Abstract
Non-additive (synergistic or antagonistic) effect, a common phenomenon for the decomposition of mixed litter in nature, is usually regulated by litter quality and environmental factors. In this study, we investigated decomposition rates and nutrient (C, N, and P) dynamics in response to water availability in six litter treatments using plant material from Dongting Lake, China. Three single-litter treatments (leaves of Carex brevicuspis, leaves of Miscanthus sacchariflorus, and stems of M. sacchariflorus) and three mixed-litter treatments (1:1 mixtures of single litters) were incubated at three levels of water availability (20%, 46%, and 100% saturation) for 120 days in a mesocosm experiment. Decomposition rates for single-litter treatments were ranked: M. sacchariflorus leaves > C. brevicuspis leaves > M. sacchariflorus stems. Decomposition rates generally increased with increasing water availability. Antagonistic or additive interactions occurred in the M. sacchariflorus leaves + M. sacchariflorus stems treatment, and synergistic interactions occurred in the other two mixed-litter treatments. N content and lignin loss rate of M. sacchariflorus leaves and M. sacchariflorus stems were increased by mixing with C. brevicuspis leaves. The magnitude of synergistic interactions increased with increasing water availability and the opposite was true for antagonistic interactions. These data suggest that the direction of non-additive effects is dependent on litter quality, while the magnitude is regulated by water availability.
Introduction
Litter decomposition plays a critical role in regulating the buildup of soil organic matter in ecosystems and it releases nutrients for plant growth and influences carbon cycling (Jiang et al. Citation2013). Studies using mixed litter from multiple species, genotypes, phenotypes, or plant parts have shown that decomposition of litter mixtures at times cannot be predicted from single litters because of non-additive effects (synergistic or antagonistic interactions; Schweitzer et al. Citation2005). Non-additive interactions are usually caused by the stimulatory or inhibitory effects of nutrient and/or secondary compound (e.g., tannins, simple phenolics) transfer among litter types (Taylor et al. Citation2007; Tiunov Citation2009). Synergistic interactions depend on the nutrient status of labile organic matter or on differences in the initial nutrient content of litter types (Liu et al. Citation2007; Schimel & Hättenschwiler Citation2007). Antagonistic interactions often occur in litter with low nutrient content (e.g., N, P, soluble substances, non-lignified carbohydrates) and high content of secondary compounds (Jiang et al. Citation2013). The overall effect of mixtures on decomposition depends on the balance between these two interactions (Li et al. Citation2013).
The magnitude of non-additive effects is usually defined as the difference between observed and expected values (Duan et al. Citation2013). The direction and magnitude of non-additive effects depend on litter properties, incubation time, soil biota, and microenvironment (e.g., temperature, exogenous nutrients; Gonçalves & Canhoto Citation2009; Duan et al. Citation2013; Jiang et al. Citation2013). In wetlands, water availability regulates the activity of decomposer communities and leaching processes (Clein & Schimel Citation1994) and litter decomposition rates can increase with increasing water availability (Liu et al. Citation2006). Studies using litter mixtures suggest that water can affect interactions among litter components by leaching and passive diffusion (Briones & Ineson Citation1996). In addition, active nutrient exchange occurs via fungal hyphae, the activity of which is limited by water availability (Xu et al. Citation2010). Although water can play an important role in the exchange of nutrients and secondary compounds, it is unclear whether water availability affects the interactions between components of mixed litter.
In this study, non-additive effects in response to water availability were investigated in litter types of different quality from Dongting Lake, the second largest lake in China. Three types of single litter (leaves of Carex brevicuspis, leaves of Miscanthus sacchariflorus, and stems of M. sacchariflorus) and three types of litter mixtures (combinations of two single litter components) were incubated at three levels of water availability. C. brevicuspis and M. sacchariflorus are dominant macrophyte species in the Dongting Lake wetlands, where they occur in different elevation and soil moisture zones. C brevicuspis prefers lower elevations (higher soil moisture), while M. sacchariflorus is found in habitats at higher elevation and with lower soil moisture content (Chen et al. Citation2014; Pan et al. Citation2014). These species also coexist in some areas and litter mixtures occur in these areas. Our preliminary analyses showed that initial N and P contents were highest in C. brevicuspis leaves and lowest in M. sacchariflorus stems. Here, we tested the following hypotheses: (1) decomposition rates of both single and mixed litter will increase with increasing water availability; (2) synergistic interactions will occur in litter mixtures that include C. brevicuspis leaves because of the high nutrient content and antagonistic interactions will occur in mixtures containing M. sacchariflorus leaves and stems because of the low nutrient and high lignin content; and (3) the magnitude of non-additive effects will increase with increasing water availability because of transfer of nutrients and secondary compounds.
