Abstract
Purpose: To test the accuracy and efficacy of the multimodality imaging-compatible insertion robot with a respiratory motion calibration module designed for ablation of liver tumors in phantom and animal models. To evaluate and compare the influences of intervention experience on robot-assisted and ultrasound-controlled ablation procedures.
Methods: Accuracy tests on rigid body/phantom model with a respiratory movement simulation device and microwave ablation tests on porcine liver tumor/rabbit liver cancer were performed with the robot we designed or with the traditional ultrasound-guidance by physicians with or without intervention experience.
Results: In the accuracy tests performed by the physicians without intervention experience, the insertion accuracy and efficiency of robot-assisted group was higher than those of ultrasound-guided group with statistically significant differences. In the microwave ablation tests performed by the physicians without intervention experience, better complete ablation rate was achieved when applying the robot. In the microwave ablation tests performed by the physicians with intervention experience, there was no statistically significant difference of the insertion number and total ablation time between the robot-assisted group and the ultrasound-controlled group. The evaluation by the NASA-TLX suggested that the robot-assisted insertion and microwave ablation process performed by physicians with or without experience were more comfortable.
Conclusion: The multimodality imaging-compatible insertion robot with a respiratory motion calibration module designed for ablation of liver tumors could increase the insertion accuracy and ablation efficacy, and minimize the influence of the physicians’ experience. The ablation procedure could be more comfortable with less stress with the application of the robot.
Introduction
Ablation is an effective method with minimal invasion for treating hepatocellular carcinoma (HCC). And, it is recommended by the AASLD, EASL, APASL and NCCN guidelines for HCC [Citation1–4]. The antenna insertion process and energy delivering are involved in the traditional liver ablation procedure. Computed tomography (CT), magnetic resonance imaging (MRI) or ultrasound (US) is generally applied as the guiding imaging. The insertion process lacks real-time monitoring when guided by CT or MRI. The US realized the real-time guiding with the possibility of some important part of liver not shown clearly. The ablation effects are generally evaluated by post-procedure imaging. Thus, the accuracy of aiming the target with the antenna point and the treating effects of ablation in the ablation procedure mostly depend on the experience of the physicians.
As the medical technology develops, robots designed for ablation of liver tumors could offer a technical solution to increase the insertion accuracy [Citation5–15] and ensure the ablation effects [Citation5,Citation9–11,Citation13,Citation16] without the influence of the experience of the physicians. The robots that were reported were single-imaging compatible, including two compatible with 3D ultrasound [Citation8,Citation10] and the others compatible with CT [Citation5,Citation7,Citation10,Citation11,Citation13,Citation15,Citation16] or MRI [Citation6]. Thus far, to our knowledge, the reported robots increased the insertion accuracy through positioning the antenna to the exact spatial coordinate according to the pre-procedure insertion plan made on the basis of pre-procedure images. But the pre-procedure plan made for the robots only considered the insertion pathways without ablation zone evaluation. The limitations of those robots were lacking of real-time monitoring and the pre- and post-procedure ablation zone evaluation. The results could also be influenced by the changes of patient’s position and respiratory movements without intra-procedure feedback to the physician. The multimodality imaging-compatible insertion robot designed by our team realized accomplishing a pre-procedure insertion and ablation plan and assisting the ablation procedure exactly according the plan with real-time multimodality imaging guiding. The real-time monitor with multimodality imaging including CT, US and 3D virtual models also provided a feedback function, including calibration feedback and insertion process feedback, to avoid injuries to the adjacent structures. A respiratory motion calibration module was also added in the software of the robot system to minimize the influence of the respiratory movements. The working principal of this module was to generate a respiratory motion calibration curve by calculating the relative spatial-coordinates changes of the three fiducial markers on the subject’s surface between the coordinates of the markers in the pre-procedure plan and those in the actual insertion process. When the value on the curve was close to zero, the insertion should be done immediately. The purpose of this study is to test the accuracy and efficacy of the robot we have designed in phantom and animal models.
