ABSTRACT
Traditional therapeutic approaches in the treatment of cancer have many side effects and are often ineffective and non-specific, leading to the development of therapy-resistant tumour cells. Recently, numerous discoveries about stem cells have given a new outlook on their application in oncology. Stem cells are unique because of their biological attributes, including self-renewal, differentiation in different types of specialized cells and synthesis of molecules that interplay with tumour niche. They are already used as an effective therapeutic option for haematological malignancies, such as multiple myeloma and leukaemia. The main goal of this study is to investigate the possible applications of different types of stem cells in cancer treatment and to summarize novel advances, as well as the limitations of their application in cancer treatment. Research and clinical trials that are underway revealed and confirmed the enormous potential of regenerative medicine in the treatment of cancer, especially when combined with different nanomaterials. Nanoengineering of stem cells has been the focus of novel studies in the area of regenerative medicine, such as the production of nanoshells and nanocarriers that enhance the transport and uptake of stem cells in their targeted tumour niche and enable the effective monitoring of stem cell effects on tumour cells. Although nanotechnology has a lot of limitations, it provides new opportunities for the development of effective and innovative stem cell therapies.
1. Introduction
While the mortality in developed countries from infectious diseases is constantly decreasing, the incidence of cancer is constantly increasing and its incidence is now just below the incidence of the diseases of the cardiovascular system. The complex process of carcinogenesis leads to the alteration of healthy cells and their mutation into tumour cells that can escape from all control mechanisms occurring throughout the cell cycle. Consequently, tumour cells differ from healthy, unaltered cells. They proliferate faster than healthy cells, avoid cell apoptosis, escape the protective mechanism of the host’s immune system, induce synthesis of inflammatory molecules. Tumour cells trigger the onset of angiogenesis, as well as the process of migration into surrounding tissues and distant parts of the host organism. Although it develops in childhood, cancer is rare before the age of thirty and its frequency increases with age (Sung et al., Citation2021).
More than 60% of cancer deaths in developed countries occur in people over 65 years old. Real data on cancer incidence depends on an accurate and extensive recording of new disease cases in a defined population, the quality of accurate diagnosing, the appropriate classification of the disease and reliable censuses or estimates of the population by gender and age. The annual number of newly diagnosed cancer patients (excluding skin cancer) is about 19.3 million worldwide and the most common cancer in women, including all age groups after the age of thirty is breast cancer (Sung et al., Citation2021). Cervical cancer occurs at a younger age, most often between the ages of 45 and 49. In men, the most common cancers are lung, prostate and colorectal cancer. According to certain statistics, today every fifth person in developed countries dies of cancer, while about a third of cancer patients are being successfully treated (Sung et al., Citation2021).
The tumours are heterogeneous, which means that they are composed of different types of cells, but their base is formed by highly resistant cancer stem cells (Patterson et al., Citation2018). When describing a tumour, the term neoplasm or new growth is used. The term neoplasm refers only to uncontrolled, autonomous and purposeless growth, i.e. the multiplication of cells during which a tumour nodule is formed.The tumour is a term used for any abnormal accumulation of tissue, which can be malignant, cancerous or benign, non-cancerous (Patterson et al., Citation2018). Benign tumours grow more slowly and their growth is limited to the place of their origin. Furthermore, they do not have the invasive ability to invade the surrounding tissue and form local or distant metastases. Such cells show some degree of autonomous growth control with unaltered differentiation. On the other hand, malignant neoplasms have very similar structures to healthy tissues or organs, but the problem arises when the cell cycle control mechanisms are changed. Malignant tumours are poorly differentiated tissues, although they can give an image of well-differentiated tissues, which is an essential characteristic of malignant neoplasms (Patterson et al., Citation2018).
The altered function of numerous proteins that have a key role in cell signalling, control of the cell cycle and programmed cell death, leads to the breakdown of regulatory mechanisms in normal cells and the uncontrolled proliferation of cancer cells. As a result, there is uncontrolled growth and multiplication of altered, small cells, dominance over healthy cells, disruption of their structure and function and eventually their death. Therefore, malignant tumours grow faster, locally invade the surrounding tissue and potentiate, through the blood vessel or lymphatic system, the occurrence of metastatic changes in other organs, bringing the patient to the stage of extended, advanced disease. Tumours in humans are of monoclonal origin that means that they arose from a single transformed cell. The main classification of both benign and malignant tumours is according to the cell types they originate from. Therefore, they are classified into carcinomas (formed in the epithelial tissue), sarcomas (solid tumours of connective tissues – muscles, bones, cartilage), leukaemias and lymphomas (malignant diseases of the cells that originate from hematopoietic system and immune system) (Patterson et al., Citation2018).
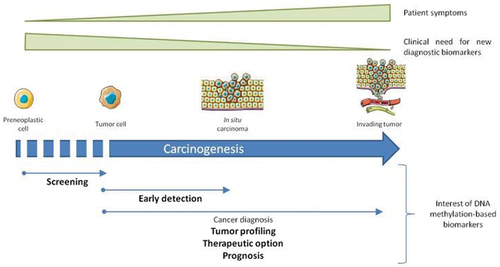
The development of cancer is a multi-step, complicated process in which the changes in the genetic material of the cells accumulate progressively over the years, giving cancer cells the ability to invade and metastasize. This period of transformation of a healthy cell into a malignant one includes numerous changes, as shown in , the transition from controlled to autonomous growth with normal differentiation, autonomous growth with disturbed differentiation, the appearance of nuclear atypia, the possibility of passing through the basal membrane, the possibility of passing through the dermis and entering blood vessels, stopping in nodes and organs, such as the liver and the lungs and the possibility of clonal expansion of metastasis at different locations (Patterson et al., Citation2018).
Figure 1. Phases of carcinogenesis – a process where a cell progresses from the preneoplastic cell into tumour cell and in situ carcinoma, leading to the invasion of blood vessels and lymph nodes, as well as surrounding tissue (Delpu et al., Citation2013).
In simple terms, the application of cell therapy involves the collection, processing and storage of different types of cells as a new therapeutic agents. Nevertheless, the cell therapy sector is still considered to be in its early stages of development – particularly in terms of wider clinical application and commercialization (Atala, Citation2021). Cell therapies and the development of regenerative medicine comprise a new field in the medical, (bio) pharmaceutical and biological sciences, based on the ‘super-powers’ that stem cells show while fighting many different diseases. Their expansion and wider clinical application are expected in the future. When using stem cells either for therapeutic purposes or for clinical research, strictly controlled conditions must be met, in order to do collection, processing, storage and transport. Each of these steps should include strictly controlled protocols that will ensure their integrity and quality (Das et al., Citation2021). The most common classification of stem cells is into two groups: embryonic and adult stem cells. Stem cells based therapy could be categorised based on several very different approaches such as the initial population of used cells (e.g. placental cells), the type of cells in which they differentiate (e.g. mesenchymal cells), the type of disease they treat (e.g. cancer, neurodegenerative or autoimmune diseases) or the level of manipulation they needed (from minimal to high manipulated) (Atala, Citation2021).
Human embryonic stem cells have been intensively used in the processes of in vitro fertilization and for research purposes. At the same time, they have attracted enormous attention from the scientific and general public due to potential dilemmas within the ethical and legal aspects of their application. Since 2006, attention has shifted again to adult stem cells, due to the discovery of the technique of genetic ‘reprogramming’ of differentiated cells of an adult organism (e.g. skin fibroblasts), whereby already differentiated cells return to their ‘parent’ state, showing the properties of pluripotent stem cells (Das et al., Citation2021). This type of cell is called an induced pluripotent stem cell (iPS). Today, iPS cells are predominantly used in research, replacing the controversial human embryonic stem cells (G. Liu et al., Citation2020).
However, cellular biotherapeutics used in medicine are still characterized as experimental therapy (G. Liu et al., Citation2020). An exception is the transplantation of stem cells from the bone marrow and peripheral blood. The protocol which has been routinely used for decades in the treatment of patients with malignant blood diseases, autoimmune disorders and some tumour diseases is constantly being improved. A little more than a decade and a half ago, the pluripotent stem cells (iPS) trait of ‘trans-differentiation’ was discovered. This ability to produce cell descendants of other cell lineages (Heart, liver, bone and others)under the influence of certain factors started the development of regenerative medicine (G. Liu et al., Citation2020). However, science cannot yet fully control the ‘behaviour’ of stem cells and that is why there is a growing need to conduct more research that will establish standardized indications, therapeutic range and dosage protocols, based on successful experimental and clinical practice.
Answers are still being sought to the questions of whether, how and to what extent stem cells can migrate outside of the part of the body weight they are targeted (Atala, Citation2021). In other words, everything new that researchers learn about ‘managing’ the differentiation (maturation or specialization) of stem cells will increase the possibilities for the treatment of various diseases, from neurological disorders to cancer. From the legal regulatory aspect, it is expected to approach the development and application of cell therapies in the same way as the production of other therapeutic agents in the pharmaceutical industry – both including the appropriate physical environment and laboratory procedures covered by complex quality systems in accordance with prescribed legal regulations. The development of cell therapies represents technological as well as organizational and legal regulatory challenges. Legislation-oriented models and modalities that include clearly defined and comprehensive complex quality systems have proven to be a sustainable approach and a prerequisite for successful projects in the development and application of cell therapies on a global level (Das et al., Citation2021; Inthagard et al., Citation2019).
Surgery as a treatment option is limited to localized tumours. Radiotherapy can destroy tumours by damaging the cancer cell’s DNA and chemotherapy does that by using highly toxic drugs (Siegel et al., Citation2022). Immunotherapy is becoming an important option for cancer treatment with quite improved clinical outcomes The major disadvantage of all the previously mentioned therapies is non-specificity, which results in suboptimal effectiveness, resistance to therapy and ultimately, cancer recurrence. In addition, even with the use of immunotherapy, many side effects have been observed (Inthagard et al., Citation2019; Siegel et al., Citation2022). However, despite rapid advances in diagnostic and treatment options, the cancer death rate has not declined as much as wanted. In 2022, 609.360 deaths from cancer are expected to occur in the United States (Society, Citation2022).
Meanwhile, stem cell therapy is a new therapeutic option that could significantly improve the effectiveness of other therapeutic modalities and reduce their side effects. Several investigational therapy protocols based on stem cells are now in the phase of preclinical trials with promising results (Das et al., Citation2021). Therefore, it is necessary to carry out further evaluation, all to prepare the application of stem cells in clinical research and provide hope to oncology patients. This review aims to summarize previous findings about stem cell-based therapy and to collect the latest information on this topic, as well as to give an overview of known mechanisms by which stem cells contribute to fighting against cancer. In addition, the paper will present recent achievements and limitations in the area of stem cell-based therapy.
1.1. Gene therapy in cancer treatment
Since malignant tumour cells accumulate a large number of gene mutations, it is assumed that gene therapy could be effective in the treatment of tumours (Yahya & Alqadhi, Citation2021). Gene therapy is defined as the administration of genetic material into the cells to have a beneficial therapeutic effect and cure for a disease. There are different approaches to tumour gene therapy, such as the replacement of mutations in oncogenes and tumour-suppressor genes, molecular chemotherapy, protection of bone marrow stem cells, immunogenic treatment, transfer of genetic material and tumour-specific expression and oncolysis (Patterson et al., Citation2018). Genetic mutations in tumour cells mostly result in either activation of oncogenes or the inactivation of tumour-suppressor genes. Gene therapy attempts to stop the activity of oncogenes and better the activity of tumour-suppressor genes (Yahya & Alqadhi, Citation2021).
