ABSTRACT
Contextual learning pervades our perception and cognition and plays a critical role in adjusting to aversive and stressful events. Our ability to memorise spatial context has been studied extensively with the contextual cueing paradigm, in which participants search for targets among simple distractor cues and show search advantages for distractor configurations that repeat across trials. Mixed evidence suggests that confrontation with adversity can enhance as well as impair the contextual cueing effect. We aimed to investigate this relationship more systematically by devising a contextual cueing task that tests spatial configuration learning within complex visual scenes that were emotionally neutral or negative (Study 1) and was preceded by the Maastricht Acute Stress Test (MAST) or a no-stress control condition (Study 2). We demonstrate a robust contextual cueing effect that was comparable across negative and neutral scenes (Study 1). In Study 2, acute stress disrupted spatial configuration learning irrespective of scene valence and endogenous cortisol reactivity to stress. Together with the emerging evidence in the literature, our findings suggest that spatial configuration learning may be subject to complex regulation as a function of spatial or temporal proximity to a stressor, with potential implications for the development of stress-related psychopathology.
Throughout all parts of life, our behaviour is guided by past experiences and by our emotions (Thompson, Citation1991). Among many benefits, this is critical for our survival when we face stressors and threatening situations. However, in our constantly changing and complex environment, responding appropriately to potential threat is often impossible without memory for the situational context. Failing to encode or retrieve contextual information from memory can lead to maladaptive responses, with dramatic consequences for our psychological well-being (Maren, Phan, & Liberzon, Citation2013). For example, post-traumatic stress disorder (PTSD) has been theorised to result from failures of appropriate memory reconstruction and contextualisation (Brewin, Gregory, Lipton, & Burgess, Citation2010; Ehlers, Citation2010; also see Rubin, Berntsen, & Bohni, Citation2008). That is, if an individual is unable to encode, embed, or retrieve contextual features of a traumatic situation, trauma-related cues may trigger a full-blown defensive reaction even in harmless situations. Therefore, the human ability to form contextual representations, especially in emotional situations, is of paramount interest for both researchers and clinicians.
Contextual learning pervades all levels of perception and cognition (Chun, Citation2000) and often occurs automatically in the absence of effort and awareness (Barrett & Kensinger, Citation2010). A striking example is the ability to memorise visuospatial regularities in our environment, which has gained considerable attention in research since the introduction of the so-called contextual cueing paradigm (Chun & Jiang, Citation1998). Here, participants search a target among spatial configurations of multiple simple distractor cues. When participants view a configuration that they have already seen before – even without consciously recognising it – their memory markedly speeds up the target search. This search advantage – the so-called contextual cueing effect – is thought to result from improved attentional guidance and/or enhanced response selection (Kunar, Flusberg, Horowitz, & Wolfe, Citation2007). It has been demonstrated in terms of attention allocation to the target region (Schankin & Schubo, Citation2009), fewer fixations during search (Harris & Remington, Citation2017), and consequently in lower reaction times (RT) for repeated compared to novel configurations. Some studies suggest that contextual cueing is relatively independent of awareness, as well as of attention and working memory allocation during in the learning phase (e.g. Colagiuri & Livesey, Citation2016; Vickery, Sussman, & Jiang, Citation2010), yet the evidence thus far remains inconclusive (e.g. Brockmole & Henderson, Citation2006b; Manginelli, Langer, Klose, & Pollmann, Citation2013; Travis, Mattingley, & Dux, Citation2013; Vadillo, Konstantinidis, & Shanks, Citation2016). Moreover, the encoded memory traces continue to facilitate visual search even after a one-week delay (Chun & Jiang, Citation2003).
Spatial context learning might be particularly adaptive in threatening and stressful situations. In other words, the presence of a stressor might both up- and downregulate the automatic detection of threat and safety cues in the spatial environment. One possibility is that the salience of threatening situations leads to enhanced attention capturing, impaired disengagement, as well as higher arousal and vigilance, which generally amplifies visual search and learning for all elements of the scene (Olatunji, Ciesielski, Armstrong, & Zald, Citation2011; Phelps, Ling, & Carrasco, Citation2006). Contrasting this view, aversive situations and the ensuing negative mood have also been theorised to narrow the attentional focus (Gable & Harmon-Jones, Citation2010), reducing attention to contextual stimuli surrounding the stressor. Indeed, various studies have shown that emotionally arousing stimuli tend to be remembered better at the expense of memory for contextual background information (e.g. Steinmetz & Kensinger, Citation2013), while degrading the learning of item-context associations (e.g. Bisby & Burgess, Citation2014; Bisby, Horner, Bush, & Burgess, Citation2018).
In addition, contextual cueing is likely to be affected by stress responses orchestrated by the autonomous nervous system (ANS) and the hypothalamus-pituitary-adrenal (HPA) axis, which result in a quick release of adrenalin and noradrenalin, and a slower release of the stress hormone cortisol (de Kloet, Joels, & Holsboer, Citation2005; Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013; Schwabe, Joëls, Roozendaal, Wolf, & Oitzl, Citation2012). These systems generally facilitate the consolidation of new memories and inhibit the retrieval of older memories (Kuhlmann, Piel, & Wolf, Citation2005; Quaedflieg & Schwabe, Citation2018; Roozendaal, Okuda, de Quervain, & McGaugh, Citation2006; Smeets, Otgaar, Candel, & Wolf, Citation2008; Wolf, Citation2017). In addition, there is emerging evidence that acute stress reduces the reliance on hippocampus-based information processing and learning (Quaedflieg & Schwabe, Citation2018; Smeets, van Ruitenbeek, Hartogsveld, & Quaedflieg, Citation2018; Wirz, Wacker, Felten, Reuter, & Schwabe, Citation2017). Spatial configuration learning may be affected by this shift in encoding, as it is known to depend on medial temporal lobe structures (Burgess, Maguire, & O’Keefe, Citation2002; Chun & Phelps, Citation1999; Manns & Squire, Citation2001; Preston & Gabrieli, Citation2008). These areas subserve the encoding of complex feature conjunctions and allocentric spatial representations (Fyhn, Hafting, Treves, Moser, & Moser, Citation2007; Murray, Bussey, & Saksida, Citation2007; van Strien, Cappaert, & Witter, Citation2009), and thus comprise major input for the construction of spatial representations in the hippocampus.
The available evidence bearing on spatial configuration learning in aversive and stressful situations is still scarce and appears to be mixed. Szekely, Rajaram, and Mohanty (Citation2017) found an amplified contextual cueing effect when the search targets consisted of schematic angry faces among configurations of neutral faces. Similarly, a recent study found amplified contextual cueing when the abstract cue configurations were accompanied by aversive pictures, while positive pictures had the opposite effect (Zinchenko, Geyer, Müller, & Conci, Citationin press). Another study embedded threatening stimuli (i.e. pictures of spiders) within the learned spatial configurations, which affected search efficiency but not configuration memory (Yamaguchi & Harwood, Citation2017). Meanwhile, Kunar, Watson, Cole, and Cox (Citation2014) exposed participants to series of neutral or negative images, immediately followed by a contextual cueing task. In this study, spatial configuration learning was impaired in the negative image condition. Finally, our own study (Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013) used the Maastricht Acute Stress Test (MAST; Smeets et al., Citation2012) to induce acute stress prior to configuration learning. We found no overall reduction in configuration learning, but a stress-induced impairment that was moderated by the endogenous stress cortisol response (see also Roozendaal, Griffith, Buranday, de Quervain, & McGaugh, Citation2003).
Together, limited evidence suggests that affective elements within the environment might amplify configuration learning (cf. Szekely et al., Citation2017; Yamaguchi & Harwood, Citation2017; Zinchenko et al., Citationin press). In contrast, tentative evidence suggests that confrontation with more aversive and stressful stimuli before configuration learning has a negative impact (Kunar, Watson, et al., Citation2014), potentially moderated by the endogenous cortisol response to stress (Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013). Still, a shortcoming of these studies is that the effects of stress and emotional valence were tested in spatial configurations that were entirely unrelated to the source of negative information. This essentially precludes conclusions about spatial context learning involving stressors within more complex visual environments.
