?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Despite an understanding that a major effect of cold exposure is a fall in core body temperature which is responsible for the observed decrements in the performance, it is surprising that thermogenic supplements are seldom evaluated to verify if they can aid in improving the performance during cold exposure. Following evidence from our previous study indicating the ability of pepper and cinnamon to improve cold endurance, we investigated further here if the improved endurance had advantages in real time where they could positively affect cognitive performance (assessed by Novel object test) when exposed to cold in albino wistar rats. In order to delineate if the observed improvement if any, was due to their cognitive enhancing ability or thermogenic potential, distinctive room temperature (RT) and cold temperature (CT) groups were used. Cold exposure impaired cognitive performance which improved following treatment with both the spices. We noted an increased rate of cold adaptive thermogenesis in CT treated group as evidenced by an elevated norepinephrine, free fatty acid levels in blood, increased expression of UCP1 in brown adipose tissue, the net effect being a decreased fall in the core body temperature. Absence of any notable effect in these parameters in the RT treated group ascertained that at least in the current experimental set up the observed improvement in performance in CT treated group is due to the thermogenic potential of the spices alone. In conclusion, our results demonstrate that the cognitive impairment caused by exposure to cold can be effectively countered by agents with thermogenic potential.
1. Introduction
The biochemical reactions governing life operate in a very narrow temperature range. Any deviation from the normal temperature homeostasis causes major changes in physiology compromising the vital functions. The impairments caused by exposure to cold temperature are well reported [Citation1]. For military personnel, wilderness survivors, workers at cold storage etc., not only is prolonged exposure to cold temperature inevitable but the nature of their work also demand that they remain vigilant and not suffer from mental fatigue. Although insulating oneself from cold remains the first line of defence for survival in cold, there are emergencies when such insulation might not be available or prove to be insufficient. The only key to survival then depends upon the physiological ability to generate heat.
A previous study from our lab demonstrated that a 70% ethanol extract of pepper and cinnamon could improve cold endurance in albino Wistar rats by increasing their cold adaptive thermogenesis [Citation2]. Further to this, we wished to evaluate if the improved endurance actually had a practical implication where in the performance of the animal was positively modulated. That thermogenic compounds can be effective performance-enhancing agents in cold temperature have not been studied previously at least to our knowledge. Rediscovery of metabolically active BAT in adult humans [Citation3] and the fact that it can be turned on by various pharmacological agents, provides compelling reasons to evaluate the already known thermogenic compounds for their ability to affect performance in cold temperatures. Based on this and on indication in literature about the ability of pepper and cinnamon to improve cognitive performance [Citation4–7] the current study was designed to evaluate if the thermogenic and performance enhancing ability of the two spices could normalise or improve performance following exposure to cold temperature in albino Wistar rats.
2. Materials and methods
2.1. Preparation of extracts
Pepper and cinnamon were purchased from the local market. They were shade dried and ground to a fine powder using a lab mill grinder. One hundred grams of the powder was suspended in 500 ml of 70% aqueous ethanol and was shaken for 24 h using a rotary shaker at room temperature. The solution was then filtered and the extraction process repeated for 3–4 times. The filtrate was concentrated under vacuum using a flash evaporator. The concentrate was then lyophilised and stored in an air tight container at −20°C until further use. Epigallocatechin gallate (EGCG) rich green tea extract was procured from Indo Vedic Nutrients Pvt. Ltd and used as positive control as its thermogenic and performance enhancing abilities are well reported.
2.2. Experimental design
Animal handling and experimentation were carried out in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). 80 male Albino Wistar rats were divided randomly into two groups, room temperature (RT, n = 40) and cold temperature (CT, n = 40). RT and CT groups were each further subdivided into four groups (n = 10), namely, control (receiving water), green tea, pepper and cinnamon. In each sub group, five animals did not undergo any test and served as the sedentary group and the remaining five animals underwent the novel object test. The extracts were administered orally through gavage at a dosage of 250 mg/kg for a period of 7 days.
