Abstract
Skeletal muscles undergo macroscopic changes, including atrophy and myosteatosis, alongside microscopic changes in their phenotype due to aging. Sarcopenia is a muscle disease that affects older people and is characterized by the progressive loss of skeletal muscle, resulting in reduced muscle function and a decrease in quality of life. Following the onset of sarcopenia, current treatment relies upon a progressive resistance training (RT) programme to promote muscle hypertrophy and improve physical performance by the upregulation of protein synthesis and the increase in type II muscle fiber size. However, RT is not appropriate for all patients, and is infrequently integrated into medicine or social care for older people. Alternative interventions to RT include multi-modal training, low-intensity blood-flow restriction training, and whole-body vibration training. Understanding the macroscopic and microscopic changes in the skeletal muscle in response to different interventions is essential to recognizing their validity and further utility as a preventative intervention.
Introduction
Sarcopenia is a muscle disease observed in older people that is characterized by the progressive loss of skeletal muscle mass, leading to impaired muscle function and physical performance.Citation1 The loss of muscle mass typically occurs at a rate of 3–8% per decade after the age of thirty,Citation2 with multiple age-related processes contributing to the development of the disease, including loss of muscle fibers (primarily type II), anabolic resistance, reduced satellite cell function, chronic low-grade inflammation, and age-related hormonal changes.Citation3 The number of older people is increasing from 727 million people older than 65 years in 2020 to an estimated 1.5 billion people worldwide by 2050, suggesting that there will be a significant increase in those affected by muscle loss during aging. Sarcopenia gained disease status in 2016, highlighting the serious implications to quality of life (QoL) that sarcopenia can present.Citation4 Modern changes in lifestyle choices, including the expansion of obesity rates, reduced physical activity, increased alcohol consumption and cigarette smoking, have put a wider age range at increased risk of muscle atrophy, increasing the likelihood of a sarcopenia diagnosis in later life.Citation5 Several genetic factors have also been associated with muscle atrophy and sarcopenia, including insulin-like growth factor-1 (IGF-1), vitamin D receptor genes, and variability in birth weight.Citation6,Citation7 It is becoming evident that chronological aging is not the only cause of sarcopenia. As unhealthy lifestyle choices thrive, and genetic variation prevails, muscle atrophy can be seen across generational boundaries. Secondary sarcopenia can also develop if chronic disease or organ failure occurs.Citation8 It is important that healthcare professionals are familiar with the terms cachexia and secondary sarcopenia because the interchangeability of these terms is contentious. Sarcopenia is defined as a loss of muscle mass and function due to aging, whereas cachexia is defined as muscle loss due to an underlying illness.Citation9 Despite this, the term sarcopenia is increasingly being used to describe the loss of muscle mass and decrease in physical function in response to both aging and disease, such as cancer. Therefore, there needs to be consideration for interventions for cachexia, primary sarcopenia, and secondary sarcopenia.Citation8 Unfortunately, as it is likely that patients will require different interventions based on their healthcare conditions, it is beyond the scope of this review to consider individual conditions which can result in cachexia or secondary sarcopenia.
The current recommended treatment for sarcopenia includes an adequate nutritional intake and a sustained physical activity level throughout aging. However, both nutritional and exercise interventions lack conclusive literature regarding the most effective therapeutic strategy, highlighting the need for the optimal exercise prescription to be established within the literature and implemented clinically.
Methods
Articles were obtained from PubMed, Google Scholar, and ScienceDirect. Search terms included: ‘sarcopenia’, ‘exercise’, ‘older people’, ‘sarcopenic’, ‘resistance training’, ‘whole-body vibration training’, multi-model training’, and ‘low-intensity blood flow restriction training’. The literature search included human and rodent studies. The articles selected were between the years 1983–2022.
Current diagnosis criterion
The earliest stage of sarcopenia development is known as pre-sarcopenia, which is the term used when an individual begins to demonstrate evidence of a decline in skeletal muscle mass.Citation10 With no intervention, pre-sarcopenia may progress to sarcopenia, which is highly correlated with frailty and a risk of falls in the older population.Citation11 The correlation observed between frailty and sarcopenia is alarming. Frailty proceeds disability and a loss of independence and premature mortality, all of which may be preventable if the sarcopenic phenotype was reversed before the onset of chronic physical decline.Citation11 Unfortunately, sarcopenia is rarely diagnosed in its early stages and does not become apparent until a critical event occurs, such as a fall or a significant physical decline.Citation12 The European Working Group on Sarcopenia in Older People (EWGSOP) developed a consensus diagnostic criterion for age-related sarcopenia categorizing ˈpre-sarcopeniaˈ, ˈsarcopeniaˈ, and ˈsevere sarcopeniaˈ.Citation13 Using the EWGSOP diagnostic guidelines, cases should first be identified using a SARC-F questionnaire ().Citation14 The SARC-F questionnaire is a five-item screening test to assess strength, walking ability, ability to rise from a chair, climb stairs, and fall frequency.Citation15 If sarcopenia is suspected following the SARC-F questionnaire, muscle strength should be assessed. Following the SARC-F and muscle strength assessment, sarcopenia can be confirmed by assessing muscle mass and quality, and severity can be established by evaluating physical performance (). The EWGSOP also provide measurement protocols to assess muscle mass, strength, and physical function alongside appropriate cutoff points to aid diagnosis.Citation13 Early diagnosis of sarcopenia in the pre-sarcopenic stage would aid the prevention of the disease. However, this would require early screening of the older population, followed by an effective intervention to reverse the pre-sarcopenic phenotype. Neither of which are currently implemented in routine clinical practice.
Figure 1. EWGSOP algorithm for case-finding, making a diagnosis and quantifying severity in clinical practice. Image adapted from Cruz-Jentoft et al.Citation13
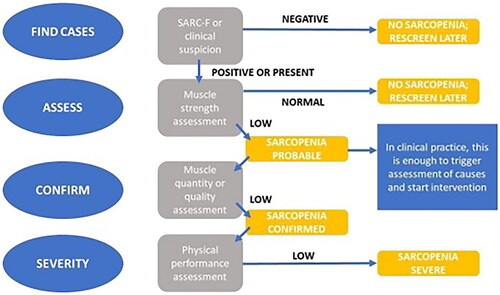
Table 1. SARC-F Questionnaire used to begin the EWGSOP diagnostic protocol for patients with suspected sarcopenia.
Macroscopic and microscopic changes
The loss of muscle mass and strength associated with aging is attributed to a combination of factors. These include a loss of motor neurons, myosteatosis, changes to muscle architecture, muscle inflammation, anabolic resistance, mitochondrial damage, endocrine alterations, and a decline in muscle regenerative capacity (). These factors all contribute to sarcopenia and a reduction in physical capability as we age.
Table 2. Summary table of the anatomical, cellular, and molecular changes observed in aging and sarcopenia.
Anatomical changes
Neurodegeneration occurs due to a loss of spinal motor neurons and a reduction in axonal myelination in the peripheral axons.Citation16,Citation17 However, it is yet to be elucidated which region of the spinal cord is primarily affected or if all regions are equally affected.