Materials and methods
The hardware of the robotic system
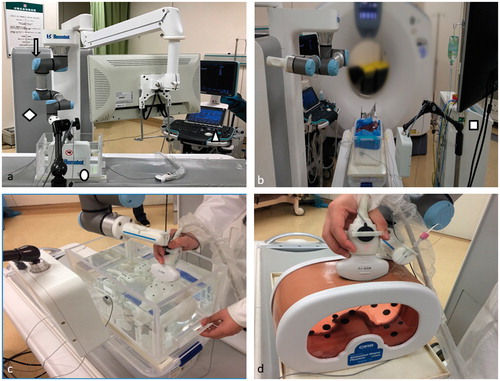
The hardware of the robotic system consisted of a robotic arm with six degree of free (DOF) (UR5/CB3; Universal Robots, Shanghai, China), ultrasonic unit (Resona 7; Minddray, Shenzhen, China), electromagnetic tracking unit (Aurora V3; NDI, Germany), ablation system (KY-2000; Kangyou, Nanjing, China), and the controller and display unit ()). The controller and display unit was assembled to the robotic arm as one mobile work platforms. The ultrasonic unit and the electromagnetic unit were connected to the controller for digital data collection. The ablation system was an independent unit with its own controlling part. The ablation system was connected to the robotic system through its antenna, which was held by the needle-holding device installed in the end of the robotic arm during the procedure.
The software of the robotic system
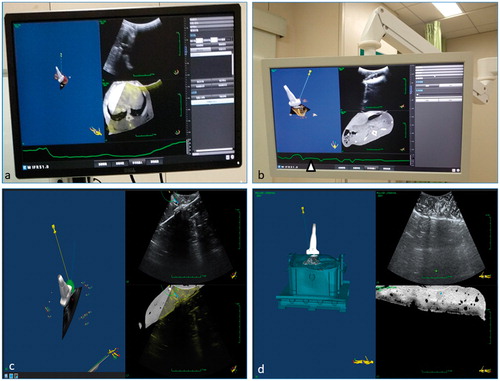
The software of the robotic system consisted of registration module, calibration module, multimodality imaging fusing module, navigation module, 3D visualization module, pre-procedure insertion–ablation plan making module, post-procedure ablation evaluation module, and respiratory motion calibration module. The real-time multimodality images including pre-procedure MR (or CT) images, US images, fusion images of MR (or CT) and US images and 3D virtual model of antenna and target were displayed on the monitor screens. The pre-procedure insertion–ablation plan and respiratory calibration curve was also displayed as the feedback for the physicians ().
Workflow of the robotic system
The workflow of robotic system included seven steps, which were registration, pre-procedure plan making, calibration, positioning, insertion, ablation and post-procedure evaluation. Firstly, the testing subject had the pre-procedure CT or MRI scanning with three electromagnetic markers sticking to its surface. The DICOM data was then imported to the controller of the robotic system. Then, the electromagnetic sensors were attached to the markers on the surface of the subject and the US probe. With the registration and calibration module, the robotic arm, the subject and the US probe were registered in one world coordinate. With the 3D visualization module, the DICOM data of the subject was transformed to a 3D virtual model, of which planes of any directions could be displayed. When the subject was scanned by the US probe, the 3D model with the probe and the real-time US image of the scanning plane within the 3D model were displayed on the screen with the navigation module (). At the meantime, the real-time US image, the CT (or MRI) image of the US scanning plane and the fusing image of US and CT (or MRI) image ()) were also displayed with the multimodality imaging fusing module. Secondly, the pre-procedure insertion and ablation plan was made by the physician with the plan making module on the 3D model and pre-procedure CT (or MRI) images with virtual antenna and ablation area (, the green area in the 3D model image). Thirdly, the physician manually scanned the subject with US probe to get the calibration feedback. Precise registration was confirmed in the fusing images by physician’s evaluation of that whether the automatically marking anatomical landmarks in US image coincided with those in CT (or MRI) image. If not, the registration and calibration process should be repeated until the precise registration was confirmed. The perfect fusion of real-time US image and the CT (or MRI) image of the US scanning plane ensured the physician the calibration is done. Fourthly, the physician gave orders to the robotic arm through the controller to position the antenna to the planning insertion path. Both the virtual planning pathway and the real-time position of the antenna were showed on the screen during the poisoning process with the navigation module. Fifthly, the respiratory calibration curve was generated according to the changes of the relative positions of the three fiducial markers on the subject’s surface with the respiratory motion calibration module. When the relative positions of the markers were in accordance with the data from pre-procedure CT images, the value of this curve was zero. When the antenna was positioned, the physician manually scanned the subject with US probe to accomplish real-time monitor. When the curve went to the lowest point, the physician held the tail part of the antenna, whose body part was held by the robotic arm, to manually insert the antenna to the target. The respiratory calibration curve could also be used as a biofeedback to help the patient retain the same breathing position in the insertion process. To avoid injuries to adjacent structures, the physicians should stop insertion when either the respiratory calibration curve went up or there were other important structures on the planning insertion pathway observed in the fusing images. The insertion process could be continued under the safe condition until the actual antenna totally overlapped the virtual antenna on the monitor screen. If the actual insertion pathway was deviated from the plan, the robotic-assisted insertion procedure should be restarted from the first step. Sixthly, physician controlled the ablation system to accomplish the ablation according pre-procedure plan. Seventhly, when ablation was finished, the subject had the CT (or MRI) scanning again. The DICOM data was imported to the controller. The original tumor and ablation zone were showed in one 3D model with the post-procedure ablation evaluation module to evaluate the efficacy of ablation procedure.
