Abstract
Objective: To evaluate the correlation between the gestational sac size and the effect and safety of high intensity focused ultrasound (HIFU) combined with ultrasound-guided suction curettage for caesarean scar pregnancy (CSP).
Methods: Seventy-six patients with CSP were enrolled. Based on their gestational sac size, patients were divided into three groups: Group 1 (n = 16, 10–20 mm), Group 2 (n = 28, 21–30 mm) and Group 3 (n = 32, >30 mm). All of them were treated by HIFU combined with ultrasound-guided suction curettage. Baseline characteristics, parameters and adverse events of HIFU, and intraoperative hemorrhage during ultrasound-guided suction curettage were recorded.
Results: The median treatment time and average treatment intensity of HIFU in Group 3 were significantly higher than Group 1 (p < .05); the median HIFU treatment power in Group 2 and Group 3 were both significantly higher than that of Group 1 (p < .05). The median sonication time of HIFU in Group 3 was significantly longer than patients in the other two groups (p < .05). The size of the gestational sac had a positive correlation with all the above-mentioned parameters of HIFU and blood loss during ultrasound-guided suction curettage (p < .05). No statistically significant differences were observed among the three groups in the duration of vaginal bleeding post-curettage and the time necessary for serum β-hCG to return to normal levels (p > .05).
Conclusions: HIFU combined with ultrasound-guided suction curettage is a safe and effective clinical approach for CSP. Gestational sac size is a meaningful factor for predicting the efficacy and safety of HIFU treatment and hemorrhage during ultrasound-guided suction curettage.
Introduction
Caesarean scar pregnancy (CSP), first reported by Larsen and Solomon in 1978, is a rare ectopic pregnancy where in the fertilized egg or the gestational sac is implanted at the scar site of the previous caesarean section (CS) [Citation1,Citation2]. The incidence of CSP is estimated between 1:1800 to 1:2216 pregnancies, which is accounting for 6.1% of ectopic pregnancy [Citation3,Citation4]. With the increased rate of CS worldwide, reports of CSP have rapidly risen in recent decades. Patients with CSP may have risks of life-threatening massive hemorrhage, hypovolemic shock, uterine rupture, disseminated intravascular coagulation and even maternal death [Citation4]. Therefore, pregnancy termination is generally recommended once the diagnosis of CSP is confirmed. However, to date, due to the rarity of this condition and the paucity of clinical data, there is no universal therapeutic guideline for the treatment of CSP. Currently, the clinical managements for CSP include: traditional dilation and curettage (D&C) alone, systemic or local methotrexate (MTX) administration, uterine artery embolisation (UAE) alone or combined with D&C, laparoscopic excision, hysteroscopic resection and hysterectomy [Citation5–7]. Traditional D&C alone is dangerous since it may cause uncontrollable massive hemorrhage or even lead to an inevitable conversion to hysterectomy. Although conservative medication with MTX can preserve patient fertility with low cost, the failure rate is more than 50% and it takes a long period for the absorption of the embryo sac. UAE alone or combined with D&C has been reported effective with high success rates in several studies, but the complication rate of UAE is also high (about 47%), in which fever, nausea and low abdominal or limb pain arecommon [Citation8]. In addition, the long-term premature ovarian failure complication caused by UAE hinders its application in CSP patients, most of whom are young. Excision through laparoscopy or operative hysteroscopy can directly remove the lesion and reduce recurrence, but it may cause post-operative tissue adhesion and affect the patient’s fertility. The effectiveness of hysterectomy for CSP is indubitable, but it is not suitable for patients of reproductive age who want to keep their fertility and preserve their uterus. Thus, hysterectomy nowadays is not commonly used in clinical practice for CSP and is only performed in critical situations, such as uncontrollable aggressive blood loss and uterine ruptures.
