Abstract
The purpose of this study was to evaluate the combined effects of heat and polylactic-co-glycolic acid (PLGA) nanoparticles, as 5-fluorouracil carriers with/without iron oxide core, on the viability and proliferation capacity of human colon cancer cell line HT-29 in the spheroid model. HT-29 spheroid cells were treated with different concentrations of 5-FU or 5-FU-loaded into both nanoparticles for 74 h. Hyperthermia was then performed at 43 °C for 60 min. Finally, the effects of the mentioned treatments on cell viability and proliferation capacity were evaluated using the trypan blue dye exclusion test and colony formation assay, respectively. Our results showed that hyperthermia, in combination with 5-FU or PLGA nanoparticles as 5-FU carriers, significantly enhanced the cytotoxic effects as compared to the control group. Considering that nanoparticles could increase the intracellular concentration of drugs in cancer cells, the extent of cytotoxic effects following treatment with 5-FU-loaded into both nanoparticles was significantly higher than that with free 5-FU. In addition, the presence of iron oxide cores in nanoparticles during hyperthermia enhanced the cytotoxic effects of hyperthermia compared with nanoparticles without iron oxide core. Based on this study, hyperthermia in combination with 5-FU-loaded PLGA nanoparticles with iron oxide core drastically reduced the proliferation capacity of HT-29 cells; therefore, it may be considered as a new direction in the treatment of colon cancer.
Introduction
In our previous study, the effect of hyperthermia and 5-fluorouracil (5-FU)-loaded magnetic nanoparticles was evaluated on the level of DNA damages in spheroid model of human colon cancer cell line HT-29 using alkaline comet assay [Citation1]. In this study, the effect of this treatment was evaluated on the clonogenic forming ability of the cells. Clonogenic ability is often considered to be the gold standard for measurement of the intrinsic sensitivity of tumour cells, because it provides information that is most relevant for treatment success or failure. Comet assay method measures DNA damage. Hence, it cannot be applied to measure the initial response to drugs that do not elicit prompt DNA damage. Therefore, there are significant differences between clonogenicity and DNA damages of the cells and these two methods provide different information.
Colon cancer is one of the most frequently detected cancers in the world [Citation2]. Based on the most recent statistics reported by the American Cancer Society in 2014, it ranks as the third most frequently diagnosed cancer and is the third leading cause of cancer death in both men and women in the US [Citation3].
At present, surgery, radiotherapy and chemotherapy are the main modalities to treat colon cancer. However, due to tumour resistance and severe side effects of chemoradiotherapy, the overall survival rate is very low [Citation3]. Therefore, it is crucial to find new therapy modalities, particularly those which kill tumour cells while causing minimal injury to normal tissues.
Hyperthermia is a cancer treatment modality used either alone or in combination with other modalities such as chemotherapy and radiotherapy [Citation4]. The tumour core is frequently resistive to conventional treatments, like radiotherapy (since it is hypoxic) and chemotherapy (due to low drug supply). However, the increased blood perfusion induced by hyperthermia can enhance the damage of tumour cells at its centre [Citation5,Citation6].
Clinical studies have demonstrated that hyperthermia, in combination with chemotherapy, drastically increases the cytotoxicity of chemotherapy agents [Citation7]. The interaction between heat and anticancer agents augments drug accumulation to the tumour by enhancing endothelial cell permeability and local blood flow [Citation8]. Under such conditions, numerous side effects of the drug could be decreased by a suitable reduction of the drug dose or drug therapeutic effect could be raised with the same drug dose [Citation9].
5-Fluorouracil is one of the extensive spectrum chemotherapy drugs used widely in the treatment of a range of cancers, particularly colorectal cancer [Citation10]. 5-FU works during the S-phase of cell division, exerting its anticancer effects through the inhibition of thymidylate synthase (TS) and the incorporation of its metabolites into RNA and DNA [Citation11,Citation12].
Although 5-FU-based chemotherapy can somewhat improve the overall survival of patients with colorectal cancer, clinical trials have shown that the response rates for 5-FU-based chemotherapy as a first-line treatment for advanced colorectal cancer are only 10–15% [Citation11,Citation13]. The reasons are numerous and varied. A short half-life, extensive distribution, lack of selectivity and severe side effects have been major obstacles, restricting its clinical applicability. To overcome the aforementioned limitations, a large number of studies have been performed on the sustained-release of drug delivery systems and magnetic drug targeting (MDT) [Citation14]. Existing reports have shown the potential of controlled release systems using polymers for 5-FU to extend its plasma circulation lifetime and to enhance its therapeutic activity at doses exhibiting acceptable toxicities. In addition, this avoids the side effects caused due to overdosing and repeated infusion of drug. Magnetic drug targeting (MDT) by using drug-loaded magnetic nanoparticles can selectively focus chemotherapeutics on tumour tissue. When a magnetic field is applied to the tumour, the injected magnetic drug carriers can be confined to the target site [Citation15].
It has been shown that the entrapment of anticancer agents in polymer-coated magnetic nanoparticles optimises their therapeutic efficacy and reduces side effects, compared with conventional anti-cancer drugs [Citation16].
