bud at the cell membrane. After release the viral protease (PRO, encoded by the pol gene) in the virion, mediates proteolytic cleavages of the viral capsid. This proteolytic cleavage turns the virion into an infectious particle (Freed, Citation2015).
Figure 1. (A) Schematic representation of the HIV-1 viral genome organization in its proviral DNA form: The long terminal repearts (LTR) act as promoter and poly-adenylation sites for the production of the full length viral genomic RNA transcript, that is ultimately incorporated in the virus particle. The gag and pol proteins are translated from the full length genomic RNA. The other viral proteins (Env,Tat, Vpu, Vif, Vpr, Rev, and Nef) are translated from mRNAs generated from the genomic RNA transcript by differential splicing. (B) Schematic representation of the HIV life cycle: HIV is an enveloped virus and upon binding to the cell, the HIV viral membrane will fuse with the cell, which allows the viral capsid to enter. The viral RNA is reverse transcribed into the proviral cDNA by the viral reverse transcriptase (RT). Subsequently, the viral integrase (IN) integrates the proviral cDNA intro the host genome. The host transcription machinery transcribes the proviral cDNA and produces various mRNAs encoding for the different HIV proteins, as well as the full length viral genomic RNA. The virus assembles at the cell surface membrane. After budding, the viral protease, mediates a proteolytic cleavage of the viral capsid, resulting in the mature infectious virus particle (virion). The currently available antiretroviral drugs target different steps in the life cycle. The steps at which the different drugs (Entry/Attachment, NRTIs, NNRTIs, Integrase, and protease inhibitors) act are indicated with circles and arrows.
Primary HIV infection manifests itself as a flu like illness, after which the individual enters a more or less asymptomatic stage that can last for several years (Levy, Citation2007). Over time HIV destroys the immune system leading to the immune deficient state HIV/AIDS. This state is defined as increasing viral load with a decline in CD4+ T cells to <200 cells/μl, and/or opportunistic infections and other HIV/AIDS defining illnesses (Levy, Citation2007). HIV infection can induce cell death directly, but the majority of dying immune cells are uninfected bystander cells. The bystander cell death is in part caused by the cytotoxic effects of released viral proteins, such as tat (trans-activator of transcription), negative factor (nef) and the envelope (env) protein (Timilsina & Gaur, Citation2016; Tsao, Guo, Jeffrey, Hoxie, & Su, Citation2016). However, the major driver of immune cell death is the HIV induced chronic inflammatory state, which results in pyroptosis (‘fiery cell death’) and deregulation of immune cells (Doitsh et al., Citation2014; Monroe et al., Citation2014).
HIV appears to have originated from simian immunodeficiency virus (SIV) through zoonotic transmission from primates to humans (Keele et al., Citation2006). There are two types of HIV, each of which are considered to have resulted from different zoonotic transmission events (Visseaux, Damond, Matheron, Descamps, & Charpentier, Citation2016). HIV type 1 (HIV-1) is the global epidemic HIV type. HIV type 2 (HIV-2), is considered less pathogenic, and is primarily found in Western Africa, but infected individuals have also been reported in different countries around the world (Visseaux et al., Citation2016). The practice of hunting bush meat is probably the most likely route by which the virus entered the human population (Faria et al., Citation2014; Hahn, Shaw, De Cock, & Sharp, Citation2000). The infection of humans by HIV ancestors does not appear to be a recent event. Humans have been exposed to the virus ancestors long before we became aware of the HIV/AIDS epidemic, and dates as early the 1920s have been proposed (Faria et al., Citation2014; Tongo, Dorfman, & Martin, Citation2016; Worobey et al., Citation2008). Western colonization, human encroachment, urbanization of Africa, and global travel are considered factors that contributed to the global spread of the virus (Faria et al., Citation2014).
HIV viral diversity
HIV is extremely diverse and many different subtypes are found around the world. In addition, there is significant diversity within subtypes and within infected individuals (Overbaugh & Bangham, Citation2001). The huge diversity is the result of the error prone replication strategy of HIV, and allows the virus to escape the immune response (Overbaugh & Bangham, Citation2001). The majority of the viral mutations are introduced during the reverse transcription process, as the viral RT enzyme does not have any proofreading activity (Overbaugh & Bangham, Citation2001). The RT enzyme can also jump between templates, resulting in recombinant HIV variants (Overbaugh & Bangham, Citation2001). The high mutation rate does not only result in high viral variability between individuals, but also manifests itself as high variability within an infected individual. HIV does not exist as a single clonal species in an infected person, but as a quasispecies. A quasispecies can be envisioned as a ‘swarm’ of numerous different viral variants (Domingo, Citation1998).
