The publishers would like to apologize for an error that occured in
Int. J. Hyperthermia, March 2007; 23(2): 141–151
Compensating for bone interfaces and respiratory motion in high-intensity focused ultrasound
M. TANTER, M. PERNOT, J.-F. AUBRY, G. MONTALDO, F. MARQUET, & M. FINK
The correct figures are shown below:
Figure 1. (a) 300-element HIFU array with water-cooling and coupling system and the 300 electronic channels driving the HIFU brain array. A curved array (128 elements—3MHz) is embedded in the vicinity of the HIFU array in order to provide a low-quality echographic image the skull surface and brain for a rough positioning of the complete system with respect to the head. (b) Quasi Random distribution of the 300 elements into a spherical mould. (c, d) 2D scan of the transcranial focal spot: distribution of the acoustic energy deposition in the focal plane of the array (z = 140 mm depth) without (c) and with (d) time reversal correction of the skull distortions.
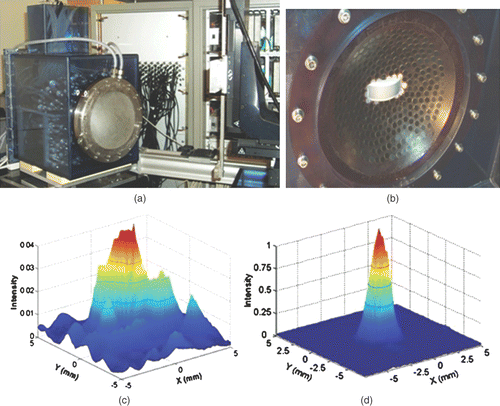
Figure 2. The different steps of the brain HIFU therapy guided by CT scans: (a) the monkey is imaged into a CT-scanner. The head is fixed on a house-made stereotactic frame. (b) A treatment software allows selecting the targeted spot. From these data, numerical simulation of the 3D transcranial ultrasonic propagation enables the estimation and correction of skull aberrations for the HIFU treatment. (c) One week after the CT scan exam, the monkey's head is again fixed into the house-made stereotactic frame. (d) The HIFU array is coupled to the monkey's head via the stereotactic frame, thus ensuring the concordance of CT scan reference frames and the HIFU experimental reference frame. The HIFU treatment is then performed using the calculated emission signals.
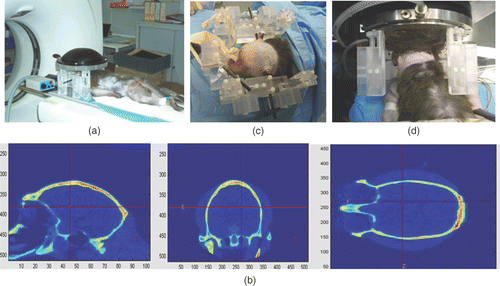
Figure 3. (a) Principle of the transcranial time reversal focusing technique guided by CT scans. (b) 3D simulation of the wave propagation (finite differences): wave field distribution at different time steps (at t = 0 µs, t = 8 µs, t = 16 µs, t = 24 µs, t = 32 µs, t = 40 µs, t = 48 µs, t = 56 µs, t = 64 µs) in a selected plane. (c) The monkey was sacrificed 1 week after treatment: the 7T MR images of the fixed brain clearly show the thermal necrosis at the targeted location using a Steady State Free Precession (SSFP) sequence. The histological examination clearly show thermal acidophilic necrosis in the area treated with time reversal correction and normal pattern (neurons, oligodendrocytes, capillaries) in the area treated with a non-corrected HIFU beam.
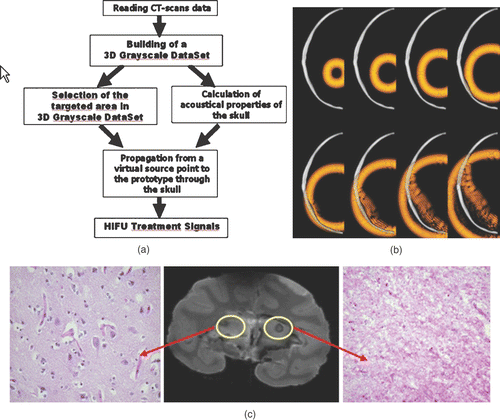
Figure 4. Spatial directivity pattern of the transcostal ultrasonic beam in the focal plane using (a) conventional focusing and (b) time reversal focusing. (c) Necrosis obtained using time reversal focusing through the rib cage in liver samples for a 5-s duration at 1000 W cm−2 and for a 5-s duration at 1600 W cm−2. (d) Temperature elevation at several locations on the rib cage with conventional and time reversal focusing. The temperature elevation is estimated using implanted thermocouples (Ironconstantan, 40 µm diameter, PhysiTemp Corp.).
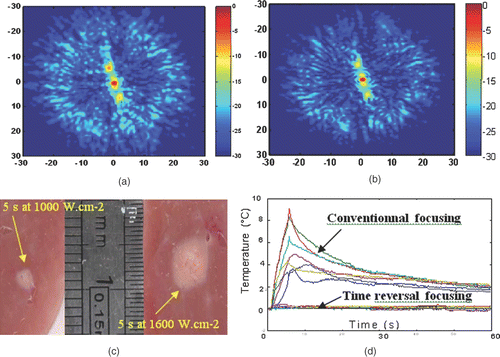
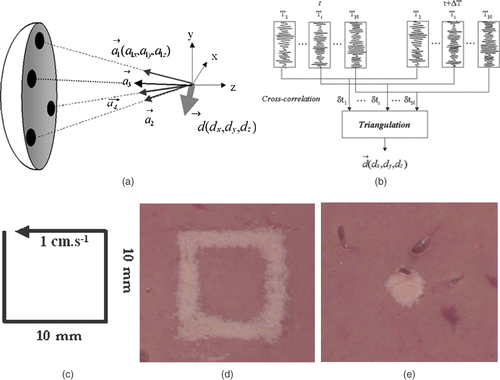
Figure 5. Basic principle of the real-time motion correction. (a) Four subapertures successively send short ultrasonic impulses focusing at the ‘tracking location’. (b) Standard 1D cross-correlations between successive sets of acquisitions permits to recover the 3D displacement vector. (c) Displacement trajectory of a liver sample mounted on three-axis linear motors. Necrosis obtained in the moving liver sample (d) without and (e) with real-time motion correction.