Oncogene activity can be terminated in several ways and one of them is at the DNA (deoxyribonucleic acid) level, where gene transcription can be prevented by using synthetic oligonucleotides that bind to the target DNA, thus building a triple DNA helix. On the level of mRNA (messenger RNA) oncogene activity can be prevented from being translated by using ‘antisense’ sequences that bind to the mRNA and thus promote its degradation, as shown in . In addition, at RNA level, ribozymes, molecules with enzymatic activity that bind to the complementary mRNA molecule and enzymatically refine it, can be used. Attempts are made to restore the function of tumour suppressor genes by introducing a healthy tumour suppressor gene into tumour cells. The disadvantage of such approaches to gene therapy in cancer treatment is that for successful treatment it is necessary to establish a normal phenotype in each tumour cell that is currently not possible (Yahya & Alqadhi, Citation2021).
Figure 2. Gene therapy in cancer treatment achieved by the use of tumour-specific promoter (TSP), altering the expression of genes specifically involved in cancer cell development, preventing tumorigenesis (Chen et al., Citation2018).
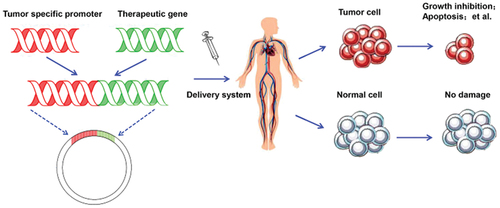
The goal of molecular chemotherapy is to direct the action of cytotoxic drugs as selectively as possible to tumour cells, consequently avoiding the side effects of systemic chemotherapy. This is achieved by targeted application of the gene that encodes the enzyme responsible for converting a non-toxic precursor into a drug that kills tumour cells, after which this precursor is systemically administered to patients (Yahya & Alqadhi, Citation2021). During the therapy, attempts are made to protect the bone marrow stem cells, which is achieved by introducing the mdr1(engl.multidrug resistance) gene. It makes the bone marrow stem cells as resistant as possible to chemotherapy. Such a form of gene therapy could enable the use of higher doses of chemotherapy drugs (Yahya & Alqadhi, Citation2021).
The main role of immunotherapy is to strengthen the immune system in its fight against tumours. Gene therapy techniques are also used to teach immune cell show to recognise antigens expressed on the surface of tumour cells or dendritic cells, which most efficiently present tumour antigens. The genes for the molecules CD80 and CD86 (Clusters of differentiation 80 and 86) that participate in the immune recognition of cells from lymphocytes are introduced into the tumour cells so that they can strengthen the immune reaction against the tumour (Yahya & Alqadhi, Citation2021). There are two basic approaches to gene therapy: ex vivo and in vivo (G. Liu et al., Citation2020). Ex vivo techniques consist of extracting cells from the patient’s body and growing these cells in culture, introducing foreign into cells by using a vector and returning them to the patient’s body. In vivo gene transfers include the direct introduction of genes into the diseased tissue, directly in the patient’s organism. In tumour-specific expression, the gene carrier can be injected directly into the tumour or into the blood vessels that supply the tumour. The genes that code the glycoprotein envelope of viral vectors can be altered to allow specific binding of the vector to tumour cells expressing certain receptors or induce oncolysis of tumour cells (G. Liu et al., Citation2020).
2. Therapeutic possibilities of parentage stem cells in cancer therapy
The use of stem cells in the treatment of tumours depends on their capacity for proliferation, migration and differentiation, as well as the potential to develop into different cell types during the early growth and formation of the organism. In addition, they serve as an internal system for regeneration, as they are capable of a practically unlimited number of divisions, thereby renewing other cells in the body (Kooreman et al., Citation2018; Li et al., Citation2018). After cell division, each new stem cell has the potential to retain the same role as a mother cell or to differentiate into another cell type with a specialized function. Two main characteristics distinguish stem cells from other cells in the body (Kooreman et al., Citation2018). First, they possess the ability to self-renew through division, sometimes even after a very long period of inactivity (Y. Li et al., Citation2018). Second, under certain physiological or experimental conditions, the development of specialized cells can be induced (Li et al., Citation2018). In some organs, such as the liver or the bone marrow, stem cells are constantly dividing and renewing the different cell types. In other organs, such as the heart or pancreas, they divide only in specific situations, mostly after injury or damage.
2.1. Types of pluripotent stem cells
There are two basic types of pluripotent stem cells (PSCs): embryonic stem cells (ESCs) and induced pluripotent cells (iPSCs). ESCs have the ability to generate all cell types except placental cells but their use in clinical trials is limited due to ethical reasons. In 2006, virally induced expression of four transcription factors octamer-binding transcription factor 4 (Oct4), SRY-Box Transcription Factor 2(Sox2), cellular Myc(c-Myc) and Kruppel-like factor 4 (Klf4), called Yamanaka factors, were discovered (Li et al., Citation2018). The use of Yamanaka factors enabled the induction of the generation of pluripotent (iPS) cells from adult fibroblasts, which led to significant progress in the field of cell biology. iPSCs produced in this way share the same characteristics as ESCs and at the same time have one extremely important advantage, they almost eliminate ethical problems due to the destruction of embryos. Today, ESCs and iPSCs are used as an important starting point for the induction of effector T cells and NK (natural killer) cells (Y. Li et al., Citation2018), as well as for the development of cancer vaccines (Ouyang et al., Citation2019) that will be discussed in more detail in the rest of the article.
2.2. Adult stem cells
Adult stem cells can be isolated from almost every adult human tissue and since they are undifferentiated, with the attribute of plasticity, they can differentiate into many different types of specialized cells. Adult stem cells (ASCs) can be found in the bone marrow, liver and other organs of both children and adults. The process of extracting these cells from the bone marrow is painful and can cause complications. On the contrary, stem cells obtained from umbilical cord blood are significantly younger and have a greater ability to adapt consequently having a better therapeutic effect. A match of 67% between the donor and the patient is sufficient for the success of the transplantation of stem cells taken from umbilical cord blood, in contrast to stem cells obtained from the bone marrow where the match should be almost 100% (Yahya & Alqadhi, Citation2021).
The three most common groups of ASCs used in cancer treatment are hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs) and neural stem cells (NSCs) (Li et al., Citation2018). HSCs, located in the bone marrow could differentiate into all types of mature blood cells in the body through the process of haematopoiesis. With the approval of the FDA (American Food and Drug Administration), HSCs have found their main application in the treatment of multiple myeloma and leukaemia (Siegel et al., Citation2022). Namely, HSCs obtained from the umbilical cord blood, the bone marrow or peripheral blood of the donor are transplanted by infusion into the recipient that is preceded by immunosuppression of the patient’s immune system (Siegel et al., Citation2022).
MSCs are multipotent cells located in many tissues and organs with an enhanced ability to rapidly proliferate and consequently regenerate different types of tissues. In vitro, these have the ability of paracrine signalling, releasing molecules with a pleiotropic effect and the ability to induce the differentiation of several specialized cell types (Christodoulou et al., Citation2018). Additionally, thanks to their ability to interact with their environment, MSCs are often used as a support system for other cancer treatments or as a carrier of therapeutic agents while treating many different cancer types (Lin et al., Citation2019). Mesenchymal stem cells are the main candidate for the treatment of spinal cord damage in Alzheimer’s disease, cerebral palsy, Parkinson’s disease, and numerous rheumatological diseases, such as psoriasis and rheumatoid arthritis, myocardial infarction and further studies should unequivocally confirm that (Lin et al., Citation2019).
NSCs are multipotent, undifferentiated cells originating primarily from the central nervous system. They have the ability to self-regenerate and transform into neurons and glial cells, both during the embryonic development of an organism, as well as in adult brain neurogenesis (Cordero et al., Citation2022). The use of NSCs in the treatment of the primary and metastatic cancers of the prostate, lung and breast is extensively tested in mouse models (Bazinet & Popradi, Citation2019; Karthaus et al., Citation2020; Mercer-Smith et al., Citation2022). Stem cell research is continuously evolving in quest of finding effective cancer treatment. In certain cases of aggressive or recurrent cancers, after other therapies failed, treatment with stem cells remains the only therapeutic option for the patient.
2.3. Development and characteristics of cancer stem cells
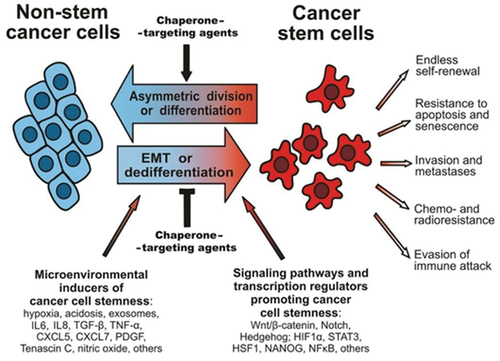
Cancer stem cells (CSCs) mutate from normal stem cells. Therefore, they share many common characteristics with stem cells – the possibility of self-renewal or uncontrolled cell division and immortality. Characteristics of cancer cells and cancer stem cells are shown in . CSCs also have an important role in the growth and survival of other tumour cells in the tumour niche, the occurrence of tumour metastases or tumour recurrence and they are the ones that take the blame for the tumour cells being resistant to chemotherapy and radiotherapy. Therefore, CSCs are a potential target in novel therapies that are being developed for various types of solid tumours as well as they are the focus of numerous oncological aspects (Juarez et al., Citation2012). Stem cells have the potential to curate many different types of cancers by using different mechanisms, such as breast cancer (Lin et al., Citation2019), gastric cancer (Karthaus et al., Citation2020), lung cancer (Mercer-Smith et al., Citation2022) and haematological neoplasms (Bazinet & Popradi, Citation2019).
Figure 3. A scheme illustrating the plasticity of cancer cell phenotypes, their main characteristics and signalling pathways that can contribute to cancer stem cell development, as well as environmental factors (synthesis of interleukins, oxygen radicals and growth factors) (Kabakov et al., Citation2020).
2.4. Bone marrow migration and tumorigenic effect of stem cells
The use of chemotherapy for cancer treatment carries numerous side effects. One of the most common side effects is damage to the hematopoietic processes and the occurrence of pancytopenia – the decrease in the number of all three bloodlines (Leukocytes, erythrocytes and platelets) that is considered an urgent and life-threatening emergency in oncology. In the case of hematologic cancers, the underlying disease very often leads to these complications even without chemotherapy. One of the potential treatment modalities for treating pancytopenia is the intravenous infusion of autologous or allogeneic HSCs (Juarez et al., Citation2012). The role of HSCs would be to carry out the process of homing – the rapid migration of cells to a defined niche of stem cells in the bone marrow (BM). During transmigration, HSCs come into contact with the endothelium of blood vessels through different molecules, such as CD44 (cluster of differentiation, LFA-1 (lymphocyte function-related antigen 1), degradable enzyme MMP-2/9 (Matrix metalloproteinase2/9) and VLA-4/5 (very late antigen-4/5) (Juarez et al., Citation2012; Okajima, Citation2013).
After entering the bone marrow (BM), transplanted HSCs start complex interactions with numerous chemokines, receptors and signalling molecules, to achieve their final effect – the formation of new and healthy specialized blood cells (Bazinet & Popradi, Citation2019). Complex molecular mechanisms stand behind the HSC homing process. One of the assumed mechanisms is an active interaction between SDF-1 (Stromal cell-derived factor 1) and CXCR4 (Chemokine receptors type 4) stem cell receptor. SDF-1 is produced by stem cells located at the BM stem cell niche. Other molecules included in the molecular signalling network are ceramide-1 phosphate, sphingosine-1-phosphate, extracellular ATP (Adenosine triphosphate) or UTP (Uridine triphosphate) and Ca 2+ and H + ions (Juarez et al., Citation2012; Okajima, Citation2013).