Among many types of nanocarriers, poly(d,l-lactic-co-glycolic acid) (PLGA)-coated iron oxide nanoparticles show the potential to be the ideal drug delivery system for cancer treatment. Some of their attractive properties are as follows: (1) biodegradability and biocompatibility, (2) in vivo and in vitro cell tracking, (3) magnetic resonance imaging (MRI) contrast enhancement, (4) high magnetic susceptibility and high accumulation in the desired target tissue or organ, (5) approval of any of the components of this nanoparticle by the Food and Drug Administration (FDA) and European Medicine Agency (EMA) in various drug delivery systems in humans and (6) accurate control of the release rate of an incorporated drug [Citation6,Citation17,Citation18].
However, a unique advantage of these nanoparticles is their application in hyperthermia in which iron oxide nanoparticles encapsulated within PLGA can absorb thermal energy, thus locally raising the temperature inside tumour cells. The temperature in heated cells containing iron oxide nanoparticles is higher than in those cells which do not contain these materials. This, in turn, results in promoting cell death by hyperthermia and enhancing the drug release. Such a system can dramatically increase the efficacy of treatments [Citation19,Citation20]. Therefore, this study was undertaken for the first time to investigate and compare the cytotoxic effects of two nanoparticles (PLGA nanoparticles with and without iron oxide core) in the presence and absence of hyperthermia in HT-29 cells.
Compared to monolayer cultures, three-dimensional culture systems such as spheroid cultures are much more similar to in vivo tumours. Moreover, in contrast to in vivo xenografts, they can easily be manipulated and exposed to specific treatment modalities. Therefore, spheroid cultures were used in the current study [Citation21].
Materials and methods
Cell line
The human colonic adenocarcinoma cell line HT-29 was obtained from Pasteur Institute of Iran. Cells were grown in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% foetal bovine serum (FBS) (PAA, PAA Laboratories Ply Ltd, Australia), 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma, St. Louis, MO). HT-29 cells were cultured as a monolayer at a density of 104 cells/cm2 in T-25 tissue culture flasks (Orange Scientific, Braine-l’Alleud, Belgium). The cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2. Cells were harvested by trypsinising cultures with 1 mM EDTA/0.25% Trypsin (w/v) in phosphate buffer saline (PBS).
Spheroid culture
Spheroids were initiated using the liquid overlay technique [Citation22]. 5 × 105 cells were seeded into 100 mm culture Petri dishes coated with a thin layer of 1% agar (Bacto Agar, Difco, Detroit, MI) with 10 ml of RPMI supplemented with 10% FBS. Cells were incubated at 37 °C in a humidified 5% CO2 atmosphere and grown for 10 days. Half of the culture medium was replaced with fresh culture medium twice per week. Ten-day spheroids with mean diameter of 100 μm were used for cytotoxicity studies.
Synthesis and characterisation of nanoparticles
Synthesis of nanoparticles
Four types of nanoparticles were used in this work. They include: (1) PLGA nanoparticles without iron oxide core; (2) PLGA nanoparticles without iron oxide core as 5-FU carrier; (3) PLGA nanoparticles with iron oxide core; (4) PLGA nanoparticles with iron oxide core as 5-FU carrier.
Synthesis of mentioned nanoparticles was based on the method of Dr. Khoee et al. [Citation23]. The biodegradable 5-FI-loaded nanoparticles with iron oxide core were synthesised through a modified multiple emulsions–solvent evaporation method oil in water, oil in water (o/w/o/w). Drug-free nanoparticles were prepared in a similar way without incorporating drug in the process.
5-Fluorouracil-loaded nanoparticles without iron oxide core were synthesised through a double emulsions method (w/o/w) [Citation24]. Drug-free nanoparticles without iron oxide core were synthesised through an emulsions inversion method (o/w) [Citation25].
Determination of particle size and morphology
The morphological investigation of nanoparticles was performed using a Zeiss LEO 906 transmission electron microscope (TEM) (Zeiss, Germany). The nanoparticles were suspended in distilled water and sonicated for 4 min using a 20 kHz ± 500 Hz (200 W) ultrasonic generator, SONOPULS Ultrasonic homogeniser, Model HF-GM 2200 (BANDELIN Electronic GmbH & Co. KG, Heinrichstraße, Berlin, Germany) and a titanium microtip MS-73 with diameter 3 mm as the probe. A drop of the nanoparticle suspension was placed on a carbon-coated grid. The grid was perfectly dried and observed under the TEM at 100 kV. Furthermore, the hydrodynamic size average of the nanoparticle was examined by dynamic light scattering (DLS) (Brookhaven Instruments, Holtsville, NY).
Zeta potential
Zeta potential is an indicator of surface charge, which influences the stability of nanoparticles. In the case of charged particles, as the zeta potential increases, the repulsive interactions will be larger, leading to the formation of more stable particles with a more uniform size distribution [Citation26]. Zeta potential of nanoparticles was determined by a zeta potential analyser (Brookhaven Instruments, Holtsville, NY). The nanoparticles were suspended in distilled water and sonicated for 4 min.