The range of tissues infected by HIV is also very diverse. Important targets are different CD4+ T-cell populations and cells of monocyte/macrophage lineage in the periphery and the gastrointestinal associated lymphoid tissue (GALT) (Kotler, Citation2005). HIV also infects the central nervous system (CNS) soon after primary infection, and can cause neurological disease (Davis et al., Citation1992; Lamers et al., Citation2016). The availability, susceptibility and activation state of the infectable cells, and immune selection pressures in the various tissues, gives rise to distinct HIV quasispecies in different tissues in an HIV infected individual (Overbaugh & Bangham, Citation2001). This diversity of HIV and ability to escape the immune system, has hampered the efforts to find a broadly protective vaccine for HIV. Although the HIV vaccine failures have been disappointing, they have taught us a lot about vaccine approaches in general (Bansal, Malaspina, & Flores, Citation2010).
HIV reservoirs
The various HIV infected tissues are not only important for their contribution to overall viral diversity. They are also important as host-pathogen interfaces, and reservoirs for HIV replication. The largest lymphoid tissue infected by HIV, is the GALT. The gut is a major site for viral replication and pathogenesis. In both the SIV model and human HIV context, primary infection results in massive immune cell depletion in the GALT. The immune cell population in the GALT recovers very slowly, even after prolonged suppressive antiretroviral therapy and with restored CD4+ T-cell levels in the periphery (Costiniuk & Angel, Citation2012). HIV replicates to high levels in the GALT, and high viral loads in the colon during early infection is linked to lower survival (van Marle, Sharkey, Gill, & Church, Citation2013), underscoring the importance of the GALT in viral pathogenesis. The chronic inflammation in the GALT not only contributes to immune depletion, it also leads to gastrointestinal barrier dysfunction and disease (Brenchley, Price, & Douek, Citation2006a). The barrier dysfunction has also been proposed to lead to translocation of bacterial cell wall products into the periphery, which in turn drives inflammation and disease progression. Even under suppressive antiviral therapy chronic inflammation in the GALT persists (Brenchley et al., Citation2006b).
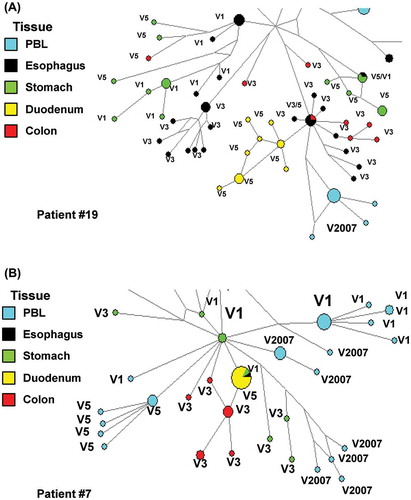
The HIV viral reservoirs in the GALT are extensive, with distinct viral quasispecies populating different parts of the GI tract (i.e. esophagus, stomach, duodenum, ileum, jejunum and colon) (Lerner et al., Citation2011; van Marle et al., Citation2007, 2010). Other studies and our published and unpublished work also suggests a dynamic viral evolution and exchange of virus between different GI tissues and blood (Figure ) (Lerner et al., Citation2011; van Marle et al., Citation2007, 2010). In addition, infection of the GI tract by different viral variants appears to affect both GI pathogenesis and systemic disease progression (van Marle et al., Citation2013), underscoring the importance of viral diversity in pathogenesis.
Figure 2. Network analyses of RT encoding proviral DNA sequences from gut and peripheral blood lymphocytes (PBL) from different visits (V). Patient #19 (A) patient #7 (B) viral sequence found in PBL in 2007 (patient on cART, V2007) where closely related to pre cART (V1–V7) sequences of different tissues. There does not appear to be one specific tissue of origin. These observations suggest highly dynamic and complex viral dynamics between the gut viral reservoirs and peripheral blood (van der Meer & van Marle (unpublished results)).
As an immune privileged tissue, the brain/CNS is probably a more unique tissue infected by HIV. In the brain innate immune cells dominate over adaptive immune cells, and the main cells infected are of monocytoid lineage (macrophages and microglia) (Gonzalez-Scarano & Martin-Garcia, Citation2005). The other abundant glial cell in the brain are astrocytes that also contain HIV proviral DNA, and although the infection is not highly productive, they contribute to neuropathology by expressing and releasing HIV accessory proteins, such as the nef protein (Gray et al., Citation2014; Ranki et al., Citation1995). Again, chronic inflammation and bystander cell death underlie CNS pathology (Gonzalez-Scarano & Martin-Garcia, Citation2005). The brain/CNS is an important reservoir throughout infection and populated by distinct viral quasispecies (Bednar et al., Citation2015; Roda et al., Citation2017; Spector & Rappaport, Citation2017).