2.3. Measurement of core body temperature
Prior to beginning the treatment, all the CT groups (both sedentary and test) were subjected to a cold challenge, where they were exposed to 4°C for 140–180 min. Changes in Core Body Temperature (CBT) were recorded continuously using rectal probes which was inserted approximately 1.5 cm into the rectum and held in place using an adhesive tape. The rectal probe was connected to a T-type thermister pod and an eight channel power lab data acquisition system (AD Instruments, Australia) for continuous recording of temperature. To hold the rectal probes in place and avoid erroneous measurement the rats were placed in a restrainer. The restrainer was placed in the cage 3–4 days before commencing the experiment with both ends open allowing free in and outward movement for the animal. This facilitated familiarisation of the animals with the restrainer and caused minimal stress during experimentation. The size of the restrainer used was such that it prevented the animal from turning around but allowed forward and backward movement. After 7 days of treatment the animals were again exposed to 4°C and the changes in CBT was recorded. Difference in the fall in CBT before and after treatment when exposed to the same period of cold was used as an index of cold endurance.
2.4. Novel object test (NOT)
This test is generally used to assess recognition memory in laboratory animals [Citation8]. The apparatus used was an open plastic box measuring 60 × 45 × 25 cm3. The objects to be discriminated were made of plastic or metal. Their weight was such that they could not be displaced by rats. The apparatus was placed in a sound isolated room. The task consisted of three phases or trials, viz habituation, familiarisation and test. During the habituation phase, the animals were allowed to explore the box initially for a period of two minutes in the absence of any object. During the familiarisation phase, two objects (referred hence forth as old objects, OA) similar in colour, texture and odour were placed closer to the rear wall of the box. The animals were reintroduced into the box and allowed to explore the objects for a period of 4–5 min. This training of the animal with the OA was performed everyday for a period of 5–7 days. It was noted that with progressive training, familiarisation of the animals with the open box and the OA considerably increased. After the training period, to assess the memory of the animal, in the test phase one of the two OA was replaced with a novel object (NO), which was different in colour, texture, odour and shape to OA. Discrimination Index (DI), was calculated using the following formulae
This value can vary between +1 and −1, where a positive score indicates more time spent with the novel object, a negative score indicates more time spent with the old object, and a zero score indicates a null preference. Memory was assessed before and after treatment immediately after RT or CT exposure. On day 7, after the test, animals were sacrificed under mild anaesthesia. Blood and other organs were separated and stored at −80°C until further analysis.
2.5. Antioxidant assays
Tissues were homogenised in 50 mM phosphate buffer (pH 7.4) at a concentration of 10% (w/v). Superoxide dismutase (SOD) activity was determined with a commercially available kit (Randox, Cat no. SD. 125) and glutathione-S-transferase (GST) by the method of Habig et al. [Citation9]. Catalase (CAT) was determined by measuring the decay of 6 mM H2O2 solution at 240 nm by spectrophotometric degradation method. An extinction coefficient of 43.6/M/cm/was used to determine the enzyme activity and values were expressed as mmol H2O2 degraded/min/mg of protein. Reduced glutathione (GSH) levels were determined by its reaction with 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) to yield a yellow chromophore which was measured using spectrophotometer at 412 nm. GSH concentration was calculated by using standard curve prepared with reduced glutathione and expressed as mg/mg protein. Lipid peroxidation was measured as malondialdehyde (MDA) and was analysed as described by [Citation10]. The amount of MDA content present was calculated using a molar extinction coefficient of 1.56 × 105/M/cm.
2.6. Estimation of NOR
NOR in plasma was quantified using High Performance Liquid Chromatography with an Electrochemical Detector [Citation11]. The levels of NOR were expressed as ng/dL of plasma.
2.7. Estimation of FFA
Free Fatty Acids (FFA) in serum was estimated as described by Falholt et al. [Citation12].