To overcome these limitations, we devised an adaptation of the contextual cueing paradigm (Chun & Jiang, Citation1998; Meyer, Krans, van Ast, & Smeets, Citation2017), in which the learned spatial patterns are based on complex naturalistic scenes rather than on configurations of abstract visual cues (e.g. Brockmole & Henderson, Citation2006a, Citation2006b). In particular, we employed visual scenes taken from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, Citation2005), albeit processed to equalise low-level spatial regularities of the stimuli (see below for details). This novel variant allowed us – in Study 1 – to systematically assess the difference between spatial configuration learning in neutral versus negative scenes. In addition, in Study 2, we used the MAST (Quaedflieg, Meyer, Van Ruitenbeek, & Smeets, Citation2017; Smeets et al., Citation2012) to assess the impact of acute stress on spatial configuration learning in neutral and negative scenes. Based on prior studies (e.g. Kunar, Watson, et al., Citation2014; Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013), we generally expected spatial configuration learning to be weaker in negative scenes compared to neutral scenes, and to be impaired under acute stress as a function of the endogenous stress cortisol response. Finally, in the absence of prior evidence, we had no firm hypotheses about the interaction between scene valence and stress.
Study 1
We employed an adapted version of the contextual cueing paradigm (Chun & Jiang, Citation1998) in which picture-based stimuli served as visual configurations that enable spatial learning. This allowed us to assess whether the typical contextual cueing effect can be replicated in more naturalistic, complex, and meaningful visual scenes. Further, this study aimed to test whether spatial configuration learning is affected by the affective valence of the scenes, expecting a smaller contextual cueing effect in negative as compared with neutral scenes.
Method
Participants
Thirty healthy participants (27 women) with a mean age of 22.1 years (SD = 2.3) were recruited at the university campus and completed this study. Inclusion criteria were current enrolment as a student and normal or corrected-to-normal vision. They received course credits or a small monetary compensation for their participation. This study was approved by the standing ethical committee of the Faculty of Psychology and Neuroscience, Maastricht University.
Spatial configuration learning in complex scenes
Based on the abbreviated Spatial Contextual Cueing Task (Bennett, Barnes, Howard, & Howard, Citation2009), we developed a task that measures spatial configuration learning within neutral and negative emotional scenes. In particular, participants were shown a series of complex visual displays consisting of grey-scale background pictures with a superimposed arrow-shaped target pointing left or right. Participants were required to find the target and indicate in which direction it pointed as fast and accurately as possible. Some of the background scenes were repeated across the task, the target location remaining constant, whereas novel scenes were shown on other trials. Since repeated scenes predict the target location, they were expected to facilitate the search and lead to faster reaction times (RTs) in comparison to trials with novel pictures. This RT difference is referred to as the contextual cueing effect. We relied on an abbreviated version of the original contextual cueing paradigm (Chun & Jiang, Citation1998) as the basis for our task, as it has been shown to produce more robust learning effects that are similarly considered to reflect implicit learning (Bennett et al., Citation2009).
The task was administered twice, once with neutral images serving as background pictures, and once with negative emotional pictures (order counterbalanced). Each task administration consisted of 10 blocks of 14 trials. Beforehand, 14 unique target positions were randomly chosen on a 6-rows by 8-columns grid. In each block, the target appeared on each position once, randomly pointing either left or right. Six out of the 14 trials consisted of displays that were repeated across blocks, in which the target position was held constant. Six other trials consisted of novel displays that appeared only once in the entire task. In order to reduce the possibility that participants become aware of the constant target position in repeated displays, two additional trials with repeated displays were inserted in each block, wherein the target position was varied across blocks. The order of trials was shuffled within each block.
Trials started with a 1 sec fixation period, followed by the display requiring participants to indicate as quickly and accurately as possible whether the arrow-shaped target stimulus pointed left or right by pressing response keys on a response box with the index or middle finger of their dominant hand. The display was presented for 10 sec or until the participant responded, followed by the next trial. Each block was followed by a break that could be ended by the participant. Before the actual task, participants were given a training block consisting of 12 trials with neutral images serving as backgrounds. After each of these training trials, participants were given feedback by displaying the words good, wrong, or missed, for 500 ms in the middle of the screen.
Sixty-eight neutral and 68 negative pictures from the International Affective Picture System (IAPS; Lang et al., Citation2005) served as the basis for the background images during the trials. Twelve additional neutral pictures were selected for the training trials.Footnote1 In order to minimise the influence of low-level stimulus attributes of the IAPS pictures (e.g. luminance, contrast, spatial frequency) on search performance, the pictures of each set were transformed to grayscale and equalised on low-level perceptual features with the SHINE toolbox for Matlab (Willenbockel et al., Citation2010). In particular, spatial frequencies and luminance histograms were equalised in one iteration, using the optimised structural similarity algorithm implemented in the toolbox. During trials, the images were displayed on full screen in 1024 × 768 resolution. The target symbol consisted of a pentagon arrow (44 × 24) filled with grey colour corresponding to the average luminosity of the background picture. Inside the pentagon arrow, a row of four small triangles pointing in the same direction as the arrow were inserted, alternatingly with higher and lower luminance (1 SD) than the average of the background picture. See for an illustration.
Figure 1. Illustration of greyscale background images, equalised for low-level perceptual features, superimposed by search targets pointing left or right. Targets are enlarged and highlighted for illustration. The displayed pictures, proportions, and luminosities do not correspond to the actual task. Images for this illustration were retrieved from www.geograph.org.uk/profile/120370, copyright (CC BY-SA 2.0) by Garry Cornes.

For data reduction, median response times (RT) were derived for accurate responses per block and array type (novel and repeated), and subsequently averaged across 3 consecutive blocks (the first block in each task administration was omitted since the first repetitions occurred in the second block). This yielded novel and repeated RT scores of 3 epochs, respectively for the task with neutral and negative images. An implicit spatial learning score was then calculated by subtracting repeated RT scores from novel RT scores per epoch, and then averaging the outcome across epochs. Accuracy scores were calculated per epoch and array type, though we omitted them from further analysis as they were too close to ceiling (on average >95%) in all conditions.
Explicit spatial memory
Following each task administration, we tested the participants’ explicit spatial memory by showing them the six repeated images once more in random order without the target symbol. For each image, they were required to indicate the position of the target symbol by moving an arrow-shaped cursor with the computer mouse and clicking the left mouse button. Once the participants indicated the target position, the next image was shown. To quantify explicit spatial memory performance, we calculated the Euclidian distance in pixels between the indicated location and the actual target position on each trial. The median distance across trials was extracted and served as an inverse index of explicit spatial memory. In addition, we calculated the proportion of trials in which participants selected a position in the correct image quadrant, allowing us to determine whether their performance exceeded chance level (i.e. 25%).
Procedure
Participants were invited to a single lab session. After giving informed consent, they were given instructions about the spatial learning task and performed the training block. Next, they completed the actual task twice, once with neutral and once with negative images, whereby the order was counterbalanced across participants, and each time followed by the explicit recognition test.
Statistical analysis
We replaced single extreme scores in the distributions of RT and spatial configuration learning scores so that their deviance from the sample mean equalled 2.5 times the sample SD (i.e. Winsorizing; Rivest, Citation1994). Our main analyses focus on the contextual cueing effect in RT using repeated measures ANOVA. All analyses below were repeated with Order of task administration as an additional factor (between subjects), but these models are only described where effects for Order emerged. When sphericity assumptions for ANOVA were violated, Greenhouse-Geisser corrected p values, along with the respective epsilon and uncorrected degrees of freedom, are reported. Alpha was set at .05 for all tests.