Neurodegeneration is the consequence of apoptosis, reduced serum IGF-1 signaling, and increased cytokine and oxidative stress levels which is observed in chronological aging. The loss of spinal motor neurons and their reduced excitability experienced during aging and/or neuro-disability results in a lack of muscle innervation, causing denervated muscle fibers and a reduction in muscle fiber number, size, and strength.Citation18 With time, a lack of muscle innervation leads to chronic atrophy accompanied by a loss of neuromuscular function, including changes in maximal motor neuron firing frequency, agonist muscle activation, antagonist muscle coactivation, force steadiness and spinal inhibitory circuitry.Citation17 Furthermore, individuals with neuro-disabilities are also at a greater risk of developing sarcopenic obesity,Citation19 which is defined as a low skeletal muscle mass coupled with high levels of adiposity and myosteatosis.Citation20
These neurodegenerative changes result in a reduction in physical function. However, exercise has been shown to induce several neuroprotection effects, which in turn sustains physical function as we age.Citation21 The most relevant finding concerning the reversal of the sarcopenic phenotype is the protective effect of exercise on motor unit maintenance which can mitigate the natural loss of motors units as we age, delaying the neurodegenerative effects associated with sarcopenia.Citation22
Myosteatosis describes the process of increased uptake of lipids into the muscle adipocytes and is frequently observed in aging muscle. Myosteatosis can result in decreased physical function and has been found to significantly correlate with frailty.Citation23,Citation24 Possible explanations for the development of myosteatosis include, the impaired function of subcutaneous depots, increased fatty acid mobilization and storage, reduced fatty acid oxidation, inhibited adipocyte differentiation, muscle disuse, altered leptin signaling, sex steroid deficiency, and age-related alterations to satellite cell differentiation.Citation25–28 Myosteatosis affects muscle quality and muscle architecture, including changes to muscle fiber orientation which leads to decreased muscle strength and function – which are key elements in the EWGSOP diagnostic criterion. Furthermore, intramuscular fat is metabolically active and has the ability to secrete inflammatory cytokines causing systemic inflammation, alongside a decline in muscle cell proliferation, differentiation, and catabolism.Citation29 Furthermore, Perkisas et al. and Farrow et al. identified that intramuscular fat is negatively correlated with muscle mass, strength, and function.Citation24,Citation30 However, exercise intervention can reduce the amount of lipid infiltration into the muscle and increase muscle attenuation in adults, indicating that exercise can improve muscle quality in groups at risk of sarcopenia.Citation31
Cellular and molecular changes
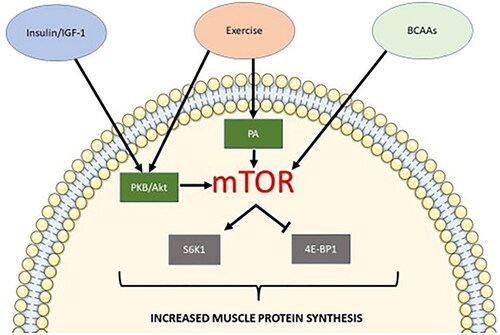
Anabolic resistance describes the reduced stimulation of muscle protein synthesis (MPS) to a given dose of anabolic stimuli, which results in muscle atrophy. Anabolic stimuli for MPS include insulin, serum IGF-1, and branched-chain amino acids (BCAAs). These anabolic signals affect MPS via the activation of the mTOR pathway and its downstream mediators, increasing ribosomal biogenesis and protein translation ().Citation32 MPS is primarily stimulated by BCAAs. However, the presence of all nine essential amino acids (EAAs) are required for significant stimulation of MPS, and the process will be severely limited by a lack of EAA availability.Citation33 In addition, insulin plays an important role in priming the muscle for protein synthesis.Citation34,Citation35 Aging and commencement of sarcopenia are often associated with physical inactivity and systemic inflammation, and these factors can induce anabolic resistance and altered proteostasis.Citation36,Citation37 However, exercise, especially in the form of resistance training, can act as an anabolic stimulus and has been shown to reset the dynamics of the MPS equilibrium, allowing MPS to exceed muscle protein breakdown.Citation38,Citation39
Figure 2. Simplified mTOR1 pathway for muscle protein synthesis. Anabolic stimuli including insulin/IGF-1, exercise (mechanical stimulation), and BCAAs can induce the mTOR1 pathway for protein synthesis. Insulin/IGF-1 and exercise allow the phosphorylation of PKB/Akt. PKB/Akt phosphorylation stimulates mTORC1 activity via the phosphorylation of TSC2 and PRAS40, both negative regulators of mTOR activity. Exercise also induces mTOR1 activation via the binding of phosphatidic acid (PA) to mTOR1. Sensing of BCAA presence in the muscle cell leads to mTOR1 lysosomal translocation leading to its subsequent activation. Activated mTOR1 phosphorylates translation repressor 4E-BP1. mTOR1 also phosphorylates S6K1, which stimulates a second phosphorylation event by PDPK1 activating S6K1. Active S6K1 can, in turn, stimulate the initiation of protein synthesis.
A second metabolic change observed in the older population is a decline in mitochondrial capacity. Observed declines exist amongst the mitochondrial number, density, and size.Citation40 Functional declines have also been demonstrated, including a decrease in ATP production, altered respiration and enzymatic actions, and an increase in reactive oxygen species (ROS) production [38].Citation40–42 Interestingly, several studies have elucidated that mitochondrial decline is not related to aging but rather by a reduction in physical activity as a symptom of aging, and can be compared to anabolic resistance.Citation43,Citation44 The ability of exercise to increase mitochondrial content and function is well documented, demonstrating that exercise stimulates mitochondrial biogenesis through increases in the peroxisome proliferator-activated receptor γ coactivator, alongside remodeling of the mitochondrial network.Citation45,Citation46
Endocrine alterations have also been implicated in aging and sarcopenia, and andropause has been strongly associated with sarcopenia in men. Testosterone replacement therapy with hypogonadism or testosterone levels below the normal range resulted in a significant increase in muscle mass, muscle strength, and MPS.Citation47,Citation48 More recently, it has been demonstrated that aerobic exercise and interval training can significantly influence basal testosterone levels in older men.Citation49 However, the magnitude of effect size was small and inconsistent with other short-term exercise programmes including resistance training (RT).
Serum IGF-1, which stimulates MPS via activation of the mTOR pathway, also exhibits a gradual decline with age.Citation50 A combination of diminished mTOR signaling and a decline in the availability of anabolic stimuli suggests that endocrine alterations may play a vital role in the development of sarcopenia.Citation32,Citation51 Testosterone has also been shown to signal via mTOR and the androgen receptor to induce hypertrophic effects.Citation52 The combination of anabolic stimuli loss and aberrant mTOR signaling with age highlights the multifactorial nature in the development of sarcopenia.
Aged muscle has also been shown to possess diminished or delayed regeneration capacity and an increased likelihood for fibrosis formation following injury.Citation53–55 The proliferative potential of satellite cells (mSCs) diminishes with age.Citation56 However, this is not thought to be related to a decrease in their number, but due to the microenvironment of the aging muscle changing from the niche specific for satellite cell function.Citation57 Furthermore, growth hormone (GH) levels decrease with age, causing an increase in myostatin expression.Citation56,Citation58 These changes cause inhibition of mSCs division, Wnt signaling alterations, mSCs differentiation into fibroblasts or adipocytes, insufficient upregulation of the Notch ligand Delta and increased oxidative stress levels.Citation59–61
Recent research has also implicated cellular senescence-associated alterations in mSCs as a causative mechanism of age-related sarcopenia, resulting in the loss of muscle regenerative potential. Knockout mice and the use of cultured myoblasts investigated factors endothelin-1, TRIM32, and GSK3α and their relation to cellular senescence. These factors are all implicated during aging and have been shown to induce mSC senescence resulting in muscle degeneration.Citation62–64 Satellite cell senescence has also been associated with chronic low-grade sterile inflammation. This is also known as inflammaging in sarcopenia, where the senescence-associated secretory phenotype (SASP) changes the surrounding muscle microenvironment. The secretion of SASP is hypothesized to disrupt tissue structure and function alongside the promotion of the inflammatory state, as observed in sarcopenia and a number of other age-related pathologies.Citation65,Citation66
Exercise has been shown to positively affect the regenerative capacity of aged muscles. Resistance and endurance training ranging from a few days to months has been shown to increase the number of mSCs in the older population. Improved muscle regeneration has been observed in trained mice with a concomitant improvement in vascularization and inflammatory response.Citation53 However, exercise-induced mSCs activation will reduce with age due to the intrinsic and extrinsic changes that occur within the mSCs.