Determination of drug content and encapsulation efficiency
In order to assess the drug concentration in all systems, UV absorption measurements were performed. 5-FU concentration was investigated by application of Beer’s law. These estimations are based on absorbance determinations of the 5-FU-loaded nanoparticles compared with free-drug naoparticles. The drug content in the nanoparticles is defined as the ratio of the encapsulated drug to the total weight of solid magnetic nanoparticles. The encapsulation efficiency is defined as the ratio of the encapsulated drug content to the total amount of drug used for nanoparticle preparation. First, 10 mg of 5-FU-loaded nanoparticles were weighed and redissolved in 10 ml of acetone (spectroscopy grade). In PLGA nanoparticles with iron oxide core, the insoluble magnetite particles were removed from the solution by magnetic separation. These steps were done on free-drug nanoparticles and this sample were used as blank in absorbance measurement. Finally, the 5-FU concentration in the acetone solution was determined, with respect to a previously established calibration curve, by UV absorption at a wavelength of 210 nm (characteristic absorption band of 5-FU), and the amount of encapsulated drug in the nanoparticles was calculated. Drug content and encapsulation efficiency were obtained using EquationEquations (1)(1) and Equation(2)
(2) , respectively:
(1)
(2)
In vitro drug release
The in vitro release of 5-FU from the nanoparticles was performed in a phosphate buffered saline (PBS) medium (pH 7.4) using the dialysis method. The dialysis bag (molecular weight cut-off 12 400 Da) was soaked in preheated double-distilled water before use. A weighed amount of freeze-dried nanoparticles was resuspended in PBS and transferred into a dialysis bag. Both ends of the tube were affixed with clamps. The bag was placed into 100 ml of preheated PBS that acted as a release medium. The release study was performed in an incubator shaker at 37 and 43 °C. At definite time intervals, a sample from the incubation medium was withdrawn and analysed using UV–visible spectrophotometer and replaced by the same quantity of fresh PBS solution. At fixed time intervals (e.g. 3 days); the medium in the vessel was completely removed and replaced with fresh release medium. The 5-FU concentration was calculated based on the absorbance intensity of 5-FU at 266 nm. In the assessment of drug release behaviour, the cumulative amount of released drug was calculated, and the percentages of released drug from PLGA nanoparticles were plotted against time. All experiments were carried out in triplicate.
Hyperthermia and 5-FU (free or loaded into PLGA nanoparticles with and without iron oxide) treatments of spheroid culture
Cells were cultured for multicellular spheroid formation. On day 10, spheroids with a mean diameter of 100 μm were treated with 1, 5 and 10 μM of free 5-FU (Sigma) or 5-FU-loaded PLGA nanoparticles with and without iron oxide cores for 74 h. Drug-free PLGA nanoparticles with/without iron oxide were used as the control of drug-loaded nanoparticles treatment. Before heat treatment, the medium was replaced with a fresh culture medium. Hyperthermia was applied at 43 °C for 60 min in a water bath (Memmert) with ±0.1 °C accuracy. Control cells were exposed to 37 °C. After heat treatment, the spheroid cells were treated with 300 μl of 1 mM EDTA/0.25% trypsin (w/v) in PBS for 5 min at 37 °C. Trypsin was neutralised by the addition of 700 μl of the culture medium containing 10% FBS. Spheroids were mechanically disaggregated. The single cells were counted and tested for viability using trypan blue dye exclusion assay and colony formation ability.
Clonogenic assay
Clonogenic assay was done to assess the long-term cytotoxicity of different treatments. Treated and control single cell suspensions from spheroid cultures were seeded at a density of 3000 cells per 60 mm Petri dish containing 5 ml of culture medium supplemented with 10% FBS. The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2. After 8 days, the colonies were fixed with 2% formaldehyde in PBS and stained with 0.5% crystal violet. The colonies were counted using an inverted phase microscope (BEL) and the plating efficiency (PE) was determined by EquationEquation (3)(3) :
(3)
The surviving fraction was given by EquationEquation (4)(4) :
(4)
Statistical analysis
All experiments were performed in triplicate. All data are expressed as mean values ± SEM (Standard Error of Mean), with “n” denoting the number of experiments. For statistical analysis, one-way ANOVA followed by Turkey’s test as the post-hoc analysis were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL). The value of p < .05 was considered to be statistically significant.
Results
Cell characteristics
The human colon carcinoma cell line HT-29 grows as a monolayer in tissue culture flasks. The population doubling time was approximately 24.16 ± 0.67 h. The HT-29 cells are able to form spheroids in liquid overlay cultures. The volume doubling time of the HT-29 spheroid was approximately 74.3 ± 5 h, which was applied as drug treatment time consequently.
Characterisation of nanoparticles
Particle size and zeta potential determination
The hydrodynamic size and zeta potentials of the four types of nanoparticles investigated with dynamic light scattering (DLS) analysis and zeta potential analyser, respectively, are presented in . The nanoparticles formulated in this study were found to be in the size range of 30–100 nm and stable in the dispersion state, possessing high absolute values of zeta potential and having negative surface charges.
Table 1. Measurement of hydrodynamic size and zeta potential of nanoparticles.
Morphological studies
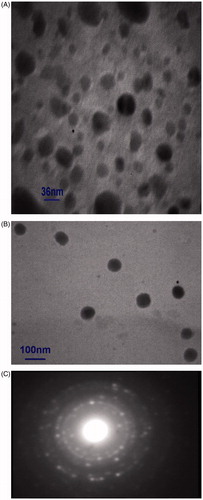
Morphologies of the particles were examined using a transmission electron microscope (TEM). show typical TEM micrographs of 5-FU-loaded PLGA-coated nanoparticles with and without iron oxide core, respectively. shows the selected area electron diffraction (SAED) pattern associated to the sample from (A). The results suggest that most of the copolymer nanoparticles had a regular spherical shape, and the magnetic nanoparticles were well dispersed inside the PLGA nanoparticles with iron oxide core.