Results
Contextual cueing
A 2 (Valence: neutral, negative) by 2 (Array type: novel, repeated) by 3 (Epoch) repeated measures ANOVA revealed a main effect of Array, F(1,29) = 154.42, p < .001, η2p = .84, indicating a strong contextual cueing effect with faster RTs in repeated (M = 804.0 ms, SE = 15.6) compared to novel trials (M = 982.5 ms, SE = 24.7; see ). There also was a main effect of Epoch, F(2,58) = 5.30, p = .008, η2p = .15, and an Epoch by Array interaction, F(2,58) = 3.60, p = .034, η2p = .11, due to reaction times decreasing over time for repeated trials, F(2,58) = 18.92, p < .001, η2p = .40 (Epoch 3 min 1 MDifference = −68.4 ms, SE = 13.2; p[LSD] < .001), but not for novel trials, F(2,58) = 0.51, p = .606, η2p = .02.
Figure 2. Reaction times for search in novel and repeated background scenes with neutral and negative valence. Error bars indicate 95% confidence intervals.
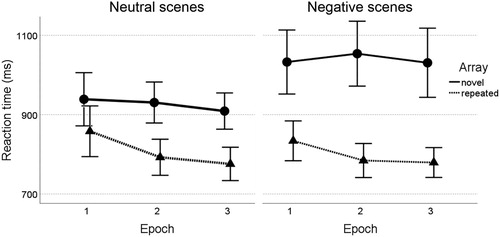
Furthermore, Valence interacted with Array, F(1,29) = 10.07, p = .004, η2p = .26, in the absence of a three-way interaction, p = .969. Follow-up ANOVAs per Array type revealed that repeated trials were unaffected by Valence, F(1,29) = 0.12, p = .735, η2p < .01, but there was a significant Valence effect on RT in novel trials, F(1,29) = 22.27, p < .001, η2p = .43, with slower RTs in negative (M = 1039.1, SE = 32.6) compared to neutral trials (M = 925.9, SE = 21.2). Consequently, the contextual cueing effect (novel – repeated) was larger in negative pictures (M = 244.5 ms, SE = 29.6) as compared to neutral pictures (M = 122.7 ms, SE = 18.7), F(1,29) = 9.80, p = .004, η2p = .25.
Explicit spatial memory
In our analyses concerning Valence effects on explicit spatial memory scores (i.e. median distance from the correct target positions), there was a two-way interaction between Task order and Valence, F(1,27) = 9.41, p = .005, η2p = .26, while Valence had no main effect F(1,27) = 1.65, p = .209, η2p = .06. Follow-up independent-samples t-tests showed that order of task administration had no influence on performance with neutral pictures, t(27) = 0.53, p = .600. However, participants who performed the task with negative pictures first displayed worse explicit spatial memory for the negative pictures (i.e. larger median distance from the target), as compared with participants who had done the task with neutral pictures beforehand, t(27) = −3.29, p = .003. A similar Task order by Valence interaction emerged for the percentage of trials in which participants chose the correct picture quadrant, F(1,27) = 11.21, p = .002, η2p = .29. Again, performance for neutral pictures was unaffected by Task order, t(27) = 0.10, p = .919, while it was for negative pictures, t(27) = 4.00, p < .001. The mean scores for both indices of memory performance can be inspected in .
Table 1. Means (SD) for explicit spatial memory performance on neutral and negative trials.
Summary
Our results demonstrate a robust contextual cueing effect within previously seen complex visual scenes. Moreover, the effect was modulated by the emotionality of the scene (neutral, negative) in which learning took place. Unlike predicted, however, search within repeated negative pictures appeared unaffected, as the responses latencies on these trials did not differ from those in repeated neutral pictures. In contrast, visual search performance was markedly slowed in novel negative scenes, leading to a relatively increased rather than decreased contextual cueing effect for negative scenes.
For explicit spatial memory, we found that participants were able to point to the correct target quadrant above chance level (i.e. 25%; see ), demonstrating some level of explicit knowledge for the scene-target associations. They showed reduced memory performance only in negative images, and only when the task with negative images was administered first. Thus, valence does not affect explicit spatial memory per se. Rather, negative images may be more distracting than neutral images, such that participants who view negative images tend to notice (and memorise) the spatial regularities later. Conversely, practice with neutral images may attenuate the distracting aspects of negative scenes. Overall, valence had no influence on explicit memory when participants performed the task with neutral images first.
Study 2
Building on the insights gained in Study 1, we aimed to test whether acute stress impairs spatial configuration learning in complex visual scenes, moderated by each individual’s endogenous cortisol stress response (Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013). Therefore, we subjected healthy individuals once to the MAST (Quaedflieg et al., Citation2017; Shilton, Laycock, & Crewther, Citation2017; Smeets et al., Citation2012) and once to a no-stress version in two separate laboratory sessions. In a within-subject cross-over design, they completed the spatial configuration learning tasks with neutral and negative images following stress induction or control task. We tested the hypotheses in a sample balanced for sex, accounting for known differences in the hormonal stress response (Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013; Smeets, Dziobek, & Wolf, Citation2009; Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, Citation2001). Based on our prior study, we did not expect sex differences in the contextual cueing effect.
Method
Participants
Thirty-two healthy participants (50% women), recruited at the Maastricht University campus, completed this study. Mean age was 21.7 years (SD = 2.3). A self-report screening form was used to check eligibility, whereby the following exclusion criteria were handled: (1) body mass index (BMI; kg/m²) below 18 or above 30, (2) cardiovascular disease, severe physical illness or endocrine disorders, (3) current psychopathology, (4) substance abuse, (5) heavy smoking (> 10 cigarettes/day), and (6) current use of medication known to affect the function of the HPA axis. For women, hormonal contraceptive use was an additional inclusion criterion, because it suppresses cortisol response variation due to the female menstrual cycle (Kudielka, Hellhammer, & Wüst, Citation2009). This study was approved by the standing ethical committee of the Faculty of Psychology and Neuroscience, Maastricht University. All participants gave informed consent and were compensated with a small financial reward or partial course credit in return for their participation.
Maastricht Acute Stress Test (MAST)
The MAST (Smeets et al., Citation2012) is a procedure that reliably induces subjective and cortisol stress responses by combining physical and mental arithmetic challenges with uncontrollability, unpredictability, and negative social evaluation. At first, participants were instructed about the procedure during a preparation phase lasting 5 min. Next, they underwent a 10 min acute stress phase, whereby they received instructions on the computer screen and were alternately prompted to immerse their left hand in ice-cold water (2 °C) or to engage in mental arithmetic (counting backwards from 2,043 in steps of 17). During mental arithmetic trials, participants were asked to direct their gaze towards a video camera (enabling them to see themselves on a TV monitor) and received negative performance feedback by the experimenter. In total, five hand immersion trials (each lasting 60 or 90 sec) were alternated with 4 mental arithmetic trials (lasting between 45 and 90 sec). The exact number and duration of the two types of trials was unbeknownst to the participants.
No-stress control condition
We employed a no-stress control condition that has an identical procedure as the MAST, but with all stressful elements removed (Smeets et al., Citation2012; Experiment 3). In particular, the water for hand-immersion trials was lukewarm (35 °C), and mental arithmetic was replaced by repeatedly counting aloud from 1 to 25 at a self-chosen pace. No feedback on participants’ performance was given, and there was no videotaping in the control task.
Spatial configuration learning
We used the same two spatial configuration learning tasks with neutral and negative images as in Study 1. The only difference was that participants were given a response box and were required to indicate the direction of the target symbol using the index and middle finger of their right hand, because participants had previously used their left hand during the water immersion trials of the MAST.
Assessment of stress responses
Mood changes in response to the MAST and control condition were measured using repeated administrations of the Positive and Negative Affect Schedule, state version (PANAS; Watson, Clark, & Tellegen, Citation1988). Its two 10-items subscales measure current positive affect (PA; all αs > .88) and negative affect (NA; all αs > .59) on five-point scales (1 = very slightly or not at all; 5 = very much). Given the emotional nature of the stress induction, only NA scores were included in the analyses. Additionally, we administered Subjective Units of Distress Scales (SUDS), asking participants to rate how distressing they found the current part of the experiment on an 11-point scale (anchors: 0 – completely relaxed, 100 – worst distress/anxiety/irritation ever experienced).