Accuracy tests with respiratory motion models
The accuracy tests were performed on a rigid body model and a phantom model placed on a moving device simulating respiratory movements (). There were nine target points with different locations in each of the model. Three electromagnetic markers were stuck to the different surfaces. The DICOM data was collected by CT scan of the model and imported to the controller. First, three physicians (A, B and C) with 5-year US-guided liver intervention experience and three physicians (D, E and F) with no US-guided liver intervention experience used the robot to insert a needle to the target points in each model. Two insertions were performed for each target point. Eighteen insertions in total were performed by one physician for either model. The insertion needle point with an electromagnetic sensor in it could be tracked by the navigation module. The distance and the angles (to the vertical, sagittal and horizontal planes) between the target point and the needle point was calculated with their spatial coordinates and recorded. The subjective assessment for working with the robot was evaluated by the NASA-Tolerance Load Index (TLX) method. The insertion time was also recorded ().
Table 1. Data and statistical results of the accuracy tests of rigid body model and phantom model.
One week after the accuracy tests with the robot, the same six physicians repeated the insertions by traditional method, which was with simple US guidance. Two insertions were performed for each target point. Eighteen insertions in total were performed by one physician for either model. The distances and the angles (to the vertical, sagittal and horizontal planes) between the target point and the needle point, the insertion time and the evaluation by the NASA-TLX method were recorded ().
Microwave ablation tests in porcine livers and rabbits
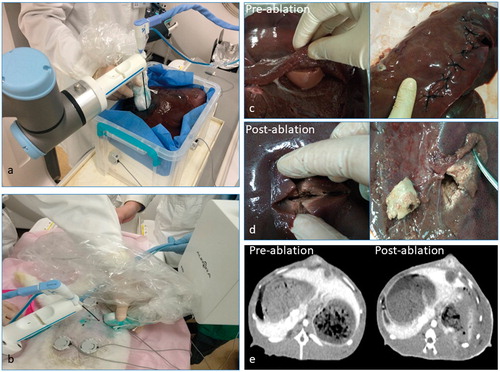
The microwave ablation tests were performed in porcine liver tumor models and rabbits VX2 liver cancer tumor model. Our study was approved by the animal care and use committee of our institution. The porcine liver tumor model was made of a whole porcine liver and several kidney tissue pieces (1 cm3). Several slits were cut in the porcine liver with the kidney tissue pieces in them (each piece for each slit). Then the slits were sewed up. The kidney tissue pieces were simulated the tumors (). Rabbit VX2 liver cancer tumor model was built with New Zealand white rabbit (two tumors in one rabbit). When the tumors grew to 1 × 1×1 cm, we applied the rabbits model for the tests ().
The porcine liver tumor model was placed in a box with three electromagnetic markers sticking on different surface. CT scan was made. Three physicians (A, B and C) with 5-year US-guided liver intervention experience and three physicians (D, E and F) with no US-guided liver intervention experience used the robot to perform ablation procedure for the simulated liver tumors. Ten tumors were ablated by each physician. After the ablation, the ablation area was cut up to evaluate the ablation effects (). The insertion number, total ablation time and the evaluation by the NASA-TLX method for each tumor were recorded. Completed ablation rate and technical success rate was calculated and recorded (). One week after the microwave ablation tests with the robot, the same six physicians repeated the ablation procedure by traditional method, which was with simple US guidance and ablation based on subjective evaluation. After the ablation, the ablation area was cut up to evaluate the ablation effects. The insertion number, total ablation time and the evaluation by the NASA-TLX method for each tumor were recorded. Completed ablation rate and technical success rate was calculated and recorded ().