In the past two decades, high-intensity focused ultrasound (HIFU) ablation, as a novel non-invasive therapy, has been increasingly used in the treatment of many solid tumours and benign diseases, especially gynecological diseases [Citation9]. Studies have shown that HIFU is safe and effective in treating uterine fibroids and adenomyosis [Citation10,Citation11]. In 2014, Huang et al. first reported their preliminary study about the application of HIFU combined with D&C on four patients with CSP [Citation12]. Xiao’s group found that after ultrasound-guided HIFU ablation for 16 patients with CSP, the serum beta human chorionic gonadotropin (β-hCG) gradually returned back to normal level within 10 weeks and the CSP mass disappeared in 14 weeks [Citation13]. Another study has reported that when compared with UAE, HIFU treatment for CSP has a lower rate of treatment-related adverse events [Citation14]. These preliminary results have shown that HIFU ablation in treating CSP is feasible and effective.However, the sample size of some studies was relatively small and the protocol they used varied. Furthermore, the question of whether gestational sac size canserve as a meaningful predictor of the effect and safety of HIFU treatment had not been answered yet. Therefore, in this study, we are trying to figure out the correlation between the size of gestational sac and the effect and safety of HIFU treatment combined with ultrasound-guided suction curettage by retrospectively analysing the results of patients with CSP using this combined therapy in our hospital, aimed to provide an optimized protocol of HIFU treatment for CSP.
Materials and methods
This research was approved by the ethics committee at Suining central hospital. A written informed consent was obtained from every patient before each treatment procedure.
Patients
From January 2015 to January 2017, 76 patients diagnosed with CSP and treated with HIFU followed by ultrasound-guided suction curettage in Suining central hospital were retrospectively analyzed. The diagnosis of CSP was based on: (1) history of amenorrhoea; (2) increased serum β-hCG level; (3) prior history of CS delivery; (4) transvaginal or transabdominal ultrasound and pelvic magnetic diagnostic imaging (MRI) (. Sonographic and colour Doppler flow findings were in accordance with CSP diagnostic criteria postulated by Godin et al. [Citation15]. All the sonographic and MRI results were comfirmed by two experienced gynecological radiologists.
Figure 1. Ultrasound image and MRI obtained from a patient with CSP. A, The transabdominal ultrasound image showed a gestational sac (white arrow) embedding at the scar site of the previous CS with empty uterus cavity and cervical canal. Color Doppler reveals a rich blood supply surrounding gestational sac. B, A saggittal view MR image showed a gestational sac (white arrow) embedding at the site of the previous CS scar.
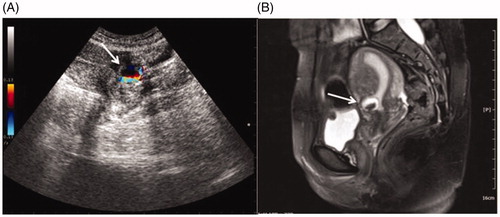
Based on the size of gestational sac embedded in the scar area, 76 CSP patients were divided into three groups: in Group 1 (n = 16), the gestational sac diameter was between 10 and 20 mm; in Group 2 (n = 28), the gestational sac diameter was between 21 to 30 mm; in Group 3 (n = 32), the gestational sac diameter was larger than 30 mm. The patient information of each group, including age, body mass index (BMI), symptoms, menolipsis days, gravidity, parity, interval since last CS, pre-treatment serum β-hCG level and uterine scar thickness, was collected from the medical records.
Pre-HIFU treatment preparation
Strict three-day diet preparation before HIFU treatment was required for bowel safety. Patients were asked to start the diet preparation with a bland diet (no fibre and no milk) 3 days prior to HIFU treatment, followed by semi-liquid and liquid food in the next two days, respectively. At least 12-h fasting was required the night before treatment day, followed by 1–3 times of enema performed on the morning of treatment day. Shaving the lower abdominal area, from the umbilicus to the upper margin of the pubic symphysis, was performed. In addition, degreasing and degassing were perfomed using 75% ethanol and a vacuum device prior to HIFU treatment. Catheterization was also performed to control bladder size and help push the bowel away from the acoustic pathway for bowel safety.
Ultrasound-guided HIFU ablation
HIFU ablation was performed using either the HIFU JC or JC200 system (Haifu Technology Co. Ltd, Chongqing, China). HIFU energy was generated by a transducer with the frequency of 1.0 MHz and the size of 20 cm in diameter, which was located in a water reservoir filled with cold degassed water. The focal region was 8 × 3 × 3 mm3 and the focal length was 15 cm. A B mode probe (MyLab 70, Esaote, Genova, Italy) was integrated in the center of the transducer to provide real-time sonographic monitoring during HIFU ablation. Movement of the transducer was achieved with a computer-controlled mechanical positioning device.