To assess hormonal stress responding of the hypothalamic–pituitary–adrenal (HPA) axis, we took salivary cortisol samples using synthetic Salivette devices (Sarstedt®, Etten-Leur, the Netherlands) at 4 time points during each session. Samples were stored at −20 °C immediately on collection. Cortisol levels were determined by a commercially available luminescence immuno assay (IBL, Hamburg, Germany). Mean intra- and inter-assay coefficients of variation are typically less than 8% and 12%, respectively, and the lower and upper detection limits were 0.015 mg/dl (0.41 nmol/l) and 4.0 mg/dl (110.4 nmol/l), respectively.
Procedure
Two laboratory sessions with an interval of one week were scheduled with each participant. Testing was restricted to the early afternoon to reduce the influence of circadian cortisol rhythms (Nicolson, Citation2008). Following the procedure of Meyer, Smeets, Giesbrecht, Quaedflieg, and Merckelbach (Citation2013), participants were instructed to come well-rested and to refrain from activities known to affect cortisol measurements before participation (e.g. no alcohol or drugs for 24 h, no eating, smoking, heavy physical activity, brushing teeth for 2 h). After giving informed consent and providing biographical information in session 1, they were subjected to the MAST or the no-stress control task, preceded and followed by administration of the PANAS. Next, participants were given the training block and the two versions of the spatial learning task. Saliva cortisol measurements were made immediately before MAST or control condition onset (tpre-stress), as well as at t+05, t+20, and t+30 relative to stress/control offset. Session 2 followed the same procedure, but MAST and control condition were substituted. The order of stress vs. control was counterbalanced across participants, and the order of the spatial task versions (neutral, negative) was counterbalanced across participants and sessions.
Statistical analysis
Our main analyses focus on the contextual cueing effect in RT using repeated measures ANOVA, with condition (MAST, control) and valence (neutral, negative) as within-subject factors. In the analyses of cortisol stress responses, biological sex was entered additionally as a between-subjects factor. We repeated all analyses with sex and session order (MAST or control in session 1 or 2) as additional factors, but report these only in the case of additional effects. When sphericity assumptions for ANOVA were violated, Greenhouse-Geisser corrected p values, along with the respective epsilon and uncorrected degrees of freedom, are reported. Alpha was set at .05 for all tests.
Results
Cortisol responses
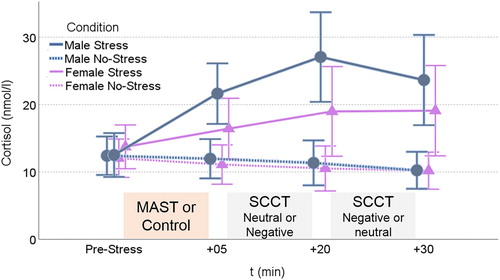
A 2 (Condition: stress, control) by 4 (Time: cortisol measurements) by 2 (Sex) repeated measures ANOVA on cortisol levels revealed a significant interaction between Time and Condition, F(3,90) = 18.62, ε = .49, p < .001, η2p = .38. As expected, follow-up ANOVAs showed a strong cortisol increase in the stress condition, F(3,90) = 13.10, ε = .48, p < .001, η2p = .30, with elevated values at all post-stress measurements compared to baseline (ps[LSD] < = .001). The control condition displayed an opposite Time effect, F(3,90) = 5.10, ε = .53, p = .015, η2p = .15, best described by a linear decrease, contrast F(1,30) = 7.81, p = .009, η2p = .21 (see ). Although Sex did not interact significantly with Time and Condition, F(3,90) = 2.50, ε = .49, p = .108, η2p = .08, the quadratic contrast for Time by Sex in the stress condition, F(1,30) = 8.09, p = .008, η2p = .21 aligns with prior findings of higher peak cortisol responses in men compared to women using contraceptives (Kudielka et al., Citation2009). Descriptively, 88% of men (14/16) and 60% of women (10/16) displayed a peak cortisol response larger than 1.5 nmol/l in the stress condition and can be classified as cortisol responders (Miller, Plessow, Kirschbaum, & Stalder, Citation2013).
Subjective stress responses
A 2 (Condition: stress, control) by 2 (Time: PANAS measurements) repeated measures ANOVA for NA revealed a significant Time by Condition interaction, F(1,31) = 19.98, p < .001, η2p = .39. Paired-samples t-tests showed that NA increased in the stress condition by 3.2 points (SD = 5.1) from pre- to post-stress, t(31) = 3.57, p = .001. In the control condition, NA decreased by 2.0 points (SD = 2.7), t(31) = −4.15, p < .001. Consequently, NA scores differed between conditions as intended post-stress (p < .001), but not pre-stress (p = .859). A similar Time by Condition interaction was evident for SUDS scores, F(1,31) = 66.35, p < .001, η2p = .68,Footnote2 with an increase of 4.0 points (SD = 2.4) in the stress condition and no change in the control condition (MDifference = 0.0, SD = 1.1).
Contextual cueing effect
In a 2 (condition: stress, control) by 2 (Valence: neutral, negative) by 2 (Array type: novel, repeated) by 3 (Epoch) repeated measures ANOVA on RTs (see ), we found no significant four-way interaction, F(2,62) = 2.08, p = .134, η2p = .06. While the Array by Epoch interaction was not significant (p = .069), the typical main effect of Array emerged, F(1,31) = 27.48, p < .001, η2p = .50, with shorter RTs in repeated compared to novel trials, while all RTs decreased over time, Epoch F(2,62) = 10.47, ε = .84, p < .001, η2p = .25. Furthermore, Condition interacted with Array, F(1,31) = 6.88, p = .013, η2p = .18. That is, stress did not affect novel arrays (M = 895.1 ms, SE = 18.7), F = 0.01, p = .916, but RTs on repeated trials were slowed in the stress condition (M = 837.0 ms, SE = 21.1) as compared with the control condition (M = 785.7 ms, SE = 14.3), F(1,31) = 4.93, p = .034, η2p = .13. Meanwhile, the Array by Valence interaction from Study 1 did not replicate across stress and control conditions, F(1,31) = 0.20, p = .660, η2p = .01, but Valence had a main effect, F(1,31) = 30.72, p < .001, η2p = .50, with shorter RTs in neutral compared to negative trials. No other effects were statistically significant, ps > .071.Footnote3,Footnote4 Mean contextual cueing scores per condition can be inspected in .
Figure 4. RT in novel and repeated neutral and negative scenes over time, in the control and in the stress condition. Error bars indicate 95% confidence intervals.
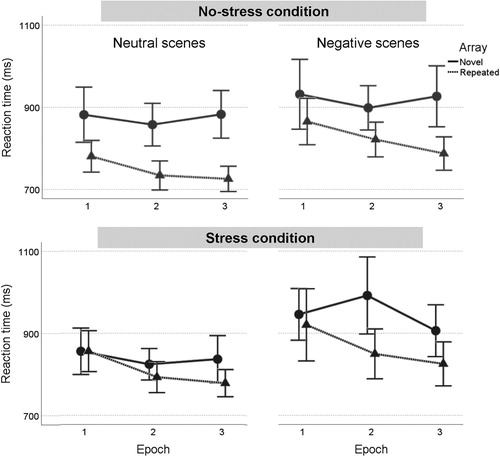
Figure 5. Contextual cueing effect (RT in novel minus repeated scenes) over time in the stress and control condition, separately for neutral and negative scenes. Error bars indicate 95% confidence intervals.