Table 2. Data and statistical results of the microwave ablation tests of porcine liver and rabbit.
Rabbits with VX2 liver cancer were under anesthesia when the tests were performed. The rabbit was fixed in a supine position. Three electromagnetic markers (one on midsection, two on hypogastrium) were stuck on the ventral surface with hair shaved. Contrast-enhanced CT scan was made by contrast agent induction through auricular vein. Three physicians (A, B and C) with 5-year US-guided liver intervention experience and three physicians (D, E and F) with no US-guided liver intervention experience used the robot to perform ablation procedure for the Rabbits with VX2 liver cancer. Ten tumors were ablated by each physician. After the ablation, contrast-enhanced CT scan was made again. The DICOM data of post-procedure contrast-enhanced CT was imported to the controller to accomplish the post-procedure ablation evaluation (). The insertion number, total ablation time and the evaluation by the NASA-TLX method for each tumor were recorded. Completed ablation rate and technical success rate was calculated and recorded (). One week after the microwave ablation tests with the robot, the same six physicians repeated the ablation procedure by traditional method, which was with simple US guidance and ablation based on subjective evaluation. After the ablation, contrast-enhanced CT scan was made again to get the ablation evaluation by the physicians. The insertion number, total ablation time and the evaluation by the NASA-TLX method for each tumor were recorded. Completed ablation rate and technical success rate was calculated and recorded ().
Data evaluation
For the accuracy tests with respiratory motion models, analysis of variance was used to compare the difference of normally distributed data, including the distances and the angles (to the vertical, sagittal and horizontal planes) from the target point to the needle point. Wilcoxon rank-sum test was used to compare the difference of the data not normally distributed, including the evaluation by the NASA-TLX method. The difference was considered statistically significant with a p values <.05.
For microwave ablation tests in porcine livers and rabbits, Wilcoxon rank sum test was used to compare the difference of the data not normally distributed, including insertion number, total ablation time and the evaluation by the NASA-TLX method for each tumor. The difference was considered statistically significant with a p values <.05.
Results
Accuracy tests
When the robot was applied (robot group), there was no statistically significant difference of the recorded data between 5-year experience physicians and physicians with no experience. When the traditional method was applied (traditional group), there were statistically significant differences of the recorded data between 5-year experience physicians and physicians with no experience (). For the 5-year experience physicians, there was no statistically significant difference of the distances and the angles (from the target point to the needle point) and the insertion time between the robot group and the traditional group. For the physicians with no experience, there was statistically significant difference of the distances and the angles (from the target point to the needle point) and the insertion time between the robot group and the traditional group, suggesting that the robot supported group was better in accuracy and insertion time. The evaluation by the NASA-TLX method was statistically significant different in both 5-year experience physicians and physicians with no experience, suggesting that the insertion process was more comfortable with less stress when using the robot ().
Microwave ablation tests
The completed ablation rates and technical success rates of different groups were shown in , suggesting that the robot could increase the ablation efficacy and minimize the influences of the physicians’ experience. When the robot was applied (robot group), there was no statistically significant difference of the recorded data between 5-year experience physicians and physicians with no experience in the microwave ablation tests. When the traditional method was applied (traditional group), there were statistically significant differences of the recorded data between 5-year experience physicians and physicians with no experience (). For the 5-year experience physicians, there was no statistically significant difference of the insertion number and total ablation time between the robot group and the traditional group. For the physicians with no experience, there were statistically significant differences of the insertion number and total ablation time between the robot group and the traditional group. There was statistically significant difference of the evaluation by the NASA-TLX method in both 5-year experience physicians and physicians with no experience between the robot group and the traditional group, suggesting that the ablation procedure was more comfortable with less stress when using the robot ().
Discussion
In our study, we established the proof of principle of a multimodality imaging-compatible insertion robot with a respiratory motion calibration module designed for ablation of liver tumors. The real-time multimodality imaging and a respiratory motion calibration module could provide the physicians with feedback of the safety and accuracy when applying the insertion robotic system. The accuracy tests and microwave ablation tests were performed with standard workflow by both physicians with 5-year intervention experience and physicians with no intervention experience. The results of our study suggested that the robot designed by our team could increase the insertion accuracy and ablation efficacy, and minimize the influences of the physicians’ experience on ablation procedure. The ablation procedure could be more comfortable with less stress with the application of the robot.