Methods
Collection and preparation of litter
We collected C. brevicuspis leaf litter and M. sacchariflorus leaf and stem litter from standing dead plants at Dongting Lake (29° 27′ 2.02″ N, 112° 47′ 32.28″ E), Hunan, China, in November 2012. After collection, the leaves and stems were air-dried to constant mass for 48 hr and cut into pieces (∼10 cm). Weighed litter samples (5 g), including three single and three mixed (1:1 mixtures, by weight, of single components) litters, were placed into 10 × 15-cm nylon bags (1-mm mesh). This mesh size would exclude macroinvertebrates but allow microbial colonization and leaching of litter fragments (Langhans et al. Citation2008). Sets (strings) of six bags (one of each litter type) were strung together by wire cables to facilitate harvest.
Experimental set-up
The experimental treatments consisted of three levels of water availability in a one-way factorial design. Strings of litterbags (n = 45, three per harvest) were placed in mesocosm tanks (1 × 3 × 0.7 m) in March 2013 at the Dongting Lake Station for Wetland Ecosystem Research. Three mesocosms were divided evenly into three sections, each of which was then filled with 0.2, 0.4, or 0.6 m of sand and water depth was maintained at 0.2 m; moisture content at 5 cm depth (relative to sand surface) was 100% (high water availability), 46% (medium water availability), and 10% (low water availability), respectively. Washed sand was chosen as a substrate to avoid confounding effects of environmental nutrients on litter decomposition (Sylvain et al. Citation2011). Moisture content was maintained at initial levels by adding water every 2 d.
Litter strings were randomly placed in the mesocosms (at 5-cm intervals) on 14 April 2013 and the litterbags were buried at 5 cm depth in the sand. A total of 270 litterbags were used (3 replicates × 6 litter types × 3 treatments × 5 harvests). Three strings per treatment were sampled after 15, 30, 60, 120, and 240 d of incubation. Prior to incubation, three samples of each single litter were prepared to measure initial litter quality.
Chemical analyses
After collection, litter samples were separated and washed gently using deionized water until the water was transparent and then oven-dried at 60 °C to constant weight (1 wk) to measure remaining dry mass (accuracy to 0.01 g). All samples were ground to powder and passed through a 0.5-mm mesh screen for analysis of litter quality. Samples of initial litter were analyzed for N, P, organic C, cellulose, and lignin contents; samples of incubated litter were analyzed for N, P, and lignin contents. Organic C content was analyzed using the H2SO4–K2Cr2O7 heat method, N and P were quantified using Kjeldahl digestion followed by colorimetric analysis, and cellulose and lignin contents were measured by hydrolysis (10% H2SO4 for cellulose, 72% H2SO4 for lignin; Graça et al. Citation2005).
Calculation and statistical analyses
Decomposition rate (k) for each litter type was calculated as follows:
where W0 is the initial litter mass and Wt is the mass remaining at time t in days (Olson Citation1963; Gingerich et al. Citation2014). Mass remaining was calculated as a percentage of the initial mass. Components (N, P, and lignin) that remained in litter were determined by multiplying litter mass by the content of each component. Decomposition rate, mass remaining, and litter components were compared among the single-litter treatments by two-way analysis of variance (ANOVA) with treatment and time as main factors.
The expected mass that remained in mixed-litter treatments was estimated based on the mass remaining in single-litter bags from the same string as follows (Hoorens et al. Citation2003):
where R1 and R2 indicate the mass remaining in litterbags containing single litters. Litter interactions were defined as deviations between the observed and expected mass remaining. Zero indicated an additive interaction; positive and negative values indicated synergistic and antagonistic interactions, respectively.
Paired t-tests were used for the three litter treatments to assess whether observed and expected mass remaining and components differed. Then, analysis of covariance (ANCOVA) was used to test whether the magnitude of litter interaction depended on water availability, with expected mass remaining as a covariate (Duan et al. Citation2013). All statistical analyses were performed using SPSS version 21.
Results
Initial litter quality
Initial N, P, organic C, cellulose, and lignin contents differed among the three litter treatments (p < 0.05; ). N and P contents were highest in C. brevicuspis leaves, intermediate in M. sacchariflorus leaves, and lowest in M. sacchariflorus stems (p < 0.05); lignin content showed the opposite trend (p < 0.05). The initial ratios of C:N, C:P, and lignin:N were lowest in C. brevicuspis leaves and highest in M. sacchariflorus stems (p < 0.05; ). Differences in nutrient contents among the treatments were largest in the C. brevicuspis leaves + M. sacchariflorus stems mixture, intermediate in C. brevicuspis leaves + M. sacchariflorus leaves, and lowest in M. sacchariflorus leaves + M. sacchariflorus stems (p < 0.05).