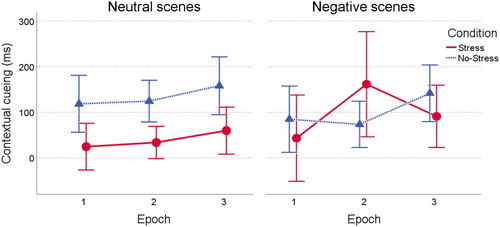
Finally, to explore a potential moderation by cortisol responses, we ran the 2 (Condition) by 2 (Valence) by 3 (Epoch) ANOVA on contextual cueing scores, entering delta-peak cortisol response as a covariate. No main or interaction effects involving the cortisol response were found, all ps > .14.
Explicit spatial memory
Note that unlike study 1, explicit spatial memory was tested only once at the end of session 2. We thus analysed the scores in a 2 × 2 ANOVA with Condition (MAST or control in session 2; between-subjects) and Valence (neutral or negative pictures in the final task; between-subjects). For distance errors, we found no main or interaction effects, all ps > .63 (overall M = 106.6 px, SD = 63.7). Similarly, there were no main or interaction effects for the percentage of correctly identified picture quadrants, all ps > .65 (overall M = 70.8%, SD = 20.7). No additional effects emerged when cortisol responding was added to the model as a covariate.
Discussion
In two experiments, we investigated whether visuospatial context learning is modulated by the valence of the scene’s content and by acute stress experienced immediately prior to learning. Both studies demonstrated clear search advantages for repeated over novel scenes, replicating a robust contextual cueing effect (Chun & Jiang, Citation1998) in more complex and meaningful visual scenes. In addition, visual search was selectively slowed in negative scenes that were novel in Study 1 (i.e. amplifying the contextual cueing effect), and in negative scenes of both types in Study 2. In Study 2, we additionally found that acute stress interfered with spatial configuration learning, as compared to the no-stress control condition. These effects were not statistically moderated by scene valence or by the endogenous cortisol stress response.
Our findings appear to contravene the idea that implicit configuration learning is impaired within affectively laden scenes (Kunar, Watson, et al., Citation2014). In particular, the slowed response latencies for novel negative scenes suggests that participants deployed greater attention to the meaningful content of novel negative scenes and were slower to disengage from them. In Study 1, this search disadvantage disappeared entirely for negative scenes that participants had seen already during the task. Indeed, this may indicate that participants can fully benefit from configuration memory to allocate their attention in repeated threatening environments. In Study 2, search times were also slowed in repeated negative scenes, as compared with repeated neutral scenes, but to a similar degree as for novel negative scenes. Since the disparity between novel and repeated scenes remained constant or even increased in both of our studies, it seems safe to conclude that spatial configuration learning was not impaired in negative compared to neutral scenes. These findings align with the studies by Yamaguchi and Harwood (Citation2017) and Szekely et al. (Citation2017) who found that affective elements within the search configuration systematically affect search performance in predicted ways, while leaving configuration learning intact or even amplifying it.
At first sight, our findings appear to contradict those by Kunar, Watson, et al. (Citation2014), who found impaired configuration learning following exposure to aversive images. More broadly, the findings may seem at odds with the view that the presence of aversive stimuli generally reduces contextual learning (Steinmetz & Kensinger, Citation2013) due to a narrowed attentional focus (Gable & Harmon-Jones, Citation2010). One possible interpretation is that contextual cueing is in fact entirely robust against factors interfering with attentional capacities. Indeed, latent learning configuration learning has been demonstrated even when visuospatial working memory capacities were tapped by a concurrent task (Pollmann, Citation2018). However, a critical difference between our Kunar and colleagues’ studies is that we assessed context learning within neutral and affective scenes rather than studying the impact of affective stimuli on learning entirely unrelated configurations. Taken together, the pattern of findings across studies suggests that a confrontation with aversive stimuli may impair the learning of unrelated spatial configurations that are encountered afterwards, but leave configuration learning intact within the aversive situation itself, or even amplifying it (cf. Szekely et al., Citation2017; Yamaguchi & Harwood, Citation2017; Zinchenko et al., Citationin press).
Strikingly, the results of Study 2 align well with this view. Here, we demonstrated that exposure a powerful laboratory stressor disrupted subsequent learning of (unrelated) spatial configurations. As such, this result can be considered a replication and extension of the Kunar, Watson, et al. (Citation2014) study. These findings also accord with the literature, since the contextual cueing effect has been associated with major input regions for the hippocampus in the entorhinal and the perirhinal cortices (Chun & Phelps, Citation1999; Manns & Squire, Citation2001; Preston & Gabrieli, Citation2008). These areas are thought to be strongly regulated by the acute ANS and HPA axis responses to acute stress, with some evidence suggesting a shift away from hippocampus-based information processing (Quaedflieg & Schwabe, Citation2018; Smeets et al., Citation2018; Wirz et al., Citation2017). However, we did not replicate the previous finding where the stress-induced impairments were modulated by the endogenous cortisol stress response, with reduced contextual cueing only in low cortisol responding individuals (Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013). This apparent disparity warrants further careful investigation. An interesting possibility is that the presence of meaningful and affective stimuli in the present study may have altered the manifestation of the ANS and HPA axis responses. In this respect, it is important to note that the present study may not have been able to detect smaller higher-level interactions. For instance, on the basis of effects reported in prior studies (e.g. Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013, found a η2p = .22 for a condition × stress responder interaction), our sample size was adequate to detect an effect size of f(U) = 0.53 (specified “as in SPSS” using G*Power v.3.1.9; α = .05, ε = 1) in a 2 × 2 mixed ANOVA with a power (1–β) = .80. However, the detection of more complex patterns should be addressed directly in the future using a more high-powered study.
In interpreting the current findings, it is important to keep in mind that we tested spatial memory for a target that was inserted in the scene, and the target location was not linked to the meaning of the scene. Therefore, different results may emerge if the target location were to be matched to central or peripheral scene details (e.g. Szekely et al., Citation2017; Yamaguchi & Harwood, Citation2017). Relatedly, our negative images likely had more attention-grabbing features than neutral images, despite being equalised on low-level perceptual dimensions, including image complexity and the salience or number of objects. In fact, segmented and salient areas of a scene have been shown to interfere with contextual cuing and reduce the configuration’s predictive impact on search (Conci & von Mühlenen, Citation2009). In a similar vein, some scene features can be more important for contextual cueing than others (e.g. configural cues versus background colours; Kunar, John, & Sweetman, Citation2014), which may have introduced uncontrolled variance and affected our findings. However, our data indicate no reduction in contextual cueing for negative scenes. Thereby, our data aligns with recent evidence that configuration learning is independent of the configuration’s meaning (e.g. Makovski, Citation2018). This further suggests that scene valence is not a part of the relevant memory traces, or that valence associations are overshadowed by the salient target-scene configuration (e.g. Sharifian, Contier, Preuschhof, & Pollmann, Citation2017). Future studies may want to include more direct measures of the visual search to disentangle these issues in more detail (e.g. number of fixations in eye-tracking; Harris & Remington, Citation2017).
A few limitations of the present studies need to be considered. First, while we controlled for low level perceptual differences between the background scenes, the transformation and grey-scaling of the IAPS pictures may have reduced the emotionality of the pictures, as well as the degree to which they were memorised. Future studies could address these possibilities by including distinct memory tests for the background pictures. Moreover, ANS measures of acute affective responses to the emotional pictures could be included, since arousal might interact with cortisol responses in modulating memory. Third, our participants displayed some degree of explicit knowledge for the scene-target associations (see, e.g. ). Interestingly, in Study 1, the task order affected explicit memory for negative scenes, but not the contextual cueing effect. While this suggests that these types of memory operate independently, we cannot exclude the possibility that explicit and semantic memory processes, in addition to implicit memory formation (Brockmole & Henderson, Citation2006b), may have contributed to our findings. Another limitation is that we tested the hypotheses in a high-functioning healthy sample and relied on the MAST to examine the effects of acute stress. While the MAST is one of the most robust experimental stress procedures (Quaedflieg et al., Citation2017; Smeets et al., Citation2012), the effects may not be comparable to real-life traumatic stressors (but see Kidd, Carvalho, & Steptoe, Citation2014). These factors may limit the generalizability to other populations, such as traumatised individuals with PTSD. Finally, our study may have been unable to detect smaller effects due to the sample size. This is particularly relevant to potential smaller interactions between valence and stress, as well as moderation effects of individual differences in the stress cortisol response (Meyer, Smeets, Giesbrecht, Quaedflieg, & Merckelbach, Citation2013). Therefore, these effects warrant to be addressed more directly in future replications.