HIV reservoirs are not only the infected tissues other than the peripheral blood. Within tissues and peripheral blood, different cellular HIV reservoirs exist. Depending on their activation state, many different immune cell populations in the blood contain latently integrated proviral DNA (Chun et al., Citation2015). An example is resting T-cells (Pomerantz, Citation2002b). Although some studies claim the majority of integrated proviral DNA appears nonfunctional, these latent reservoirs still produce significant amounts of infectious virus upon activation (Bui et al., Citation2017). During all stages of HIV infection, both latent and active replicating virus exists simultaneously. Recently, latently infected T-cells have been show to express a cell surface marker CD32a (Descours et al., Citation2017). This cell surface receptor, that normally binds to the Fc part of Immunoglobuline G (IgG), may actually help us to facilitate targeting the latent HIV cell reservoir as part of the HIV cure efforts (Pillai & Deeks, Citation2017). Regardless of the functional, latent or replicative state of HIV in the different cell reservoirs, each contains different viral quasispecies, adding to the overall complexity of the viral reservoirs in the HIV infected individual.
HIV reservoirs and antiretroviral therapy and drug resistance
HIV reservoirs are also important in the context of antiretroviral therapy. Currently, cART remains the best treatment option available for an HIV infected individual (Arts & Hazuda, Citation2012). The antiviral therapies target different steps in the HIV life cycle (Figure (B)). The first antiretrovirals were NRTIs (nucleoside RT inhibitors) targeting the RT. With the development of more NRTIs, NNRTIs (nonnucleoside RT inhibitors) and protease inhibitors, cART became possible. The standard of care in the developed world is cART. The arsenal has been strengthened with integrase inhibitors and entry inhibitors. cART consists of using drugs from different classes (either RT inhibitors, protease, integrase and entry inhibitors) which reduces the chance of drug resistance (Arts & Hazuda, Citation2012). The first generation antiretrovirals and cARTs were plagued by drug toxicity and large pill burden, which can lead to therapy nonadherence (Troya & Bascunana, Citation2016). The newer drug regimens have reduced pill burden and toxicity. The cARTs have turned HIV infection from a deadly disease to a chronic disease. For a long time, cART was largely out of reach for lower income countries, such as sub-Saharan Africa, who still bear the brunt of the epidemic. Global efforts have now made cART available to these countries. They may not have access to the full arsenal of antiretroviral treatments, but cART has led to a worldwide reduction in HIV mortality and infection (Eholie, Aoussi, Ouattara, Bissagnene, & Anglaret, Citation2012).
Despite the success of cART, drug resistance mutations remain prevalent under therapy and successful viral suppression (Gill, Lynch, Ramazani, & Krentz, Citation2017). Drug resistance can develop under suboptimal therapeutic conditions, due to for instance patient incompliance, but other factors are at play too. Viral reservoirs can act as sanctuary sites. Sanctuary sites are defined as tissues or cells inefficiently targeted by the antiretroviral drugs (Lowe et al., Citation2004). Lots of data suggests continuous HIV-1 replication in various reservoirs under antiretroviral therapy. The brain has long been recognized as an important sanctuary site for HIV, as the different antiretroviral drugs differ in their ability to cross the blood brain barrier (Cardenas et al., Citation2009). Apart from the drugs being unable to pass the endothelium of the tissue, they might also not get modified appropriately into a biologically active form (Bourry et al., Citation2010; Owen & Khoo, Citation2004). Drug transport/efflux mechanisms employed by the endothelial cells ‘protect’ the virus by efficiently transporting the drugs from the tissue back into the blood stream (Taylor, Citation2002). Recent modeling studies, suggest that with cART the brain reservoir may deplete over time. However, the models suggest this may take a long time, with estimates ranging from 3 to even-52 years depending on the patient and start of treatment (Roda et al., Citation2017). This suggests that even with cART the brain remains an important long-lived reservoir.
Individual cells also have drug transport/efflux mechanisms, and HIV proteins have been shown to upregulate these systems in infected cells (Owen & Khoo, Citation2004). Also the gut has many of these drug transport mechanisms at its disposal, and the different drugs accumulate to the different levels in different gut tissues (Bourry et al., Citation2010; De Rosa et al., Citation2013). Several studies, including ours, suggest that the gut tissue reservoir may act as a ‘hide out’ for HIV from antiretroviral therapy (Guadalupe et al., Citation2006; van Marle et al., Citation2010). High HIV-1 RNA viral loads have been observed in the rectal tissues, even though blood viral loads were successfully reduced by antiretroviral therapy (Zuckerman et al., Citation2004). In HIV/AIDS patients who received antiretroviral therapy, viral sequences continued to evolve over time in genes not targeted by the drugs, while plasma viral loads were suppressed to undetectable levels (Frost et al., Citation2001). Our work on samples of patients on monotherapies before the era of cART (van Marle et al., Citation2010), also showed that antiretroviral drug resistance mutations in proviral DNA vary highly over time and between gut compartments. The colon actually appeared to select for mutations resulting in higher drug resistance in patients on zidovudine (AZT) therapy. These data support the notion that different parts of the gut can act as reservoirs and even select for drug resistant viral variants, which may also be the case for other tissue and cell reservoirs (Arts & Hazuda, Citation2012).