The procedure of HIFU ablation was conducted under conscious sedation with fentanyl (1 μg/kg) and midazolam hydrochloride (20 μg/kg). Each patient was positioned prone on the HIFU system, with the abdominal wall soaked in degassed water over the transducer in the sealed reservoir. During treatment, the focus was placed close to the embedding area of the gestational sac in the center slice at the beginning of the HIFU ablation procedure. Sonication power ranged from 300 to 400 W. Termination of HIFU ablation for CSP occurred when: (1) obvious grey scale change in the target area was observed through a real-time US-monitor; (2) blood flow signal of gestational sac embedding area disappeared or was significantly reduced in the colour Doppler. Contrast-enhanced micro-bubble agent (SonoVue, Bracco, Milan, Italy) was used 10 min pre- and immediately post-HIFU treatment to evaluate the ablation area and changes of blood supply around the gestational sac. The therapeutic effect was confirmed by contrast-enhanced ultrasound post-HIFU: (1) a non-perfused volume was observed in gestational sac embedding area; (2) blood supply around the gestational sac, especially the embedding area, disappeared or significantly reduced ().
Figure 2. Contrast-enhanced ultrasound obtained from a patient treated with HIFU. A, abundant blood supply in myometrium of CSP scar (white arrow) around gestational sac before HIFU ablation; B, significantly reduced blood supply in myometrium of CSP scar (white arrow) around gestational sac after HIFU ablation.
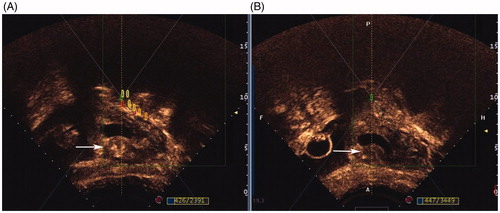
Ultrasound-guided suction curettage
Ultrasound-guided suction curettage under general anaesthesia was performed on each patient 3 days after HIFU ablation. Patients were placed in the lithotomy position. Firstly, transabdominal ultrasound (DC-60, Mindray Medical Co. Ltd, Shenzhen, China) was used to determine uterine morphology and gestational sac location. The depth of the uterus was measured before suction curettage. During the procedure, a 6.5 mm suction cannula with a vacuum pressure of 40 Pa was placed in uterine isthmus. The cannula was carefully moved around the gestational sac embedding area to detach the ablated pregnancy tissues. Then, a curette was used to gently scrape the residue. If active uterine bleeding occurred after curettage, oxytocin of 5 units in 1 mL saline was injected into the cervix at the 12 o’clock position. If necessary, antibiotics and hemostats were used after the procedure.
Adverse events observation and follow-up
Incidences of any discomfort or adverse events during HIFU ablation, including sciatic/buttock pain, lower abdominal pain, leg pain and skin burn, were recorded. Seven days after ultrasound-guided suction curettage, the post-curettage serum β-hCG was measured and then patients were discharged from hospital. The serum β-hCG was monitored weekly until it returned to normal levels at gynecology and obstetrics clinic follow-ups. The duration of vaginal bleeding post-curettage was also followed-up and recorded.
Statistical analysis
SPSS software (SPSS 22.0, IBMCompany, Chicago, IL) was used for statistical analysis. For quantitative data, the Kolmogorov-Smirnov test was first used to obtain the probability distribution of each group. If a normal distribution is fitted, the mean ± SD was reported. Variance analysis was used for inter-group analysis. If a non-normal distribution was fitted, the median and inter-quartile range (P25-P75) were reported. The Mann-Whitney U test or Kruskal Wallis test was used for inter-group analysis. For categorical data, a constituent ratio was reported, and the Chi square test was used for inter-group analysis. Spearson rank analysis was used for correlation analysis. A p values< .05 indicated a significant difference.