Together with the emerging pattern of findings in the literature (Kunar, Watson, et al., Citation2014; Yamaguchi & Harwood, Citation2017), our findings may suggest that spatial configuration learning functions well – or even better – during confrontation with aversive stimuli in the environment. However, exposure to aversive stimuli and acute stress may undermine spatial configuration learning in subsequently encountered environments. This interpretation can potentially reconcile our findings with the idea that negative affect leads to a narrowed attentional focus (Gable & Harmon-Jones, Citation2010), which would increase attention and memory for aversive stimuli at the expense of (subsequent) unrelated context (e.g. Steinmetz & Kensinger, Citation2013). At this point, it is also important to keep in mind that the contextual cueing paradigm measures ongoing configuration learning and thus simultaneously invokes encoding, consolidation, and retrieval phases of memory formation. These phases may be hypothesised to be affected differentially by valence and by acute stress, in a time-dependent manner (e.g. Quaedflieg et al., Citation2015; Quaedflieg & Schwabe, Citation2018; Quaedflieg, Schwabe, Meyer, & Smeets, Citation2013). For instance, the stress-induced impairments in configuration learning might be due to a failure to retrieve spatial memory despite latent learning (Pollmann, Citation2018). Interestingly, this would align with the view that the ANS and HPA axis responses to acute stress generally facilitate the consolidation of new memories whilst inhibiting the memory retrieval (Smeets et al., Citation2008; Wolf, Citation2017). The issues await to be tested directly, e.g. by separating learning from long-term configuration memory (Chun & Jiang, Citation2003). Future studies may address the idea that spatial configuration learning is modulated under stress as a function of proximity to the stressor. This would add to the emerging evidence that the human stress response differentially modulates memory, depending on type of memory (e.g. Smeets et al., Citation2018) and temporal proximity of stress (e.g. Quaedflieg et al., Citation2015).
More broadly, our findings contribute to our understanding of contextual memory formation in emotional situations, which features prominently in information theories of PTSD (e.g. Ehlers, Citation2010). For instance, the dual representation model (Brewin et al., Citation2010) postulates that failures to form contextualised memories in the hippocampal area allows sensation-based, egocentric representations of trauma to be activated in isolation from the spatiotemporal context, resulting in intrusive “reliving” of the trauma. Spatial configuration learning heavily relies on the hippocampal area (e.g. Chun & Phelps, Citation1999; Preston & Gabrieli, Citation2008). Based on the dual representation model, this could suggest an intimate relationship of configuration learning with the development of hippocampus-based memory contextualisation, which should be associated with fewer intrusive memories. However, contextual cueing may rely on egocentric spatial memory (Chua & Chun, Citation2003), and its relationship with other forms of contextual memory integration associations (e.g. Bisby et al., Citation2018; Bisby & Burgess, Citation2014; Meyer et al., Citation2017; Zhang, van Ast, Klumpers, Roelofs, & Hermans, Citation2018) remains poorly understood.
Indeed, individuals with superior spatial configuration have been found to develop fewer intrusive memories after viewing a trauma film (Meyer, Smeets, Giesbrecht, Quaedflieg, Girardelli, et al., Citation2013). However, another study by our lab found the exact opposite pattern (Meyer et al., Citation2017). Interestingly, in the latter study, spatial configuration learning was tested following a different memory task with aversive pictures. Speculatively, this may suggest that contextual cueing can both promote and suppress intrusive memories, depending on the response to aversive stimuli in the environment. Accordingly, spatial configuration learning may be critical for the construction of both contextual and sensation-based memories of aversive events (Brewin et al., Citation2010), orchestrated by the acute stress response. Future research may want to explore this idea, based on the groundwork laid in the present studies.
Conclusions
Contextual learning pervades all levels of perception and cognition (Chun, Citation2000) and is critical for everyday functioning and psychological well-being (Maren et al., Citation2013). Our study demonstrates that individuals are able to memorise spatial regularities in complex visual scenes. This ability was not compromised in scenes with an aversive emotional content. However, when our participants were acutely stressed, they were less able learn spatial configurations and use this information to guide their attention during visual search. Future studies should directly address the possibilities that spatial configuration learning can be up- and downregulated as a function of spatial or temporal proximity to a stressor. Moreover, it seems promising to further explore the interaction between stress, contextual cueing, and contextual memory formation in the development of stress-related psychopathology.
Acknowledgements
We are especially thankful to Ruchira Suresh and Doriana Marchetti for her help in collecting the data and to Ron Hellenbrand (Maastricht University) for implementing the spatial learning paradigm.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Thomas Meyer http://orcid.org/0000-0001-7228-5365
Additional information
Funding
Notes
1 The 68 neutral images were IAPS numbers: 2102, 2104, 2190, 2191, 2200, 2215, 2221, 2383, 2396, 2397, 2575, 2745, 2850, 2870, 5130, 5395, 5455, 5471, 5500, 5520, 5530, 5532, 5740, 7000, 7004, 7010, 7020, 7025, 7031, 7035, 7036, 7037, 7038, 7041, 7043, 7046, 7050, 7052, 7054, 7055, 7056, 7057, 7059, 7060, 7080, 7090, 7100, 7130, 7150, 7170, 7175, 7211, 7217, 7224, 7233, 7234, 7242, 7500, 7546, 7547, 7560, 7595, 7700, 7710, 7920, 7950, 9080. Negative images: 1052, 1070, 1090, 1120, 1220, 1280, 1300, 1932, 2055, 2120, 2661, 2683, 2688, 2691, 2694, 2703, 2730, 2811, 2981, 3000, 3010, 3016, 3022, 3030, 3053, 3064, 3068, 3069, 3071, 3100, 3110, 3120, 3130, 3150, 3180, 3225, 3230, 3250, 3261, 3500, 3530, 3550, 6010, 6020, 6190, 6200, 6210, 6211, 6212, 6230, 6231, 6312, 6313, 6315, 6370, 6510, 6540, 6550, 6560, 6821, 9040, 9042, 9181, 9254, 9410, 9423, 9490, 9594. Additional neutral images for training trials: 2206, 2372, 2441, 2840, 6150, 7040, 7053, 7110, 7491, 7493, 7550, 7705.
2 In addition, when Order and Sex was added to the model, there was a trivial four-way interaction involving Sex and Order, p = .045, η2p = .14, driven by higher baseline distress in the control condition for women who had previously experienced the stress condition, as compared with men, t(14) = 2.26, p = .040.
3 Two trend-level three-way interactions were observed, namely interactions of Condition and Array with Valence, F(1,31) = 3.34, p = .077, η2p = .10, and with Epoch, F(2,62) = 2.76, p = .071, η2p = .08, suggesting that the negative impact of stress on learning might be dampened in negative scenes. However, these trends may have been driven largely by one influential case, despite Winsorizing (after removing this case ps > .13). Moreover, in none of the conditions, we found no support for an Array × Valence interaction, Fs(1,31) < 2.3, ps > .138, η2ps < .07.
4 When condition Order and Sex were included in the model, an Order by Condition interaction emerged, F(1,28) = 81.46, p < .001, η2p = .75, partly qualified by Valence, F(1,28) = 10.10, p = .004, η2p = .27. These effects were driven by generally faster RTs in the second compared to the first session, which effect was more pronounced for negative images.