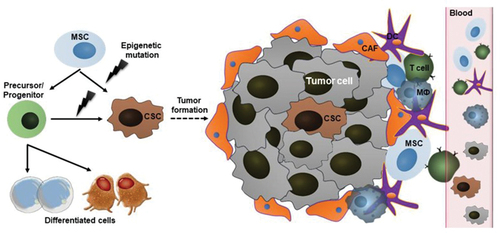
The mutations inside tumour cells are favoured by chronic inflammation, a state of long-term cell hypoxia, the formation of reactive oxygen radicals and oxidative stress of cells (T. Huang et al., Citation2020). Therefore, it is assumed that the migration of MSCs into the tumour tissue is started by the release of the same molecule from the tumour microenvironment that is being released when other pathological processes deploy in other tissues, such as inflammation that occurs in myocardial ischemia (Jiang et al., Citation2019). The secretion of numerous chemoattracting factors is also the main communication pathway occurring between tumour cells and the immune system of the human organism (Atiya et al., Citation2020). The key regulators in that process are nano-sized exosomes (30–150 nm) - the vesicles that contain molecules with pro-regenerative effects and with the ability to affect important cellular processes, such as proliferation, angiogenesis, oxidative stress, inflammation and immunotolerance (Liesveld et al., Citation2020). A very simplified representation of a complex tumour environment is shown in . It has been proven that various molecules, such as IL-6 (Interleukin 6), CXCL16 (chemokine ligand 16) and CCL-25 (CC - motif 25 (Chemokine ligand 25)), mostly released from the cells of prostate cancer, osteosarcoma, breast cancer and multiple myeloma, provoke the MSCs migration into the tumour niche (Liesveld et al., Citation2020; Zhang et al., Citation2020). MSCs in tumour niche transform into endothelial cells or myofibroblasts, thereby contributing to the development of the tumour stroma. The key role in that process of transformation has proinflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin − 1β (IL-1β) that are released by tumour-associated immune cells (Suman et al., Citation2019; Vakhshiteh et al., Citation2019; Wu et al., Citation2016).
Figure 4. Tumor niche contains tumour cells, cancer stem cells, different immune cells (T-cells, dendritic cells, macrophages, etc.) and their products - chemoattractants, which have the main role in process of carcinogenesis (Chu et al., Citation2020).
2.5. CSC molecular signalling pathways
The development of CSCs is mostly caused by the changes in the molecular signalling pathways that regulate normal stem cell proliferation, including Notch, NF-κB (nuclear factor kappa), Wnt/β-catenin, PI3K/PTEN (phosphoinositide 3-kinase), JAK/STAT (Janus kinase/signal transducers of transcription) and Hedgehog (Garcia-Mayea et al., Citation2020; Li et al., Citation2021). In practice, different methods of CSCs isolation are used. Scientists have found a way to target the protein markers on the cell surface and to detect cells’ metabolic characteristics (Najafi et al., Citation2019; Ruiu et al., Citation2019). Some markers expressed on CSCs surface, such as Lgr5 (leucine-rich repeat 5-containing G-protein-coupled receptor), CD133 (prominin-1, HSC marker) and EpCAM (epithelial cell adhesion molecule) are often used to identify CSCs from highly heterogeneous cell types in tumours (Najafi et al., Citation2019). CD133 cells have been successfully extracted from brain, lung, colorectal, liver and gastrointestinal tumours (Najafi et al., Citation2019).
Another example of the successful use of markers to identify the CSCs in tumours is the CD44 marker, combined with CD133 or CD24. In the NOD/SCID breast cancer model in mice, a small population of stem cells expressing CD44 + CD24 −/low cells were identified and it was proven that they accelerate tumour growth or completely form the original tumour tissue (Najafi et al., Citation2019). CSC is also recognized by assessing their metabolic activity. The activity of enzyme aldehyde dehydrogenase (ALDH), whose main role in physiological processes is to catalyze the oxidation of aldehydes is often measured. The breast cancer cell populations with high ALDH activity and expressing CD44, CD24 and CD133 have higher tumour potential than corresponding low/negative controls. By using the flow cytometry-based ALDEFLUOR assay, it was shown that high levels of ALDH were found in cell populations with high metabolic activity, which included normal SCs and CSCs (Ruiu et al., Citation2019). The antigens that are associated with tumours (TAAs) and expressed on CSCs are also good target molecules for immunotherapy. They are characteristic of germ cells but they can also be found on the surface of CSCs (Ruiu et al., Citation2019).
3. Potential applications of stem cells in cancer
To this date, many different approaches for treating cancer using stem cell therapy have been developed. , gives an insight into some of these approaches. HSC transplantation in haematological diseases was previously mentioned. Furthermore, ESCs and iPSCs can be used to produce CAR-based therapy (chimeric antigen receptor T cells). Based on specific markers on the surface of CSCs, specially engineered CAR-T cells can recognize and target CSCs, starting the cascade of their destruction. In addition, ESCs and iPSCs are potentially important factors in the development of cancer vaccines (Ruiu et al., Citation2019). Furthermore, MSC/NSCs express a pronounced intrinsic tropism towards tumour cells. Therefore, they are effective for gene delivery to the tumour environment. In addition, exosomes extracted from cultured MSCs/NSCs carrying a drug can be used in targeted tumour therapy. Moreover, MSCs can reduce graft vs. host disease (GVHD) as one of the main side effects that can appear in the transplantation of HSCs (Ruiu et al., Citation2019).
Figure 5. Possible applications of stem cell therapy in oncology; (1) Transplantation of HSC after chemotherapy and radiotherapy for the regeneration of blood marrow cells and leukocytes, (2) the use of ESCs and iPscs for the development of vaccines against cancer and (3) the use of MSCs for the transportation of genes and drugs to targeted tumour environment (Chu et al., Citation2020).
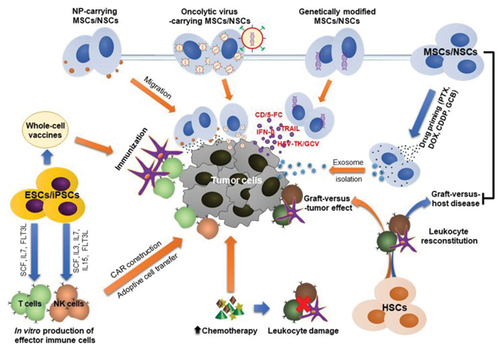
3.1. Application of HSCs transplantation in cancer
HSC transplantation is primarily approved by FDA for the treatment of leukaemia, lymphoma and multiple myeloma, usually after a course of radiotherapy or chemotherapy today. This type of therapy represents an established concept for the treatment of haematological cancers. However, side effects of this therapy with allogenic HSC, primarily graft-versus-host complications (GVHD), remain a challenge during treatment but thanks to the matching of donor and recipient tissue, dangerous graft rejection reactions are minimized (Chu et al., Citation2020; Toledo-Guzmán et al., Citation2018). Furthermore, numerous clinical trials are underway examining the use of HSC transplantation, in combination with chemotherapy or immunotherapy, for the treatment of other types of cancer, such as sarcomas, different types of brain tumours and breast cancer (Espinosa-Cotton & Cheung, Citation2021). The study for the treatment of pancreatic cancer using stem cell therapy has begun at the University Clinic in Heidelberg: after the tumour removal and chemotherapy, patients were transplanted with blood stem cells from the bone marrow of a healthy relative, primarily a sibling (Jurado et al., Citation2017). Consequently, stem cells automatically take over the role of defending the patient’s body against tumour cells, since the immune system is significantly weakened. Stem cell transplantation in an attempt to treat pancreatic cancer has shown promising results but additional studies and clinical research are needed for confirmation (Jurado et al., Citation2017).
3.2. Application of MSCs transplantation after cancer treatment
Highly invasive chemotherapy or immunotherapy, with consequent damage to healthy tissues, especially the hematopoietic system, are still the main therapy regimens applied in cancer patients. The infusion of MSCs increases the proliferation of HSCs, thus improving the end outcome of the treatment (F. Yin et al., Citation2014; Muroi et al., Citation2016). In addition, because of the immunomodulatory effects of MSCs, GVHD as one of the most common complications of HSC transplantation could be effectively reduced or prevented. shows recent clinical trials with promising outcomes of co-transplantation of MSCs and HSCs, without significant side effects. It has also been established that MSCs potentiate the recovery of injured organs and improve the tolerability of high doses of chemotherapy drugs (Muroi et al., Citation2016).
Table 1. Clinical trials focused on the efficacy of MSCs in decreasing the onset of GVDH occurrence, induced by allogeneic HSC transplantation in cancer patients.
3.3. Genetically engineered stem cells
‘Gene therapy of suicide’ represents the newer approach where stem cells are viral engineered to magnify the expression and the release of tumour-toxic cytokines/chemokines or enzymes that activate prodrugs (Alekseenko et al., Citation2021). When stem cells release enzymes, the enzymes can help activate prodrugs into active molecules that are toxic to cancer cells (Gutova et al., Citation2017). One example of this process is the efficient conversion of 5-fluorocytosine (5-FC) to the tumour-toxic 5-fluorouracil (5-FU) after infusion of MSCs or NSCs with cytosine deaminase (CD) expressed on their surface (von Einem et al., Citation2019). It was also shown that irinotecan and NSC expressing carboxylesterase (CE)is quite effective because it helps irinotecan to metabolize into a 1000 times more toxic compound. In studies performed on neuroblastoma mice, tumour grafts appeared to be more sensitive to the simultaneous administration of NSCs with CD expression and irinotecan, compared to treatment with irinotecan alone (von Einem et al., Citation2019). gives an insight into clinical research with stem cells. Furthermore, autologous MSCs expressing Herpes simplex virus thymidine kinase enzyme (HSV-TK) have been used in the treatment of progressed gastrointestinal cancer in humans (EudraCT number 2012–003741–15). It was proven that HSV-TK transforms ganciclovir from the less potent monophosphorylated form to the more potent triphosphate form with a stronger cytotoxic effect (Sage et al., Citation2018). The combination of prodrugs and transduced MSCs is safe and tolerable and that could stop the further progression of the tumour (Rosenblum et al., Citation2018). NSCs used as carriers of the gene for sustained release of apoptosis-inducing ligand (TRAIL), minimize brain tumour metastasis and lengthen the life of treated mice. This form of administration of NSC was used in a clinical trial that included subjects with lung adenocarcinoma (NCT03298763, recruiting). Co-expression of a suicidal gene (CD) and a cytotoxic gene (i.e. Interferon- β (IFN-β) that promotes tumour cell apoptosis) in stem cells is promising for improving treatment efficacy (Y. Liu et al., Citation2020).
Table 2. Presentation of clinical research investigating the effects of stem cell therapy in cancer treatment.
3.4. Stem cells as carriers of nanoparticles
The complex process of nanoengineering of stem cells has been recently investigated. Methods and techniques based on nanotechnology have a great potential to enhance the production of special nanoshells and nanocarriers that would enhance the uptake of the stem cells in their targeted tumour niche as well as the monitoring of their differentiation and fate in vivo. Nanomaterials have also enhanced drugs (Khalid et al., Citation2023), stem cells or gene delivery and the monitoring of micro-environmental signals. The nanocarriers have to interact with the tumour niche to protect stem cells from their aggressive inflammatory products and defence mechanisms, consequently improving the drug’s or stem cells’ therapeutic effects. Although nanotechnology has a lot of limitations (Iftikhar et al., Citation2023), this provides new opportunities for the development of innovative stem cell therapies (Dong et al., Citation2021). One of the most important challenges in the use of stem cells for the treatment of different diseases, such as cancer, is the inability to in vivo monitor the differentiation of engrafted stem cells. Different imaging techniques in the domain of nanotechnology have been developed for the follow-up of stem cell fate, such as quantum dots and gold nanorods.