Recently, we reported on a unique case with extremely delayed seroconversion (>4 years after primary infection) to HIV, while having robust immune responses to other antigens (Siemieniuk, van der Meer, van Marle, & Gill, Citation2016). This patient received continuous cART, but had a continuous HIV viremia, without detectable signs of antiviral drug resistance. This viremia became undetectable upon HIV seroconversion. This case illustrated the importance of a humoral response for complete viral control on cART. However, it strongly supports the presence of long lived viral reservoirs that keep producing significant amounts of viral progeny, despite considerable pressure of intense integrase inhibitors containing cART regimens.
HIV reservoirs and the implications for the cure strategies
HIV reservoirs also have significant implications for the HIV cure strategies. Until now the best documented individual potentially cured from HIV is the ‘Berlin Patient’ (Allers et al., Citation2011; Hutter et al., Citation2009). This patient received a bone marrow transplant from a CCR5-Δ32 homozygous donor to treat acute myelogenous leukemia. This supplied the patient with immune cells that are highly resistant to infection by CCR5 receptor using HIV variants, which are the majority of the circulating HIV variants. Although HIV may still be present in reservoirs, such as the brain, the patient has up until now not required therapy and virus has been hard to detect (Yukl et al., Citation2013). Similar bone marrow transplants treatments may not be practical for large scale role out. Studies on HIV patients with bone marrow transplants of non CCR5-Δ32 cells, revealed a period of low or undetectable virus without antiviral treatment. However, the virus always returned (Deeks et al., Citation2016). The latter shows that the bone marrow transplant by itself is not a cure, but together with the ‘Berlin patient’ they do show that gene editing approaches of genes such as ccr5 in hematopoietic stem cells could be a viable avenue for large scale use. Mouse models for HIV have further shown proof of principle (Holt et al., Citation2010; Xu et al., Citation2017). However, there are some issues. CCR5-Δ32 is more common among Northern European populations (up to 16% allele frequency), but it is very rare in African populations (Solloch et al., Citation2017), which creates a logistical issue for deploying a ‘Berlin patient’ approach globally. Moreover, CCR5-Δ32 is not totally benign as it appears to be associated with increased risk for disease upon West Nile Virus infection (Glass et al., Citation2006).
Although antiretroviral therapy is considered a treatment and not a cure, it is still considered a major component of the cure strategies (Deeks et al., Citation2016). Studies have shown that intense early treatment can significantly reduce the size of the early viral reservoir and alter the time to viral rebound. Some patients showed long term posttreatment viral control (Cockerham, Hatano, & Deeks, Citation2016; Deeks et al., Citation2016). In addition, the ‘Mississippi baby’ case showed a prolonged period of undetectable virus after cessation of treatment (Persaud & Luzuriaga, Citation2014). These results give support to the idea of ‘waking up’ HIV reservoirs using latency reversing agents (LRAs), and then ‘hitting it’ with intense antiretroviral therapy. The expectation is that after activating the latent reservoirs the immune system will come in and destroy infected cells. The subsequent antiretroviral therapy would then prevent the establishment of new viral reservoirs. This so-called ‘shock and kill’ approach would deplete the viral reservoirs and ultimately be curative (Deeks, Citation2012). The structured treatment interruptions, of the early 2000s were founded on a similar idea, but not successful at curing the infection (Pomerantz, Citation2002a). However, with the newer better LRAs and more potent antiretroviral drugs, such as the integrase inhibitors, more success is anticipated (Deeks et al., Citation2016).
Although the ‘shock and kill’ approach has merit it can still have pitfalls. Apart from not eliminating the whole viral reservoir, what are other risks associated with it? Will we be waking up a ‘sleeping giant’? Although, integrated proviral DNA is assumed largely non-functional, functional virus can be easily recovered from latently infected cells (Chargin et al., Citation2015). This virus could be resistant to the available antiretroviral drugs, and potentially more pathogenic. It has been proposed that drug resistant HIV variants are less pathogenic, because they replicate less efficiently. However, it has been shown that they can also have increased replication ability (Hu & Kuritzkes, Citation2014). Moreover, reduced pathogenicity is not a safe assumption as shown by the 2005 case of a patient in New York City that rapidly progressed to HIV/AIDS upon infection by a multidrug resistant HIV variant (Markowitz et al., Citation2005).