Table 1. Initial quality of the three types of plant litter used in the study.
Decomposition of single-litter treatments
All single-litter types decayed most quickly in the initial two weeks of the experiment, after which decomposition rates slowed down (). Decomposition rates of single litters were highest in M. sacchariflorus leaves at high water availability and lowest in M. sacchariflorus stems at low water availability (). Two-way ANOVA showed significant effects of water availability on decomposition rate for all single-litter treatments (p < 0.001; ).
Figure 1. Percentage (mean ± SE) of mass, nitrogen, phosphorus, and lignin remaining in three single litters in three water availability treatments. LW, IW, and HW indicate low, intermediate, and high water availability, respectively.
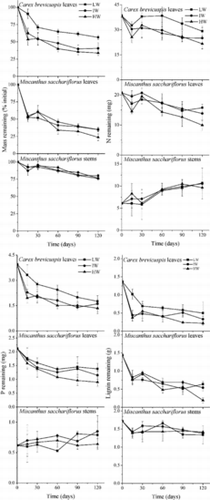
Table 2. Regression statistics (r2) for exponential rates of decomposition (k).
Both leaf litters released N and P at the end of incubation, but M. sacchariflorus stems accumulated these nutrients (). P content decreased more rapidly than N content in both leaf litters. The release of N and P from leaf litter was significantly promoted by increased water availability (p < 0.01) but N and P accumulation in M. sacchariflorus stems was not affected by water availability (p > 0.05). The effects of water availability on lignin content of the single-litter treatments were significant (p < 0.05) and lignin content decreased more quickly in leaf litter than in M. sacchariflorus stems.
Decomposition of mixed litters
The effects of water availability on decomposition rates of the three litter mixture treatments were significant (p < 0.001; ). Similar to single-litter types, decomposition of mixed litters was most rapid initially and was enhanced by increased water availability. For a given level of water availability, decomposition rate among the mixed litters was, from fastest to slowest: C. brevicuspis leaves + M. sacchariflorus leaves > C. brevicuspis leaves + M. sacchariflorus stems > M. sacchariflorus leaves + M. sacchariflorus stems.
Figure 2. Expected (Exp) and observed (Obs) mass, nutrients, and lignin remaining in the three litter mixtures in the three water availability treatments (mean ± SE). LW, IW, and HW indicate low, intermediate, and high water availability, respectively. The letters a, b, and c indicate C. brevicuspis leaves + M. sacchariflorus leaves, C. brevicuspis leaves + M. sacchariflorus stems, and M. sacchariflorus leaves + M. sacchariflorus stems mixture, respectively.
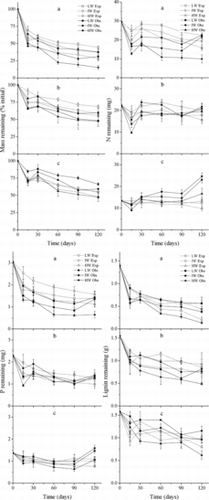
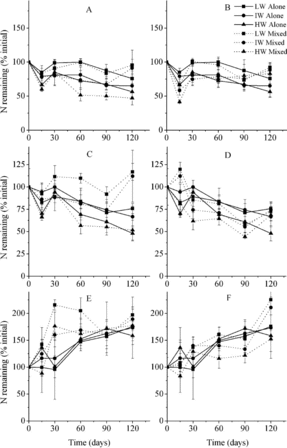
Similar to single-litter types, the leaf litters in mixtures released N and P, while the stem litter accumulated these nutrients at the end of the incubation ( and ). N content of M. sacchariflorus leaves and M. sacchariflorus stems was higher when mixed with C. brevicuspis leaves (p < 0.05; ). P content of M. sacchariflorus leaves was lower when mixed with C. brevicuspis leaves (p < 0.05). Lignin loss rates of M. sacchariflorus leaves were higher when mixed with C. brevicuspis leaves but lower when mixed with M. sacchariflorus stems (, p < 0.05). Lignin loss rates of M. sacchariflorus stems were higher when mixed with C. brevicuspis leaves and mixed with M. sacchariflorus leaves at high water availability (p < 0.05). Other litter component dynamics were not affected.