Drug content and encapsulation efficiency of nanoparticles
The drug content and encapsulation efficiency of PLGA nanoparticles with iron oxide core were 11.5 and 71.8%, respectively. For the nanoparticles without iron oxide core, they were 10.1 and 63.1%, respectively. Based on our previous data [Citation27], the intracellular iron content was measured using atomic absorption spectroscopy (AAS). The weight of each nanoparticle and the weight of iron oxide in each particle were calculated. Each particle contains approximately 0.37 × 10−16 g iron oxide. Iron concentrations of the cells were determined 74 h after incubation of spheroids with different concentrations of 5-FU-loaded PLGA-coated iron oxide nanoparticles, using atomic adsorption spectroscopy. shows iron contents of incubated spheroids with 5-FU-loaded PLGA-coated iron oxide nanoparticles and the calculated particle number in each cell.
Table 2. Iron content of the cells after 74 h incubation with free and different concentrations of 5-FU-loaded PLGA-coated iron oxide nanoparticles. N(0) mean free nanoparticles and N(1), N(5), N(10) mean the nanoparticles release 1, 5 and 10 μM 5-FU respectively during 74 h, Mean ± STD of three independent experiments. The particle number per cell has been quantitatively calculated.
In vitro release profile of 5-FU
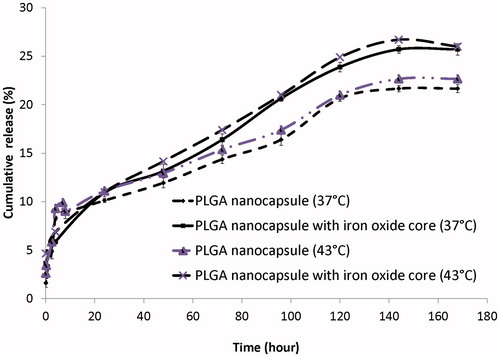
shows the cumulative in vitro release profiles of 5-FU from the PLGA nanoparticles with and without iron oxide core at 37 and 43 °C. As can be seen, temperature had no effect on release rate. Furthermore, 5-FU was released to the extent of 17 and 14.5% from PLGA nanoparticles with and without iron oxide core, respectively, within approximately 74 h in a phosphate buffer at 37 °C.
Effect of 5-FU alone or in combination with hyperthermia on colony-forming ability
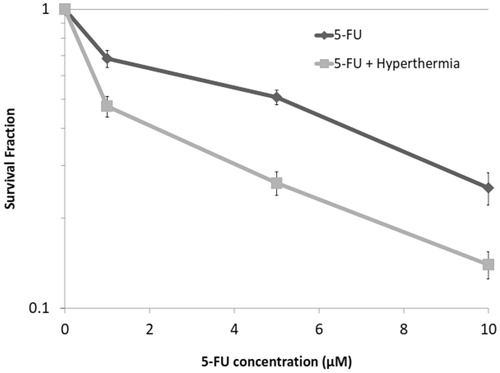
The cell response to 5-FU in the presence and absence of hyperthermia on the colony-forming ability was studied by applying different concentrations of 5-FU plus hyperthermia at 43 °C for 1 h. Plots of survival fraction versus 5-FU concentrations are shown in . As can be seen, the proliferation capacity of the cells in both groups of 5-FU and 5-FU + hyperthermia significantly reduced by increasing 5-FU concentrations. However, when the cells were treated with 5-FU for 74 h, followed by hyperthermia, the extent of reduction in the colony-forming ability was significantly more than when treated with 5-FU alone (p < .05).
Effect of 5-FU-loaded PLGA nanoparticles with and without iron oxide core alone or in combination with hyperthermia on colony-forming ability
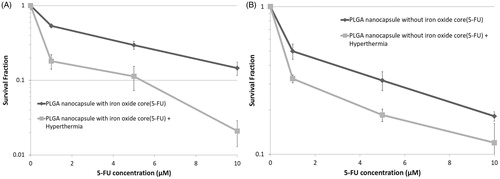
presents the cytotoxic effects of different concentrations of 5-FU-loaded PLGA nanoparticles, with and without iron oxide core, alone or in combination with hyperthermia at 43 °C for 1 h, respectively. As can be seen, survival fraction decreased by increasing the concentration of 5-FU released from nanoparticles. Furthermore, the interaction between hyperthermia and 5-FU-loaded nanoparticles could significantly decrease the survival fraction in comparison with 5-FU-loaded nanoparticles alone.
Figure 4. Effects of different concentrations of 5-FU released from (A) PLGA nanoparticles with iron oxide core, (B) PLGA nanoparticles without iron oxide core, alone and in combination with hyperthermia at 43 °C for 1 h, on the survival fraction of HT-29 cells in spheroid culture. Mean ± SEM of three independent experiments (n = 9).
Cytotoxic effects of 5-FU and 5-FU-loaded PLGA nanoparticles with and without iron oxide core
shows the effect of 5-FU and 5-FU-loaded PLGA nanoparticles with and without iron oxide core on colony-forming ability. Results indicate that the cytotoxic damages in cells treated with 5-FU and 5-FU-loaded PLGA nanoparticles increased by increasing the concentration of 5-FU. However, the extent of reduction in the survival fraction following treatment with 5-FU-loaded nanoparticles was significantly more than 5-FU.
Figure 5. Survival fraction of HT-29 cells in spheroid culture versus different concentrations of free 5-FU or 5-FU-loaded PLGA nanoparticles with and without iron oxide core. Mean ± SEM of three independent experiments (n = 9).