That activating the latent reservoir is not without risk, is illustrated by a recent study using new LRAs in the SIV model. This study clearly showed activation of virus in the brain, as well as signs of neurological disease (Gama et al., Citation2017). This raises concerns about the pathogenic effects in other tissue or cell reservoirs upon massive activation of latent HIV (Spector & Rappaport, Citation2017). Further research will be needed to unravel the potential sequela of HIV reservoir activation.
Conclusion
The HIV cure appears to have remained elusive, but significant progress has been made. The consensus is that for eradication, we will need multiple approaches, consisting of combinations of antiretroviral therapies with latency activating and immunization/immune control strategies, as well as gene therapy editing and transplantation protocols (Deeks et al., Citation2016). However, these approaches will need to take into account the complexity of the HIV reservoirs in order to combat them (Coiras, Lopez-Huertas, Perez-Olmeda, & Alcami, Citation2009). Continued study of the dynamics of the HIV viral reservoirs, will therefore have to remain an essential component of the HIV cure activities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Norquay Endowment for HIV/AIDS research, and the Canadian Institutes for Health Research (CIHR) [grant number HIV/AIDS HOP-75354], [grant number HIV/AIDS HOP-90184].
References
- Allers, K., Hutter, G., Hofmann, J., Loddenkemper, C., Rieger, K., Thiel, E., & Schneider, T. (2011). Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood, 117(10), 2791–2799.
- Arts, E. J., & Hazuda, D. J. (2012). HIV-1 antiretroviral drug therapy. Cold Spring Harbor Perspectives in Medicine, 2(4), a007161.
- Bansal, G. P., Malaspina, A., & Flores, J. (2010). Future paths for HIV vaccine research: Exploiting results from recent clinical trials and current scientific advances. Current Opinion in Molecular Therapeutics, 12(1), 39–46.
- Bednar, M. M., Sturdevant, C. B., Tompkins, L. A., Arrildt, K. T., Dukhovlinova, E., Kincer, L. P., & Swanstrom, R. (2015). Compartmentalization, viral evolution, and viral latency of HIV in the CNS. Current HIV/AIDS Reports, 12(2), 262–271.10.1007/s11904-015-0265-9
- Bourry, O., Mannioui, A., Sellier, P., Roucairol, C., Durand-Gasselin, L., Dereuddre-Bosquet, N., … Le Grand, R. (2010). Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology, 7, 78.10.1186/1742-4690-7-78
- Brenchley, J. M., Price, D. A., & Douek, D. C. (2006a). HIV disease: Fallout from a mucosal catastrophe? Nature Immunology, 7(3), 235–239.10.1038/ni1316
- Brenchley, J. M., Price, D. A., Schacker, T. W., Asher, T. E., Silvestri, G., Rao, S., … Douek, D. C. (2006b). Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine, 12(12), 1365–1371.10.1038/nm1511
- Bui, J. K., Halvas, E. K., Fyne, E., Sobolewski, M. D., Koontz, D., Shao, W., … Mellors, J. W. (2017). Ex vivo activation of CD4+ T-cells from donors on suppressive ART can lead to sustained production of infectious HIV-1 from a subset of infected cells. PLoS Pathogens, 13(2), e1006230.10.1371/journal.ppat.1006230
- Cardenas, V. A., Meyerhoff, D. J., Studholme, C., Kornak, J., Rothlind, J., Lampiris, H., et al. (2009). Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. Journal of Neurovirology, 15(4), 324–333.10.1080/13550280902973960
- Chargin, A., Yin, F., Song, M., Subramaniam, S., Knutson, G., & Patterson, B. K. (2015). Identification and characterization of HIV-1 latent viral reservoirs in peripheral blood. Journal of Clinical Microbiology, 53(1), 60–66.10.1128/JCM.02539-14
- Chiozzini, C., & Toschi, E. (2016). HIV-1 tat and immune dysregulation in AIDS pathogenesis: A therapeutic target. Current Drug Targets, 17(1), 33–45.
- Chun, T. W., Moir, S., & Fauci, A. S. (2015). HIV reservoirs as obstacles and opportunities for an HIV cure. Nature Immunology, 16(6), 584–589.10.1038/ni.3152
- Cockerham, L. R., Hatano, H., & Deeks, S. G. (2016). Post-treatment controllers: Role in HIV “cure” research. Current HIV/AIDS Reports, 13(1), 1–9.10.1007/s11904-016-0296-x
- Coiras, M., Lopez-Huertas, M. R., Perez-Olmeda, M., & Alcami, J. (2009). Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nature Reviews Microbiology, 7(11), 798–812.10.1038/nrmicro2223
- Collins, D. R., & Collins, K. L. (2014). HIV-1 accessory proteins adapt cellular adaptors to facilitate immune evasion. PLoS Pathogens, 10(1), e1003851.