References
- Barrett, L. F., & Kensinger, E. A. (2010). Context is routinely encoded during emotion perception. Psychological Science, 21(4), 595–599. doi: 10.1177/0956797610363547
- Bennett, I. J., Barnes, K. A., Howard, J. H., & Howard, D. V. (2009). An abbreviated implicit spatial context learning task that yields greater learning. Behavior Research Methods, 41(2), 391–395. doi: 10.3758/brm.41.2.391
- Bisby, J. A., & Burgess, N. (2014). Negative affect impairs associative memory but not item memory. Learning & Memory, 21(1), 21–27. doi: 10.1101/lm.032409.113
- Bisby, J. A., Horner, A. J., Bush, D., & Burgess, N. (2018). Negative emotional content disrupts the coherence of episodic memories. Journal of Experimental Psychology: General, 147(2), 243–256. doi: 10.1037/xge0000356
- Brewin, C. R., Gregory, J. D., Lipton, M., & Burgess, N. (2010). Intrusive images in psychological disorders: Characteristics, neural mechanisms, and treatment implications. Psychological Review, 117(1), 210–232. doi: 10.1037/a0018113
- Brockmole, J. R., & Henderson, J. M. (2006a). Recognition and attention guidance during contextual cueing in real-world scenes: Evidence from eye movements. Quarterly Journal of Experimental Psychology, 59(7), 1177–1187. doi: 10.1080/17470210600665996
- Brockmole, J. R., & Henderson, J. M. (2006b). Using real-world scenes as contextual cues for search. Visual Cognition, 13(1), 99–108. doi: 10.1080/13506280500165188
- Burgess, N., Maguire, E. A., & O’Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron, 35(4), 625–641. doi: 10.1016/s0896-6273(02)00830-9
- Chua, K. P., & Chun, M. M. (2003). Implicit scene learning is viewpoint dependent. Perception & Psychophysics, 65(1), 72–80.
- Chun, M. M. (2000). Contextual cueing of visual attention. Trends in Cognitive Sciences, 4(5), 170–178. doi: 10.1016/S1364-6613(00)01476-5
- Chun, M. M., & Jiang, Y. H. (1998). Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cognitive Psychology, 36(1), 28–71.
- Chun, M. M., & Jiang, Y. H. (2003). Implicit, long-term spatial contextual memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29(2), 224–234. doi: 10.1037/0278-7393.29.2.224
- Chun, M. M., & Phelps, E. A. (1999). Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience, 2(9), 844–847.
- Colagiuri, B., & Livesey, E. J. (2016). Contextual cuing as a form of nonconscious learning: Theoretical and empirical analysis in large and very large samples. Psychonomic Bulletin & Review, 23(6), 1996–2009. doi: 10.3758/s13423-016-1063-0
- Conci, M., & von Mühlenen, A. (2009). Region segmentation and contextual cuing. Attention, Perception, & Psychophysics, 71(7), 1514–1524. doi: 10.3758/APP.71.7.1514
- de Kloet, E. R., Joels, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. doi: 10.1038/nrn1683
- Ehlers, A. (2010). Understanding and treating unwanted trauma memories in posttraumatic stress disorder. Zeitschrift Fur Psychologie-Journal of Psychology, 218(2), 141–145. doi: 10.1027/0044-3409/a000021
- Fyhn, M., Hafting, T., Treves, A., Moser, M.-B., & Moser, E. I. (2007). Hippocampal remapping and grid realignment in entorhinal cortex. Nature, 446(7132), 190–194.
- Gable, P., & Harmon-Jones, E. (2010). The motivational dimensional model of affect: Implications for breadth of attention, memory, and cognitive categorisation. Cognition and Emotion, 24(2), 322–337.
- Harris, A. M., & Remington, R. W. (2017). Contextual cueing improves attentional guidance, even when guidance is supposedly optimal. Journal of Experimental Psychology: Human Perception and Performance, 43(5), 926–940. doi: 10.1037/xhp0000394
- Kidd, T., Carvalho, L. A., & Steptoe, A. (2014). The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biological Psychology, 99, 34–40. doi: 10.1016/j.biopsycho.2014.02.010
- Kudielka, B. M., Hellhammer, D. H., & Wüst, S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34(1), 2–18. doi: 10.1016/j.psyneuen.2008.10.004
- Kuhlmann, S., Piel, M., & Wolf, O. T. (2005). Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience, 25(11), 2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005
- Kunar, M. A., Flusberg, S., Horowitz, T. S., & Wolfe, J. M. (2007). Does contextual cuing guide the deployment of attention? Journal of Experimental Psychology: Human Perception and Performance, 33(4), 816–828. doi: 10.1037/0096-1523.33.4.816
- Kunar, M. A., John, R., & Sweetman, H. (2014). A configural dominant account of contextual cueing: Configural cues are stronger than colour cues. Quarterly Journal of Experimental Psychology, 67(7), 1366–1382. doi: 10.1080/17470218.2013.863373
- Kunar, M. A., Watson, D. G., Cole, L., & Cox, A. (2014). Negative emotional stimuli reduce contextual cueing but not response times in inefficient search. Quarterly Journal of Experimental Psychology, 67(2), 377–393. doi: 10.1080/17470218.2013.815236
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2005). International affective picture system (IAPS): Instruction manual and affective ratings. Technical report A-6. Gainesville, FL: University of Florida.
- Makovski, T. (2018). Meaning in learning: Contextual cueing relies on objects’ visual features and not on objects’ meaning. Memory & Cognition, 46(1), 58–67. doi: 10.3758/s13421-017-0745-9
- Manginelli, A. A., Langer, N., Klose, D., & Pollmann, S. (2013). Contextual cueing under working memory load: Selective interference of visuospatial load with expression of learning. Attention, Perception, & Psychophysics, 75(6), 1103–1117. doi: 10.3758/s13414-013-0466-5
- Manns, J. R., & Squire, L. R. (2001). Perceptual learning, awareness, and the hippocampus. Hippocampus, 11(6), 776–782. doi: 10.1002/hipo.1093
- Maren, S., Phan, K. L., & Liberzon, I. (2013). The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14, 417–428. doi: 10.1038/nrn3492
- Meyer, T., Krans, J., van Ast, V., & Smeets, T. (2017). Visuospatial context learning and configuration learning is associated with analogue traumatic intrusions. Journal of Behavior Therapy and Experimental Psychiatry, 54, 120–127. doi: 10.1016/j.jbtep.2016.07.010
- Meyer, T., Smeets, T., Giesbrecht, T., Quaedflieg, C. W. E. M., Girardelli, M. M., Mackay, G. R. N., & Merckelbach, H. (2013). Individual differences in spatial configuration learning predict the occurrence of intrusive memories. Cognitive, Affective, & Behavioral Neuroscience, 13(1), 186–196. doi: 10.3758/s13415-012-0123-9
- Meyer, T., Smeets, T., Giesbrecht, T., Quaedflieg, C. W. E. M., & Merckelbach, H. (2013). Acute stress differentially affects spatial configuration learning in high and low cortisol-responding healthy adults. European Journal of Psychotraumatology, 4(1), 19854. doi: 10.3402/ejpt.v4i0.19854
- Miller, R., Plessow, F., Kirschbaum, C., & Stalder, T. (2013). Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: Evaluation of salivary cortisol pulse detection in panel designs. Psychosomatic Medicine, 75(9), 832–840. doi: 10.1097/psy.0000000000000002
- Murray, E. A., Bussey, T. J., & Saksida, L. M. (2007). Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Annual Review of Neuroscience, 30, 99–122. doi: 10.1146/annurev.neuro.29.051605.113046
- Nicolson, N. A. (2008). Measurement of cortisol. In L. J. Luecken & L. C. Gallo (Eds.), Handbook of physiological research methods in health psychology (pp. 37–74). Los Angeles: Sage.
- Olatunji, B. O., Ciesielski, B. G., Armstrong, T., & Zald, D. H. (2011). Emotional expressions and visual search efficiency: Specificity and effects of anxiety symptoms. Emotion, 11(5), 1073–1079.