Results
Baseline characteristics of CSP patients
As shown in , the average age of CSP patients in Group 1, Group 2 and Group 3 was 30.81 ± 5.31 years, 31.68 ± 5.01 years and 31.78 ± 4.28 years, respectively. The mean BMI was 21.62 ± 3.27 kg/m2 in Group 1, 22.68 ± 2.78 kg/m2 in Group 2 and 21.97 ± 3.47 kg/m2 in Group 3. Among these patients, 18.75% in Group 1, 53.57% in Group 2 and 21.88% in Group 3 were asymptomatic. For the rest of them, 3.57% in Group 2 complained of abdominal pain; 81.25% in Group 1, 32.14% in Group 2 and 68.75% in Group 3 complained of painless vaginal bleeding; and 10.71% in Group 2 and 9.38% in Group 3 complained of both symptoms. The median duration of amenorrhoea in three groups were 40.5, 42.0 and 48.5 days, respectively. The median pre-treatment serum β-hCGs were 18994.95mIU/mL in Group 1, 32226.8 mIU/mL in Group 2and 42248.6 mIU/mL in Group 3. The median thickness of the gestational sac embedding myometrium in each group was 4.8, 4.2 and 4.1 mm, respectively. The median interval from last CS to CSP was 7.5 years in Group 1, 5.0 years in Group 2 and 3.5 years in Group 3. Gravidity, parity and times of previous caesarean pregnancy are also listed in . We found that except days of amenorrhoea (p = .034), there was no significant difference in the other baseline characteristics among the three groups (p > .05).
Table 1. Comparison of baseline characteristics of CSP patients in three groups.
HIFU ablation results and safety evaluation
All patients received only one session of HIFU treatment. The median treatment power used was 390 W in Group 1, 399 W in Group 2 and 400 W in Group 3 (). Compared with Group 1, the power used in Group 2 and 3 was significantly higher (p < .05). The median treatment time of these three groups were 39.5, 48.5 and 65 min, respectively. Inter-group analysis showed that treatment time used in Group 3 was significantly longer than in Group1 (p < .05). The median sonication time was 556 s in Group 3, which was remarkably longer than the other two groups (290 s in Group 1 and 400 s in Group 2) (p < .05). The average treatment intensity was 449.55 ± 121.34 s/h in Group 1, 474.21 ± 109.78 s/h in Group 2 and 540.17 ± 112.82 s/h in Group 3. As shown in , the significant difference of average treatment intensity between Group 3 and Group 1 was observed (p < .05).
Table 2. Comparison of HIFU treatment results for CSP patients in three groups.
The common adverse events during HIFU were sciatic/buttock pain and treatment area pain (). No significant differences were observed between the three groups in the above-mentioned adverse effects (p > .05). One patient (3.6%) in Group 2 vomited during HIFU. In this study, neither leg pain nor skin burn were observed as HIFU-related adverse events.
Table 3. Adverse events during HIFU treatment in three groups.
Ultrasound-guided suction curettage results and follow-up
showed us that the median blood loss in ultrasound-guided suction curettage procedure was 20 mL in Group 1, 20 mL in Group 2 and 30 mL in Group 3. The blood loss in Group 3 was significantly higher than that in Group 1 and Group 2 (p < .05). One patient in Group 3 had massive vaginal bleeding (500 mL) during curettage, the procedure was terminated, and then compression with a balloon was used and a second curettage was conducted successfully 5 days laterwith blood loss of 20 mL. In the follow-up period, another patient in group 3 had massive vaginal bleeding (about 500 mL) 1 month after she was discharged from hospital. She then received a transabdominal lesionectomy. Except these two patients, all the other patients in our study did not have severe complications during the procedure or follow-up.
Table 4. Comparison of USg-suction curettage results and follow-up in three groups.
We also found that no significant difference in median duration of vaginal bleeding post-curettage (p > .05) was observed between groups (6.0 d in Group 1, 5.5 d in Group 2 and 7.0 d in Group 3). Median serum β-hCG 1 week post-curettage was 826.20 mIU/mL in Group 1, 1497.05 mIU/mL in Group 2 and 2249.10mIU/mL in Group 3. There was no statistical difference among the groups (p > .05). The average time for serum β-hCGto return to nomal levels in these three groups was 28.6 ± 5.6 d, 31.4 ± 7.8 d and 33.6 ± 10.8 d, respectively. There was also no statistical difference observed among the groups (p > .05).
Correlation analysis
To further explore whether gestational sac size has an impact on HIFU treatment and ultrasound-guided suction curettage, we pooled patient data of three groups together and carried out a correlation analysis to find relationships between size of gestational sac, parameters of HIFU treatment and ultrasound-guided suction curettage. As shown in , we found that the gestational sac size had a correlation with HIFU treatment power, treatment time, sonication time, treatment intensity and blood loss during ultrasound-guided suction curettage. The R values were 0.452, 0.312, 0.385, 0.337 and 0.419, respectively (p < .05).