- Costiniuk, C. T., & Angel, J. B. (2012). Human immunodeficiency virus and the gastrointestinal immune system: Does highly active antiretroviral therapy restore gut immunity? Mucosal Immunology, 5(6), 596–604.10.1038/mi.2012.82
- Davis, L. E., Hjelle, B. L., Miller, V. E., Palmer, D. L., Llewellyn, A. L., Merlin, T. L., … Wiley, C. A. (1992). Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology, 42(9), 1736–1739.10.1212/WNL.42.9.1736
- De Rosa, M. F., Robillard, K. R., Kim, C. J., Hoque, M. T., Kandel, G., Kovacs, C., … Bendayan, R. (2013). Expression of membrane drug efflux transporters in the sigmoid colon of HIV-infected and uninfected men. The Journal of Clinical Pharmacology, 53(9), 934–945.10.1002/jcph.132
- Deeks, S. G. (2012). HIV: Shock and kill. Nature, 487(7408), 439–440.10.1038/487439a
- Deeks, S. G., Lewin, S. R., Ross, A. L., Ananworanich, J., Benkirane, M., Cannon, P., … Zack, J. (2016). International AIDS society global scientific strategy: Towards an HIV cure 2016. Nature Medicine, 22(8), 839–850.10.1038/nm.4108
- Descours, B., Petitjean, G., Lopez-Zaragoza, J. L., Bruel, T., Raffel, R., Psomas, C., … Benkirane, M. (2017). CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature, 543(7646), 564–567.10.1038/nature21710
- Doitsh, G., Galloway, N. L., Geng, X., Yang, Z., Monroe, K. M., Zepeda, O., … Greene, W. C. (2014). Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature, 505(7484), 509–514.10.1038/nature12940
- Domingo, E. (1998). Quasispecies and the implications for virus persistence and escape. Clinical and Diagnostic Virology, 10(2–3), 97–101.10.1016/S0928-0197(98)00032-4
- Eholie, S. P., Aoussi, F. E., Ouattara, I. S., Bissagnene, E., & Anglaret, X. (2012). HIV treatment and care in resource-constrained environments: Challenges for the next decade. Journal of the International AIDS Society, 15(2), 17334.
- Faria, N. R., Rambaut, A., Suchard, M. A., Baele, G., Bedford, T., Ward, M. J., … Lemey, P. (2014). HIV epidemiology. The early spread and epidemic ignition of HIV-1 in human populations. Science, 346(6205), 56–61.10.1126/science.1256739
- Freed, E. O. (2015). HIV-1 assembly, release and maturation. Nature Reviews Microbiology, 13(8), 484–496.10.1038/nrmicro3490
- Frost, S. D., Gunthard, H. F., Wong, J. K., Havlir, D., Richman, D. D., & Leigh Brown, A. J. (2001). Evidence for positive selection driving the evolution of HIV-1 env under potent antiviral therapy. Virology, 284(2), 250–258.10.1006/viro.2000.0887
- Gama, L., Abreu, C. M., Shirk, E. N., Price, S. L., Li, M., Laird, G. M., … Clements, J. E. (2017). Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS, 31(1), 5–14.10.1097/QAD.0000000000001267
- Gill, V. C., Lynch, T., Ramazani, S., & Krentz, H. B. (2017). Reporting on the prevalence of antiretroviral drug resistance in a regional HIV population over 20 years: A word of caution. Antiviral Therapy, 22(4), 277–286.