Additionally, nanoparticles (NPs) have been used for a long time as a vehicle for the delivery of anticancer drugs (Khalid et al., Citation2023). However, rapid elimination from the system and non-specificity in targeting tumour cells are important limiting factors of their application. It is believed that the solution to these problems could be brought by the use of stem cells for the transportation of NPs to the tumour niche. NPs can be infiltrated in the tumour cells or they can conjugate to their surface. The entry of NPs can take place through the passive or active process called endocytosis. It mostly depends on the size and concentration of NPs, their hydrophobicity and surface charge. One of the potential problems is the possibility of too rapid exocytosis of NPs from stem cells, and consequently uncontrolled release of drugs outside the targeted tumour area (Layek et al., Citation2018; Y. Liu et al., Citation2020). Layek and co-authors examined the use of nano-engineered MSCs, which served as a carrier for paclitaxel (PTX-NP), a chemotherapy drug, in developed A549 orthotopic lung tumours in mice. It was shown that the anticancer effect of paclitaxel manifested and depots of it were formed at the target locations (X. Wang et al., Citation2019). In addition, nano-engineered MSCs significantly inhibited tumour growth and improved the survival of mice. Furthermore, MSCs first stopped in lung parenchymal tissue and then, due to their affinity for tumour cells, migrated towards tumour niche (Mokuet al., Citation2019). Additional improvement of therapeutic results would be achieved with increased uptake of NPs into cells. The transcriptional transactivator, TAT peptide, could enhance the internalization of NPs in MSCs (Layek et al., Citation2019).
Another technique by which NPs can target tumour cells is anchoring on stem cells through interaction and contact with groups located on the cell surface, such as thiol and amine groups. However, this technique often results in suboptimal drug delivery to the target site. Therefore, Layek et al. (Suryaprakash et al., Citation2019) proposed a new method – conjugation of NPs with dibenzyl cyclooctyne to azide functional groups on the MSC surface, to form a stable complex. The results showed that the content of PTX in the cell (~48 pg) was higher than the one recorded when other techniques were used (~1–20 pg per cell). In another study, the hybrid spheroids MSCs and NPs that are expressing TRAIL and carrying combinations of drugs for the treatment of glioblastoma were engineered. When injected into the tumour, these spheroids significantly improved the retention and delivery of NPs at the tumour site (Tobias et al., Citation2013).
The nanoparticles are also often used as carriers of different drugs to the targeted cells because they improve the oral bioavailability of the drug. These nanocarriers have more precise ability to target a specific region or specific cells. Therefore, the possibility of encapsulation of stem cells by different types of nanomaterials and their transport in that complex unity has been discussed in recent studies (Zhu et al., Citation2022). The main goal of this therapeutic approach is to enhance the oral administration of stem cells. Namely, oral digestion of drugs is a preferred route, due to its non-invasive characteristics, as well as the increased patient compliance. Yet, there are still many challenges to be solved. The use of stem cells, after oral administration, is challenged by the actions of gut microbiota and its enzymes, as well as the action of bile salts and low pH levels in the gut (Džidić-Krivić et al., Citation2023). The use of nanoparticles for stem cell encapsulation and protection is a promising approach to solving this existing obstacle. Therefore, new advanced techniques are still being developed, such as the use of the metal-phenolic network (MPN). It is a nanofilm that has semipermeable characteristics and strong anti-inflammatory and antioxidative effects. This protects and helps to maintain the stem cell quality, which is being compromised by the multiple stressors from the inflammatory and hostile tumour niche. However, it was not fully successful in delivering stem cells safely to their targets when ingested orally, mostly because it is acting only as a physical barrier and not interacting with the aggressive tumour niche. There are still many unknowns about MPN and further studies are required, to investigate the process of the encapsulation of stem cells by different nanomaterials, as well as the transport of these compositions to the targeted cells (Zhao et al., Citation2022). The biggest success of nanoparticle utilization in stem cell therapy for tracking (H. Huang et al., Citation2021) and facilitating stem cell therapy, mimicking the extracellular matrix environment and utilising a safe non-viral gene delivery tool (Sun et al., Citation2020).
In addition, the use of nanotechnology has a great impact on the treatment of many different diseases besides malignant, such as neurodegenerative disorders. Neurodegenerative diseases are characterised by the progressive damage of neuron structure and function, with the precise cause usually being unknown. Successful therapies have not been discovered yet, consequently making neurodegenerative disorders an important burden for healthcare systems all around the world. The main goal of regenerative therapy based on the use of stem cells in the central nervous system (CNS) is to protect the further damage of the neurons, as well as to increase the regeneration of already damaged nerve cells. The positive effects of the SCs transplantation were detected in Alzheimer’s dementia and Parkinson’s disease (Nguyen et al., Citation2019), as well as spinal muscular atrophy and amyotrophic lateral sclerosis. Further studies should investigate stem cell treatment in neurodegenerative disorders and their use in daily clinical practice (Bonaventura et al., Citation2021), such as the use of stem cells in the treatment of peripheral neuropathy caused by Diabetes Mellitus (Emina, K. et al., Citation2022; Karahmet, Prnjavorac, Bego, Meseldžić, E. et al., Citation2021, Citation2021).
Stem cells also have a great potential to be used as a therapy for skin regeneration and wound healing, as well as in the treatment of osteoarthritis (OA). It is a chronic disease of a joint caused by damage to the joint cartilage, causing the disability of patients in daily activities. One of the treatment options for OA is the use of embryonic stem cells and MSCs, which differentiate into chondrocytes. The stem cells are injected into the joint cavity affected by OA and they improve the recovery of the joint cartilage. However, further studies are needed to prove the long-term effects of this therapy in the treatment of OA (Jang et al., Citation2021). In addition, the stem cells can differentiate into neurons, consequently replacing the damaged cells after traumatic injury of the spinal cord. They can decrease the production of inflammatory molecules and synthesised neurotrophic factors, such as nerve growth factor (NGF), brain-derived- and glial-derived neurotrophic factors (BDNF, GDNF). Further investigations are required for the use of stem cells in the treatment of injured spinal cord in daily clinical practice (Shao et al., Citation2019).
3.5. Stem cells as carriers of oncolytic viruses
One of the essential characteristics of oncolytic viruses (OV) is selective replication in cancer cells, induction of tumour cell lysis and alarming of the immune system about the existence of tumour cells and the need for their elimination. However, while OVs travel to tumour cells, immune cells often recognize them as a potential danger and quickly eliminate them from the body. A potential solution to this problem lies precisely in stem cells, which could be new carriers of OV to tumours, providing them with needed protection from the immune system. The combination of ionizing radiation and temozolomide and NSCs transduced with CRAd-Survivin-pk7 OV increased the in vitro cytotoxic effect of the therapy on glioma cells, while also increasing the survival time of mice suffering from aggressive glioblastoma multiforme (GBM) (Miska & Lesniak, Citation2015). However, the effectiveness of stem cells as virus carriers rely on the relationship between the carrier and tumour cells. MSCs and NSCs are both proven to back up the process of viral replication, but the advantage of NSCs is that more types of viruses can be released from them. In addition, the use of transduced NSCs showed greater efficacy in prolonging the survival time of mice with glioma (Brossa et al., Citation2021).
3.6. Exosomes derived from stem cells as potential drug carriers
One of the potential roles of stem cells in cancer therapy is the role of a drug carrier, where they have numerous advantages compared to previous methods of targeted drug delivery, as shown in . They slow down the rapid biological degradation of therapeutic agents, reduce the frequency of systemic side effects of therapy and boost the concentration of therapeutic agents exactly at the targeted position. The antitumor efficiency of this therapeutic delivery system depends on the number of stem cells delivered in the tumour niche (Rahbarghazi et al., Citation2019). Exosomes are the natural carriers obtained from stem cells and they can encapsulate different types of therapeutic agents, mostly RNAs, proteins or molecule drugs of small size (Rahbarghazi et al., Citation2019). They have many advantages over synthetic nanoparticles, including greater biocompatibility, stability, high cargo storage capacity and more successful uptake of desired materials into tumour cells (Tripolszky et al., Citation2019). In addition, adding specific proteins or ligands to their surface can improve the interaction with the tumour microenvironment (J. Wang et al., Citation2017; Xu et al., Citation2019). The traditional transfection technique has been used for years to package genetic material, such as antitumor mRNA or micro-RNA (miR), into exosomes derived from stem cells. Xu H et al. (Xu et al., Citation2019)researched the use of exosomes that marrow stromal cells with microRNA-133b expressed and released in a rat model with a primary brain tumour. When these exosomes are direct injected into tumours, a significant reduction in the growth of glioma xenografts has been shown (Lou et al., Citation2020).
Figure 6. Engineered extracellular vesicles (EVs) for delivering therapeutic entities by the process of exogenous loading after their isolation or endogenous loading during EVs biogenesis, protecting them from damage and increasing the success of therapy (Yin, L. Liu et al., Citation2020).
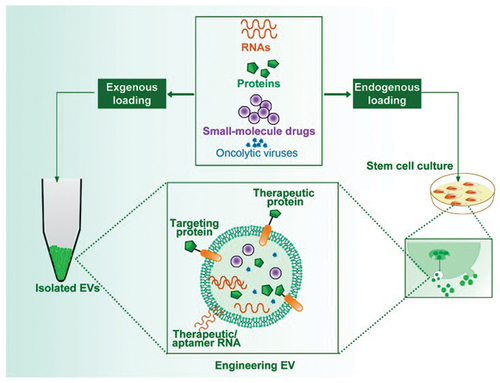
Another study examined the effect of miR-expressing exosomes secreted from miR-199a, on a tumour model of hepatocellular carcinoma. Exosomes encapsulated sorafenib, an antitumor drug, which effect was increased when delivered to the target site via exosomes (Tong et al., Citation2021). Similarly, exosomes secreted from MSCs efficiently delivered siRNA to bladder cancer cells, targeting the kinase 1 gene and silencing it (Lisini et al., Citation2020). The MSCs, when exposed to large amounts of antitumor drugs, can take up that drug and later express it at the target site, most often by forming exosomes in which they package the drug. Lisini Det al. (Lisini et al., Citation2020) reported that exosomes loaded with paclitaxel drug molecules, extracted from MSCs, significantly decreased the proliferation of tumour cells in human pancreatic cancer. In addition, it was found that these exosomes effectively transport drug molecules to the bone marrow and that they inhibit the growth of tumour cells (Pakravan et al., Citation2017). MSCs have also been used as a carrier of some other drugs, including cisplatin, doxorubicin and gemcitabine (Miliotou & Papadopoulou, Citation2018). Moreover, the encapsulation of therapeutic drugs into exosomes could happen after extraction from the stem cell culture medium by using different techniques, such as dialysis, extrusion, and electroporation. Consequently, the loading of both hydrophilic and hydrophobic drugs will have greater efficiency and more precise control (Miliotou & Papadopoulou, Citation2018).
3.7. Application of stem cells in production of immune cells
In immunotherapy, scientists have succeeded to harvest natural killer (NK) cells and CAR (chimeric antigen receptor) from a patient by genetically transducing with CAR and then reinfusing them into the patient to fight cancer cells (Acharya & Walter, Citation2020). However, the precision of this method remains challenging, especially in patients who have undergone aggressive chemotherapy in elderly patients. In addition, there is one main limiting factor for successful in vivo antitumor activity of CAR immune cells and that is their fast transformation into short-lived effector cells (Acharya & Walter, Citation2020). It is thought that the use of other sources of CAR cells would enable the application of immunotherapy in a larger number of patients. Potential candidates are human pluripotent stem cells (Iriguchi & Kaneko, Citation2019). The process involves incubating the stem cells for a month in a growth medium, which contains NK cells or cytokines that potentiate the generation of T cells. The growth medium contains numerous cytokines, such as interleukins −3,-7,-15, stem cell factor (SCF) and FMS-like receptor tyrosine kinase receptor-3 ligand (FLT3L). It is interesting that the induction of CAR on HSC gives good results in the treatment of cancer. The transplanted CAR-HSCs implanted in the bone marrow produced various immune cells that express CAR, such as NK cells, T cells and neutrophils, which resulted in the strengthening of the patient’s immunity in the fight against cancer (Iriguchi & Kaneko, Citation2019).