Table 5. Correlation between the size of gestational sac (mm) and parameters of HIFU treatment and USg-suction curettage (n = 76).
Discussion
In recent years, the application of HIFU on the management of CSP has been reported in several preliminary studies. During HIFU ablation, the ultrasound beams generated from the transducer can penetrate through abdominal skin, subcutaneous tissue and the bladder, then focused at the targeted tissue around the CSP lesion. When temperature at the target increases to over 60 °C, coagulate necrosis occurs. The cavitation effect of HIFU may also help increase the temperature of the targeted tissue and loosen the adhesion of the gestational sac to the myometrium at the uterine scar [Citation16]. It also has been reported that HIFU can destroy small blood vessels with a diameter smaller than 2 mm [Citation17]. Huang et al. reported four patients with CSP treated by HIFU combined with D&C. Among them, three patients were successfully treated with HIFU combined with D&C. In the same study, another patient with a 52 mm largest gestational sac diameter initially underwent a failed blind D&C, HIFU was then performed as second remedy. However, a laparotomy was conducted 16 days after HIFU due to persistent viginal bleeding [Citation12]. In another study, Zhu et al. performed HIFU combined with suction curettage under hysteroscopic guidance to treat patients with CSP and found that HIFU combined with suction curettage under hysteroscopic guidance had fewer adverse effects compared with those in UAE [Citation14]. However, they also found 4 out of 76 patients in the HIFU group that had excessive vaginal bleeding during D&C, two of whom had 54 and 60 mm in the largest diameter of CSP lesions. The cause of heavy bleeding during D&C may have been due to insufficient sonication energy relative to the larger gestational sac size. A study in 2017 hypothesized that having a menolipsis time longer than 51 days and a gestational sac larger than 27 mm were risk factors for CSP patients with intraoperative hemorrhage [Citation18]. Furthermore, in another study there was no serious adverse effects found, including heavy hemorrhaging, in which the HIFU treatment group had a small average gestational sac/mass diameter of 25.03 ± 8.75 mm [Citation19].
In this study, we found that the median treatment time and average treatment intensity of HIFU for patients with a gestational size larger than 30 mm were significantly higher than those of patients with gestational size of 10–20 mm (p < .05); and median treatment power of HIFU for patients with gestational size of 21–30 mm and larger than 30 mm were both significantly higher than patients with gestational size of 10–20 mm (p < .05). The median sonication time of HIFU for patients with a gestational size larger than 30 mm was significantly longer than patients in the other two groups (p < .05). Further correlation analysis () demonstrated that the size of the gestational sac had a positive correlation with all the above-mentioned parameters of HIFU. As the size of the gestational sac increases, the range of implantation at the scar area becomes larger and the chance of placental maturation is higher. Furthermore, higher peritrophoblastic vascularisation and more perforator vessels connecting the lesion and uterus are formed. During HIFU treatment, fast blood flow may easily take heat away, affecting energy depositionin the focal area, which requires a longer sonication time and treatment time, and a higher treatment power and intensity to cause coagulative necrosis in a gestational sac with a larger size. Therefore, we concluded that gestational size could be a meaningful predictor of HIFU therapeutic effect.
In regards to perform ultrasound-guided suction curettage following HIFU, since HIFU can cause reduction of blood flow in the trophoblast tissue, the risk of heavy hemorrhaging decreased during the suction curettage procedure under the guidance of ultrasound. In this study, the median volume of blood loss in patients with gestational size larger than 30 mm was more than that of patients in the other two groups (, p < .05). As we mentioned before, 2 patients had excessive hemorrhage (500 mL) in this group, while the other patients had intraoperative hemorrhaging within 50 mL. A positive correlation between gestational size and blood loss during ultrasound-guided suction curettage was found, which indicates that gestational size may be a risk factor for CSP patients with an intraoperative hemorrhage. Our result was consistent with previous reports [Citation18]. A meta-analysis of 31 different treatment regimens for CSP conducted on 2012 found that the highest success rate was 90.1% [Citation20]. In this study, the success rate of HIFU combined with ultrasound-guided suction curettage as a first regime was 97.4% (74/76), indicating this combined therapy was effective for CSP.