Currently, the reported robotic system designed for liver intervention were compatible with simple one imaging technologies. The researches of the robotic system were mostly focused on testing the insertion accuracy on static rigid models, phantoms or liver tissues with a small sample and lack of a control group. For now, there were no researches of a robotic system applied for ablation in animal liver cancer models. The robot in our study was with real-time multimodality imaging-compatible and a respiratory motion calibration module, with which the physician could evaluate the influence of the respiratory movement and the adjacent important structures to avoid unnecessary injuries. With the feedback function of the insertion robot designed by our team mentioned above, the results of the accuracy and ablation tests with a large sample on a rigid body model and a phantom model placed on a moving device simulating respiratory movements in our study were consistent with the those of the researches of other teams [Citation10,Citation13,Citation14,Citation16–18]. And we first evaluated the influences of the physicians’ experience on ablation procedure and use NASA-TLX method to describe the subjective attitude to the procedure of the physicians both with and without the robot’s assistant in our study.
The limitations of the robot of our team included three aspects. First, the electromagnetic tracking system was easily interfered with medical tools and bed. Second, the calibration error caused by organ deformation and displacement was not corrected. And in the process of the procedure, the physician should be aware of the patient skin deformation caused by pressing the US probe onto the skin to have maximized the coupling for the ultrasound waves, which was inevitably produced with US imaging. Organ deformation and displacement and skin deformation meant that the images taken from the CT (or MRI scan) were not perfectly the same as the one seen in the US image. Third, the prototype of the robotic system occupied too much space. Improvements both for the hardware and software of the insertion robot in our study should be done. Considering the more precise pre-procedure plan, the more accurate and stable of needle insertion process and the better curative effect, the insertion robot designed for liver ablation will play an important role assisting the physicians in clinical medicine.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022.
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474.
- Benson AB III, D’Angelica MI, Abbott DE, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15:563–573.
- Beyer LP, Pregler B, Niessen C, et al. Robot-assisted microwave thermoablation of liver tumors: a single-center experience. Int J Cars. 2016;11:253–259.
- Franco E, Ristic M, Rea M, et al. Robot-assistant for MRI-guided liver ablation: a pilot study. Med Phys. 2016;43:5347.
- Patriciu A, Awad M, Solomon SB, et al. Robotic assisted radio-frequency ablation of liver tumors-randomized patient study. Med Image Comput Comput Assist Interv. 2005;8(Pt 2):526–533.
- Boctor EM, Choti MA, Burdette EC, et al. Three-dimensional ultrasound-guided robotic needle placement: an experimental evaluation. Int J Med Robot. 2008;4:180–191.
- Mundeleer L, Wikler D, Leloup T, et al. Computer-assisted needle positioning for liver tumour radiofrequency ablation (RFA). Int J Med Robotics Comput Assist Surg. 2009;5:458–464.
- Xu J, Jia ZZ, Song ZJ, et al. Three-dimensional ultrasound image-guided robotic system for accurate microwave coagulation of malignant liver tumours. Int J Med Robotics Comput Assist Surg. 2010;6:256–268.
- Abdullah BJ, Yeong CH, Goh KL, et al. Robot-assisted radiofrequency ablation of primary and secondary liver tumours: early experience. Eur Radiol. 2014;24:79–85.
- Abdullah BJ, Yeong CH, Goh KL, et al. Robotic-assisted thermal ablation of liver tumours. Eur Radiol. 2015;25:246–257.
- Mbalisike EC, Vogl TJ, Zangos S, et al. Image-guided microwave thermoablation of hepatic tumours using novel robotic guidance: an early experience. Eur Radiol. 2015;25:454–462.
- Wang W, Shi Y, Goldenberg AA, et al. Experimental analysis of robot-assisted needle insertion into porcine liver. BME. 2015;26(Suppl. 1):S375–S380.
- Beyer LP, Pregler B, Michalik K, et al. Evaluation of a robotic system for irreversible electroporation (IRE) of malignant liver tumors: initial results. Int J Cars. 2017;12:803–809.
- Solomon SB, Patriciu A, Stoianovici DS. Tumor ablation treatment planning coupled to robotic implementation: a feasibility study. J Vasc Interv Radiol. 2006;17:903–907.
- Wallach D, Toporek G, Weber S, et al. Comparison of freehand-navigated and aiming device-navigated targeting of liver lesions. Int J Med Robotics Comput Assist Surg. 2014;10:35–43.
- Crocetti L, Lencioni R, Debeni S, et al. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol. 2008;43:33–39.