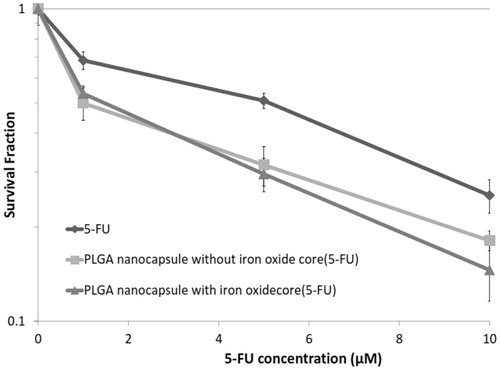
Furthermore, there was no significant difference between the survival fraction of both nanoparticles, 5-FU-loaded PLGA nanoparticles with and without iron oxide core, at the same concentration (p > .05).
Cytotoxic effects of 5-FU-loaded PLGA nanoparticles with and without iron oxide core in the presence of hyperthermia
To evaluate the role of iron in enhancing cytotoxic damages of hyperthermia, cytotoxic effects of both nanoparticles (5-FU-loaded into PLGA nanoparticles with and without iron oxide core) in combination with hyperthermia were compared. According to , the proliferation capacity of the cells after treatment with both nanoparticles reduced by increasing 5-FU amount released from nanoparticles. However, when the cells were treated with 10 μM of 5-FU-loaded PLGA nanoparticles with iron oxide core followed by hyperthermia, the extent of reduction in the colony-forming ability was significantly more than when treated with similar concentration of 5-FU-loaded PLGA nanoparticles without iron oxide core and hyperthermia (p < .05).
Figure 6. survival fraction of HT-29 cells in spheroid culture versus different concentrations of 5-FU-loaded PLGA nanoparticles with and without iron oxide core in the presence of hyperthermia at 43 °C for 1 h. Mean ± SEM of three independent experiments (n = 9).

A summary of the overall cytotoxic effect observed in HT-29 cells after all treatments are described in . As can be seen, hyperthermia in combination with 5-FU-loaded PLGA nanoparticles with iron oxide core had the most cytotoxic effect on cells as compared to the other treatments.
Table 3. The extent of cytotoxic effects as compared to the control observed in HT-29 cells on treatment with all forms of 5-FU, both types of nanoparticles alone or in combination with hyperthermia. Mean ± SD of 3 independent experiments (n = 9).
Discussion
The current study was designed to evaluate and compare the cytotoxic effects of 5-FU (free or loaded into PLGA nanoparticles with and without iron oxide) alone or in combination with hyperthermia on spheroids of HT-29 cell lines in vitro.
This study can be evaluated in three ways:
the role of hyperthermia in increasing the cytotoxicity effects of 5-FU (free or loaded PLGA nanoparticles with and without iron oxide cores);
the role of polymeric nanoparticles as 5-FU carriers in enhancing cytotoxicity effects compared with 5-FU alone;
a comparison of two nanoparticles and the role of iron oxide cores in enhancing the cytotoxic damage by hyperthermia.
Concerning the combined effects of heat and 5-FU, our result showed that 1 h exposure to hyperthermia at 43 °C had no significant toxicity on HT-29 cells in spheroid culture (). This finding is in agreement with the published report by Dobrucki and Bleehen [Citation28]. However, the cytotoxicity of 5-FU was significantly enhanced in the presence of hyperthermia. Based on these data, it may be concluded that hyperthermia can dramatically improve the efficacy of anticancer agents and reduce the required effective dose of the anticancer drug.
Maehara et al. [Citation29] showed that hyperthermia causes 5-FU to be more rapidly metabolised to active forms. Furthermore, Kido et al. [Citation30] reported that 2 h exposure to hyperthermia at 43 °C along with 1 μg/ml 5-FU increased cellular sensitivity to 5-FU in Chinese hamster V-79 cells. They argued that the pre-treatment with 5-FU blocks the cells at the S-phase. Because cells in this phase are more sensitive to hyperthermia than those in other phases, subsequent treatment with hyperthermia may selectively kill cells in the S-phase.
The advantage of combining hyperthermia and chemotherapy to treat cancer is well-recognized. Nonetheless, due to the lack of significant cell sensitivity to heat, a short biological half-life and extensive biodistribution of drugs, combined therapy has not yet reached a standard-of-care status [Citation19]. The use of PLGA-coated iron oxide nanoparticles may overcome the limitations of both hyperthermia treatment and chemotherapy using 5-FU.
The results from TEM and DLS demonstrated that the mean diameter of the nanoparticles ranged from 36 to 83 nm (). Appropriate nanoparticle size is very important for efficient drug delivery. Generally, 10–100 nm is considered as the optimal size for nanoparticle drug carriers [Citation31].
In vitro release investigations showed that 5-FU has a sustained release profile when loaded into PLGA nanoparticles with and without iron oxide core (). Our colleagues reported that the non-encapsulated 5-FU is released to the extent of 100% within approximately 6 h [Citation23]. However, 5-FU is released for approximately 33 days in a phosphate buffer at 37 °C when it is encapsulated in magnetic nanoparticles [Citation15]. Our results showed that heating at 43 °C had no significant effect on release rate of 5-FU as compared with 37 °C. Abulateefeh et al. [Citation32] compared the release rate of paclitaxel from PLGA at two pH values and two temperatures. They showed that release rate of paclitaxel is faster at lower pH; but temperature (42 °C) had no effect on the release rate of drug.