Regardless, the literature has demonstrated that lifelong exercise should be pursued as the effects of sarcopenia are dramatically diminished via the promotion of muscle mass, structure, function, metabolism, and regeneration.
Exercise training and the future of exercise interventions for sarcopenia At present, the primary treatment for sarcopenia is exercise in the form of resistance training (RT).Citation67 RT is a type of exercise that requires the muscle to work against a force leading to increased muscle mass and strength.Citation68 The force could be present in the form of a physical weight or an individual’s body weight using repetition of the same exercises on a regular basis. The literature has demonstrated a positive correlation between RT and increased physical function, muscle strength, and improved body composition.Citation68–70 Demonstrating this, a 2021 study identified that females with sarcopenia exhibited increased hand grip strength (HGS), gait speed, and isometric muscle strength following a sixteen-week RT programme.Citation71 However, conflicting evidence exists regarding an increase in muscle mass post-RT in sarcopenic individuals.Citation72,Citation73
High-intensity interval resistance training (HIIRT) has been shown to increase muscle mass and function.Citation74 HIIRT is similar to high-intensity-interval training (HIIT), involving short periods of high-intensity exercise at maximum heart rate. HIIT focuses on cardiovascular fitness; however, HIIRT focusses on strength and RT. Moro et al. compared traditional RT to HIIRT in older adults between 60 and 80 years of age. An increase in muscle strength was observed using both training methods. The study shows that HIIRT is at least as beneficial as traditional RT for muscle strength improvement. However, HIIRT presented a greater increase in testosterone in the female participants and a lesser decrease in GH which are important factors in the regulation of skeletal muscle mass in response to exercise.Citation75
RT increases muscle hypertrophy by the upregulation of MPS, alongside increasing the size of the fast-twitch muscle fibers.Citation76 These increases allow maximum production of muscle power and improvements to physical performance, thereby reducing clinical features of the sarcopenic phenotype. A 12-week exercise trial implemented in a nursing home demonstrated that exercising for as little as forty-five minutes a week can improve physical performance and the likelihood of falls in the older population.Citation77 A recent systematic review also confirmed that low-intensity RT (≤50% 1RM) is sufficient to elicit strength gains in sarcopenic individuals, however high intensity RT (80% 1RM) provides maximal results.Citation78 However, high intensity RT is unlikely to be suitable for the majority of the sarcopenic population and may only be advisable for pre-sarcopenic individuals with the physical capability for this type of training.
Despite its positive outcomes, RT struggles to retain participation rates due to its repetitive nature and non-acceptability for all patients, especially those with chronic immobility or health issues, alongside the lack of integration into medicine and social care. A personalized approach to training should be considered for sarcopenic individuals, incorporating RT while encompassing a variety of exercise and rehabilitation methods. Multiple alternative interventions have been included in the literature including multi-model training (MMT), whole-body vibration training (WBVT), and low-intensity blood flow restriction training (LIBFRT).
Multi-model training
MMT is based on a combination of physical exercises typically incorporating traditional RT, aerobic exercises, flexibility, and plyometric training. MMT programmes train a large dimension of physical skills applicable to daily life and contribute to fall prevention in the elderly. To date, a combination of RT and plyometric training has been demonstrated to be the most effective intervention for increased muscle mass and power in the older population.Citation79 In 2013, 117 older people (aged 71–90) participated in a separate multifaceted training programme. The training included a combination of aerobic and strength training twice a week for six months and identified positive effects on mobility, motor balance, muscle strength and endurance.Citation80 Furthermore, the incorporation of aerobic training with RT demonstrated the ability to ameliorate mitochondrial dysfunction via Sestrin2 in an AMPKα2 dependent manner and boasts a number of other health benefits, including reducing the risk of heart disease, high blood pressure, obesity, type 2 diabetes, metabolic syndromes, and mental health problems.Citation81,Citation82 Aerobic training alone provides a partial solution for sarcopenia reversal due to the amelioration of mitochondrial-derived ROS but it does not effectuate maximum MPS or muscle strength development thus the requirement of RT in a MTT programme aimed at ameliorating sarcopenia.Citation83,Citation84
At present MMT presents itself as the most appropriate intervention for those with secondary sarcopenia, especially for individuals who have experienced significant muscle loss alongside cardiovascular health depletion during a period of chronic illness. The implementation of RT should develop muscle strength and aerobic exercise can provide an improvement in cardiovascular function.Citation85 Currently, the combination of exercises provided by a professional would be personalized on an individual basis dependent on a patient’s current ability and current goals. However, a recent comprehensive review of the literature has concluded that the limited number of studies involving patients with secondary sarcopenia cannot confirm that exercise reverses the sarcopenic phenotype in individuals with other comorbidities.Citation8
Using a MMT as a sarcopenia intervention provides a more comprehensive approach to muscle aging therapy, alongside, improving key skills such as balance and coordination to help older individuals maintain independence and contribute to the prevention of frailty and disability. The variety of activities may also encourage participation/long-term adherence to an exercise programme whilst making exercise more accessible to less-able patients.
Whole-body vibration training
WBVT involves the participant standing or sitting on a vibrating platform whilst partaking in strength and balance exercises.Citation86 WBVT has been infrequently trialed in patients diagnosed with sarcopenia.Citation87–89 The most recent study demonstrated improvements in physical capability following twelve weeks of WBVT. Improvements in one- foot balance, shoulder and arm flexibility, gait speed, HGS, sit-to-stand repetitions, and an increase in muscle mass index were all observed.Citation89 Twelve weeks of WBVT has also demonstrated its ability to facilitate voluntary activation (the level of neural drive to the muscle during exercise) of the quadriceps muscle.Citation88
Changes to the spinal motor neuron population, denervated muscle fibers and alterations to the neuromuscular junctions have all been reported in aged muscle, resulting in a reduction of force production per unit of muscle fiber size.Citation90–93 The success of WBVT has been mainly ascribed to a complex spinal and supraspinal neurophysiological mechanism. It has been shown to elicit a reflex loop known as the tonic vibration reflex leading to motor unit activation via the 1-a afferent-α-motor neuron pathway.Citation94 Vibration can alter the efficacy of the pathway leading to positive and negative effects on muscle spindle activity and motor neuron output.Citation95 In 2017 Wei et al. investigated whether the frequency/exposure time during voluntary contraction influenced WBVT effectiveness in older people with sarcopenia. The chosen mid-range exposure of 360 s at a vibration frequency of 40 Hz over twelve-weeks had the best outcome on physical performance amongst the participants, demonstrating improvements in all outcome variables including the sit-to-stand test, 10- metre-walking test, and the timed-up-and-go (TUG) test.Citation96
The low-intensity nature of WBVT makes it an ideal intervention for those with mobility issues. However, skilled staff may be required to ensure WBVT is delivered correctly. Finally, contradictory literature has proven a need for further research into WBVT to establish its effects on motor neuron activation at several frequencies and vibration exposure times, and during voluntary and involuntary muscle contraction to finalize appropriate rehabilitation protocols.