3.8. Cancer vaccines based on stem cells
With the undeniable role of CSCs in tumorogenesis, CSC-targeted therapies would significantly improve cancer therapy (Iriguchi & Kaneko, Citation2019). One appealing possibility is the production of a cancer vaccine, either using oncofetal peptides or using CSC/ESC/iPSC. The one-time use of a vaccine based on oncofetal peptides does not produce an adequate immune response due to the heterogeneity of tumour cells, their constant mutations and the successful development of a rapid defence mechanism and escape from therapy. Cancer vaccines based on tumour stem cells could result in better outcomes (Katsukawa et al., Citation2016). Another potential approach is the production of vaccines produced from multiple ESC/iPSC-derived antigens (Iriguchi & Kaneko, Citation2019). Vaccines based on allogeneic ESCs and autologous iPSC (Katsukawa et al., Citation2016) have been proven to be more efficient in preventing tumour recurrence in test mice than vaccines from xenogeneic sources (Inui et al., Citation2017), as evidenced by the increase in the proliferation of the immune cells (Qiao et al., Citation2020) and the production of cytotoxic cytokines (Malta et al., Citation2018).
Li Y. et al. (Li et al., Citation2018) through their research showed that injecting treated iPSC cells into mice could prevent tumour recurrence after resection and slow the development of breast, lung and skin cancer in mice. These early studies using embryonic stem cells as a source for anti-tumour vaccines proved to be very effective. However, the strong immunosuppressive environment of already formed tumours limits this type of vaccine application only to prophylactic and not for therapeutic purposes. Furthermore, additional limitations to the use of these vaccines are the potential development of teratoma and the induction of autoimmune diseases (Papaccio et al., Citation2017). However, more recent studies are approaching the use of iPS cells in a way that potentially eliminates the above problems. The ultimate goal of the research was to separate blood or skin cells from the patient’s body and create autologous iPS cells that have been treated with radiation and therefore cannot promote the formation of new tumours in other tissues (Ouyang et al., Citation2019).
The team of scientists used two groups of mice: a control, unvaccinated group and a group of experimental mice vaccinated with iPS cells derived from mouse fibroblasts, combined with CpG (Immune-enhancing molecule). A few weeks later, breast, skin or lung cancer cells were injected into unvaccinated and vaccinated mice. As a result, it was found that a significant tumour formed in the control group several weeks after the injection of the cancer cells. In vaccinated mice with iPSCs, although tumours appeared, these were smaller or degenerated, which confirms the preventive effect of that vaccine. To confirm that the vaccinated mice developed specific immunity against cancer, the scientists transferred T cells from the vaccinated to unvaccinated animals with breast cancer, after which their breast cancer began to regress (Ouyang et al., Citation2019). Therefore, the possibility of using iPSCs as prophylactic agents in the elderly to reduce the incidence of tumours in that population emerges. It is hoped that in the future, any 70-year-old will be able to be vaccinated against cancer with a vaccine based on the use of iPSCs (Lohan et al., Citation2017). Currently, it remains to be determined whether this vaccine will have similar efficiency and safety in humans. Although animal testing provides a solid scientific basis, further research is needed to determine the clinical potential of such vaccines.
4. Common side effects and limitations of stem cell therapy
The use of stem cell therapy has not been yet included in standard protocols for therapeutic regimens for many diseases, as they are still many problems occurring in clinical practice. One of the remaining problems is ethical and the use of embryonal stem cells (ESCs) is limited in clinical trials, although they generate all cell types except placental cells. However, progress has been made through the use of Yamanaka factors, because it allowed the induction of the generation of pluripotent (iPS) cells from adult fibroblasts. This almost eliminates ethical problems caused by the destruction of embryos. In addition, the expectations based on the potential of stem cell therapy and their characteristics have not matched yet the observed therapeutic outcomes. Even in haematology, where stem cells have been used successfully for the treatment of lymphoma and leukaemias, several factors limit clinical benefit for the patient, such as the development of GvHD (Graft vs. host disease), as well as limited availability of donor and long-term outcomes. One of the biggest fears after therapy with umbilical cord stem cells is transplant rejection when the patient’s immune system fights against stem cells that it considers foreign, or when the stem cells begin to destroy their host. Thanks to the progress made in the process of determining the compatibility of the donor and the recipient, as well as the progress of immunosuppressive therapy, the frequency of this unwanted reaction is decreasing. However, when it comes to an elderly population, in which numerous comorbidities are common, complications are more likely to occur. Therefore, in elderly patients could the application of the so-called reduced-intensity therapy or ‘mini’ stem cell transplantation, which implies the application of a reduced dose of chemotherapy drugs and radiation, before the application of stem cells could be considered. This puts less strain on the patient’s body and allows stem cells to grow and fight against cancer (Sung et al., Citation2021).
4.1. Potential transformation of healthy stem cells into tumour stem cells
One of the main common features of healthy stem cells is the signalling they use to communicate with their microenvironment. Changes in the tumour microenvironment can potentiate the migration of CSCs which later leads to the formation of the entire tumour tissue. Healthy endogenous stem cells are under the strict control of the surrounding cells, which prevents their malignant alteration (Y. Wang et al., Citation2019). Before their transplantation and during their cultivation, transplanted stem cells are exposed to external conditions, which can change their genotype and phenotype. The longer the cultivation time, the greater the possibility of the changes in stem cells, which was proven when it was found that almost half of the MSCs (45.8%) have spontaneously mutated and turned into malignant cells after cultivation for more than 1 month. The PSCs have a higher potential for malignant alteration than HSCs, but also that there are many ways to minimize their tumorigenic potential. Therefore, Katsukawa et al. (Inui et al., Citation2017) tried to expose iPSCs to a deadly dose of gamma radiation, which resulted in reduced teratoma formation in mice.
4.2. Potential side effects of HSC transplantation
Allogeneic HSC transplantation is one of the approved procedures for the treatment of haematological and lymphoid cancers. However, it has been shown through practice that it can result in the appearance of certain side effects, including chronic GVHD, infections associated with a weakened immune response of the body and the recurrence of original cancer or the growth of a new one (Papaccio et al., Citation2017). To improve the outcomes of this therapy, additional clinical studies should be conducted, focusing on the source from which HSCs are obtained prior to transplantation. By using the umbilical cord blood as the source of stem cells, the frequency and severity of chronic GVHD can be reduced. In addition, different clinical trials have proven the efficacy of co-transplantation HSCs and MSCs in preventing or reducing chronic GVHD and some other side effects associated with HSC transplantation (Papaccio et al., Citation2017).
4.3. Inaccuracy of drug delivery using stem cells as carriers
The success of engineered stem cells as gene and drug carriers mostly depends on the number of these cells found inside the tumour and the precision of drug delivery to the exact target site. Papaccio et al. (Lohan et al., Citation2017) stated that only a few percents (2–5%) of stem cells reach the tumour location after they are applied in the system. Most of the cells injected intravenously first stopped in the lung tissue, then went out to the spleen, liver and lymph nodes and were eventually eliminated from the body. This is precisely the reason for concern because the delivery of therapeutics carried by stem cells in the wrong place can result in damage to healthy tissues and increase the risk of developing resistance to the action of these drugs. Finding ways to improve the delivery and accuracy of stem cells only to the targeted area of the tumour, can minimize these problems and enable engineered stem cell application in clinical settings.
4.4. Alteration of immune response and potential development of autoimmunity
When stem cells are obtained from inadequate donors, it can provoke the host’s immune system (Butt et al., Citation2022; Hu et al., Citation2018). This can trigger the activation of a specific T-cell response and production of the humoral antibodies by B-cells, to destroy the donor antigens, which the immune system of the recipient recognizes as a foreign body (Westhoff & Rahorst, Citation2022). At the same time, anti-donor memory cells are created, which could potentially damage the next transplant, resulting in graft rejection. In addition, the use of iPSC-based autologous vaccines may result in the occurrence of autoimmune diseases in recipients because the vaccines contain both antigens that are specific for CSCs and antigens associated with normal tissue. Yet, Kooreman et al. (Kooreman et al., Citation2018) did not observe the development of autoimmune cells after the patients were treated with an autologous iPSC vaccine. Anyhow, further clinical trials are needed to confirm the safety of this approach.
Additionally, because of the immunosuppressive characteristics of the tumour niche and many different molecules interacting with cells, combining stem cells and immunotherapy, such as using the checkpoint inhibitors, such as programmed cell death ligand 1 (PD-1)antibodies and cytotoxic T-lymphocyte associated protein 4 (CTLA-4), may give better results. Hu Q et al. (Butt et al., Citation2022), designed the HSCs especially for treating recurrent leukaemia in mice. Therefore, their surface is covered with platelets with attached PD-1 antibodies to them. HSCs, after being intravenously injected, quickly migrated into the BM, inducing the release of PD-1 antibodies, attracted by the inflammatory molecules in the tumour microenvironment. As a consequence, the response of activated T cells in the fight against tumour cells increased and the survival time of the mice improved.
4.5. Viral transfection activates viral infection after stem cells modified with viruses
Modification of stem cells, to transfer certain genes, is effectively carried out by viral transfection. However, there is a possibility that a viral infection will develop in the recipient, especially if it is a highly immunogenic virus. This can activate a cascade of toxin release and result in the elimination of transduced cells and even the death of the donor due to an overactive immune response (Westhoff & Rahorst, Citation2022). Therefore, viral vectors need to be engineered in a way that specific sequences that trigger the activation of the immune system are deleted, while targeted sequences that help to fight cancer are induced. Detailed preclinical evaluation is necessary to confirm the efficacy and safety of viral vectors before translating the therapy into the clinical setting. Despite the success that viral transfection as a method has in preclinical and clinical models, stem cell therapy is facing many challenges that must be overcome. Further experiments are needed for scientists to be able to safely and successfully apply an appropriate strategy for stem cell engineering.
5. Conclusion
Recently, numerous discoveries about stem cells have given a new outlook on their possible application in oncology. HSC transplantation has been proven as an effective treatment for different types of haematological cancers, such as lymphomas, leukaemia and multiple myeloma. However, side effects, especially GVHD, remain an important limitation of HSC transplantation. ESCs and iPSCs are used for the development of CAR-based therapy (Chimeric antigen receptor T cells). Stem cells enable the recognition of specific markers on the surface of CSCs and their elimination from hosts organism by specially engineered CAR-T cells. Additionally, stem cells play an important role in the development of cancer vaccines. The studies based on animal models highlighted the use of iPSCs-based cancer vaccines as possible prophylactic agents for lowering the incidence of tumours in an elderly population. However, further studies and clinical trials on human beings are required to determine the efficacy and safety of these vaccines. In addition, MSC/NSCs have been proven, in preclinical models, as an effective transport mechanism for the delivery of genes to the tumour niche, as well as the delivery of drugs by the exosomes extracted from stem cells in targeted tumour therapy. The standardization of engineering exosomes and anti-tumour vaccines should be conducted to achieve adequate effects and safety. In summary, the current results of stem cell therapies are very encouraging when it comes to treating tumours, but further efforts are needed to improve their efficacy and minimize side effects before they enter clinical trials. Nanoengineering of stem cells has been the focus of novel research in the area of regenerative medicine such as the production of nanoshells and nanocarriers that enhance the transport and uptake of stem cells in their targeted tumour niche and enable the effective monitoring of stem cells’ effects on tumour cells. Although nanotechnology has a lot of limitations, however, this provides new opportunities for the development of effective and innovative stem cell therapies. Furthermore, this is an optimistic approach to stem cell therapy in further research and clinical trials.