One of the most important properties of iron oxide nanoparticles is the possibility to track cells using different ways both in vivo and in vitro. To evaluate cytotoxic effects of PLGA nanoparticles with iron oxide core, it was essential to confirm cellular uptake of nanoparticles. In our previous study, the TEM images showed PLGA nanoparticles with iron oxide core were taken up by HT-29 cells [Citation1]. On the same lines, our colleagues evaluated the uptake of these nanoparticles into the prostate carcinoma cell line (DU145) in vitro using atomic absorption spectroscopy analysis [Citation27] and Prussian blue staining [Citation33]. In addition, magnetic resonance imaging confirmed that the magnetic nanoparticles were successfully targeted to the tumour [Citation15].
The cytotoxicity studies revealed that PLGA nanoparticles with/without iron oxide core containing 5-FU effectively inhibited the proliferation capacity of the cells compared with 5-FU alone (p < .05) (). Because 5-FU is hydrophilic, it cannot easily cross the cell membrane, which is composed of phospholipids. However, loading of this drug into polymeric nanoparticles facilitates its entry into cells through phagocytosis. In addition, the sustained release and prolonged lifetime of 5-FU-loaded polymeric nanoparticles () brings about the release of a great deal of drug into cells. Therefore, the required effective dose of the anticancer drug can be reduced, and minimised systemic toxicity ensues. Similar behaviour has recently been reported by our colleagues for the same polymeric nanoparticle-containing 5-FU [Citation27]. Sutar and Joshi [Citation34] reported that the treatment of HT-29 cells with 5-FU-loaded PLGA nanoparticles brings about cell lysis at a rate of 80%. In addition, Li et al. [Citation35] demonstrated that uncoated magnetic iron oxide nanoparticles (MIONP) can be toxic at a high concentration (0.40 mg/ml) to HeLa and RPE cells. However, the cytotoxicity of uncoated MIONPs at low concentrations is cell-type specific, and RPE cells are more susceptible to MIONPs than HeLa cells. This study showed that blank iron oxide nanoparticles coated with PLGA had no significant cytotoxic effect as compared to the control (p > .05) (). Based on our data and other published reports, it can be deduced that PLGA-coated iron oxide nanoparticles are efficient drug carriers for 5-FU. PLGA-coated iron oxide nanoparticles are biocompatible, and this coating is a suitable surface to penetrate into the cells [Citation36–38].
Concerning the combined effects of heat and nanoparticles, our result showed that the cytotoxic effects of 5-FU loaded into both nanoparticles was significantly enhanced in the presence of hyperthermia (). The increase in the concentration of 5-FU released from nanoparticles as well as the higher S-phase arrest in cells may be the major factors of damage caused [Citation14].
In this paper, cytotoxic effects of two nanoparticles (5-FU-loaded PLGA into nanoparticles with and without iron oxide core) in the presence and absence of hyperthermia have been compared for the first time. Our result showed that in the absence of hyperthermia, there was no significant difference between cytotoxic effects of both nanoparticles at the same concentration (). Therefore, iron oxide cores, owing to a biocompatible PLGA coating, had no cytotoxic effect, and the damage observed is only due to 5-FU released from nanoparticles.
However, in the presence of hyperthermia, cytotoxic effects of two nanoparticles were completely different, which can be attributed to the role of iron oxide core in increasing thermal sensitisation of cancer cells to thermo-chemotherapy [Citation20]. Obtained results showed that when the cells were treated with 5-FU-loaded PLGA nanoparticles with iron oxide core followed by hyperthermia, the extent of reduction in the colony-forming ability was significantly more than 5-FU-loaded PLGA nanoparticles without iron oxide core and then hyperthermia (). In fact, the iron oxide cores encapsulated within PLGA can increase cell death by hyperthermia. This also swells up the polymer coating and leads to the release of the drug [Citation39]. Recently, we reported a temperature rise profile of sonicated tumours with and without magnetic nanoparticles, which can confirm the role of iron oxide cores in increasing the temperature. We found that the temperature of CT26 tumours exposed to 3-MHz ultrasound waves at a nominal intensity of 1 W/cm2 for 10 min increased up to 3.9 ± 0.2 °C, while the combination of accumulated magnetic nanoparticles and ultrasound, under the same conditions, increased the tumour temperature up to 6.1 ± 0.4 °C [Citation40]. In addition, the antitumor studies revealed that the targeted therapy with magnetic nanoparticles containing 5-FU effectively inhibited the growth of tumours compared with 5-FU alone [Citation15]. Jordan et al. [Citation26,Citation41] demonstrated that clonogenic survival was three-folds lower after magnetic fluid hyperthermia versus water bath hyperthermia alone. They showed that the use of ferrofluid during hyperthermia, either using water bath or MFH heating, reduces the shoulder of the dose response curves of cells preincubated at 43 °C compared with heating using a water bath in a normal medium. Freeman et al. [Citation15] found that l mM iron oxide that is not toxic to CHO cells at 37 °C became cytotoxic as the temperature increased to 43 °C (relative toxicity factor, 2–4), which is roughly in accordance with our results () (difference between water bath hyperthermia with or without the iron oxide core). This thermosensitising effect of the iron oxide core at 43 °C may be because of oxidative stress induced by ferric ions, which promotes the formation of hydroxyl radicals in aqueous solution.
In comparison between the cytotoxic effect of synthesised nanoparticles on normal cells and their cancer counterparts, Yang et al. [Citation42] have indicated that although it was difficult to fully differentiate cellular responses of normal fibroblasts and their cancer counterparts to various magnetic nanoparticles (MNPs) but normal cells were shown to be more vulnerable to internalised MNPs than cancer cells.