Low-intensity blood flow restriction training
LIBFRT combines RT exercises with blood flow occlusion. A tourniquet is applied around the training limb, disrupting the blow flow, creating an ischemic environment. Under ischemic conditions, fast-twitch muscle fibers are recruited during low-intensity exercise, leading to the promotion of muscle hypertrophy.Citation97,Citation98 LIBFRT has been suggested as a potential intervention for sarcopenia due to its low-intensity nature. Multiple studies have shown that low-intensity exercise combined with moderate blood flow occlusion can promote muscle hypertrophy at a training intensity of only 20% 1RM.Citation99,Citation100 A 2019 case study of a 91-year- old sarcopenic male demonstrated improvements in muscle mass, HGS, isokinetic peak torque and the upregulation of important regulars for muscle metabolism (IL-6 and serum IGF-1), following three months of LIBFRT training compared to low- intensity RT alone.Citation101
It has been hypothesized that the success of LIBFR is reliant on several elusive mechanisms; however, metabolic by-product accumulation currently presents itself as the primary process. Whole blood lactate, plasma lactate and muscle cell lactate accumulation have been observed, resulting in an acidic intramuscular environment.Citation102–104 The change in pH in the ischemic environment leads to the increased expression of GH, causing an upregulation in MPS.Citation105
Heat shock protein 72 (HSP 72), nitric oxide synthase 1 (NOS-1), myostatin and REDD1 regulation have also been suggested to contribute to the muscle hypertrophy observed during LIBFRT. HSP72 is induced in response to stress including ischemia and has been shown to reduce muscle atrophy.Citation106 NOS-1 is upregulated, facilitating an increase in satellite cell activation.Citation107 Myostatin and REDD1 are downregulated, suggesting a possible upregulation of the mTOR pathway leading to stimulation of the MPS pathway.Citation99,Citation104,Citation108 Whilst much of this literature concerns young, healthy individuals, favorable outcomes have also been shown in a few studies undertaken within an older population.Citation101,Citation109 LIBFRT may be a highly appropriate intervention for those with sarcopenia. The novel intervention makes muscle hypertrophy, strength, and endurance more accessible to those less physically able due to its low-load and low-intensity approach, similar to WBVT.
The current barriers to exercise intervention for sarcopenia
There is much positive research to suggest that exercise interventions can provide an effective treatment for sarcopenia. However, there are several anatomical, molecular, cellular, and societal barriers to the implementation of exercise interventions for the treatment of sarcopenia (). The epicenter of the exercise intervention dilemma is not a lack of intervention possibilities, but rather that sarcopenia often remains undiagnosed until a critical stage.Citation12 The first step for improving sarcopenia management could include screening all older people regardless of their current physical presentation. Early diagnosis would be made possible using EWGSOPˈs consensus diagnostic criterion and measurement protocols or via a biomarker or medical imaging alternative, which is currently unavailable.Citation13 Screening the older population may permit early diagnosis of the disease in its pre-sarcopenic phase, ensuring that intervention can be implemented before a further loss of muscle mass or function occurs. If mass screening remains infeasible, screening could begin within an established age range or at-risk populations. Muscle mass decreases at a rate of approximately 3–8% a decade after age thirty and can accelerate to rates as fast as 15% per decade from age sixty with noticeable physical decline.Citation2 Therefore, routine screening should ideally begin before this acceleration period of muscle loss. Preventative screening could improve QoL for countless older people, alongside the potential to save the NHS an estimated £2.5 billion spent annually treating muscle weakness and sarcopenia.Citation110
Table 3. Summary table of the current barriers and future possibilities for exercise intervention to reverse muscle aging.
Following diagnosis, those identified as sarcopenic need to be provided with an effective intervention to reverse the phenotype, which is not yet implemented in routine clinical practice or care facilities. Skilled staff are often required to assist in safe exercise intervention, which presents a large-scale staffing and infrastructure requirement.
Furthermore, poor adherence to exercise programmes is well-documented due to a number of barriers that may hinder participation.Citation111 These include affordability, motivation, and concerns about other negative health outcomes.Citation112 To retain adherence rates, exercise ˈmotivatorsˈ need to be considered and incorporated into exercise programmes. The MIlk Intervention Muscle Aging (MIlkMAN) study identified five motivators for intervention engagement: self-perceived improved health, knowledge acquisition, social wellbeing, professional support in a fun environment and positive reported outcomes. The study also identified that peer encouragement and social bonds are the motivators that encourage prolonged engagement in exercise following intervention completion.Citation112 To address the identified patient barriers and exploit the ˈmotivatorsˈ, health care providers should incorporate advice about the importance of exercise for sarcopenia prevention in routine clinical visits to empower patients and help develop their knowledge about safety, effectiveness, and limitations. Social innovation is also required to implement the infrastructure required to provide exercise initiatives for those diagnosed or at risk of sarcopenia.
Health care professionals also face barriers to implementing exercise intervention in the clinical setting as there is limited guidance on physical activity advice from official bodies. There is also a lack of high-quality studies to determine patient opinion regarding exercise intervention for sarcopenia. Current advice suggests an RT programme with plyometric training as the most effective intervention for sarcopenia; however, this is not acceptable for all patients due to its high- intensity nature. The recent literature regarding MMT, WBVT and LIBFRT provides promising alternatives to RT alone, but there are still elusive elements to their application. These factors make it hard to advise physicians on the best exercise protocol to follow.
For physicians to provide a holistic approach to sarcopenia management the association between sarcopenia and polypharmacy must be considered. It has been recently reported that sarcopenia is associated with an increased prevalence of polypharmacy.Citation113 Alongside the acknowledgement that some prescription medication may affect muscle mass and function. For example, corticosteroids have been associated with muscle weakness and low appendicular lean mass.Citation114,Citation115 Malnutrition has also been associated with polypharmacy due to negative implications for the gastrointestinal microenvironment.Citation116 Malnutrition is often observed in older individuals and is thought to be a key contributor to the development of sarcopenia.Citation117 Therefore, the impact of specific medications and polypharmacy in populations at risk of sarcopenia requires further insight alongside the development of targeted interventions to reduce polypharmacy. This knowledge would help to advise physicians when prescribing medications to older individuals.
Overcoming the barriers to exercise intervention for sarcopenia
The initial barrier to sarcopenia management and the opportunity for prevention is delayed diagnosis. The EWGSOP diagnostic criterion provides a detailed diagnostic process; however, it is multistep, time-consuming, and costly. Currently, there are no established biomarkers for sarcopenia used in clinical practice, highlighting the unmet need to understand the molecular mechanisms underpinning the disease. Identification of a singular biomarker of disease has been deemed unreliable due to the multifactorial nature of the sarcopenic phenotype and the involvement of multiple signaling pathways. Therefore, it is believed that the combined use of multiple biomarkers would allow increased diagnostic accuracy.Citation118 Current research highlights the utility of a several biomarkers including inflammatory factors interleukin-6 (IL-6) and tumor necrosis factor- α (TNF-α), alongside procollagen type III N-terminal peptide (P3NP) as an indicator of muscle loss.Citation119 Existing biomarkers should be further investigated to demonstrate adequate sensitivity and specificity to predict sarcopenia, alongside investigation into additional biomarker candidates. Recent studies have implicated reactive oxygen species (ROS) in the pathophysiology of skeletal muscle aging, providing an example of a novel candidate. If a panel of accurate biomarkers are established, screening those at risk of sarcopenia could be made easily accessible via routine blood testing in the clinic. Extensive biomarker identification would not only allow the diagnosis of sarcopenia in its early stages, but also enhance our understanding of the pathophysiology of the disease to develop the most effective interventions to reverse the sarcopenic phenotype.
There is also minimal understanding of the macroscopic and microscopic changes induced by the variety of available exercise interventions. The challenges include the lack of an established biomarker as discussed, and the inconsistent assessment of myosteatosis and muscle atrophy pre- and post-intervention in the literature. For the most effective exercise prescription to be established, each exercise intervention must be compared by matching the programmes frequency, duration, and intensity. The success of each intervention could be assessed using QoL questionnaires, medical imaging, muscle biopsies, blood analysis and a multitude of physical function assessments. This approach would allow a global assessment of muscle health and any improvements to the sarcopenic phenotype post- intervention, including improvements to physical function, biomolecular changes, and gross anatomical changes that each intervention has effectuated. However, it must be considered that one prescriptive intervention may not be appropriate for all patients, as demonstrated by RT. To avoid this barrier, a prescriptive and personalized rehabilitation management protocol is required to aid and advise physicians on the effective treatment and prevention of sarcopenia for all. Ultimately the research is likely to conclude the need for a MMT approach to ensure inclusivity and optimum health benefits that expand beyond muscle health alone.
