Abstract
Thermosensitive liposomal doxorubicin (LTSL-Dox) combined with mild hyperthermia enhances the localized delivery of doxorubicin (Dox) within a heated region. The optimal heating duration and the impact of extended heating on systemic drug distribution are unknown. Here we evaluated local and systemic Dox delivery with two different mild hyperthermia durations (42 °C for 10 or 40 minutes) in a Vx2 rabbit tumor model. We hypothesized that longer duration of hyperthermia would increase Dox concentration in heated tumors without increasing systemic exposure. Temporally and spatially accurate controlled hyperthermia was achieved using a clinical MR-HIFU system for the prescribed heating durations. Forty-minutes of heating resulted in a nearly 6-fold increase in doxorubicin concentration in heated vs unheated tumors in the same animals. Therapeutic ratio, defined as the ratio of Dox delivered into the heated tumor vs the heart, increased from 1.9-fold with 10 minutes heating to 4.4-fold with 40 minutes heating. MR-HIFU can be used to guide, deliver and monitor mild hyperthermia of a Vx2 tumor model in a rabbit model, and an increased duration of heating leads to higher Dox deposition from LTSL-Dox in a target tumor without a concomitant increase in systemic exposure. Results from this preclinical study can be used to help establish clinical treatment protocols for hyperthermia mediated drug delivery.
Introduction
Chemotherapy is vital in the treatment of many malignancies, and intensification of cytotoxic chemotherapy has been largely responsible for the improvement in outcomes of children with cancer over the last 50 years. However, systemic toxicities limit the dose that can be safely administered and even these limited doses result in potentially severe acute and life-long side effects of therapy. Anthracyclines are widely used for the treatment of many cancers, including most pediatric cancers, but higher cumulative doses can result in irreversible cardiomyopathy and is an important cause of early mortality in childhood cancer survivors [Citation1]. In particular, the anthracycline doxorubicin (Dox) is a mainstay of sarcoma treatment in children and adults [Citation2,Citation3]. One approach for making doxorubicin more selective has been to encapsulate it within liposomes to prevent extravasation into healthy tissue [Citation4,Citation5] and more selectively target tumor tissue through enhanced microvascular permeability and retention [Citation6,Citation7]. This has led to a reduction in cardiotoxicity but has not been widely adopted in the treatment of pediatric cancer [Citation8–10].
To enhance peritumoral and intra-tumor release, novel formulations of Dox have been developed using lysolipid thermally sensitive liposomes (LTSL-Dox) [Citation11–13]. While passing through a region of mild hyperthermia (∼40–42 °C), the permeability of the liposomes increases and unloads the encapsulated chemotherapeutic intravascularly, allowing it to be taken up by the surrounding tumor [Citation14]. In preclinical studies, this results in marked increases in doxorubicin concentrations within the tumor [Citation15–18]. A clinical trial in adult patients with liver cancer suggested prolonged event-free and overall survival when LTSL-Dox is combined with prolonged radiofrequency ablation [Citation19].
In addition to achieving release of chemotherapy from liposomes, mild hyperthermia can augment the cytotoxic effect of chemotherapy and has been shown to improve survival in adults with soft tissue sarcoma [Citation20]. The hypothesized mechanisms include increases in blood flow, increased drug uptake, inhibition of DNA-damage repair pathways and reversal of drug resistance [Citation21,Citation22]. Local heating can be applied through a variety of modalities including radiofrequency, light, water bath and ultrasound, with radiofrequency and ultrasound being the most commonly used in patients [Citation23]. The success of hyperthermia depends upon sustained and uniform heating of the tissue for a set duration.
Magnetic resonance guided high intensity focused ultrasound (MR-HIFU) [Citation24] is a noninvasive technique that allows precise ultrasound-mediated heating of tissues within the body using MR guidance for anatomic localization. Real-time MR temperature mapping based on the proton resonance frequency (PRF) technique provides feedback for automated control of ultrasound power and location to achieve precise spatially uniform heating [Citation25]. MR-HIFU has been primarily used for various applications of noninvasive thermal ablation [Citation26]. More recently, lower intensity heating using MR-HIFU and other heating modalities has been used to trigger drug release from TSLs [Citation15–18,Citation27–29]. Combining MR-HIFU with LTSL-Dox has been shown to increase Dox concentration at the heated site and improve tumor response in preclinical models [Citation16,Citation17,Citation30,Citation31].