- Glass, W. G., McDermott, D. H., Lim, J. K., Lekhong, S., Yu, S. F., Frank, W. A., … Murphy, P. M. (2006). CCR5 deficiency increases risk of symptomatic West Nile virus infection. The Journal of Experimental Medicine, 203(1), 35–40.10.1084/jem.20051970
- Gonzalez-Scarano, F., & Martin-Garcia, J. (2005). The neuropathogenesis of AIDS. Nature Reviews Immunology, 5(1), 69–81.10.1038/nri1527
- Gray, L. R., Turville, S. G., HItchen, T. L., Cheng, W. J., Ellett, A. M., Salimi, H., … Churchill, M. J. (2014). HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS ONE, 9(2), e90620.10.1371/journal.pone.0090620
- Guadalupe, M., Sankaran, S., George, M. D., Reay, E., Verhoeven, D., Shacklett, B. L., … Dandekar, S. (2006). Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. Journal of Virology, 80(16), 8236–8247.10.1128/JVI.00120-06
- Hahn, B. H., Shaw, G. M., De Cock, K. M., & Sharp, P. M. (2000). AIDS as a zoonosis: Scientific and public health implications. Science, 287(5453), 607–614.10.1126/science.287.5453.607
- Holt, N., Wang, J., Kim, K., Friedman, G., Wang, X., Taupin, V., … Cannon, P. M. (2010). Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nature Biotechnology, 28(8), 839–847.10.1038/nbt.1663
- Hu, Z., & Kuritzkes, D. R. (2014). Altered viral fitness and drug susceptibility in HIV-1 carrying mutations that confer resistance to nonnucleoside reverse transcriptase and integrase strand transfer inhibitors. Journal of Virology, 88(16), 9268–9276.10.1128/JVI.00695-14
- Hutter, G., Nowak, D., Mossner, M., Ganepola, S., Mussig, A., Allers, K., … Thiel, E. (2009). Long-term control of HIV by CCR5 Δ32/Δ32 stem-cell transplantation. New England Journal of Medicine, 360(7), 692–698.10.1056/NEJMoa0802905
- Keele, B. F., Van Heuverswyn, F., Li, Y., Bailes, E., Takehisa, J., Santiago, M. L., … Hahn, B. H. (2006). Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science, 313(5786), 523–526.10.1126/science.1126531
- Kotler, D. P. (2005). HIV infection and the gastrointestinal tract. AIDS, 19(2), 107–117.10.1097/00002030-200501280-00002
- Lamers, S. L., Rose, R., Maidji, E., Agsalda-Garcia, M., Nolan, D. J., Fogel, G. B., … Singer, E. J. (2016). HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy-treated patients with undetectable viral loads. Journal of Virology, 90(20), 8968–8983.10.1128/JVI.00674-16
- Lerner, P., Guadalupe, M., Donovan, R., Hung, J., Flamm, J., Prindiville, T., … Dandekar, S. (2011). The gut mucosal viral reservoir in HIV-infected patients is not the major source of rebound plasma viremia following interruption of highly active antiretroviral therapy. Journal of Virology, 85(10), 4772–4782.10.1128/JVI.02409-10
- Levy, J. A. (2007). HIV and the pathogenesis of AIDS (3rd ed.). Washington, DC: American Society of Microbiology.10.1128/9781555815653
- Lowe, S. H., Sankatsing, S. U., Repping, S., van der Veen, F., Reiss, P., Lange, J. M., & Prins, J. M. (2004). Is the male genital tract really a sanctuary site for HIV? Arguments that it is not AIDS, 18(10), 1353–1362.
- Markowitz, M., Mohri, H., Mehandru, S., Shet, A., Berry, L., Kalyanaraman, R., ... Ho, D. D. (2005). Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: A case report. The Lancet, 365(9464), 1031–1038.10.1016/S0140-6736(05)74227-6
- Monroe, K. M., Yang, Z., Johnson, J. R., Geng, X., Doitsh, G., Krogan, N. J., & Greene, W. C. (2014). IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science, 343(6169), 428–432.10.1126/science.1243640
- Nisole, S., & Saib, A. (2004). Early steps of retrovirus replicative cycle. Retrovirology, 1, 9.10.1186/1742-4690-1-9
- Overbaugh, J., & Bangham, C. R. (2001). Selection forces and constraints on retroviral sequence variation. Science, 292(5519), 1106–1109.10.1126/science.1059128
- Owen, A., & Khoo, S. H. (2004). Intracellular pharmacokinetics of antiretroviral agents. Journal of HIV Therapy, 9(4), 97–101.
- Persaud, D., & Luzuriaga, K. (2014). Absence of HIV-1 after treatment cessation in an infant. The New England Journal of Medicine, 370(7), 678.
- Pillai, S. K., & Deeks, S. G. (2017). Signature of the sleeper cell: A biomarker of HIV latency revealed. Trends in Immunology, 38(7), 457–458.10.1016/j.it.2017.04.007
- Pomerantz, R. J. (2002a). Eliminating HIV-1 reservoirs. Current Opinion in Investigational Drugs, 3(8), 1133–1137.
- Pomerantz, R. J. (2002b). HIV-1 reservoirs. Clinics in Laboratory Medicine, 22(3), 651–680, vi.10.1016/S0272-2712(02)00005-7
- Ranki, A., Nyberg, M., Ovod, V., Haltia, M., Elovaara, I., Raininko, R., … Krohn, K. (1995). Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS, 9(9), 1001–1008.10.1097/00002030-199509000-00004
- Roda, W. C., Li, M. Y., Akinwumi, M. S., Asahchop, E. L., Gelman, B. B., Witwer, K. W., & Power, C. (2017). Modeling brain lentiviral infections during antiretroviral therapy in AIDS. Journal of NeuroVirology.
- Siemieniuk, R. A., van der Meer, F., van Marle, G., & Gill, M. J. (2016). A case of long-term seronegative human immunodeficiency virus (HIV) infection: The importance of the humoral response to HIV. Open Forum Infectious Diseases, 3(1), ofv209.