Once the most effective exercise interventions are established, the best way to implement it into healthcare needs to be considered. Successful implementation would be defined as ensuring that prescriptive or personalized exercise programmes are accessible to all at-risk or diagnosed with sarcopenia. Exercise referral is currently limited to health-care professionals once sarcopenia is identified.Citation120 This presents an infrastructure barrier that must be removed. To allow access to all at risk, social innovation to overcome infrastructure and staffing shortages need to be addressed. Sarcopenia management training is required for all allied health professionals and physicians to help bridge the gap in service provision.
Additional possibilities include ensuring that sport and exercise professionals have the skills to develop individualized and safe exercise interventions for older patients with sarcopenia. Once an improvement in staffing infrastructure and training occurs, patient organizations, community groups, or third-sector exercise professionals could be introduced to conduct free, face-to-face, social, and engaging exercise sessions.
Recent research has also assessed the use of home-based exercise programmes for sarcopenia.Citation121 Home-based exercise has many advantages, including minimal cost and time expenditure for patients and healthcare providers. It is also accessible to all, and it can fit into the patient’s routine. Tsekoura et al. demonstrated that group-based exercise is more effective than home-based exercise for improving muscle mass and functional performance.Citation122 However, their home-based group still saw significant improvements in gait speed, TUG, skeletal muscle mass index (SMMI), HGS, knee strength, and QoL compared to a control group. In the only published study exploring home-based exercise for sarcopenic community-dwelling individualsˈ, the results are comparable, demonstrating increases in muscle strength and function following a six-month home exercise programme.Citation123 Therefore, both home- and group-based approaches can be considered effective for sarcopenia management. Unsurprisingly, adherence rates for home-based exercise programmes are often problematic. However, the Tsekoura study maintained a high adherence rate and no intervention drop-outs by providing regular visits by a member of the study team and telephone counseling providing ongoing support and motivation. Telephone counseling is considered effective for promoting increased physical activity and exercise participation in older people and could provide a low-cost alternative to face-to-face exercise sessions.Citation124 In addition, the future looks to the use of telehealth which involves the use of internet-based platforms such as Zoom or Microsoft Teams for live online home-based exercise to encourage motivation and adherence (). However, the above solutions only protect and accommodate those at risk or diagnosed with age- related sarcopenia in later life. Therefore, they do not resolve or reverse the likelihood of sarcopenia development in future generations. Nutrition, health, and exercise education for young people requires global improvement. Education systems require stricter legislation to ensure that young people are educated on how to live a healthy lifestyle to promote healthy aging. Comprehensive exercise education in schools detailing exercise programmes and their associated physiological effects may be a good place to start.
Allowing young people an understanding of how to exercise and the physiological adaptions it provides could encourage participation but may also promote a commitment to life-long exercise. Several studies have highlighted the success of life-long exercise for healthy aging, especially concerning sarcopenia. Effects include a sustained muscle fiber size, force, and improved physical function due to the prevention of excessive apoptosis, defective autophagy, and mitochondrial dysfunction.Citation125,Citation126 Data from the British National Survey of Health and Development has also demonstrated the cumulative benefits of increased leisure time physical activity for maintaining HGS at ages 60–64. Individuals in the upper third of lifelong exercise participation had a mean grip strength of 2.11 kg greater than the lowest third.Citation127 If life-long exercise was adhered to amongst the global population, it could reverse a multitude of modern health concerns related to poor lifestyle choices, alongside the prevention of muscle atrophy in all age groups. Education surrounding the importance of health and exercise is paramount to ensure healthy aging in future generations and improving QoL for patients.
Conclusions
Research and society are not doing enough for the population at-risk of sarcopenia. A more coherent and integrated approach to sarcopenia is required, with earlier identification and diagnosis of disease in the pre-sarcopenic phase. Screening of all aging and at-risk patientsˈ needs to become routine practice to allow preventative interventions to be implemented. These actions will help contribute to the achievement of a healthy aging society. There are several key barriers to this, including a lack of knowledge regarding exercise prescription, patient adherence and identification of suitable exercise programmes. Scientific research also needs to provide a more coherent assessment of the efficacy of the variety of possible exercise interventions on skeletal muscle mass and function throughout aging, to help identify the most appropriate interventions for sarcopenia management. The advancement of this knowledge could lead to optimal prescriptive and personalized exercise protocols in clinical practice. Consequently, sustaining physical function, productivity and improved QoL as our global society continues to grow older.
Authors’ contributions
All authors participated in the conception of the review article. HE conducted the literature review. HE and MF prepared figures and drafted the manuscript. All authors edited and revised the manuscript and approved the final version.
Disclosure statement
No funding was received to assist the preparation of this manuscript. The authors have no conflicts of interest or competing interests to disclose.
Additional information
Funding
References
- Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. Sep 2014;11(3):177–180.
- Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. Jul 2004;7(4):405–410. doi:10.1097/01.mco.0000134362.76653.b2.
- Wackerhage H. Sarcopenia: Causes and Treatments. Dtsch Z Sportmed. 07/01 2017;2017(07–08):178–184. doi. doi:10.5960/dzsm.2017.289.
- Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. Dec 2016;7(5):512–514. doi:10.1002/jcsm.12147.
- Rom O, Kaisari S, Aizenbud D, Reznick AZ. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med J. Oct 2012;3(4):e0024. doi:10.5041/RMMJ.10091.
- Tan LJ, Liu SL, Lei SF, Papasian CJ, Deng HW. Molecular genetic studies of gene identification for sarcopenia. Hum Genet. Jan 2012;131(1):1–31. doi:10.1007/s00439-011-1040-7.
- Dodds R, Denison HJ, Ntani G, et al. Birth weight and muscle strength: a systematic review and meta-analysis. J Nutr Health Aging. Jul 2012;16(7):609–615. doi:10.1007/s12603-012-0053-9.
- Supriya R, Singh KP, Gao Y, Gu Y, Baker JS. Effect of Exercise on Secondary Sarcopenia: A Comprehensive Literature Review. Biology (Basel). Dec 30 2021;11(1):51. doi:10.3390/biology11010051.
- Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60(4):294–305. doi:10.1159/000356760.
- Yeh WS, Chiang PL, Kee KM, et al. Pre-sarcopenia is the prognostic factor of overall survival in early-stage hepatoma patients undergoing radiofrequency ablation. Medicine (Baltimore). Jun 5 2020;99(23):e20455. doi:10.1097/MD.0000000000020455.
- Cederholm T. Overlaps between Frailty and Sarcopenia Definitions. Nestle Nutr Inst Workshop Ser. 2015;83:65–69. doi:10.1159/000382063.
- Visvanathan R, Chapman I. Preventing sarcopaenia in older people. Maturitas. Aug 2010;66(4):383–388. doi:10.1016/j.maturitas.2010.03.020.
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. Jan 1 2019;48(1):16–31. doi:10.1093/ageing/afy169.
- Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. Aug 2013;14(8):531–532. doi:10.1016/j.jamda.2013.05.018.
- Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. Mar 2016;7(1):28–36. doi:10.1002/jcsm.12048.
- Mittal KR, Logmani FH. Age-related reduction in 8th cervical ventral nerve root myelinated fiber diameters and numbers in man. J Gerontol. Jan 1987;42(1):8–10. doi:10.1093/geronj/42.1.8.
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. Feb 2010;20(1):49–64. doi:10.1111/j.1600-0838.2009.01084.x.