While LTSL-Dox with MR-HIFU has demonstrated enhanced local delivery of Dox, the optimal heating duration and the effect of heating duration on systemic exposure to doxorubicin and thus potential toxicity remain unknown [Citation16,Citation18,Citation32–37]. The objective of this study was to investigate two different heating durations (10 and 40 min) on Dox delivered from LTSLs to tumors using a Vx2 rabbit tumor model. We hypothesized that the longer heating duration protocol would increase Dox concentration in heated tumors without increasing systemic exposure.
Method and materials
Animal preparation
Female New Zealand white rabbits (3.5–4.5 kg, n = 39) were used in this study. The animals were assigned randomly into two treatment groups: 10 min hyperthermia (n = 9) and 40 min hyperthermia (n = 7); the other 23 animals were used to develop the heating protocol to adapt the clinical hyperthermia system for animal treatment, or excluded due to insufficient tumor size for identification on MRI or unintended tumor location (adjacent to bone or skin, embedded in fat), or unreliable drug measurements (large variabilities among replicates). All procedures were approved by the UT Southwestern Institutional Animal Care and Use Committee. During tumor inoculation, the Vx2 carcinoma tumor cell suspension was diluted with Hank’s balanced salt solution (HBSS) to prepare an injection of 1–2 million cells in a volume of 0.5–1 ml, which was injected into both posterior thigh muscles of each rabbit, 12–13 days prior to MR-HIFU treatment. Body weight at the time of tumor inoculation was 3.5–4 kg.
On the day of treatment, animals were intubated and anesthetized with a mixture of 2–3.5% isoflurane and 1–2 L/min of 100% oxygen. An intravenous catheter was placed in the ear vein of the rabbit for the administration of drugs and contrast agents. A pulse oximeter was attached to the animal’s tongue to monitor heart rate and oxygen saturation, and a rectal temperature probe (T1, Neoptix, Quebec, Canada) was used to monitor and record the core body temperature throughout the treatment. Hair over the animal’s thighs was removed using an electric trimmer and depilatory cream to allow ultrasound penetration. After preparation, the animal was transferred to the MR-HIFU tabletop, which was equipped with a temperature-controlled water bath designed to position the animal at an appropriate height above the HIFU transducer and maintain the animal’s body temperature during treatment. The animal was positioned on its side, with ultrasound gel applied on the thigh facing the acoustic window, and between the hind legs to avoid undesired reflections of the ultrasound beam at the skin interface between the legs. When heating into the other leg was a possibility, a saline bag was placed between the legs as added precaution to prevent any heating of the tumor in the contralateral leg. Two extra temperature probes were placed in the water bath and on the animal’s skin. Special care (adjusting the water bath temperature, addition/removal blanket) was taken to ensure the animal's body temperature was between 36 and 37 °C before the treatment started.
LTSL-Dox (ThermoDox®, Celsion Corporation, Lawrenceville, NJ, USA) was diluted for injection in an equal volume of 5% dextrose. An infusion of 2.5 mg/kg of doxorubicin followed by 2–3 ml of 5% dextrose flush was administrated intravenously through the ear vein at a rate of 0.04 ml/s (5–6 min total) using an MR-compatible power injector (Medrad Spectris Solaris EP, Bayer Healthcare, Whippany, NJ, USA). During the hyperthermia treatment, the infusion was initiated once the average temperature within the targeted location reached 41 °C. Three hours after LTSL-Dox infusion, animals were euthanized and unabsorbed drug was cleared from the vasculature by transcardiac perfusion with saline.
MR-HIFU hyperthermia treatment
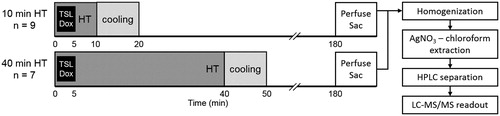
The experiment protocol is illustrated in . Mild hyperthermia treatment was performed on the tumor in one leg of the rabbit using an MR-HIFU system (Sonalleve V2, Profound Medical Inc, Mississauga, Canada) incorporated into a 3.0 T MRI scanner (Ingenia, Philips Healthcare, Best, The Netherlands), using treatment control software customized for mild hyperthermia [Citation38]. The tumor on the contralateral leg served as an unheated control. Tumors of at least 8 mm in their largest dimension were detectable on T2-weighted MR images as well-defined regions of heterogeneous signal increase against homogeneous signal from rabbit thigh muscle. A mild hyperthermia treatment cell with diameter of 10 mm was prescribed to cover the identified tumor. The angulation of the ultrasound beam was adjusted to avoid undesired absorption by bones or reflections at air interfaces. The output power was 40–60 W and the ultrasound sonication frequency was 1.2 MHz. The treatment duration was either 10 or 40 min, and temperature mapping was continued for 10 min after treatment to observe tissue cooling.