We also evaluated the safety of this combined therapy and the prognosis of patients with CSP. Sciatic/buttock pain and treatment area pain were the two most common responses of CSP patients during HIFU ablation, and all of these feelings were relieved within 3–7 daysafter treatment. No additional treatment was necessary. During follow-up, serum β-hCGof all the patients in the three groups returned to normal levels in ∼30 days, which was similar to previous reports [Citation13,Citation14,Citation19]. Although uterine perforation at a previous caesarean scar during D&C procedure has been reported in other studies[Citation14], this complication didn’t occur in our study.
This study also had some limitations. First, the sample size was relatively small because of strict inclusion criteria and data from a single center. Secondly, as a retrospective study, several unexpected factors may affect the results, especially influence the result of correlation analysis. Thus, further prospective studies in multicenter will be necessary to validate our findings.
Conclusions
In conclusion, although guidelines for CSP have yet to be established, combined therapy of HIFU and ultrasound-guided suction curettage presented a safe and efficient clinical approach for CSP. According to the size of the gestational sac, different ultrasound energy is required for HIFU treatment. Especially for CSP with a gestational sac larger than 30 mm, to reduce the risk of hemorrhage during ultasound-guided suction curettage, it is strongly recommended to give higher sonication power and longer sonication time for HIFU treatment to generate coagulation necrosis of trophoblastic tissue and reduce the risk of massive bleeding during the subsequent ultrasound-guided suction curettage procedure.
Disclosure statement
Jin Bai and Lian Zhang are senior consultants to Chongqing Haifu company. The other authors have no potential conflict of interest to report.
Additional information
Funding
References
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus-an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978;53:142–143.
- Zhai JF, Xu M, Zhang B, et al. Treatments of caesarean scar pregnancy and the corresponding results in ten years. Eur Rev Med Pharmacol Sci. 2015;19:2523–2527.
- Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114:253–263.
- Litwicka K, Greco E. Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol. 2013;25:456–461.
- Yin XH, Yang SZ, Wang ZQ, et al. Injection of MTX for the treatment of cesarean scar pregnancy: comparison between different methods. Int J Clin Exp Med. 2014;7:1867–1872.
- Shu SR, Luo X, Wang ZX, et al. Cesarean scar pregnancy treated by curettage and aspiration guided by laparoscopy. Ther Clin Risk Manag. 2015;11:1139–1141.
- Uysal F, Uysal A, Adam G. Cesarean scar pregnancy: diagnosis, management, and follow-up. J Ultrasound Med. 2013;32:1295–1300.
- Hois EL, Hibbeln JF, Alonzo MJ, et al. Ectopic pregnancy in a cesarean section scar treated with intramuscular methotrexate and bilateral uterine artery embolization. J Clin Ultrasound. 2008;36:123–127.
- Orsi F, Arnone P, Chen W, et al. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Can Res Ther. 2010;6:414–420.
- Xie B, Zhang C, Xiong C, et al. High intensity focused ultrasound ablation for submucosal fibroids: A comparison between type I and type II. Int J Hyperthermia. 2015;31:593–599.
- Zhang X, Li K, Xie B, et al. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124:207–211.
- Huang L, Du Y, Zhao C. High-intensity focused ultrasound combined with dilatation and curettage for Cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2014;43:98–101.
- Xiao J, Zhang S, Wang F, et al. Cesarean scar pregnancy: noninvasive and effective treatment with high-intensity focused ultrasound. Am J Obstet Gynecol. 2014;211:356.e1–357.
- Zhu X, Deng X, Xiao S, et al. A comparison of high-intensity focused ultrasound and uterine artery embolisation for the management of caesarean scar pregnancy. Int J Hyperthermia. 2016;32:144–150.
- Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997;67:398–400.
- Copelan A, Hartman J, Chehab M, et al. High-Intensity focused ultrasound: current status for image-guided therapy. Semin Intervent Radiol. 2015;32:398–415.
- Wu F, Chen WZ, Bai J, et al. Tumor vessel destruction resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med Biol. 2002;28:535–542.
- Ma Y, Shao M, Shao X. Analysis of risk factors for intraoperative hemorrhage of cesarean scar pregnancy. Medicine (Baltimore). 2017;96:e7327.
- Xiao J, Shi Z, Zhou J, et al. Cesarean scar pregnancy: comparing the efficacy and tolerability of treatment with high-intensity focused ultrasound and uterine artery embolization. Ultrasound Med Biol. 2017;43:640–647.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207:14–29.