Recently, Attaluri et al. [Citation43] evaluated the effect of magnetic nanoparticle hyperthermia (mNPH) and radiation (RT) on two human prostate cancer cell lines, PC-3 and LAPC-4 in two different conditions; in vitro and in vivo. In vitro study showed that ionising radiation significantly decreased the survival of LAPC-4 and PC3 cells. Moreover, LAPC-4 cells demonstrate a significantly greater sensitivity to the ionising radiation and combined therapy than do PC3 cells. In vivo study showed that LAPC-4 tumours retained higher MIONP (magnetic iron oxide nanoparticle) concentration and more uniform distribution than did PC3 tumours. They measured temperature and effects on tumour growth delay of mNPH ± RT following percutaneous delivery of MIONPs into two mouse models of prostate cancer. While the tumours generated from these cell lines proved useful for comparative experiments of mNPH with respect to MIONP distribution, the cell lines also displayed different sensitivity to heat and radiation that were recapitulated in the tumour models, thus introducing a confounding element for interpretation of results [Citation43]. Compared to this study, our recent study on the effect of magnetic drug targeting (MDT) using 5-FU-loaded magnetic nanoparticles and tumour irradiation by ultrasound on CT26 tumours in BALB/c mice showed that the combination of magnetic nanocapsules containing 3 mg kg−1 5-FU +0.18T magnetic field +10 min exposure with 0.3 W/cm2, 3 MHz ultrasound effectively inhibits the growth of CT26 tumours compared with injection of 5-FU alone or nanocapsules-MDT [Citation44]. Both studies show more research and study should be done to translate in vitro results in animal or human.
Conclusion
In summary, this study has demonstrated that hyperthermia increases the cytotoxic effects of 5-FU. On the other hand, PLGA-coated nanoparticles can deliver 5-FU more efficiently into the cells. Finally, we showed that the iron oxide core of PLGA nanoparticle can act as a thermosensitiser and increase cytotoxic effects of hyperthermia. Therefore, this method could significantly improve the efficacy of treatments and reduce the 5-FU dose needed for tumour control.
Disclosure statement
The authors report no conflicts of interest.
Funding
This work was supported by a grant (No. 24073) from the School of Medicine at Iran University of Medical Sciences (IUMS).
References
- Esmaelbeygi E, Khoei S, Khoee S, Eynali S. (2015). Role of iron oxide core of polymeric nanoparticles in the thermosensitivity of colon cancer cell line HT-29. Int J Hyperther 31:489–97.
- Labianca R, Beretta GD, Kildani B, et al. (2010). Colon cancer. Crit Rev Oncol Hematol 74:106–33.
- Siegel R, Desantis C, Jemal A. (2014). Colorectal cancer statistics, 2014. Cancer J Clinicians 64:104–17.
- Habash RW, Bansal R, Krewski D, Alhafid HT. (2006). Thermal therapy, part 2: hyperthermia techniques. Crit Rev Biomed Eng 34:491–542.
- Hildebrandt B, Wust P, Ahlers O, et al. (2002). The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43:33–56.
- Jhaveri A. (2009). Magnetic nanomedicine and hyperthermia for the treatment of thyroid cancer. MSc Thesis. Northeastern University Boston, Massachusetts. p. 20.
- Cherukuri P, Glazer ES, Curley SA. (2010). Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev 62:339–45.
- Al-Ahmady ZS, Al-Jamal WT, Bossche JV, et al. (2012). Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 6:9335–46.
- Streffer C, Vaupel P, Hahn G. (1990). Biological basis of oncologic thermotherapy. Berlin, Heidelberg, New York, London, Paris, Tokyo, Hong Kong: Springer Verlag.
- Pettersen HS, Visnes T, Vågbø CB, et al. (2011). UNG-initiated base excision repair is the major repair route for 5-fluorouracil in DNA, but 5-fluorouracil cytotoxicity depends mainly on RNA incorporation. Nucleic Acids Res 39:8430–44.
- Longley DB, Harkin DP, Johnston PG. (2003). 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Rev Cancer 3:330–8.
- Takeda H, Haisa M, Naomoto Y, et al. (1999). Effect of 5-fluorouracil on cell cycle regulatory proteins in human colon cancer cell line. Jpn J Cancer Res 90:677–84.
- Johnston P, Kaye S. (2001). Capecitabine: a novel agent for the treatment of solid tumors. Anti-Cancer Drugs 12:639–46.
- Nair L, Jagadeeshan S, Nair SA, Kumar GV. (2011). Biological evaluation of 5-fluorouracil nanoparticles for cancer chemotherapy and its dependence on the carrier, PLGA. Int J Nanomed 6:1685.
- Shakeri-Zadeh A, Shiran MB, Khoee S, et al. (2014). A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumour, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl 29:548–56.
- Zhu L, Ma J, Jia N, et al. (2009). Chitosan-coated magnetic nanoparticles as carriers of 5-fluorouracil: preparation, characterisation and cytotoxicity studies. Colloids Surfaces B 68:1–6.
- Mahmoudi M, Laurent S, Shokrgozar MA, Hosseinkhani M. (2011). Toxicity evaluations of superparamagnetic iron oxide nanoparticles: cell “vision” versus physicochemical properties of nanoparticles. ACS Nano 5:7263–76.