Figure 1. Experiment protocol for drug delivery in rabbits with Vx2 tumors using LTSL-Dox and MR-HIFU mild hyperthermia (HT) administered for either 10 or 40 min. Temperature mapping was continued for 10 min after treatment to observe tissue cooling (during which period the temperature of the heated region returned to baseline). The heated tumors, contralateral unheated tumor, and other organs were harvested 3 h after the start of LTSL-Dox infusion for drug quantification using silver nitrate/chloroform extraction with LC-MS readout.
During hyperthermia treatment, temperature within the targeted region was continuously monitored with a dynamic scan using temperature maps calculated based on PRF-shift MR thermometry and a 3 D linear drift correction algorithm [Citation38]. Additional scan strategies of center frequency stabilization and pre-scan imaging were also applied to achieve more stable temperature measurements [Citation39]. Six images were acquired (FFE-EPI, TE = 16 ms, TR = 38 ms, FOV = 40 × 40 cm, voxel size = 2.5 × 2.5 × 7.0 mm) for each dynamic scan: one slice along the ultrasound beam, three slices across the beam placed at the center of the treatment cell, and two more slices to provide information from the near and far field across the ultrasound beam.
Temperature maps were used by the MR-HIFU therapy system to automatically control the delivered power, with a goal of achieving a mean temperature of 42 °C within the treatment cell, while limiting the maximum temperature in any treatment slice to below 43 °C [Citation38]. This target temperature was selected to maintain temperatures above a drug release threshold of 40 °C, while avoiding potentially damaging exposures of greater than 44 °C. In previous studies performed with a QA phantom, the temperature accuracy achieved using this MR-HIFU system was 0.57 ± 0.58 °C in the heated region and 0.54 ± 0.42 °C in the unheated region [Citation39]. Thermal damage was also predicted using the concept of thermal dose (measured in cumulative equivalent minutes at 43 °C, CEM43), which represents the biological effect of a heat exposure accounting for the exponential relationship between duration and temperature [Citation40,Citation41]. Allowing for ±1 °C of temporal and spatial fluctuations, the 42 °C target temperature and 40 min maximum duration was expected to approach 30 CEM43, a threshold that predicts the initial onset of tissue damage [Citation42].
Quantification of doxorubicin concentration in tissue
After sacrifice, both tumors and tissue samples from various organs (heart, liver, lung, spleen, kidney, and thigh muscle) were collected in 1.5 ml microcentrifuge tubes, snap-frozen in liquid nitrogen, and stored at −80 °C. Prior to drug quantification, the frozen tissue tubes were thawed on ice, measured to 75 mg samples on disposable weighing boats, then diced and cut with scissors and scalpels. A median of six samples (range 2–59) was collected and measured for tumors, and 3–6 samples for each of the other organs. Each sample was added to a bead homogenizer vial with 1.5 ml of cell lysis buffer (0.25 M Sucrose, 1 mM CaCl2, 1 mM MgSO4 and 5 mM Tris-HCl). Bead homogenization was performed using 1.5 grams of 1 mm and 2 mm zirconia beads for three 1 min cycles (Mini-Beadbeater-16, BioSpec Products Inc., Bartlesville, OK, USA). To improve extraction of doxorubicin bound to DNA [Citation43], 100 µl of the homogenate was vortexed with 40 µl of 1.94 M AgNO3 and incubated at room temperature for 10 min. Doxorubicin was extracted through precipitation of proteins by vortexing with an added 1 ml of 2:1 v/v chloroform:isopropanol, followed by centrifugation at 1220 g for 10 min. From the resulting organic phase, 200 µl was dessicated under flowing nitrogen, and then re-suspended in a mobile phase of acetonitrile:H2O (50:50) with 0.1% formic acid and daunorubicin (100 ng/ml) as an internal standard.