Acknowledgements
The authors are grateful for the financial support from the International Society of Engineering Science and Technology (ISEST), UK.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Emina Karahmet Sher
Dr. Emina Karahmet Sher is having Master of Pharmacy (MPharm) and PhD in Pharmaceutical Sciences. She is having more than 10 years of multi-level experience in Pharmaceutical Sciences, Research and Teaching. Dr. Emina Sher is a senior member of the International Society of Engineering Science and Technology, UK (ISEST). She is leading a research group of Biochemistry and Biomedical Engineering at ISEST. Dr. Emina Sher is the author/co-author of several high-impact research papers. She is also serving as a Reviewer of many international multidisciplinary journals. She has participated in multiple international conferences and workshops. Apart from this, Dr. Emina Sher has been invited speaker at different national and international level discussion panels covered by media and TV channels.
Azra Kalić
Azra Kalic is a PhD candidate at Faculty of Pharmacy, University of Modern Sciences - CKM, Mostar, Bosnia and Herzegovina. She previously earned a Master of Pharmacy (MPharm) degree at the same University. She has also gained work experience at Brckopharm Pharmacy. Ms Kalic research interests are in the field of biotechnology and genetics.
Amina Džidić-Krivić
Dr. Amina Džidić-Krivić graduated from the Faculty of Medicine, University of Sarajevo, Bosnia and Herzegovina as a Medical Doctor (MD). Currently, she is serving as Neurology Resident at Cantonal Hospital Zenica, Bosnia and Herzegovina. Dr. Džidić-Krivić is a member of the International Society of Engineering Science and Technology, UK (ISEST). She is holding a Research Assistant position at ISEST. She has published several Research papers on stem cells, nanotechnology and gut microbiota. Dr. Džidić-Krivić research work is mainly focused on neurology, nanotechnology, immunology and pharmacology.
Merima Beća- Zećo
Merima Beća-Zećo is serving as a Senior Assistant at University of Modern Sciences CKM Mostar, Bosnia and Herzegovina. Simultaneously, she is pursuing her PhD in Pharmacy at the Faculty of Pharmacy, University of Modern Sciences - CKM, Mostar, Bosnia and Herzegovina. She is holding a degree in Master of Pharmacy (MPharm). She has also obtained additional qualifications from the University of Dzemal Bijedic, Mostar. Ms Beća-Zećo work is mostly focused on pharmacology, clinical pharmacy, immunology, pharmacokinetics and biopharmacy.
Emma Pinjić
Dr. Emma Pinjic has over twenty years of experience in the clinical research field. In her career, Dr. Pinjic worked as a physician, academic researcher and clinical instructor. She has been committed to evidence-based medicine, patient safety, and driven by a passion for academic medical research and education. Dr. Pinjic graduated from the Sarajevo University School of Medicine with M.D. Degree. She also holds a Master’s degree in Public Health and Graduate Project Management Certificate from Boston University. In her current role at the Department of Radiology research leadership team, Dr. Pinjic is responsible for research operations, strategy, personnel management, and research process improvement for the Department of Radiology.
Farooq Sher
Dr. Farooq Sher is a Senior Lecturer (Assistant Professor) at the Department of Engineering, Nottingham Trent University, UK. His undergraduate degree was in Chemical Engineering, followed by MSc Chemical Engineering from the University of Leeds, UK. He was awarded PhD in Chemical Engineering from the University of Nottingham, UK. Dr. Sher is President of the International Society of Engineering Science and Technology, UK (ISEST). ISEST is a non-profit independent organisation founded in 2007. The ISEST is an organisation of compatible scientists, professionals, engineers, academicians, technologists, students and freelancers that promotes education and research activities in the field of Science Engineering and Technology worldwide to outfit the needs for a better future of society. Dr. Sher has published several book chapters, reviews and editorials apart from this he is serving as a scientific committee member of different international conferences, workshops and panels. Dr. Sher is the author/co-author of more than 200 research and scientific papers in SCI journals. He has chaired several internal conferences and been a committee member of multiple scientific organisations. Dr. Sher is the editor of several scientific journals; including Editor in Chief of Science and Technology Journal, Associate Editor of Cleaner Chemical Engineering Journal, Associate Editor of Journal of Engineered Fibers and Fabrics and Associate Editor of Environment, Development and Sustainability. He is also an editorial board member of different journals; Experimental Results, Frontiers in Chemical Engineering, Sustainability, Energies, Processes, Environmental Health Insights, Air, Soil and Water Research Journal and Encyclopaedia etc. Apart from this, Dr. Sher is a peer reviewer for hundreds of high impact journals from diverse scientific disciplines. Dr Sher is a Fellow of the Higher Education Academy (HEA) UK.
References
- Acharya, U. H., & Walter, R. B. (2020). Chimeric Antigen Receptor (CAR)-modified immune effector cell therapy for Acute Myeloid Leukemia (AML). Cancers, 12(12), 3617. https://doi.org/10.3390/cancers12123617
- Alekseenko, I., Kuzmich, A., Kondratyeva, L., Kondratieva, S., Pleshkan, V., & Sverdlov, E. (2021). Step-by-step immune activation for suicide gene therapy reinforcement. International Journal of Molecular Sciences, 22(17), 9376. https://doi.org/10.3390/ijms22179376
- Atala, A. (2021). STEM CELLS translational medicine: A decade of evolution to a vibrant stem cell and regenerative medicine global community. Stem Cells Translational Medicine, 10(2), 157–159. https://doi.org/10.1002/sctm.21-0016
- Atiya, H., Frisbie, L., Pressimone, C., & Coffman, L. (2020). Mesenchymal stem cells in the tumor microenvironment. Tumor Microenvironment, 9(3) , 31–42.
- Baron, F., Lechanteur, C., Willems, E., Bruck, F., Baudoux, E., Seidel, L., Vanbellinghen, J. -F., Hafraoui, K., Lejeune, M., & Gothot, A., Fillet, G., Beguin, Y. (2010). Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biology of Blood and Marrow Transplantation, 16(6), 838–847. https://doi.org/10.1016/j.bbmt.2010.01.011
- Bazinet, A., & Popradi, G. (2019). A general practitioner’s guide to hematopoietic stem-cell transplantation. Current Oncology, 26(3), 187–191. https://doi.org/10.3747/co.26.5033
- Bonaventura, G., Munafò, A., Bellanca, C. M., La Cognata, V., Iemmolo, R., Attaguile, G. A., DiMauro, R., DiBenedetto, G., Cantarella, G., Barcellona, M. L., Cavallaro, S., & Bernardini, R. (2021). Stem cells: Innovative therapeutic options for neurodegenerative diseases? Cells, 10(8), 1992. https://doi.org/10.3390/cells10081992
- Brossa, A., Tapparo, M., Fonsato, V., Papadimitriou, E., Delena, M., Camussi, G., & Bussolati, B. (2021). Coincubation as mir-loading strategy to improve the anti-tumor effect of stem cell-derived evs. Pharmaceutics, 13(1), 76. https://doi.org/10.3390/pharmaceutics13010076
- Butt, M. H., Zaman, M., Ahmad, A., Khan, R., Mallhi, T. H., Hasan, M. M., Khan, Y. H., Hafeez, S., Massoud, E. E. S., Rahman, M. H., & Cavalu, S. (2022). Appraisal for the potential of viral and nonviral vectors in gene therapy: A review. Genes (Basel), 13(8), 1370. https://doi.org/10.3390/genes13081370
- Chen, C., Yue, D., Lei, L., Wang, H., Lu, J., Zhou, Y., Liu, S., Ding, T., Guo, M., & Xu, L. (2018). Promoter-operating targeted expression of gene therapy in cancer: Current stage and prospect. Molecular Therapy-Nucleic Acids, 11, 508–514. https://doi.org/10.1016/j.omtn.2018.04.003
- Christodoulou, I., Goulielmaki, M., Devetzi, M., Panagiotidis, M., Koliakos, G., & Zoumpourlis, V. (2018). Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Research & Therapy, 9(1), 1–38. https://doi.org/10.1186/s13287-018-1078-8
- Chu, D. T., Nguyen, T. T., Tien, N. L. B., Tran, D. K., Jeong, J. H., Anh, P. G., Thanh, V. V., Truong, D. T., & Dinh, T. C. (2020). Recent progress of stem cell therapy in cancer treatment: Molecular mechanisms and potential applications. Cells, 9(3), 563. https://doi.org/10.3390/cells9030563
- Cordero, A., Ramsey, M. D., Kanojia, D., Fares, J., Petrosyan, E., Schwartz, C. W., Burga, R., Zhang, P., Rashidi, A., & Castro, B., Xiao, T., Lee-Chang, C., Miska, J., Balyasnikova, I V., Ahmed, A U., Lesniak, M S. (2022). Combination of tucatinib and neural stem cells secreting anti-HER2 antibody prolongs survival of mice with metastatic brain cancer. Proceedings of the National Academy of Sciences, 119(1), e2112491119. https://doi.org/10.1073/pnas.2112491119
- Das, M. K., Lunavat, T. R., Miletic, H., & Hossain, J. A. (2021). The potentials and pitfalls of using adult stem cells in cancer treatment. Cell Biology and Translational Medicine, 12, 139–157.