- Steindl A, Leitner J, Schwarz M, et al. Sarcopenia in Neurological Patients: Standard Values for Temporal Muscle Thickness and Muscle Strength Evaluation. JCM. Apr 28 2020;9(5):1272. doi:10.3390/jcm9051272.
- Dionyssiotis Y, Prokopidis K, Trovas G, et al. Sarcopenic Obesity in Individuals With Neurodisabilities: The SarcObeNDS Study. Front Endocrinol (Lausanne). 2022;13:868298. doi:10.3389/fendo.2022.868298.
- Atkins JL. Chapter 7 - Effects of Sarcopenic Obesity on Cardiovascular Disease and All-Cause Mortality. In: Walrand S, ed. Nutrition and Skeletal Muscle. London: Academic Press; 2019. p. 93–103.
- Vecchio LM, Meng Y, Xhima K, Lipsman N, Hamani C, Aubert I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. Dec 12 2018;4(1):17–52. doi:10.3233/BPL-180069.
- Allen MD, Dalton BH, Gilmore KJ, et al. Neuroprotective effects of exercise on the aging human neuromuscular system. Exp Gerontol. Sep 2021;152:111465. doi:10.1016/j.exger.2021.111465.
- Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. doi:10.1155/2014/309570.
- Farrow M, Biglands J, Tanner SF, et al. The effect of ageing on skeletal muscle as assessed by quantitative MR imaging: an association with frailty and muscle strength. Aging Clin Exp Res. Feb 2021;33(2):291–301. doi:10.1007/s40520-020-01530-2.
- Miljkovic I, Kuipers AL, Cauley JA, et al. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. J Gerontol A Biol Sci Med Sci. Sep 2015;70(9):1133–1140. doi:10.1093/gerona/glv027.
- Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne). 2016;7:69. doi:10.3389/fendo.2016.00069.
- Perkisas S, De Cock AM, Verhoeven V, Vandewoude M. Intramuscular Adipose Tissue and the Functional Components of Sarcopenia in Hospitalized Geriatric Patients. Geriatrics (Basel). Feb 22 2017;2(1):11. doi:10.3390/geriatrics2010011.
- Sinanan AC, Buxton PG, Lewis MP. Muscling in on stem cells. Biol Cell. Apr 2006;98(4):203–214. doi:10.1042/BC20050050.
- Zamboni M, Gattazzo S, Rossi AP. Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med. Feb 2019;10(1):5–6. doi:10.1007/s41999-018-0134-3.
- Perkisas S, Lamers S, Degerickx R, et al. The relation between mortality, intramuscular adipose tissue and sarcopenia in hospitalized geriatric patients. Eur Geriatr Med. Dec 2018;9(6):801–807. doi:10.1007/s41999-018-0110-y.
- Ramirez-Velez R, Ezzatvar Y, Izquierdo M, Garcia-Hermoso A. Effect of exercise on myosteatosis in adults: a systematic review and meta-analysis. J Appl Physiol (1985). Jan 1 2021;130(1):245–255. doi:10.1152/japplphysiol.00738.2020.
- Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda). Oct 2006;21:362–369. doi:10.1152/physiol.00024.2006.
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. Aug 2003;78(2):250–258. doi:10.1093/ajcn/78.2.250.
- Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. Mar 2005;19(3):422–424. doi:10.1096/fj.04-2640fje.
- Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. Jun 2003;52(6):1377–1385. doi:10.2337/diabetes.52.6.1377.
- Morton RW, Traylor DA, Weijs PJM, Phillips SM. Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care. Apr 2018;24(2):124–130. doi:10.1097/MCC.0000000000000488.
- Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces "anabolic resistance" of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. Jun 2013;98(6):2604–2612. doi:10.1210/jc.2013-1502.
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. Jan 2004;286(1):E92–101. doi:10.1152/ajpendo.00366.2003.
- Heath GW, Gavin JR, 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol Respir Environ Exerc Physiol. Aug 1983;55(2):512–517. doi:10.1152/jappl.1983.55.2.512.
- Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. Feb 2010;65(2):119–128. doi:10.1093/gerona/glp179.
- Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. Nov 2008;57(11):2933–2942. doi:10.2337/db08-0349.
- Kumaran S, Panneerselvam KS, Shila S, Sivarajan K, Panneerselvam C. Age-associated deficit of mitochondrial oxidative phosphorylation in skeletal muscle: role of carnitine and lipoic acid. Mol Cell Biochem. Dec 2005;280(1-2):83–89. doi:10.1007/s11010-005-8234-z.
- Barrientos A, Casademont J, Rotig A, et al. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem Biophys Res Commun. Dec 13 1996;229(2):536–539. doi:10.1006/bbrc.1996.1839.
- Brierley EJ, Johnson MA, James OF, Turnbull DM. Effects of physical activity and age on mitochondrial function. QJM. Apr 1996;89(4):251–258. doi:10.1093/qjmed/89.4.251.
- Geng T, Li P, Okutsu M, et al. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. Mar 2010;298(3):C572–9. doi:10.1152/ajpcell.00481.2009.
- Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol (1985). Oct 2011;111(4):1190–1198. doi:10.1152/japplphysiol.00429.2011.
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. May 2001;56(5):M266–72. doi:10.1093/gerona/56.5.m266.
- Morley JE, Perry HM, 3rd, Kaiser FE, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. Feb 1993;41(2):149–152. doi:10.1111/j.1532-5415.1993.tb02049.x.
- Hayes LD, Elliott BT. Short-Term Exercise Training Inconsistently Influences Basal Testosterone in Older Men: A Systematic Review and Meta-Analysis. Front Physiol. 2018;9:1878. doi:10.3389/fphys.2018.01878.
- Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol (1985). Dec 1996;81(6):2509–2516. doi:10.1152/jappl.1996.81.6.2509.
- Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. Oct 17 1997;278(5337):419–424. doi:10.1126/science.278.5337.419.
- Basualto-Alarcon C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc. Sep 2013;45(9):1712–1720. doi:10.1249/MSS.0b013e31828cf5f3.
- Joanisse S, Nederveen JP, Baker JM, Snijders T, Iacono C, Parise G. Exercise conditioning in old mice improves skeletal muscle regeneration. Faseb J. Sep 2016;30(9):3256–3268. doi:10.1096/fj.201600143RR.
- Shavlakadze T, McGeachie J, Grounds MD. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology. Jun 2010;11(3):363–376. doi:10.1007/s10522-009-9260-0.
- Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. Aug 10 2007;317(5839):807–810. doi:10.1126/science.1144090.
- Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol (1985). Oct 2000;89(4):1365–1379. doi:10.1152/jappl.2000.89.4.1365.
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. Feb 17 2005;433(7027):760–764. doi:10.1038/nature03260.
- Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. Aug 2008;9(4):213–228. doi:10.1007/s10522-008-9131-0.
- Jones AE, Price FD, Le Grand F, et al. Wnt/beta-catenin controls follistatin signalling to regulate satellite cell myogenic potential. Skelet Muscle. 2015;5:14. doi:10.1186/s13395-015-0038-6.
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. Nov 28 2003;302(5650):1575–1577. doi:10.1126/science.1087573.
- Conley KE, Amara CE, Jubrias SA, Marcinek DJ. Mitochondrial function, fibre types and ageing: new insights from human muscle in vivo. Exp Physiol. Mar 2007;92(2):333–339. doi:10.1113/expphysiol.2006.034330.
- Kudryashova E, Kramerova I, Spencer MJ. Satellite cell senescence underlies myopathy in a mouse model of limb-girdle muscular dystrophy 2H. J Clin Invest. May 2012;122(5):1764–1776. doi:10.1172/JCI59581.
- Zhou J, Freeman TA, Ahmad F, et al. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. Apr 2013;123(4):1821–1832. doi:10.1172/JCI64398.