2.8. Immunoblotting
UCP-1, BDNF, and GAPDH expression was analysed by sodium dodecyl sulphate -polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Ten percent BAT and brain homogenate was prepared in lysis buffer, pH 7.4 and total protein levels were measured by the method of [Citation13]. Tissue homogenates containing 60 mg of proteins were separated on SDS-PAGE and transferred onto a nitrocellulose membrane using an electro blotting apparatus (Bio Rad systems, UK). After transfer, the membranes were probed with primary antibodies against UCP-1 (sc-6529), BDNF (sc-20981) and GAPDH (sc-166574) (Santa Cruz Biotechnology, CA, USA) at 1: 1000 dilutions and incubated at room temperature for 3 h. The membranes were then washed 7 times in PBST at 5 min interval for a period of 35 min followed by incubation for 80 min in horse radish peroxidase conjugated respective secondary antibody used at 1: 10 000 dilution. The membranes were washed again and developed in a Syngene G-box apparatus using ECL reagent. Western blot band intensity was measured using NIH image J software.
2.9. Statistical analysis
The data are expressed as mean ± standard deviation (SD). Green tea, pepper and cinnamon treated groups were compared with control group. Within each group, differences, if any, before and after treatment were also compared. In addition, where applicable, respective room and cold temperature groups were compared. Data were analysed by One-way ANOVA followed by Tukey’s Post hoc test using Graphpad prism 5 for Windows software. Differences at p < .05 were considered significant.
3. Results
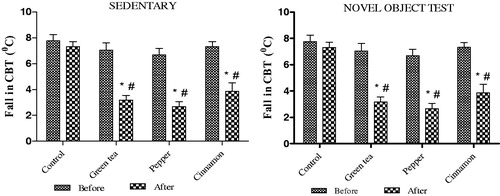
3.1. Effect of cold exposure on core body temperature before and after spice treatment following NOT
Consistent with our previous findings [Citation2], we observed that in the cold exposed animals the fall in CBT was lower in the treated groups in comparison to the control group. This difference was noted irrespective of whether the animals belonged to the sedentary or NOT group. The results are summarised in
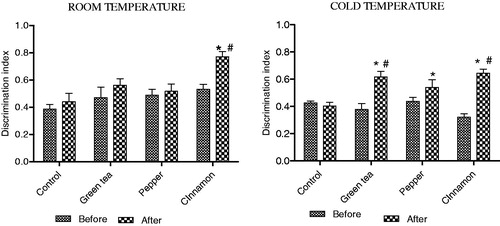
3.2. Effect of cold exposure and green tea/spice treatment on cognitive performance
In the RT group, treatment with cinnamon alone was found to improve performance as indicated by a higher discriminative index in comparison to the control group. In the CT group, treatment with green tea and both the spices improved performance in comparison to the control group. Results are summarised in .
3.3. Changes in nor and FFA levels
NOR initiates the process of NST in BAT eventually leading to the breakdown of triglycerides to release FFA [Citation14]. Hence NOR and FFA levels were used as an index of NST. NOR and FFA level was elevated significantly after treatment in both sedentary and NOT groups in comparison to control groups. Increased levels were observed between respective RT and CT groups as well. The results are summarised in .
Table 1. Effect of cold exposure and spice/green tea treatment on changes in norepinephrine and free fatty acid levels in rats subjected to NOT.
3.4. Effect of cold exposure and spice/green tea treatment on anti-oxidant status in BAT, brain, muscle and liver
Two aspects have been considered while quantifying the antioxidant status. Firstly, the effect of treatment (green tea or spices), which requires a comparison between the control and treated groups. Secondly, the effect of temperature and hence a comparison between the RT and CT control groups weather sedentary or NOT group (i.e.,) RT sedentary vs. CT sedentary and RT NOT vs. CT NOT groups. While we did observe an effect of treatment which is discussed in detail below, no consistent pattern of change was noted when RT and CT groups were compared in any of the antioxidant parameters measured here. For example, in MDA levels when sedentary RT control vs CT control was compared, it was found to be significantly different, where as for CAT there was no difference. In case of SOD, significant increase in activity was noted in the muscle and liver only following treatment. No effect was seen in BAT and brain. Hence only a comparison between the control and treated is discussed in detail.