- Delpu, Y., Cordelier, P., Cho, W. C., & Torrisani, J. (2013). DNA methylation and cancer diagnosis. International Journal of Molecular Sciences, 14(7), 15029–15058. https://doi.org/10.3390/ijms140715029
- Dong, Y., Wu, X., Chen, X., Zhou, P., Xu, F., & Liang, W. (2021). Nanotechnology shaping stem cell therapy: Recent advances, application, challenges, and future outlook. Biomedicine & Pharmacotherapy, 137, 111236. https://doi.org/10.1016/j.biopha.2021.111236
- Džidić-Krivić, A., Kusturica, J., Sher, E. K., Selak, N., Osmančević, N., Karahmet Farhat, E., & Sher, F. (2023). Effects of intestinal flora on pharmacokinetics and pharmacodynamics of drugs. Drug Metabolism Reviews, 55(1–2), 1–14. https://doi.org/10.1080/03602532.2023.2186313
- Emina, K., Prnjavorac, B., Softić, A., Srabović, N., Tamer, B., Sher, F., Lekić, L., Farhat, E. K., Meseldzic, N., & Imamović, S. (2022). IDF21-0423 Michigan neuropathy screening for assessing diabetes in participants and correlation to the immune response. Diabetes Research and Clinical Practice, 186, 109682. https://doi.org/10.1016/j.diabres.2022.109682
- Espinosa-Cotton, M., & Cheung, N. -K.V. (2021). Immunotherapy and radioimmunotherapy for desmoplastic small round cell tumor. Frontiers in Oncology, 11. https://doi.org/10.3389/fonc.2021.772862
- Garcia-Mayea, Y., Mir, C., Masson, F., Paciucci, R., & LLeonart, M. (2020). Insights into new mechanisms and models of cancer stem cell multidrug resistance. Seminars in Cancer Biology, 60, 166–180. https://doi.org/10.1016/j.semcancer.2019.07.022
- Gutova, M., Goldstein, L., Metz, M., Hovsepyan, A., Tsurkan, L. G., Tirughana, R., Tsaturyan, L., Annala, A. J., Synold, T. W., & Wan, Z., Seeger, R., Anderson, C., Moats, R A., Potter, P M., Aboody, K S. (2017). Optimization of a neural stem-cell-mediated carboxylesterase/irinotecan gene therapy for metastatic neuroblastoma. Molecular Therapy-Oncolytics, 4, 67–76. https://doi.org/10.1016/j.omto.2016.11.004
- Huang, H., Du, X., He, Z., Yan, Z., & Han, W. (2021). Nanoparticles for stem cell tracking and the potential treatment of cardiovascular diseases. Frontiers in Cell and Developmental Biology, 9, 662406. https://doi.org/10.3389/fcell.2021.662406
- Huang, T., Song, X., Xu, D., Tiek, D., Goenka, A., Wu, B., Sastry, N., Hu, B., & Cheng, S. -Y. (2020). Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics, 10(19), 8721. https://doi.org/10.7150/thno.41648
- Hu, Q., Sun, W., Wang, J., Ruan, H., Zhang, X., Ye, Y., Shen, S., Wang, C., Lu, W., & Cheng, K., Dotti, G., Zeidner, J F., Wang, J., Gu, Z. (2018). Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nature Biomedical Engineering, 2(11), 831–840. https://doi.org/10.1038/s41551-018-0310-2
- Iftikhar, M., Noureen, A., Jabeen, F., Uzair, M., Rehman, N., Sher, E. K., Katubi, K. M., Américo-Pinheiro, J. H. P., & Sher, F. (2023). Bioinspired engineered nickel nanoparticles with multifunctional attributes for reproductive toxicity. Chemosphere, 311, 136927. https://doi.org/10.1016/j.chemosphere.2022.136927
- Inthagard, J., Edwards, J., & Roseweir, A. K. (2019). Immunotherapy: Enhancing the efficacy of this promising therapeutic in multiple cancers. Clinical Science, 133(2), 181–193. https://doi.org/10.1042/CS20181003
- Inui, S., Minami, K., Ito, E., Imaizumi, H., Mori, S., Koizumi, M., Fukushima, S., Miyagawa, S., Sawa, Y., & Matsuura, N. (2017). Irradiation strongly reduces tumorigenesis of human induced pluripotent stem cells. Journal of Radiation Research, 58(4), 430–438. https://doi.org/10.1093/jrr/rrw124
- Iriguchi, S., & Kaneko, S. (2019). Toward the development of true “off‐the‐shelf” synthetic T‐cell immunotherapy. Cancer Science, 110(1), 16–22. https://doi.org/10.1111/cas.13892
- Jang, S., Lee, K., & Ju, J. H. (2021). Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. International Journal of Molecular Sciences, 22(5), 2619. https://doi.org/10.3390/ijms22052619
- Jiang, Y., Wells, A., Sylakowski, K., Clark, A. M., & Ma, B. (2019). Adult stem cell functioning in the tumor micro-environment. International Journal of Molecular Sciences, 20(10), 2566. https://doi.org/10.3390/ijms20102566
- Juarez, J. G., Harun, N., Thien, M., Welschinger, R., Baraz, R., Dela Pena, A., Pitson, S. M., Rettig, M., DiPersio, J. F., & Bradstock, K. F., Bendall, L J. (2012). Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood, the Journal of the American Society of Hematology, 119(3), 707–716. https://doi.org/10.1182/blood-2011-04-348904
- Jurado, M., De La Mata, C., Ruiz-García, A., López-Fernández, E., Espinosa, O., Remigia, M. J., Moratalla, L., Goterris, R., García-Martín, P., & Ruiz-Cabello, F., Garzón, S., Pascual, M J., Espigado, I., Solano, C. (2017). Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: A phase I/II study. Cytotherapy, 19(8), 927–936. https://doi.org/10.1016/j.jcyt.2017.05.002
- Kabakov, A., Yakimova, A., & Matchuk, O. (2020). Molecular chaperones in cancer stem cells: Determinants of stemness and potential targets for antitumor therapy. Cells, 9(4), 892. https://doi.org/10.3390/cells9040892
- Karahmet, E., Prnjavorac, B., Bego, T., Meseldžić, N., Imamović, S., Karahmet, E., Sher, F., Lekić, L., & Begić, E. J. A. S. M. S. (2021). IL-1β in correlation to the common diabetic complications. J Acta Scientific MEDICAL SCIENCES 5(9), 25–29. https://doi.org/10.31080/ASMS.2020.05.0999
- Karahmet, E., Prnjavorac, B., Bego, T., Softić, A., Begić, L., Begić, E., Karahmet, E., Prnjavorac, L., & Prnjavorac, I. (2021). Clinical use of an analysis of oxidative stress and IL-6 as the promoters of diabetic polyneuropathy. Medicinski Glasnik: Official Publication of the Medical Association of Zenica-Doboj Canton, Bosnia and Herzegovina, 18(1), 12–17. https://doi.org/10.17392/1279-21
- Karthaus, W. R., Hofree, M., Choi, D., Linton, E. L., Turkekul, M., Bejnood, A., Carver, B., Gopalan, A., Abida, W., & Laudone, V., Biton, M., Chaudhary, O., Xu, T., Masilionis, I., Manova, K., Mazutis, L., Pe’er, D., Regev, A., Sawyers, C L. (2020). Regenerative potential of prostate luminal cells revealed by single-cell analysis. Science, 368(6490), 497–505. https://doi.org/10.1126/science.aay0267
- Katsukawa, M., Nakajima, Y., Fukumoto, A., Doi, D., & Takahashi, J. (2016). Fail-safe therapy by gamma-ray irradiation against tumor formation by human-induced pluripotent stem cell-derived neural progenitors. Stem Cells and Development, 25(11), 815–825. https://doi.org/10.1089/scd.2015.0394
- Khalid, A. D., Ur-Rehman, N., Tariq, G. H., Ullah, S., Buzdar, S. A., Iqbal, S. S., Sher, E. K., Alsaiari, N. S., Hickman, G. J., & Sher, F. (2023). Functional bioinspired nanocomposites for anticancer activity with generation of reactive oxygen species. Chemosphere, 310, 136885. https://doi.org/10.1016/j.chemosphere.2022.136885
- Kooreman, N. G., Kim, Y., de Almeida, P. E., Termglinchan, V., Diecke, S., Shao, N. -Y., Wei, T. -T., Yi, H., Dey, D., & Nelakanti, R., Brouwer, T P., Paik, D T., Sagiv-Barfi, I., Han, A., Quax, P.H.A., Hamming, J F., Levy, R., Davis, M M., Wu, J C. (2018). Autologous Ipsc-based vaccines elicit anti-tumor responses in vivo. Cell Stem Cell, 22(4), 501–513. e507. https://doi.org/10.1016/j.stem.2018.01.016
- Layek, B., Sadhukha, T., Panyam, J., & Prabha, S. (2018). Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Molecular Cancer Therapeutics, 17(6), 1196–1206. https://doi.org/10.1158/1535-7163.MCT-17-0682
- Layek, B., Sehgal, D., Argenta, P. A., Panyam, J., & Prabha, S. (2019). Nanoengineering of mesenchymal stem cells via surface modification for efficient cancer therapy. Advanced Therapeutics, 2(9), 1900043. https://doi.org/10.1002/adtp.201900043
- Liesveld, J. L., Sharma, N., & Aljitawi, O. S. (2020). Stem cell homing: From physiology to therapeutics. Stem Cells, 38(10), 1241–1253. https://doi.org/10.1002/stem.3242
- Li, Y., Hermanson, D. L., Moriarity, B. S., & Kaufman, D. S. (2018). Human Ipsc-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell, 23(2), 181–192. e185. https://doi.org/10.1016/j.stem.2018.06.002
- Lin, W., Huang, L., Li, Y., Fang, B., Li, G., Chen, L., & Xu, L. (2019). Mesenchymal stem cells and cancer: Clinical challenges and opportunities. BioMed Research International, 2019, 1–12. https://doi.org/10.1155/2019/8148156
- Lisini, D., Nava, S., Frigerio, S., Pogliani, S., Maronati, G., Marcianti, A., Coccè, V., Bondiolotti, G., Cavicchini, L., & Paino, F., Petrella, F., Alessandri, G., Parati, E A., Pessina, A. (2020). Automated large-scale production of paclitaxel loaded mesenchymal stromal cells for cell therapy applications. Pharmaceutics, 12(5), 411. https://doi.org/10.3390/pharmaceutics12050411
- Liu, G., David, B. T., Trawczynski, M., & Fessler, R. G. (2020). Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Reviews and Reports, 16(1), 3–32. https://doi.org/10.1007/s12015-019-09935-x
- Liu, Y., Wang, J., Xiong, Q., Hornburg, D., Tao, W., & Farokhzad, O. C. (2020). Nano–bio interactions in cancer: From therapeutics delivery to early detection. Accounts of Chemical Research, 54(2), 291–301. https://doi.org/10.1021/acs.accounts.0c00413
- Li, Y., Wang, Z., Ajani, J. A., & Song, S. (2021). Drug resistance and cancer stem cells. Cell Communication and Signaling, 19(1), 1–11. https://doi.org/10.1186/s12964-020-00627-5
- Lohan, P., Treacy, O., Griffin, M. D., Ritter, T., & Ryan, A. E. (2017). Anti-donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: Are we still learning? Frontiers in Immunology, 8, 1626. https://doi.org/10.3389/fimmu.2017.01626
- Lou, G., Chen, L., Xia, C., Wang, W., Qi, J., Li, A., Zhao, L., Chen, Z., Zheng, M., & Liu, Y. (2020). MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. Journal of Experimental & Clinical Cancer Research, 39(1), 1–9. https://doi.org/10.1186/s13046-019-1512-5
- Malta, T. M., Sokolov, A., Gentles, A. J., Burzykowski, T., Poisson, L., Weinstein, J. N., Kamińska, B., Huelsken, J., Omberg, L., & Gevaert, O., Colaprico, A., Czerwińska, P., Mazurek, S., Mishra, L., Heyn, H., Krasnitz, A., Godwin, A K., Lazar, A J., Stuart, J M., Hoadley, K A., Mariamidze, A.…. (2018). Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell, 173(2), 338–354. e315. https://doi.org/10.1016/j.cell.2018.03.034
- Mercer-Smith, A. R., Buckley, A., Valdivia, A., Jiang, W., Thang, M., Bell, N., Kumar, R. J., Bomba, H. N., Woodell, A. S., Luo, J., Floyd, S R., Hingtgen, S D. (2022). Next-generation tumor-homing induced neural stem cells as an adjuvant to radiation for the treatment of metastatic lung cancer. Stem Cell Reviews and Reports.18 (7), 1–20. https://doi.org/10.1007/s12015-022-10375-3
- Miliotou, A. N., & Papadopoulou, L. C. (2018). CAR T-cell therapy: A new era in cancer immunotherapy. Current Pharmaceutical Biotechnology, 19(1), 5–18. https://doi.org/10.2174/1389201019666180418095526
- Miska, J., & Lesniak, M. S. (2015). Neural stem cell carriers for the treatment of glioblastoma multiforme. EBioMedicine, 2(8), 774–775. https://doi.org/10.1016/j.ebiom.2015.08.022
- Moku, G., Layek, B., Trautman, L., Putnam, S., Panyam, J., & Prabha, S. (2019). Improving payload capacity and anti-tumor efficacy of mesenchymal stem cells using TAT peptide functionalized polymeric nanoparticles. Cancers, 11(4), 491. https://doi.org/10.3390/cancers11040491
- Muroi, K., Miyamura, K., Okada, M., Yamashita, T., Murata, M., Ishikawa, T., Uike, N., Hidaka, M., Kobayashi, R., & Imamura, M., Tanaka, J., Ohashi, K., Taniguchi, S., Ikeda, T., Eto, T., Mori, M., Yamaoka, M., Ozawa, K. (2016). Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: A phase II/III study. International Journal of Hematology, 103(2), 243–250. https://doi.org/10.1007/s12185-015-1915-9
- Najafi, M., Mortezaee, K., & Ahadi, R. (2019). Cancer stem cell (a) symmetry & plasticity: Tumorigenesis and therapy relevance. Life Sciences, 231, 116520.https://doi.org/10.1016/j.lfs.2019.05.076
- Nguyen, H., Zarriello, S., Coats, A., Nelson, C., Kingsbury, C., Gorsky, A., Rajani, M., Neal, E. G., & Borlongan, C. V. (2019). Stem cell therapy for neurological disorders: A focus on aging. Neurobiology of Disease, 126, 85–104. https://doi.org/10.1016/j.nbd.2018.09.011
- Okajima, F. (2013). Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cellular Signalling, 25(11), 2263–2271. https://doi.org/10.1016/j.cellsig.2013.07.022
- Ouyang, X., Telli, M. L., & Wu, J. C. (2019). Induced pluripotent stem cell-based cancer vaccines. Frontiers in Immunology, 10, 1510. https://doi.org/10.3389/fimmu.2019.01510
- Pakravan, K., Babashah, S., Sadeghizadeh, M., Mowla, S. J., Mossahebi-Mohammadi, M., Ataei, F., Dana, N., & Javan, M. (2017). MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cellular Oncology, 40(5), 457–470. https://doi.org/10.1007/s13402-017-0335-7
- Papaccio, F., Paino, F., Regad, T., Papaccio, G., Desiderio, V., & Tirino, V. (2017). Concise review: Cancer cells, cancer stem cells, and mesenchymal stem cells: Influence in cancer development. Stem Cells Translational Medicine, 6(12), 2115–2125. https://doi.org/10.1002/sctm.17-0138
- Patterson, A. D., Gonzalez, F. J., Perdew, G. H., & Peters, J. M. (2018). Molecular regulation of carcinogenesis: friend and foe. Toxicological Sciences: An Official Journal of the Society of Toxicology, 165(2), 277–283. https://doi.org/10.1093/toxsci/kfy185
- Qiao, Y., Agboola, O. S., Hu, X., Wu, Y., & Lei, L. (2020). Tumorigenic and immunogenic properties of induced pluripotent stem cells: A promising cancer vaccine. Stem Cell Reviews and Reports, 16(6), 1049–1061. https://doi.org/10.1007/s12015-020-10042-5
- Rahbarghazi, R., Jabbari, N., Sani, N. A., Asghari, R., Salimi, L., Kalashani, S. A., Feghhi, M., Etemadi, T., Akbariazar, E., & Mahmoudi, M., Rezaie, J. (2019). Tumor-derived extracellular vesicles: Reliable tools for Cancer diagnosis and clinical applications. Cell Communication and Signaling, 17(1), 1–17. https://doi.org/10.1186/s12964-019-0390-y
- Rosenblum, D., Joshi, N., Tao, W., Karp, J. M., & Peer, D. (2018). Progress and challenges towards targeted delivery of cancer therapeutics. Nature Communications, 9(1), 1–12. https://doi.org/10.1038/s41467-018-03705-y
- Ruiu, R., Tarone, L., Rolih, V., Barutello, G., Bolli, E., Riccardo, F., Cavallo, F., & Conti, L. (2019). Cancer stem cell immunology and immunotherapy: Harnessing the immune system against cancer’s source. Progress in Molecular Biology and Translational Science, 164, 119–188.