- Alcalde-Estevez E, Asenjo-Bueno A, Sosa P, et al. Endothelin-1 induces cellular senescence and fibrosis in cultured myoblasts. A potential mechanism of aging-related sarcopenia. Aging (Albany NY). Jun 22 2020;12(12):11200–11223. doi:10.18632/aging.103450.
- Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. Nov 2 2011;479(7372):232–236. doi:10.1038/nature10600.
- Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. May 2017;35:200–221. doi:10.1016/j.arr.2016.09.008.
- Dent E, Morley JE, Cruz-Jentoft AJ, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging. 2018;22(10):1148–1161. doi:10.1007/s12603-018-1139-9.
- Chilibeck PD, Calder AW, Sale DG, Webber CE. A comparison of strength and muscle mass increases during resistance training in young women. Eur J Appl Physiol Occup Physiol. 1998;77(1-2):170–175. doi:10.1007/s004210050316.
- Morley JE. Weight loss in older persons: new therapeutic approaches. Curr Pharm Des. 2007;13(35):3637–3647. doi:10.2174/138161207782794149.
- Chen N, He X, Feng Y, Ainsworth BE, Liu Y. Effects of resistance training in healthy older people with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Eur Rev Aging Phys Act. 2021/11/11 2021;18(1):23. doi:10.1186/s11556-021-00277-7.
- Seo MW, Jung SW, Kim SW, Lee JM, Jung HC, Song JK. Effects of 16 Weeks of Resistance Training on Muscle Quality and Muscle Growth Factors in Older Adult Women with Sarcopenia: A Randomized Controlled Trial. IJERPH. Jun 23 2021;18(13):6762. doi:10.3390/ijerph18136762.
- Escriche-Escuder A, Fuentes-Abolafio IJ, Roldán-Jiménez C, Cuesta-Vargas AI. Effects of exercise on muscle mass, strength, and physical performance in older adults with sarcopenia: A systematic review and meta-analysis according to the EWGSOP criteria. Exp Gerontol. 2021/08/01/2021;151:111420. doi:10.1016/j.exger.2021.111420.
- Bao W, Sun Y, Zhang T, et al. Exercise Programs for Muscle Mass, Muscle Strength and Physical Performance in Older Adults with Sarcopenia: A Systematic Review and Meta-Analysis. Aging Dis. Jul 2020;11(4):863–873. doi:10.14336/ad.2019.1012.
- Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol (1985). Jan 2007;102(1):368–373. doi:10.1152/japplphysiol.00789.2006.
- Moro T, Tinsley G, Bianco A, et al. High intensity interval resistance training (HIIRT) in older adults: Effects on body composition, strength, anabolic hormones and blood lipids. Exp Gerontol. Nov 2017;98:91–98. doi:10.1016/j.exger.2017.08.015.
- Johnston AP, De Lisio M, Parise G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl Physiol Nutr Metab. Feb 2008;33(1):191–199. doi:10.1139/H07-141.
- Brett L, Stapley P, Meedya S, Traynor V. Effect of physical exercise on physical performance and fall incidents of individuals living with dementia in nursing homes: a randomized controlled trial. Physiother Theory Pract. Jan 2021;37(1):38–51. doi:10.1080/09593985.2019.1594470.
- Beckwée D, Delaere A, Aelbrecht S, et al. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J Nutr Health Aging. 2019;23(6):494–502. doi:10.1007/s12603-019-1196-8.
- Franchi MV, Monti E, Carter A, et al. Bouncing Back! Counteracting Muscle Aging With Plyometric Muscle Loading. Front Physiol. 2019;10:178. doi:10.3389/fphys.2019.00178.
- Gudlaugsson J, Aspelund T, Gudnason V, et al. [The effects of 6 months’ multimodal training on functional performance, strength, endurance, and body mass index of older individuals. Are the benefits of training similar among women and men?]. Laeknabladid. Jul 2013;99(7-8):331–337. manaetha fjolthornaettrar thornjalfunar a hreyfigetu, voethvakraft, thornol og likamsthornyngdar stuethul eldri einstaklinga. Eru ahrif thornjalfunar sambaerileg hja konum og korlum? doi:10.17992/lbl.2013.0708.504.
- Liu S, Yu C, Xie L, Niu Y, Fu L. Aerobic Exercise Improves Mitochondrial Function in Sarcopenia Mice Through Sestrin2 in an AMPKalpha2-Dependent Manner. J Gerontol A Biol Sci Med Sci. Jun 14 2021;76(7):1161–1168. doi:10.1093/gerona/glab029.
- Mersy DJ. Health benefits of aerobic exercise. Postgrad Med. Jul 1991;90(1):103–7, 110-102. doi:10.1080/00325481.1991.11700983.
- Bagheri R, Robinson I, Moradi S, et al. Muscle Protein Synthesis Responses Following Aerobic-Based Exercise or High-Intensity Interval Training with or Without Protein Ingestion: A Systematic Review. Sports Med. 2022/11/01 2022;52(11):2713–2732. doi:10.1007/s40279-022-01707-x.
- Yoo SZ, No MH, Heo JW, et al. Role of exercise in age-related sarcopenia. J Exerc Rehabil. Aug 2018;14(4):551–558. doi:10.12965/jer.1836268.134.
- Taylor RS, Long L, Mordi IR, et al. Exercise-Based Rehabilitation for Heart Failure: Cochrane Systematic Review, Meta-Analysis, and Trial Sequential Analysis. JACC Heart Fail. Aug 2019;7(8):691–705. doi:10.1016/j.jchf.2019.04.023.
- Fischer M, Vialleron T, Laffaye G, et al. Long-Term Effects of Whole-Body Vibration on Human Gait: A Systematic Review and Meta-Analysis. Front Neurol. 2019;10:627. doi:10.3389/fneur.2019.00627.
- Chang SF, Lin PC, Yang RS, Yang RJ. The preliminary effect of whole-body vibration intervention on improving the skeletal muscle mass index, physical fitness, and quality of life among older people with sarcopenia. BMC Geriatr. Jan 17 2018;18(1):17. doi:10.1186/s12877-018-0712-8.
- Wei N, Ng GYF. The effect of whole body vibration training on quadriceps voluntary activation level of people with age-related muscle loss (sarcopenia): a randomized pilot study. BMC Geriatr. Oct 11 2018;18(1):240. doi:10.1186/s12877-018-0923-z.
- Lin P-C, Chang S-F, Ho H-Y. Effect of Whole-Body Vibration Training on the Physical Capability, Activities of Daily Living, and Sleep Quality of Older People with Sarcopenia. Applied Sciences. 2020;10(5):1695. doi:10.3390/app10051695.
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. Nov 1977;34(2):213–219. doi:10.1016/0022-510x(77)90069-7.
- Carlson BM. The Biology of Long-Term Denervated Skeletal Muscle. Eur J Transl Myol. Mar 31 2014;24(1):3293. doi:10.4081/ejtm.2014.3293.
- Jang YC, Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp Gerontol. Feb-Mar 2011;46(2-3):193–198. doi:10.1016/j.exger.2010.08.029.
- Weisleder N, Brotto M, Komazaki S, et al. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J Cell Biol. Aug 28 2006;174(5):639–645. doi:10.1083/jcb.200604166.
- Xu L, Negro F, Rabotti C, Farina D, Mischi M. Investigation of The Neural Drive During Vibration Exercise by High-density Surface-electromyography. Annu Int Conf IEEE Eng Med Biol Soc. Jul 2019;2019:1944–1947. doi:10.1109/EMBC.2019.8857922.
- Pope ZK, DeFreitas JM. The effects of acute and prolonged muscle vibration on the function of the muscle spindle’s reflex arc. Somatosens Mot Res. 2015;32(4):254–261. doi:10.3109/08990220.2015.1091770.