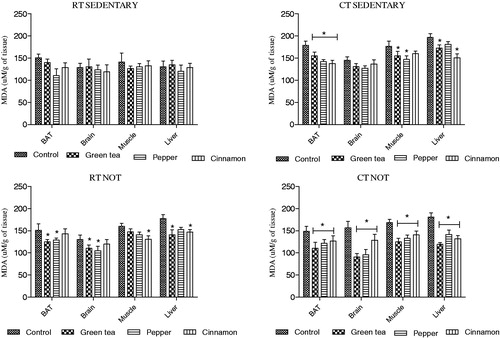
3.4.1. Changes in MDA levels
Degree of lipid peroxidation in brain was measured in terms of quantity of MDA released. We noted that in the RT group of animals treatment with green tea or spices did not lead to any significant decrease in MDA levels in comparison to control group. This was true for both sedentary and NOT group. In the CT group, an elevated level of MDA was noted in the control animals which decreased upon treatment with green tea and spices. This was noted in both sedentary and NOT groups ().
3.4.2. Changes in reduced glutathione levels
Reduced glutathione is one of the important antioxidants produced by the cells, participating directly in the neutralisation of free radicals and peroxides. As a result during a stressful challenge there is a need for increased production of GSH [Citation15]. However, it is also true that when the stress is greater than the available antioxidants, the cell suffers oxidative stress. We noted that cold exposure causes oxidative stress and treatment with green tea and spices relieved the stress by increasing the levels of GSH.
RT group: In the sedentary group, treatment with green tea and spices increased the level of GSH in BAT and liver in comparison to the control group. Whereas in the NOT group, an elevated level was noted in BAT, brain and liver of treated animals.
CT group: In both the sedentary and NOT group elevated GSH level was observed in all four organs following treatment with green tea and both the spices in comparison to their controls.
Interestingly, unlike other antioxidant parameters that were measured, we noted a significant increase in GSH levels in all four organs when respective RT and CT groups were compared (belonging to either sedentary or NOT groups). Results are summarised in .
Table 2. Effect of cold exposure and green tea/spice treatment on the levels of reduced glutathione in BAT, brain, muscle and liver of rats subjected to NOT.
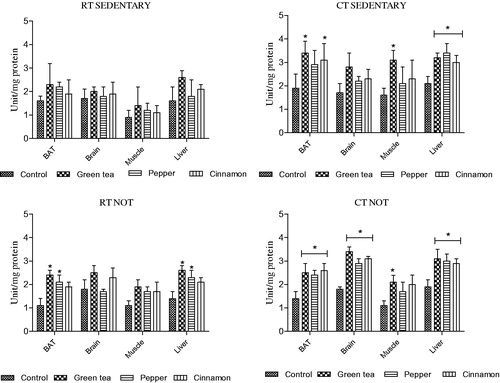
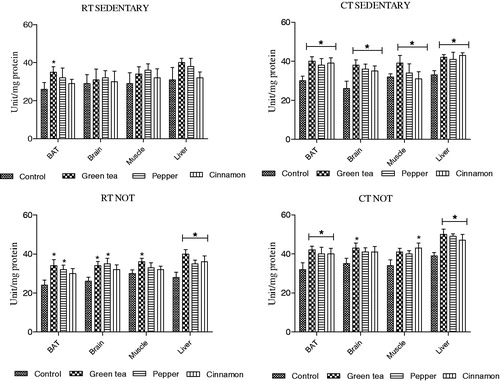
3.4.3. Changes in superoxide dismutase (SOD) activity
Superoxide dismutase is an essential antioxidant defence system required in all cells exposed to oxygen. In RT sedentary and NOT groups, treatment with either green tea or spices did not increase the SOD activity in comparison to their respective controls.