Doxorubicin levels were quantitated by LC-MS/MS using a high-performance liquid chromatograph (Prominence, Shimadzu Scientific Instruments Inc., Columbia, MD) coupled to a mass spectrometer (AB SCIEX 4000 QTRAP, Applied Biosystems, Foster City, CA). Doxorubicin and daunorubicin were first separated on a C18 column (ZORBAX Eclipse XDB-C18, 50 × 4.6 mm with 5 micron packing, Agilent, Santa Clara, CA) under the following gradient conditions: Buffer A: dH20 + 0.1% formic acid, Buffer B: acetonitrile + 0.1% formic acid, 0–2 min 5% B, 2.0–3.5 min gradient to 60% B, 3.5–5.0 min 60% B, 5.0 to 5.1 min gradient to 5% B, 5.1 to 7.5 min 5% B. Doxorubicin was then detected with the mass spectrometer in multiple reaction monitoring mode by following the precursor to fragment ion transition 544.17 to 397.0. The internal standard, daunorubicin, was monitored using the transition 528.15 to 321.0. Instrument settings were optimized for each compound as follows: dwell time 150 ms; declustering potential 91.0 volts; entrance potential 8 volts (doxorubicin) 14 volts (daunorubicin); collision energy 21 volts (doxorubicin), 23 volts (daunorubicin); collision cell exit potential 10 volts (doxorubicin), 8 volts (daunorubicin); curtain gas 25 psi; collision gas medium; ion spray voltage 5000 V; turbo heater temperature 500 °C; nebulizing gas 70 psi; auxiliary gas 70 psi.
Standard curves for each tissue were prepared by addition of doxorubicin to 100 µl of blank tissue homogenate from untreated animals prior to addition of AgNO3. A value three-fold above the analyte peak area obtained in blank tissue homogenate was designated the limit of detection (LOD). The limit of quantitation (LOQ) was defined as the lowest concentration at which back calculation yielded a concentration within 20% of the theoretical value and above the LOD signal area.
Statistical analysis
The capability of the MR-HIFU system to achieve precisely controlled hyperthermia within a targeted region was evaluated based on the temporal mean and standard deviation of the spatial mean temperature within the target region (Tmean), the temperature that 10% of the target region exceeds (T10), and the temperature that 90% of the target region exceeds (T90). The overall efficacy of mild hyperthermia was quantified by the duration that targeted tissues experienced temperatures higher than the drug release temperature of 40 °C (Time >40 °C), and the thermal dose coverage to 50% (CEM43T50) and 90% (CEM43T90) of the target area. The risk of unintended tissue necrosis by overheating was quantified by the duration of damaging temperatures higher than 44 °C (Time >44 °C), and the number of animals in which the thermal dose delivered to 10% of the target area (CEM43T10) was greater than 30 CEM43.
Localized Dox deposition and systemic exposure were evaluated based on Dox concentrations in the heated tumors, unheated tumors, and unheated normal tissues. The specificity of Dox deposition towards tumor vs heart tissue (the target of its cumulative dose-limiting toxicity) was expressed by the therapeutic ratio, defined as the ratio of Dox delivered into the heated tumor vs the heart.
Differences in treatment parameters, Dox deposition, and therapeutic ratio were compared between heating groups by t-tests for each parameter or tissue type. Dox concentrations were similarly compared between targeted and untargeted tumors within each heating group. Bonferroni-corrected p values were compared to a significance threshold of p = 0.05.
Results
MR-HIFU hyperthermia treatment
Examples of MR treatment planning and thermometry images acquired during MR-HIFU mild hyperthermia treatment are demonstrated in . The two heating groups did not have significant differences in terms of the weight of the animals, the size of the heated tumors, or the core body temperature at the start of treatment ().
Figure 2. MR-HIFU mild hyperthermia in rabbit Vx2 tumors. Treatment planning images along (A) and across (B) the ultrasound beam (cone shape), with tumor and targeted location indicated by white arrows. MR temperature maps confirm adequate hyperthermia in both the 10 min (C) and 40 min (D) heating groups. White circle indicates the treated ROI, and black contour in (C) and (D) is the 42 °C isotherm. Bar = 2 cm.
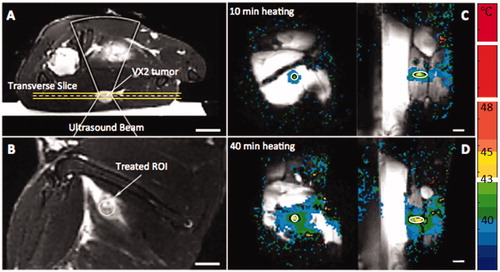
Table 1. Characteristics and heating quality in rabbits administered MR-HIFU mild hyperthermia for 10 min (HT10) vs 40 min (HT40). (Student’s t-test, significance threshold p < .05.).