- Solloch, U. V., Lang, K., Lange, V., Bohme, I., Schmidt, A. H., & Sauter, J. (2017). Frequencies of gene variant CCR5-Δ32 in 87 countries based on next-generation sequencing of 1.3 million individuals sampled from 3 national DKMS donor centers. Human Immunology, 78(11–12), 710–717.10.1016/j.humimm.2017.10.001
- Spector, S. A., & Rappaport, J. (2017). HIV cure strategists: Ignore the central nervous system at your patients’ peril. AIDS, 31(1), 167–168.10.1097/QAD.0000000000001268
- Suhasini, M., & Reddy, T. R. (2009). Cellular proteins and HIV-1 rev function. Current HIV Research, 7(1), 91–100.10.2174/157016209787048474
- Taylor, E. M. (2002). The impact of efflux transporters in the brain on the development of drugs for CNS disorders. Clinical Pharmacokinetics, 41(2), 81–92.10.2165/00003088-200241020-00001
- Timilsina, U., & Gaur, R. (2016). Modulation of apoptosis and viral latency – An axis to be well understood for successful cure of human immunodeficiency virus. Journal of General Virology, 97(4), 813–824.10.1099/jgv.0.000402
- Tongo, M., Dorfman, J. R., & Martin, D. P. (2016). High degree of HIV-1 group M (HIV-1M) genetic diversity within circulating recombinant forms: Insight into the early events of HIV-1M evolution. Journal of Virology, 90(5), 2221–2229.10.1128/JVI.02302-15
- Troya, J., & Bascunana, J. (2016). Safety and tolerability: Current challenges to antiretroviral therapy for the long-term management of HIV infection. AIDS Reviews, 18(3), 127–137.
- Tsao, L. C., Guo, H., Jeffrey, J., Hoxie, J. A., & Su, L. (2016). CCR5 interaction with HIV-1 Env contributes to Env-induced depletion of CD4 T cells in vitro and in vivo. Retrovirology, 13, 22.10.1186/s12977-016-0255-z
- van Marle, G., Church, D. L., Nunweiler, K. D., Cannon, K., Wainberg, M. A., & Gill, M. J. (2010). Higher levels of Zidovudine resistant HIV in the colon compared to blood and other gastrointestinal compartments in HIV infection. Retrovirology, 7, 74.10.1186/1742-4690-7-74
- van Marle, G., Gill, M. J., Kolodka, D., McManus, L., Grant, T., & Church, D. L. (2007). Compartmentalization of the gut viral reservoir in HIV-1 infected patients. Retrovirology, 4, 87.10.1186/1742-4690-4-87
- van Marle, G., Sharkey, K. A., Gill, M. J., & Church, D. L. (2013). Gastrointestinal viral load and enteroendocrine cell number are associated with altered survival in HIV-1 infected individuals. PLoS ONE, 8(10), e75967.10.1371/journal.pone.0075967
- Visseaux, B., Damond, F., Matheron, S., Descamps, D., & Charpentier, C. (2016). Hiv-2 molecular epidemiology. Infection, Genetics and Evolution, 46, 233–240.10.1016/j.meegid.2016.08.010
- WHO. (2016a). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach (2nd ed.). Retrieved from http://www.who.int/hiv/pub/arv/arv-2016/en/
- WHO. (2016b, July 2016). HIV/AIDS data and statistics. Retrieved September 1, 2017, from http://www.who.int/hiv/data/en/
- Worobey, M., Gemmel, M., Teuwen, D. E., Haselkorn, T., Kunstman, K., Bunce, M., … Wolinsky, S. M. (2008). Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature, 455(7213), 661–664.10.1038/nature07390
- Xu, L., Yang, H., Gao, Y., Chen, Z., Xie, L., Liu, Y., ... Deng, H. (2017). CRISPR/Cas9-mediated CCR5 ablation in human hematopoietic stem/progenitor cells confers HIV-1 resistance in vivo. Molecular Therapy, 25(8), 1782–1789.10.1016/j.ymthe.2017.04.027
- Yukl, S. A., Boritz, E., Busch, M., Bentsen, C., Chun, T. W., Douek, D., ... Deeks, S. G. (2013). Challenges in detecting HIV persistence during potentially curative interventions: A study of the Berlin patient. PLoS Pathogens, 9(5), e1003347.10.1371/journal.ppat.1003347
- Zuckerman, R. A., Whittington, W. L., Celum, C. L., Collis, T. K., Lucchetti, A. J., Sanchez, J. L., … Coombs, R. W. (2004). Higher concentration of HIV RNA in rectal mucosa secretions than in blood and seminal plasma, among men who have sex with men, independent of antiretroviral therapy. The Journal of Infectious Diseases, 190(1), 156–161.10.1086/jid.2004.190.issue-1