In CT sedentary group, treatment with both the spices and green tea increased SOD activity only in liver. Whereas in NOT group, an elevated SOD activity was observed in all four organs following treatment with both the spices and green tea ().
3.4.4. Changes in catalase (CAT) activity
Hydrogen peroxide is a normal by product of numerous metabolic reactions but highly toxic at very low concentrations as it can cause oxidative damage to the cells. It is neutralised into water and hydrogen by one of the important antioxidant enzymes, catalase [Citation16].
RT group: No effect of treatment was observed in the sedentary group on any organ. In the NOT group, no effect of treatment was observed in muscles. In BAT and brain, green tea and pepper increased the CAT activity whereas cinnamon had no effect. In liver, treatment with green tea and both the spices increased the CAT activity.
CT group: Treatment with both the spices and green tea increased CAT activity in all four organs in the sedentary group. In the NOT group, significant increase in CAT activity was observed following treatment with both spices and green tea in BAT and liver. In brain and muscle although we noted an increased CAT activity, it was not statistically significant ().
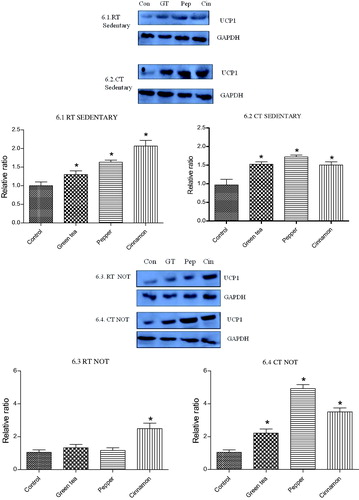
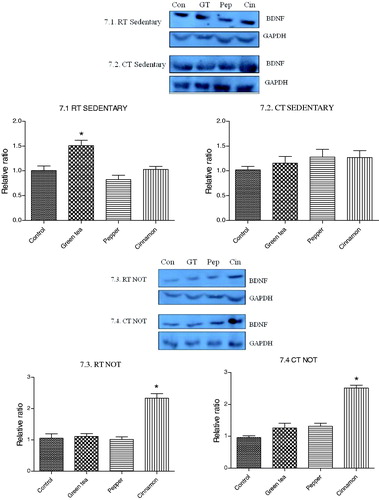
3.5. Effect of cold exposure and green tea/spice treatment on the expression of UCP1 and BDNF
Both the spices and green tea up regulated the expression of UCP1 in BAT in RT as well as CT groups. This was noted in both sedentary and NOT groups. The expression of BDNF in brain was up regulated only in cinnamon treated RT and CT groups ( and ).
4. Discussion
A previous study from our laboratory demonstrated that an ethanol extract of pepper and cinnamon can enhance cold adaptive thermogenesis. Also, the whole extract was found to be more effective than the major components alone viz, piperine and cinnamamdehyde, [Citation2]. Hence the current study was also carried out with whole extracts only. Distinctive RT and CT groups for all four treatments viz., water, green tea, pepper, and cinnamon were used to identify if the observed improvement was due to the two spices acting as thermogenic agents or as performance enhancing agents. For the same reason, parameters indicative of both cold adaptive thermogenesis and cognitive performance was measured. Reports show that exposure to cold can result in a decrease in hippocampal temperature, suggesting that central cooling may be responsible for performance decrements [Citation17]. It may be that a decrease in brain temperature slows hippocampal synaptic transmission, which could impair cognition [Citation18]. Specifically, cold exposure can lead to decrements in memory [Citation19,Citation20], vigilance [Citation21], reaction time [Citation22], decision making [Citation23], etc.