In both the 10 min (HT10) and 40 min (HT40) heating groups, adequate heating above the drug release temperature (40 °C) was achieved in the target region for the intended duration, with minimal overheating (>44 °C). For both groups, the system demonstrated high temporal accuracy and stability of mean temperatures within the tumor around the desired 42 °C, with uniform spatial control demonstrated by a small range between T10 and T90. A potentially damaging thermal dose greater than 30 CEM43 was observed in 10% of the target area in 4/7 animals of the HT40 group; all rabbits in the HT10 group had a CEM43T10 below 15 equivalent minutes. During treatment, the core body temperature (rectal) increased slightly more during the prolonged heating duration of the HT40 group compared to the HT10 group (HT10: 37.2 ± 0.8 °C vs HT40: 38.3 ± 0.6 °C, p = 0.642).
Doxorubicin concentrations in tumors and other organs
Dox quantification in tissues using silver nitrate/chloroform extraction with LC-MS readout had a limit of quantitation of 0.1 ng/mg in the rabbit tissues evaluated (heart, kidney, liver, lung, muscle, spleen, heated/unheated tumor). The assay showed a linear fit over a dynamic range of three to four orders of magnitude for all matrices evaluated.
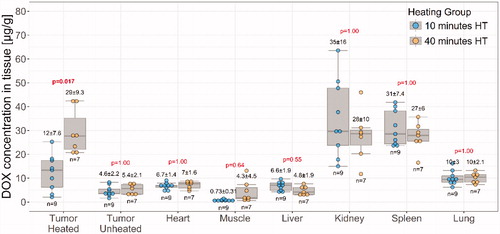
Dox biodistribution demonstrated similar tissue drug concentrations in the heart (6.7 ± 1.4 µg/g vs 7 ± 1.6 µg/g, p = 1) and all other non-tumor locations in both treatment groups (). In targeted tumors, heating for 40 min instead of 10 min resulted in a significant increase in Dox concentration (29 ± 9.3 µg/g vs 12 ± 7.6 µg/g, Bonferroni-adjusted p values = .017). The heated tumor demonstrated a significant increase in Dox compared to the contralateral unheated tumor at both 10 min of heating (2.7 ± 1.4 fold, Bonferroni-corrected paired t-test p = .015) and 40 min of heating (5.8 ± 1.6 fold, Bonferroni-corrected paired t-test p = .0005).
Figure 3. Doxorubicin biodistribution in rabbits administered LTSL-Dox and MR-HIFU hyperthermia. Comparisons between heating durations of 10 min vs. 40 min were made for each tissue type by t-test, Bonferroni-corrected p values are indicated.
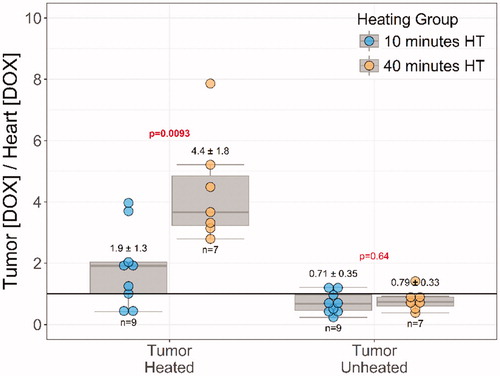
The therapeutic ratio in the unheated tumors was smaller than one in both groups (HT10: 0.7 ± 0.4, HT40: 0.8 ± 0.3, ). In the heated tumors, however, the therapeutic ratio was doubled for HT10 group (1.9 ± 0.3), while showing a more than four-fold increase in the HT40 group (4.4 ± 1.8). A significant difference was observed between HT10 and HT40 groups (p = .0093, ).
Discussion
In rabbits with bilateral Vx2 tumors, extending the duration of mild hyperthermia delivered to one of the tumors from 10 min to 40 min at 42 °C increased the therapeutic ratio from 1.9-fold to 4.4-fold. This increase in therapeutic ratio resulted from an increase in doxorubicin delivery to tumors with longer heating duration, without increasing doxorubicin concentrations in the heart or other major organs. Forty minutes of heating resulted in a nearly 6-fold increase in doxorubicin concentration vs unheated tumors in the same animals. Temporally and spatially accurate hyperthermia was achieved using a clinical MR-HIFU system for the prescribed heating durations of 10 and 40 min.