- Phelps, E. A., Ling, S., & Carrasco, M. (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science, 17(4), 292–299.
- Pollmann, S. (2018). Working memory dependence of spatial contextual cueing for visual search. British Journal of Psychology, doi: 10.1111/bjop.12311
- Preston, A. R., & Gabrieli, J. D. E. (2008). Dissociation between explicit memory and configural memory in the human medial temporal lobe. Cerebral Cortex, 18(9), 2192–2207. doi: 10.1093/cercor/bhm245
- Quaedflieg, C. W. E. M., Meyer, T., Van Ruitenbeek, P., & Smeets, T. (2017). Examining habituation and sensitization across repetitive laboratory stress inductions using the MAST. Psychoneuroendocrinology, 77, 175–181. doi: 10.1016/j.psyneuen.2016.12.009
- Quaedflieg, C. W. E. M., & Schwabe, L. (2018). Memory dynamics under stress. Memory (Hove, England), 26(3), 364–376. doi: 10.1080/09658211.2017.1338299
- Quaedflieg, C. W. E. M., Schwabe, L., Meyer, T., & Smeets, T. (2013). Time dependent effects of stress prior to encoding on event-related potentials and 24h delayed retrieval. Psychoneuroendocrinology, 38(12), 3057–3069. doi: 10.1016/j.psyneuen.2013.09.002
- Quaedflieg, C. W. E. M., van de Ven, V., Meyer, T., Siep, N., Merckelbach, H., & Smeets, T. (2015). Temporal dynamics of stress-induced alternations of intrinsic amygdala connectivity and neuroendocrine levels. PLoS ONE, 10(5), e0124141. doi: 10.1371/journal.pone.0124141
- Rivest, L. P. (1994). Statistical properties of Winsorized means for skewed distributions. Biometrika, 81(2), 373–383. doi: 10.1093/biomet/81.2.373
- Roozendaal, B., Griffith, Q. K., Buranday, J., de Quervain, D. J. F., & McGaugh, J. L. (2003). The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proceedings of the National Academy of Sciences, 100(3), 1328–1333. doi: 10.1073/pnas.0337480100
- Roozendaal, B., Okuda, S., de Quervain, D. J. F., & McGaugh, J. L. (2006). Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience, 138(3), 901–910. doi: 10.1016/j.neuroscience.2005.07.049
- Rubin, D. C., Berntsen, D., & Bohni, M. K. (2008). Memory-based model of posttraumatic stress disorder: Evaluating basic assumptions underlying the PTSD diagnosis. Psychological Review, 115(4), 985–1011. doi: 10.1037/a0013397
- Schankin, A., & Schubo, A. (2009). Cognitive processes facilitated by contextual cueing: Evidence from event-related brain potentials. Psychophysiology, 46(3), 668–679. doi: 10.1111/j.1469-8986.2009.00807.x
- Schwabe, L., Joëls, M., Roozendaal, B., Wolf, O. T., & Oitzl, M. S. (2012). Stress effects on memory: An update and integration. Neuroscience & Biobehavioral Reviews, 36(7), 1740–1749. doi: 10.1016/j.neubiorev.2011.07.002
- Sharifian, F., Contier, O., Preuschhof, C., & Pollmann, S. (2017). Reward modulation of contextual cueing: Repeated context overshadows repeated target location. Attention, Perception, & Psychophysics, 79(7), 1871–1877. doi: 10.3758/s13414-017-1397-3
- Shilton, A. L., Laycock, R., & Crewther, S. G. (2017). The Maastricht Acute Stress Test (MAST): Physiological and subjective responses in anticipation, and post-stress. Frontiers in Psychology, 8(567), doi: 10.3389/fpsyg.2017.00567
- Smeets, T., Cornelisse, S., Quaedflieg, C. W. E. M., Meyer, T., Jelicic, M., & Merckelbach, H. (2012). Introducing the Maastricht Acute Stress Test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology, 37(12), 1998–2008. doi: 10.1016/j.psyneuen.2012.04.012
- Smeets, T., Dziobek, I., & Wolf, O. T. (2009). Social cognition under stress: Differential effects of stress-induced cortisol elevations in healthy young men and women. Hormones and Behavior, 55(4), 507–513. doi: 10.1016/j.yhbeh.2009.01.011
- Smeets, T., Otgaar, H., Candel, I., & Wolf, O. T. (2008). True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology, 33(10), 1378–1386. doi: 10.1016/j.psyneuen.2008.07.009
- Smeets, T., van Ruitenbeek, P., Hartogsveld, B., & Quaedflieg, C. W. E. M. (2018). Stress-induced reliance on habitual behavior is moderated by cortisol reactivity. Brain and Cognition, doi: 10.1016/j.bandc.2018.05.005
- Steinmetz, K. M., & Kensinger, E. (2013). The emotion-induced memory trade-off: More than an effect of overt attention? Memory & Cognition, 41(1), 69–81. doi: 10.3758/s13421-012-0247-8
- Szekely, A., Rajaram, S., & Mohanty, A. (2017). Context learning for threat detection. Cognition and Emotion, 31(8), 1525–1542. doi: 10.1080/02699931.2016.1237349
- Thompson, R. (1991). Emotional regulation and emotional development. Educational Psychology Review, 3(4), 269–307.
- Travis, S. L., Mattingley, J. B., & Dux, P. E. (2013). On the role of working memory in spatial contextual cueing. Journal of Experimental Psychology: Learning, Memory, and Cognition, 39(1), 208–219. doi: 10.1037/a0028644
- Vadillo, M. A., Konstantinidis, E., & Shanks, D. R. (2016). Underpowered samples, false negatives, and unconscious learning. Psychonomic Bulletin & Review, 23(1), 87–102. doi: 10.3758/s13423-015-0892-6
- van Strien, N. M., Cappaert, N. L. M., & Witter, M. P. (2009). The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nature Reviews Neuroscience, 10(4), 272–282. doi: 10.1038/nrn2614
- Vickery, T. J., Sussman, R. S., & Jiang, Y. V. (2010). Spatial context learning survives interference from working memory load. Journal of Experimental Psychology: Human Perception and Performance, 36(6), 1358–1371.
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. doi: 10.1037/0022-3514.54.6.1063
- Willenbockel, V., Sadr, J., Fiset, D., Horne, G., Gosselin, F., & Tanaka, J. (2010). Controlling low-level image properties: The SHINE toolbox. Behavior Research Methods, 42(3), 671–684. doi: 10.3758/BRM.42.3.671
- Wirz, L., Wacker, J., Felten, A., Reuter, M., & Schwabe, L. (2017). A deletion variant of the α2b-adrenoceptor modulates the stress-induced shift from “cognitive” to “habit” memory. The Journal of Neuroscience, 37(8), 2149–2160. doi: 10.1523/jneurosci.3507-16.2017
- Wolf, O. T. (2017). Stress and memory retrieval: Mechanisms and consequences. Current Opinion in Behavioral Sciences, 14, 40–46. doi: 10.1016/j.cobeha.2016.12.001
- Wolf, O. T., Schommer, N. C., Hellhammer, D. H., McEwen, B. S., & Kirschbaum, C. (2001). The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology, 26(7), 711–720. doi: 10.1016/s0306-4530(01)00025-7
- Yamaguchi, M., & Harwood, S. L. (2017). Threat captures attention but does not affect learning of contextual regularities. Cognition and Emotion, 31(3), 564–571. doi: 10.1080/02699931.2015.1115752
- Zhang, W., van Ast, V. A., Klumpers, F., Roelofs, K., & Hermans, E. J. (2018). Memory contextualization: The role of prefrontal cortex in functional integration across item and context representational regions. Journal of Cognitive Neuroscience, 30(4), 579–593. doi: 10.1162/jocn_a_01218
- Zinchenko, A., Geyer, T., Müller, H., & Conci, M. (in press). Affective modulation of memory-based guidance in visual search: Dissociative role of positive and negative emotions.