The present results are in agreement with those demonstrating cognitive impairments following cold exposure [Citation4–7]. Treatment with green tea and both the spices significantly improved performance, but only in the cold exposed group. We reasoned that it may be due to the following factors. Firstly, the enhanced heat generation and hence a lowered fall in CBT helps restore temperature homeostasis to a large extent. This implies that the major problem of central cooling or hypothermia is primarily addressed which in turn prevents the slowing down of physiological processes that are required to maintain cognition. The spices increase physiological heat generation by activating or potentiating NST in BAT. The sympathetic nervous system responds by releasing NOR which in turn result in an enhanced expression of UCP1 in BAT. UCP1 is the protein responsible for uncoupling oxidative phosphorylation resulting in an increased heat generation. Although the cue from SNS activating BAT is the primary reaction, an orchestral response from other organs which release factors that activate BAT directly or indirectly are now known [Citation24–26]. It is of interest to note that animals with decreased fall in CBT were more mobile and active. Hence the chances of them making any choice between the novel and old object were high when compared to the control animals which were mostly immobile or seemed to forget the task itself.
Secondly, treatment with spices increased the level of NOR, which is one of the neurotransmitters majorly involved in cognition and known to be depleted in cold exposed individuals. Compounds that enhance catecholamine levels have been used previously to improve cognitive impairments in cold exposed conditions [Citation27]. The observed elevated level of NOR is due to a decreased rate of degradation as these spices effectively inhibit catechol-O-methyl-transferase, the enzyme that regulates the level of NOR. Interestingly, we also noted that the increased NOR level in the RT treated group did not result in any improvement in performance in comparison to their respective control group. We could not reason out this observation. We speculate that the observed fold increase in NOR levels in the CT treated group may be essential to achieve any improvement in cognition.
Thirdly, during cold exposure, due to the increased rate of oxygen consumption, the levels of free radicals generated increase considerably [Citation28]. Hence, a pro-oxidant status is a commonly observed effect of cold exposure and oxidative stress is known to disrupt normal cellular functioning. Considering the high levels of polyunsaturated fatty acids in brain, it is susceptible for attack by the free radicals, increasing the oxidative stress and affecting cognitive performance. An elevated MDA level observed in brain and other organs viz., BAT, muscle, liver of the control group is an indicator of increased lipid peroxidation and a decreased activity of the antioxidant machinery. Treatment with both the spices increased activities of SOD, CAT and elevated GSH content.
Brain-derived neurotrophic factor (BDNF) is involved in cell survival, growth and differentiation of the nervous system. It is also found to be involved in synaptic and structural plasticity [Citation29], learning and memory [Citation30] and reported to have neuroprotective effects [Citation31]. Effect of piperine and cinnamaldehyde on increasing the expression of BDNF in the hippocampal region and hence improving cognition has been reported by many groups [Citation32–34]. Contrary to these indications we did not observe any change in the expression level of BDNF following treatment with pepper and cinnamon. Although the pepper and cinnamon extracts used in our study contained piperine and cinnamldehyde as their major components respectively, using the whole extract and not piperine or cinnmaldehyde alone may have been the reason for the unaltered expression in BDNF level.
In conclusion our results demonstrate that the cognitive impairment caused by exposure to cold can be effectively countered by agents with thermogenic potential. The most obvious reason being their ability to restore temperature homeostasis by enhancing the physiological heat production. This was indicated by a lowered fall in CBT which we believe was achieved by an increased level of UCP1 expression in BAT and an increased level of NOR and FFA in circulation. Absence of any notable effect in the RT treated group strengthened our hypothesis that at least in the current experimental set up the spices did not affect any parameters that enhances cognition. Hence, we propose that it is logical and most obvious that while addressing the problem of improving performance in cold temperatures, compounds with thermogenic ability will prove most effective. Hence a revaluation of the known and existing thermogenic compounds for their ability to affect performance in cold temperatures may provide newer ways of normalising performance, physical or cognitive, during prolonged cold exposure.
Disclosure of interest
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors gratefully acknowledge the constant support provided by Director, Dr. R. K. Sharma, Defence Food Research Laboratory, DRDO, Mysore.