One limitation of our study is the lack of pharmacokinetic (PK) data. Unfortunately, collecting blood samples for PK evaluation during and immediately after the 5–6 min LTSL-Dox infusion was not possible because the drug infusion was performed during delivery of MR-HIFU hyperthermia and the manipulation of the animal will alter the reliability of MR thermometry. However, previous studies of LTSL-Dox plasma PK in rabbits [Citation30,Citation44], pigs [Citation37], humans [Citation45–47], and numerical simulations [Citation32] have indicated that plasma concentrations decrease following a Z model at a rate of approximately 45.7 to 957.1 ng/ml/min immediately after infusion. The rate of decay of plasma Dox concentrations predicted by these datasets aligns with the observed difference in tumor Dox concentrations in the 10 and 40 min groups.
Our study demonstrated significant increases in Dox concentrations with MR-HIFU, but the results were less pronounced than the nearly 26-fold increase in Dox in heated vs unheated tumors reported in an early report by Staruch et al [Citation16]. We hypothesize that this may have been due a difference in the animals’ core body temperature, which was 34–37 °C in the previous study vs. a more consistent and physiologic 36–37 °C here. Higher body temperature in the current study may have led to increased nonspecific release of LTSL-Dox, but is likely a better representation of the clinical situation. While the majority of LTSL-Dox is uncoupled at target temperatures of 39–42 °C, close to 30% has been shown to be released at 37 °C [Citation48]. Other preclinical studies demonstrated a roughly 4–6 fold increase in Dox with hyperthermia, more consistent with our results [Citation17,Citation30]. In 10 adult patients with unresectable liver cancer, the combination of LTSL-Dox followed by partial tumor heating with ultrasound-guided HIFU demonstrated 3–7 fold enhancement of local doxorubicin concentrations in biopsy samples from the heated region immediately after HIFU vs. after drug infusion but before HIFU [Citation49].
The ideal heating protocol for combination with LTSL-Dox is unknown. Previous work demonstrated drug delivery at particular durations of mild hyperthermia that ranged from a few minutes to an hour [Citation32–34,Citation36,Citation50], but our study is the first to demonstrate increased drug deposition and no significant changes in the systemic biodistribution with increased heating duration under the same experimental protocol. Recent computer simulations [Citation51] and in vivo studies [Citation37] demonstrated a similar conclusion to our work, with increased duration of radiofrequency ablation leading to more drug deposition at the hyperthermic margins of the ablation zone in liver tissue. An in vivo study using MR-HIFU [Citation29] demonstrated that ablative heating (greater than 240 CEM43) induced vessel damage that limited drug deposition only to the tumor rim, while mild hyperthermia (30 min at 41 °C) achieved a uniform distribution across the entire tumor. They found that the combination of mild hyperthermia followed by ablation resulted in the highest intratumoral drug concentrations (likely due to post-delivery vessel damage preventing drug efflux from the tumor), and improved tumor control. While thermal ablation directly induces tumor necrosis and may enhance drug delivery when applied after mild hyperthermia, prolonged mild hyperthermia-mediated drug delivery alone may be preferable in tumors involving critical neurovascular structures that would be damaged by ablation [Citation52], a common reason that sarcomas are unresectable at diagnosis and require neoadjuvant therapy.
The objective of this work was to study the effect of increased heating duration on doxorubicin distribution. We did not evaluate the therapeutic effect of higher drug concentration in tumor. In a lung cancer model in vitro, intracellular Dox concentrations greater than 60 ng/mg are required for greater than 99% cell kill [Citation53]. However, it is not possible to directly correlate doxorubicin concentrations in these in vitro experiments with the values measured in our study as doxorubicin concentrations in vivo are expected to change dynamically with the initial uptake and subsequent clearance of doxorubicin from tumor cells. In vivo, several studies have shown improved anti-tumor efficacy of LTSL formulations plus localized mild hyperthermia compared with animals treated with LTSL drug alone [Citation31,Citation54]. Previous studies combining mild hyperthermia with LTSL-Dox [Citation31,Citation55] and traditional liposomal Dox [Citation56] point to a correlation between Dox tumor drug concentration and therapeutic effect.