Figure 3. N remaining in litter according to mixture types (mean ± SE). LW, IW, and HW indicate low, intermediate, and high water availability, respectively. The letters A, B, C, D, E, and F indicate C. brevicuspis leaves mixed with M. sacchariflorus leaves and M. sacchariflorus stems, M. sacchariflorus leaves mixed with C. brevicuspis leaves and M. sacchariflorus stems, and M. sacchariflorus stems mixed with C. brevicuspis leaves and M. sacchariflorus leaves, respectively.
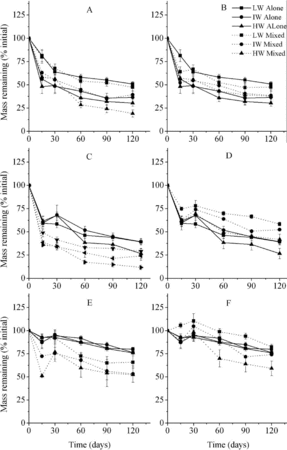
Figure 4. P remaining in litter according to mixture types (mean ± SE). LW, IW, and HW indicate low, intermediate, and high water availability, respectively. The letters A, B, C, D, E, and F indicate C. brevicuspis leaves mixed with M. sacchariflorus leaves and M. sacchariflorus stems, M. sacchariflorus leaves mixed with C. brevicuspis leaves and M. sacchariflorus stems, and M. sacchariflorus stems mixed with C. brevicuspis leaves and M. sacchariflorus leaves, respectively.
The lignin:N and lignin:P ratios for mixtures increased for all mixtures and the N:P ratios decreased (). The lignin:N and N:P ratios in the C. brevicuspis leaves + M. sacchariflorus treatment and the lignin:N ratio in the M. sacchariflorus leaves + M. sacchariflorus stems treatment were significantly affected by increasing water availability (p < 0.01; ). Other litter-component ratios were not affected (p > 0.05).
Figure 5. Lignin remaining in litter according to mixture types (mean ± SE). LW, IW, and HW indicate low, intermediate, and high water availability, respectively. The letters A, B, C, D, E, and F indicate C. brevicuspis leaves mixed with M. sacchariflorus leaves and M. sacchariflorus stems, M. sacchariflorus leaves mixed with C. brevicuspis leaves and M. sacchariflorus stems, and M. sacchariflorus stems mixed with C. brevicuspis leaves and M. sacchariflorus leaves, respectively.
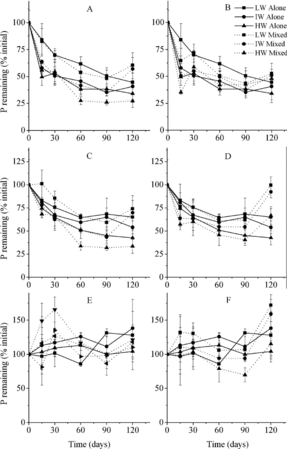
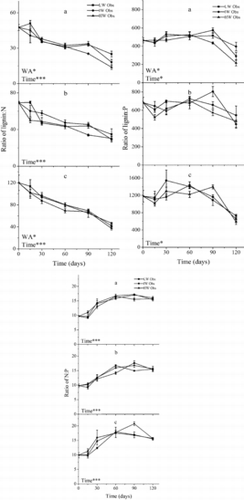
Figure 6. Observed (Obs) lignin:N, lignin:P, and N:P ratios (mean ± SE) in litter mixtures according to water availability. LW, IW, and HW indicate low, intermediate, and high water availability, respectively. The letters a, b, and c indicate mixtures of C. brevicuspis leaves + M. sacchariflorus leaves, C. brevicuspis leaves + M. sacchariflorus stems, and M. sacchariflorus leaves + M. sacchariflorus stems, respectively. *p < 0.05; ***p < 0.001.
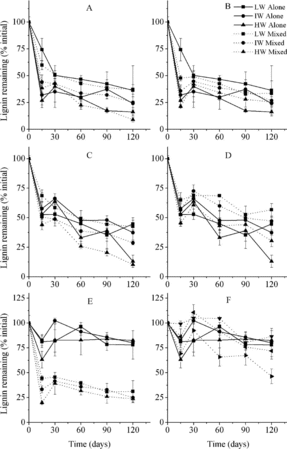
Figure 7. Mass remaining in litter according to mixture types (mean ± SE). LW, IW, and HW indicate low, intermediate, and high water availability, respectively. The letters A, B, C, D, E, and F indicate C. brevicuspis leaves mixed with M. sacchariflorus leaves and M. sacchariflorus stems, M. sacchariflorus leaves mixed with C. brevicuspis leaves and M. sacchariflorus stems, M. sacchariflorus stems mixed with C. brevicuspis leaves and M. sacchariflorus leaves, respectively.