Additional information
Funding
References
- Mäkinen TM, Palinkas LA, Reeves DL, et al. Effect of repeated exposures to cold on cognitive performance in humans. Physiol Behav. 2006;87:166–176.
- Pandit C, Anilakumar KR. Cold adaptive thermogenesis following consumption of certain pungent spice principles: a validation study. J Therm Biol. 2017;64:35–40.
- Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525.
- Zoladz PR, Raudenbush B. Cognitive enhancement through stimulation of the chemical senses. N Am J Psychol. 2005;7:125–140.
- Wattanathorn J, Chonpathompikunlert P, Muchimapura S, et al. Piperine, the potential functional food for mood and cognitive disorders. Food Chem Toxicol. 2008;46:3106–3110.
- Parachikova A, Green KN, Hendrix C, et al. Formulation of a medical food cocktail for Alzheimer’s disease: Beneficial effects on cognition and neuropathology in a mouse model of the disease. PLoS One. 2010;5:e14015.
- Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011;6:e16564–e16511.
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-Transferases the first enzymatic step in mercapturic acid formation*. J Biol Chem. 1974;249:7130–7139. Available from http://www.jbc.org/content/249/22/7130.
- Buege J. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. Available from http://www.sciencedirect.com/science/article/pii/S0076687978520326.
- Alburges ME, Narang N, Wamsley JK. A sensitive and rapid HPLC-ECD method for the simultaneous analysis of norepinephrine, dopamine, serotonin and their primary metabolites in brain tissue. Biomed Chromatogr. 1993;7:306–310.
- Falholt K, Lund B, Falholt W. An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin Chim Acta. 1973;46:105–111.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
- Li Q, Yu S, Wu J, et al. Sulfiredoxin‐1 protects PC12 cells against oxidative stress induced by hydrogen peroxide. J Neurosci Res. 2013;91:861–870.
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716.
- Ahlers ST, Thomas JR, Berkey DL. Hippocampal and body temperature changes in rats during delayed matching-to-sample performance in a cold environment. Physiol Behav. 1991;50:1013–1018.
- Moser EI, Andersen P. Conserved spatial learning in cooled rats in spite of slowing of dentate field potentials. J Neurosci. 1994;14:4458–4466.
- Thomas JR, Ahlers ST, House JF, et al. Repeated exposure to moderate cold impairs matching-to-sample performance. Aviat Space Environ Med. 1989;60:1063–1067.
- Patil PG, Apfelbaum JL, Zacny JP. Effects of a cold-water stressor on psychomotor and cognitive functioning in humans. Physiol. Behav. 1995;58:1281–1286.
- Flouris AD, Westwood DA, Cheung SS. Thermal balance effects on vigilance during 2-hour exposures to -20 degrees C. Aviat Space Environ Med. 2007;78:673–679.
- Ellis HD. The effects of cold on the performance of serial choice reaction time and various discrete tasks. Hum Factors. 1982;24:589–598.
- Watkins SL, Castle P, Mauger AR, et al. The effect of different environmental conditions on the decision-making performance of soccer goal line officials. Res Sport Med. 2014;22:425–437.
- Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489.
- Hondares E, Rosell M, Gonzalez FJ, et al. Hepatic FGF21 expression is induced at birth via PPARα in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212.
- Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468.
- Mahoney CR, Castellani J, Kramer FM, et al. Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiol Behav. 2007;92:575–582.
- Quiroga GD. Brown fat thermogenesis and exercise: two examples of physiological oxidative stress? Free Radic Biol Med. 1992;13:325–340.
- Hu X, Ballo L, Pietila L, et al. BDNF-induced Increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J Neurosci. 2011;31:15597–15603.
- Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269.
- Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes Brain Behav. 2008;7:906–914.
- Li S, Wang C, Wang M, et al. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381.
- Mao QQ, Huang Z, Zhong XM, et al. Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 2014;261:140–145.
- Jana A, Modi KK, Roy A, et al. Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol. 2013;8:739–755.