Future in vivo studies should examine the proposed benefits of even longer heating durations [Citation32]. However, in this study, evidence of early-stage thermal damage was observed at 40 min, which might become more pronounced at longer duration. Moreover, the stability of the magnet and the accuracy of MR thermometry were confirmed for hyperthermia treatment up to 40 min; heating for longer durations will be more challenging [Citation39]. Other factors including patient comfort level should also be considered in longer duration heating. With these conclusions, 40 min is expected to be a safe limit for localized hyperthermia treatment with the system described in this study. To take advantage of prolonged drug release without inducing necrosis, slightly lower target temperatures should be used in order to limit the accumulation of thermal dose to approximately 30 CEM43 [Citation42]. Studies should investigate the effect of various doses of LTSL-Dox on systemic Dox exposure and therapeutic ratio. The cumulative systemic dose of Dox has been suggested as an independent risk factor for the development of cardiomyopathy [Citation57,Citation58] but even smaller amounts may lead to morphologic and clinical changes [Citation59], suggesting there is no completely safe systemic dose of Dox.
In summary, we have demonstrated that MR-HIFU can be used to guide, deliver and monitor mild hyperthermia of Vx2 tumors in a rabbit model, and that an increased duration of heating leads to higher Dox deposition from LTSLs in a target tumor without increase in systemic exposure. These results provide direction for clinical protocols of hyperthermia-mediated drug delivery.
Acknowledgments
We also would like to thank Mr. Cecil Futch for helping set up the experiment and build equipment/parts used in this study.
Disclosure statement
Robert M Staruch is a paid employee of Profound Medical Inc. Rajiv Chopra is a shareholder of Profound Medical Inc.
Additional information
Funding
References
- Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842.
- Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497.
- Hawkins DS, Spunt SL, Skapek SX. Children's oncology group's 2013 blueprint for research: soft tissue sarcomas. Pediatr Blood Cancer. 2013;60:1001–1008.
- Abraham SA, Waterhouse DN, Mayer LD, et al. The liposomal formulation of doxorubicin. Meth Enzymol. 2005;391:71–97.
- Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomed. 2012;7:49–60.
- Wu NZ, Da D, Rudoll TL, et al. Increased microvascular permeability contributes to preferential accumulation of Stealth liposomes in tumor tissue. Cancer Res. 1993;53:3765–3770.
- Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate Chem. 2010;21:797–802.
- Drummond DC, Meyer O, Hong K, et al. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–743.
- Batist G, Barton J, Chaikin P, et al. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Exp Opin Pharmacother. 2002;3:1739–1751.
- Petersen GH, Alzghari SK, Chee W, et al. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J Control Rel. 2016;232:255–264.
- Yatvin MB, Weinstein JN, Dennis WH, et al. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293.
- Kneidl B, Peller M, Winter G, et al. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomed. 2014;9:4387–4398.
- Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev. 2001;53:285–305.
- Landon CD, Park JY, Needham D, et al. Nanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed J. 2011;3:38–64.
- Partanen A, Yarmolenko PS, Viitala A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia. 2012;28:320–336.
- Staruch RM, Ganguly M, Tannock IF, et al. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia. 2012;28:776–787.
- de Smet M, Heijman E, Langereis S, et al. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. J Control Rel. 2011;150:102–110.
- Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–5575.
- Tak WY, Lin SM, Wang Y, et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res. 2018;24:73–83.
- Issels RD, Lindner LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4: 483–492.
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56.
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–2554.
- Datta NR, Ordonez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41:742–753.
- Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50:221–229.
- Enholm JK, Kohler MO, Quesson B, et al. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng. 2010;57:103–113.
- Kobus T, McDannold N. Update on clinical magnetic resonance-guided focused ultrasound applications. Magn Reson Imaging Clin N Am. 2015;23:657–667.
- Hijnen NM, Heijman E, Kohler MO, et al. Tumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-up. Int J Hyperthermia. 2012;28:141–155.
- Grull H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Rel. 2012;161:317–327.
- Hijnen N, Kneepkens E, de Smet M, et al. Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proc Natl Acad Sci UsaU S A. 2017;114:E4802–e4811.
- Ranjan A, Jacobs GC, Woods DL, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Rel.
- Staruch RM, Hynynen K, Chopra R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: therapeutic effect in rabbit Vx2 tumours. Int J Hyperthermia. 2015;31:118–133.