- Sage, E., Davies, A., Kolluri, K., Patrick, S., Weil, B., Rego, R. V. T. P., Edwards, A., Bain, O., Santilli, G., & Thakrar, R., Champion, K., Day, A., Popova, B., Fullen, D., Thrasher, A., Kalber, T., Forster, M., Lythgoe, M., Lowdell, M., Janes, S.M. (2018). Targeted stem cells expressing TRAIL as a therapy for lung Cancer TACTICAL: A phase I/II trial. Lung Cancer, 115(Supp. 1), S87. https://doi.org/10.1016/S0169-5002(18)30222-8
- Shao, A., Tu, S., Lu, J., & Zhang, J. (2019). Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Research & Therapy,10(1), 1–13. https://doi.org/10.1186/s13287-019-1357-z
- Siegel, R., Miller, K., Fuchs, H., & Jemal, A. (2022). Cancer statistics, 2022. CA 384. CA: A Cancer Journal for Clinicians, 72(1), 385. https://doi.org/10.3322/caac.21708
- Society, A. C. (2022). “Cancer facts & figures 2022.” Retrieved 10/23/2022, from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf.
- Suman, S., Domingues, A., Ratajczak, J., & Ratajczak, M. Z. (2019). Potential clinical applications of stem cells in regenerative medicine. Stem Cells, 1–22. https://link.springer.com/chapter/10.1007/978-3-030-31206-0_1
- Sung, H., Ferlay, J., & Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 Cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
- Sun, Y., Lu, Y., Yin, L., & Liu, Z. (2020). The roles of nanoparticles in stem cell-based therapy for cardiovascular disease. Frontiers in Bioengineering and Biotechnology, 8, 947. https://doi.org/10.3389/fbioe.2020.00947
- Suryaprakash, S., Lao, Y. -H., Cho, H. -Y., Li, M., Ji, H. Y., Shao, D., Hu, H., Quek, C. H., Huang, D., & Mintz, R. L., Bagó, J R., Hingtgen, S D., Lee, K-B., Leong, K W. (2019). Engineered mesenchymal stem cell/nanomedicine spheroid as an active drug delivery platform for combinational glioblastoma therapy. Nano Letters, 19(3), 1701–1705. https://doi.org/10.1021/acs.nanolett.8b04697
- Tobias, A. L., Thaci, B., Auffinger, B., Rincón, E., Balyasnikova, I. V., Kim, C. K., Han, Y., Zhang, L., Aboody, K. S., & Ahmed, A. U., Lesniak, M S. (2013). The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Translational Medicine, 2(9), 655–666. https://doi.org/10.5966/sctm.2013-0039
- Toledo-Guzmán, M. E., Bigoni-Ordóñez, G. D., Hernández, M. I., & Ortiz-Sánchez, E. (2018). Cancer stem cell impact on clinical oncology. World Journal of Stem Cells, 10(12), 183. https://doi.org/10.4252/wjsc.v10.i12.183
- Tong, Y., Liu, X., Xia, D., Peng, E., Yang, X., Liu, H., Ye, T., Wang, X., He, Y., Xu, H., Ye, Z., Chen, Z., Tang, K. (2021). Biological roles and clinical significance of exosome-derived noncoding RNAs in bladder cancer. Frontiers in Oncology, 11, 704–703. https://doi.org/10.3389/fonc.2021.704703
- Tripolszky, A., Németh, K., Szabó, P. T., & Bálint, E. (2019). Synthesis of (1, 2, 3-triazol-4-yl) methyl phosphinates and (1, 2, 3-triazol-4-yl) methyl phosphates by copper-catalyzed azide-alkyne cycloaddition. Molecules, 24(11), 2085. https://doi.org/10.3390/molecules24112085
- Vakhshiteh, F., Atyabi, F., & Ostad, S. N. (2019). Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. International Journal of Nanomedicine, 14, 2847–2860.https://doi.org/10.2147/IJN.S200036
- von Einem, J. C., Guenther, C., Volk, H. D., Grütz, G., Hirsch, D., Salat, C., Stoetzer, O., Nelson, P. J., Michl, M., & Modest, D. P., Holch, J W., Angele, M., Bruns, C., Niess, H., Heinemann, V. (2019). Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT‐ME‐1 trial. International Journal of Cancer, 145(6), 1538–1546. https://doi.org/10.1002/ijc.32230
- Wang, X., Chen, H., Zeng, X., Guo, W., Jin, Y., Wang, S., Tian, R., Han, Y., Guo, L., & Han, J., Wu, Y., Mei, L. (2019). Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharmaceutica Sinica B, 9(1), 167–176. https://doi.org/10.1016/j.apsb.2018.08.006
- Wang, J., Li, W., Zhang, L., Ban, L., Chen, P., Du, W., Feng, X., & Liu, B. -F. (2017). Chemically edited exosomes with dual ligand purified by microfluidic device for active targeted drug delivery to tumor cells. ACS Applied Materials & Interfaces, 9(33), 27441–27452. https://doi.org/10.1021/acsami.7b06464
- Wang, Y., Tian, M., Wang, F., Heng, B. C., Zhou, J., Cai, Z., & Liu, H. (2019). Understanding the immunological mechanisms of mesenchymal stem cells in allogeneic transplantation: From the aspect of major histocompatibility complex class I. Stem Cells and Development, 28(17), 1141–1150. https://doi.org/10.1089/scd.2018.0256
- Wei, W., Huang, Y., Li, D., Gou, H. -F., & Wang, W. (2018). Improved therapeutic potential of MSCs by genetic modification. Gene Therapy, 25(8), 538–547. https://doi.org/10.1038/s41434-018-0041-8
- Westhoff, C. M., & Rahorst, L. (2022). Immunohematology and compatibility testing. Rossi’s Principles of Transfusion Medicine, 118–130.
- Wu, X. -B., Liu, Y., Wang, G. -H., Xu, X., Cai, Y., Wang, H. -Y., Li, Y. -Q., Meng, H. -F., Dai, F., & Jin, J. -D. (2016). Mesenchymal stem cells promote colorectal cancer progression through AMPK/AMPK/Mtor-mediated NF-κB activation. Scientific Reports, 6(1), 1–12. https://doi.org/10.1038/srep21420
- Xu, H., Zhao, G., Zhang, Y., Jiang, H., Wang, W., Zhao, D., Hong, J., Yu, H., & Qi, L. (2019). Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Research & Therapy, 10(1), 1–14. https://doi.org/10.1186/s13287-019-1446-z
- Yahya, E. B., & Alqadhi, A. M. (2021). Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sciences, 269, (0024–3205),119087 . https://doi.org/10.1016/j.lfs.2021.119087
- Yin, F., Battiwalla, M., Ito, S., Feng, X., Chinian, F., Melenhorst, J. J., Koklanaris, E., Sabatino, M., Stroncek, D., & Samsel, L., Klotz, J., Hensel, N F., Robey, P G., Barrett, A J. (2014). Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: Correlation of biological markers with clinical responses. Stem Cells, 32(5), 1278–1288. https://doi.org/10.1002/stem.1638
- Yin, L., Liu, X., Shi, Y., Ocansey, D. K. W., Hu, Y., Li, X., Zhang, C., Xu, W., & Qian, H. (2020). Therapeutic advances of stem cell-derived extracellular vesicles in regenerative medicine. Cells, 9(3), 707. https://doi.org/10.3390/cells9030707
- Zhang, G., Miao, F., Xu, J., & Wang, R. (2020). Mesenchymal stem cells from bone marrow regulate invasion and drug resistance of multiple myeloma cells by secreting chemokine CXCL13. Bosnian Journal of Basic Medical Sciences, 20(2), 209.https://doi.org/10.17305/bjbms.2019.4344
- Zhao, Y., Shi, Y., Yang, H., Liu, M., Shen, L., Zhang, S., Liu, Y., Zhu, J., Lan, J., & Li, J., Ge, S. (2022). Stem cell microencapsulation maintains stemness in inflammatory microenvironment. International Journal of Oral Science, 14(1), 48. https://doi.org/10.1038/s41368-022-00198-w
- Zhou, H., Guo, M., Bian, C., Sun, Z., Yang, Z., Zeng, Y., Ai, H., & Zhao, R. C. (2010). Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: Clinical report. Biology of Blood and Marrow Transplantation, 16(3), 403–412. https://doi.org/10.1016/j.bbmt.2009.11.006
- Zhu, R., Zhang, F., Peng, Y., Xie, T., Wang, Y., & Lan, Y. (2022). Current progress in cancer treatment using nanomaterials. Frontiers in Oncology, 12, 930125. https://doi.org/10.3389/fonc.2022.930125