- Wei N, Pang MY, Ng SS, Ng GY. Optimal frequency/time combination of whole body vibration training for developing physical performance of people with sarcopenia: a randomized controlled trial. Clin Rehabil. Oct 2017;31(10):1313–1321. doi:10.1177/0269215517698835.
- Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol (1985). Jun 2000;88(6):2097–2106. doi:10.1152/jappl.2000.88.6.2097.
- Moritani T, Sherman WM, Shibata M, Matsumoto T, Shinohara M. Oxygen availability and motor unit activity in humans. Eur J Appl Physiol Occup Physiol. 1992;64(6):552–556. doi:10.1007/BF00843767.
- Fry CS, Glynn EL, Drummond MJ, et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985). May 2010;108(5):1199–1209. doi:10.1152/japplphysiol.01266.2009.
- Yasuda T, Fujita S, Ogasawara R, Sato Y, Abe T. Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: a pilot study. Clin Physiol Funct Imaging. Sep 2010;30(5):338–343. doi:10.1111/j.1475-097X.2010.00949.x.
- Lopes KG, Bottino DA, Farinatti P, et al. Strength training with blood flow restriction - a novel therapeutic approach for older adults with sarcopenia? A case report. Clin Interv Aging. 2019;14:1461–1469. doi:10.2147/CIA.S206522.
- Loenneke JP, Kim D, Fahs CA, et al. The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging. Nov 2017;37(6):734–740. doi:10.1111/cpf.12367.
- Nitzsche N, Schulze R, Weigand F, Hummer N, Schulz H. Comparison of an Acute Resistance Training on the Lactate Concentration with and without Blood Flow Restriction at Different Loads. Dtsch Z Sportmed. 11/01 2018;Volume 2018(11):337–343. doi. doi:10.5960/dzsm.2018.351.
- Kawada S, Ishii N. Skeletal muscle hypertrophy after chronic restriction of venous blood flow in rats. Med Sci Sports Exerc. Jul 2005;37(7):1144–1150. doi:10.1249/01.mss.0000170097.59514.bb.
- Patterson SD, Hughes L, Warmington S, et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front Physiol. 2019;10:533. doi:10.3389/fphys.2019.00533.
- Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol (1985). Jan 2000;88(1):359–363. doi:10.1152/jappl.2000.88.1.359.
- Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell. Aug 2002;13(8):2909–2918. doi:10.1091/mbc.e02-01-0062.
- Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. Apr 2008;40(4):691–698. doi:10.1249/MSS.0b013e318160ff84.
- Hackney KJ, Brown LWJ, Stone KA, Tennent DJ. The Role of Blood Flow Restriction Training to Mitigate Sarcopenia, Dynapenia, and Enhance Clinical Recovery. Techniques in Orthopaedics. 2018;33(2):98–105. doi:10.1097/BTO.0000000000000271.
- Pinedo-Villanueva R, Westbury LD, Syddall HE, et al. Health Care Costs Associated With Muscle Weakness: A UK Population-Based Estimate. Calcif Tissue Int. Feb 2019;104(2):137–144. doi:10.1007/s00223-018-0478-1.
- Robison JI, Rogers MA. Adherence to exercise programmes. Recommendations. Sports Med. Jan 1994;17(1):39–52. doi:10.2165/00007256-199417010-00004.
- Dismore L, Hurst C, Sayer AA, Stevenson E, Aspray T, Granic A. Study of the Older Adults’ Motivators and Barriers Engaging in a Nutrition and Resistance Exercise Intervention for Sarcopenia: An Embedded Qualitative Project in the MIlkMAN Pilot Study. Gerontol Geriatr Med. Jan-Dec 2020;6:2333721420920398. doi:10.1177/2333721420920398.
- Prokopidis K, Giannos P, Reginster JY, et al. Sarcopenia is associated with a greater risk of polypharmacy and number of medications: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. Apr 2023;14(2):671–683. doi:10.1002/jcsm.13190.
- Jensen LD, Andersen O, Hallin M, Petersen J. Potentially inappropriate medication related to weakness in older acute medical patients. Int J Clin Pharm. Jun 2014;36(3):570–580. doi:10.1007/s11096-014-9940-y.
- Pasco JA, Mohebbi M, Holloway KL, Brennan-Olsen SL, Hyde NK, Kotowicz MA. Musculoskeletal decline and mortality: prospective data from the Geelong Osteoporosis Study. J Cachexia Sarcopenia Muscle. Jun 2017;8(3):482–489. doi:10.1002/jcsm.12177.
- Wang X, Wu M. Research progress of gut microbiota and frailty syndrome. Open Med (Wars). 2021;16(1):1525–1536. doi:10.1515/med-2021-0364.
- Verstraeten LMG, van Wijngaarden JP, Pacifico J, Reijnierse EM, Meskers CGM, Maier AB. Association between malnutrition and stages of sarcopenia in geriatric rehabilitation inpatients: RESORT. Clin Nutr. 2021;40(6):4090–4096. doi:10.1016/j.clnu.2021.02.007.
- Curcio F, Ferro G, Basile C, et al. Biomarkers in sarcopenia: A multifactorial approach. Exp Gerontol. Dec 1 2016;85:1–8. doi:10.1016/j.exger.2016.09.007.
- Berry SD, Ramachandran VS, Cawthon PM, et al. Procollagen type III N-terminal peptide (P3NP) and lean mass: a cross-sectional study. J Frailty Aging. 2013;2(3):1–6. doi:10.14283/jfa.2013.19.
- Brown WJ, McCarthy MS. Sarcopenia: What Every NP Needs to Know. The Journal for Nurse Practitioners. 2015/09/01/2015;11(8):753–760. doi:10.1016/j.nurpra.2015.05.017.
- Prescott M, Lilley-Kelly A, Cundill B, et al. Home-based Extended Rehabilitation for Older people (HERO): study protocol for an individually randomised controlled multi-centre trial to determine the clinical and cost-effectiveness of a home-based exercise intervention for older people with frailty as extended rehabilitation following acute illness or injury, including embedded process evaluation. Trials. Nov 8 2021;22(1):783. doi:10.1186/s13063-021-05778-5.
- Tsekoura M, Billis E, Tsepis E, et al. The Effects of Group and Home-Based Exercise Programs in Elderly with Sarcopenia: A Randomized Controlled Trial. JCM. Nov 26 2018;7(12):480. doi:10.3390/jcm7120480.
- Maruya K, Asakawa Y, Ishibashi H, Fujita H, Arai T, Yamaguchi H. Effect of a simple and adherent home exercise program on the physical function of community dwelling adults sixty years of age and older with pre-sarcopenia or sarcopenia. J Phys Ther Sci. Nov 2016;28(11):3183–3188. doi:10.1589/jpts.28.3183.
- Eakin E, Reeves M, Lawler S, et al. Telephone counseling for physical activity and diet in primary care patients. Am J Prev Med. Feb 2009;36(2):142–149. doi:10.1016/j.amepre.2008.09.042.
- Liang J, Zhang H, Zeng Z, et al. Lifelong Aerobic Exercise Alleviates Sarcopenia by Activating Autophagy and Inhibiting Protein Degradation via the AMPK/PGC-1alpha Signaling Pathway. Metabolites. May 18 2021;11(5):323. doi:10.3390/metabo11050323.
- Zampieri S, Pietrangelo L, Loefler S, et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J Gerontol A Biol Sci Med Sci. Feb 2015;70(2):163–173. doi:10.1093/gerona/glu006.
- Dodds R, Kuh D, Aihie Sayer A, Cooper R. Physical activity levels across adult life and grip strength in early old age: updating findings from a British birth cohort. Age Ageing. Nov 2013;42(6):794–798. doi:10.1093/ageing/aft124.