- Gasselhuber A, Dreher MR, Partanen A, et al. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: computational modelling and preliminary in vivo validation. Int J Hyperthermia. 2012;28:337–348.
- Li L, ten Hagen TL, Hossann M, et al. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J Control Rel. 2013;168:142–150.
- Dromi S, Frenkel V, Luk A, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727.
- Hauck ML, LaRue SM, Petros WP, et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res. 2006;12:4004–4010.
- Willerding L, Limmer S, Hossann M, et al. Method of hyperthermia and tumor size influence effectiveness of doxorubicin release from thermosensitive liposomes in experimental tumors. J Control Rel. 2016;222:47–55.
- Swenson CE, Haemmerich D, Maul DH, et al. Increased duration of heating boosts local drug deposition during radiofrequency ablation in combination with thermally sensitive liposomes (ThermoDox) in a porcine model. PLoS One. 2015;10:e0139752.
- Tillander M, Hokland S, Koskela J, et al. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med Phys. 2016;43:1539–1549.
- Bing C, Staruch RM, Tillander M, et al. Drift correction for accurate PRF-shift MR thermometry during mild hyperthermia treatments with MR-HIFU. Int J Hyperthermia. 2016;32:673–687.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiation Oncol Biol Phy. 1984;10:787–800.
- Perez CA, Sapareto SA. Thermal dose expression in clinical hyperthermia and correlation with tumor response/control. Cancer Res. 1984;44:4818s–4825s. PubMed PMID: 6380716; eng.
- McDannold NJ, King RL, Jolesz FA, et al. Usefulness of MR imaging-derived thermometry and dosimetry in determining the threshold for tissue damage induced by thermal surgery in rabbits. Radiology. 2000;216:517–523.
- Ibsen S, Su Y, Norton J, et al. Extraction protocol and mass spectrometry method for quantification of doxorubicin released locally from prodrugs in tumor tissue. J Mass Spectrom. 2013;48:768–773.
- Staruch RM, Ganguly M, Tannock IF, et al. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. Int J Hyperther. 2012;28:776–787.
- Poon RT, Borys N. Lyso-thermosensitive liposomal doxorubicin: a novel approach to enhance efficacy of thermal ablation of liver cancer. Exp Opin Pharmacother. 2009;10:333–343.
- Wood BJ, Poon RT, Locklin JK, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol. 2012;23:248–255 e7.
- Lyon PC, Gray MD, Mannaris C, et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single-centre, open-label, phase 1 trial. Lancet Oncol. 2018;19:1027–1039.
- Gasselhuber A, Dreher MR, Negussie A, et al. Mathematical spatio-temporal model of drug delivery from low temperature sensitive liposomes during radiofrequency tumour ablation. Int J Hyperthermia. 2010;26:499–513.
- Lyon PC, Gray MD, Mannaris C, et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single-centre, open-label, phase 1 trial. Lancet Oncol. 2018;19: 1027–1039.
- Andriyanov AV, Koren E, Barenholz Y, et al. Therapeutic efficacy of combining pegylated liposomal doxorubicin and radiofrequency (RF) ablation: comparison between slow-drug-releasing, non-thermosensitive and fast-drug-releasing, thermosensitive nano-liposomes. PLoS One. 2014;9:e92555.
- Rossmann C, McCrackin MA, Armeson KE, et al. Temperature sensitive liposomes combined with thermal ablation: Effects of duration and timing of heating in mathematical models and in vivo. PLoS One. 2017;12:e0179131.
- Haveman J, Van Der Zee J, Wondergem J, et al. Effects of hyperthermia on the peripheral nervous system: a review. Int J Hyperthermia. 2004;20:371–391.
- Kerr DJ, Kerr AM, Freshney RI, et al. Comparative intracellular uptake of adriamycin and 4'-deoxydoxorubicin by non-small cell lung tumor cells in culture and its relationship to cell survival. Biochem Pharmacol. 1986;35:2817–2823.
- Tagami T, Ernsting MJ, Li SD. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J Control Rel. 2011;152:303–309.
- Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60:6950–6957.
- Huang SK, Stauffer PR, Hong K, et al. Liposomes and hyperthermia in mice: increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res. 1994;54:2186–2191.
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879.
- Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. JCO. 2005;23:2629–2636.
- Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes–a report from the Children's Oncology Group. JCO. 2012;30:1415–1421.