- Danhier F, Ansorena E, Silva JM, et al. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–22.
- Petryk A, Giustini A, Ryan P, et al. (2009). Iron oxide nanoparticle hyperthermia and chemotherapy cancer treatment. Proc Soc Photo-Optical Instrument Eng 7181:71810N.
- Torres-Lugo M, Rinaldi C. (2013). Thermal potentiation of chemotherapy by magnetic nanoparticles. Nanomedicine (Lond) 8:1689–707.
- Friedrich J, Eder W, Castaneda J, et al. (2007). A reliable tool to determine cell viability in complex 3-d culture: the acid phosphatase assay. J Biomol Screen 12:925–37.
- Yuhas JM, Li AP, Martinez AO, Ladman AJ. (1977). A simplified method for production and growth of multicellular tumor spheroids. Cancer Res 37:3639–43.
- Ashjari M, Khoee S, Mahdavian AR. (2012). A multiple emulsion method for loading 5‐fluorouracil into a magnetite‐loaded nanocapsule: a physicochemical investigation. Polym Int 61:850–9.
- Khoee S, Yaghoobian M. (2009). An investigation into the role of surfactants in controlling particle size of polymeric nanocapsules containing penicillin-G in double emulsion. Eur J Med Chem 44:2392–9.
- Khoee S, Hassanzadeh S, Goliaie B. (2007). Effects of hydrophobic drug–polyesteric core interactions on drug loading and release properties of poly (ethylene glycol)–polyester–poly (ethylene glycol) triblock core–shell nanoparticles. Nanotechnology 18:175602.
- Hans M, Lowman A. (2002). Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci 6:319–27.
- Hajikarimi Z, Khoei S, Khoee S, Mahdavi SR. (2014). Evaluation of the cytotoxic effects of PLGA coated iron oxide nanoparticles as a carrier of 5-fluorouracil and mega-voltage X-ray radiation in DU145 prostate cancer cell line. IEEE Trans Nanobiosci 13:403–8.
- Dobrucki J, Bleehen N. (1985). Cell-cell contact affects cellular sensitivity to hyperthermia. Brit J Cancer 52:849.
- Maehara Y, Sakaguchi Y, Takahashi I, et al. (1992). 5-Fluorouracil's cytotoxicity is enhanced both in vitro and in vivo by concomitant treatment with hyperthermia and dipyridamole. Cancer Chemother Pharmacol 29:257–60.
- Kido Y, Kuwano H, Maehara Y, et al. (1991). Increased cytotoxicity of low-dose, long-duration exposure to 5-fluorouracil of V-79 cells with hyperthermia. Cancer Chemother Pharmacol 28:251–4.
- Davis ME. (2008). Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Rev Drug Discov 7:771–82.
- Abulateefeh SR, Spain SG, Thurecht KG, et al. (2013). Enhanced uptake of nanoparticle drug carriers via a thermoresponsive shell enhances cytotoxicity in a cancer cell line. Biomater Sci 1:434–42.
- Mohammadi S, Khoei S, Mahdavi SR. (2012). The combination effect of poly(lactic-co-glycolic acid) coated iron oxide nanoparticles as 5-fluorouracil carrier and X-ray on the level of DNA damages in the DU 145 human prostate carcinoma cell line. J Bionanosci 6:23–7.
- Sutar P, Joshi V. (2013). Preparation and characterisation of 5-fluorouracil loaded PLGA nanoparticles for colorectal cancer therapy. Unique J Pharmaceut Biol Sci 1:52–8.
- Li L, Mak K, Shi J, et al. (2012). Comparative in vitro cytotoxicity study on uncoated magnetic nanoparticles: effects on cell viability, cell morphology, and cellular uptake. J Nanosci Nanotechnol 12:9010–17.
- Tansık G, Yakar A, Gündüz U. (2014). Tailoring magnetic PLGA nanoparticles suitable for doxorubicin delivery. J Nanoparticle Res 16:1–13.
- Muthu M. (2009). Nanoparticles based on PLGA and its co-polymer: an overview. Asian J Pharmaceut 3:266–73.
- Schleich N, Sibret P, Danhier P, et al. (2013). Dual anticancer drug/superparamagnetic iron oxide-loaded PLGA-based nanoparticles for cancer therapy and magnetic resonance imaging. Int J Pharmaceut 447:94–101.
- Naik S, Carpenter EE. (2008). Poly (D,L-lactide-co-glycolide) microcomposite containing magnetic iron core nanoparticles as a drug carrier. J Appl Phys 103:07A313.
- Shakeri-Zadeh A, Khoei S, Khoee S, et al. (2015). Combination of ultrasound and newly synthesized magnetic nanocapsules affects the temperature profile of CT26 tumors in BALB/c mice. J Med Ultrason 42:9–16.
- Jordan A, Scholz R, Wust P, et al. (1999). Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J Magnet Magnetic Mater 194:185–96.
- Yang WJ, Lee JH, Hong SC, et al. (2013). Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 6:4689–706.
- Attaluri A, Kandala SK, Wabler M, et al. (2015). Magnetic nanoparticle hyperthermia enhances radiation therapy: a study in mouse models of human prostate cancer. Int J Hyperther 31:359–74.
- Shakeri-Zadeh A, Khoee S, Shiran MB, et al. (2015). Synergistic effects of magnetic drug targeting using a newly developed nanocapsule and tumour irradiation by ultrasound on CT26 tumours in BALB/c mice. J Mater Chem B 3:1879–87.