Mass remaining of C. brevicuspis leaves was not affected by mixing (p < 0.01; and ). Mass remaining of M. sacchariflorus leaves was lower when mixed with C. brevicuspis leaves but higher when mixed with M. sacchariflorus stems (p < 0.01). Mass remaining of M. sacchariflorus stems was lower when mixed with C. brevicuspis leaves and mixed with M. sacchariflorus leaves at higher water availability but higher when mixed with M. sacchariflorus leaves at low and intermediate water availability (p < 0.01). The mixtures of C. brevicuspis leaves + M. sacchariflorus leaves and C. brevicuspis leaves + M. sacchariflorus stems decayed faster than expected and the mixture of M. sacchariflorus leaves + M. sacchariflorus stems decayed more slowly than expected in all but the low-water-availability treatments (). ANCOVA showed that the magnitude of synergistic interactions increased and that of antagonistic interactions decreased, with increasing water availability ( and ). For example, from low to high water availability, litter mass loss was increased from +6.67% to +12.93% for mixtures of C. brevicuspis leaves + M. sacchariflorus leaves, and was reduced from −10.30% to +0.68% for mixtures of M. sacchariflorus leaves + M. sacchariflorus stems.
Table 5. Results of t-tests between expected values and observed values of mass remaining in mixed-litter treatments after incubation (df = 14).
Table 3. One-way ANOVA results of mass, N, P, and lignin remaining in single litters in single-and mixed- litter bags as well as ratio of lignin:N, lignin:P, and N:P in mixed- litter bags in three water availability treatments (df = 2).
Table 4. Results of t-tests between values at decay alone and values at decay mixed of litter mass, nutrients, and lignin remaining for three litter types after incubation (df = 44).
Table 6. ANCOVA of magnitude of non-additive effects.
Discussion
Decomposition rates for all single and mixed litters increased with increasing water availability, which is consistent with our first hypothesis and with the findings of Liu et al. (Citation2006). This facilitation effect of high water availability might result from high decomposer activity in moist substrates because water was essential for microbial growth (Osono et al. Citation2003). Rapid leaching might be another explanation. Langhans et al. (Citation2008) have observed that high water availability stimulates initial decomposition in C. brevicuspis leaves.
Synergistic interactions were found in the mixtures of C. brevicuspis leaves + M. sacchariflorus leaves and C. brevicuspis leaves + M. sacchariflorus stems and antagonistic or addictive interactions occurred in the M. sacchariflorus leaves + M. sacchariflorus stems mixture. These results indicated that the direction of non-additive effects might be determined by litter quality rather than by water availability (Schimel & Hättenschwiler Citation2007). These results are consistent with our second hypothesis. Synergistic interactions might be attributed to highly efficient translocation of nutrients released from C. brevicuspis leaves because a large amount of N and P were released from C. brevicuspis leaves in mixture. Antagonistic interactions might be a result of secondary compounds and lower nutrient contents (Hättenschwiler et al. Citation2005; Liu et al. Citation2007). Berglund and Ågren (Citation2012) have noted that synergistic interactions occur when high-quality litter releases nitrogen rapidly. After mixed with C. brevicuspis leaves, the N and P contents of M. sacchariflorus leaves and M. sacchariflorus stems were higher than expected with no change in the nutrient dynamics of C. brevicuspis leaves, which suggested that a large proportion of released nutrients from C. brevicuspis leaves remained in the litter mixture (Liu et al. Citation2007; Duan Citation2013). According to the resource-complementarity mechanism for synergistic interactions, nutrient transfer from nutrient-rich litter might be able to satisfy the growth and development requirements of ligninolytic fungi in litters with low nutrient or high organic carbon contents (Gessner et al. Citation2010). Antagonistic interactions might have been caused by secondary compounds such as phenolics in M. sacchariflorus stems (Parveen et al. Citation2013), which retain N in litter by formation of phenol–protein complexes and inactivation of microbial enzymes (Aerts & Chapin Citation2000). Accumulation of N and P in stem litter suggested that decomposers could not assimilate adequate nutrients from this litter. This demand would not be satisfied by M. sacchariflorus leaves, which released lower quantities of nutrients compared with C. brevicuspis leaves. As a result, inhibition by secondary compounds might dominate litter interactions, leading to less lignin loss than expected.
The magnitude of synergistic interactions increased but the magnitude of antagonistic interactions decreased, with increasing water availability, which was partially consistent with our third hypothesis. The promotional effect of high water availability on decomposition might result from leaching of nutrients from C. brevicuspis leaves or from stimulation of nutrient transfer, since water is involved in passive diffusion and active transport of nutrients by fungal hyphae (Taylor et al. Citation2007). Because more nutrients are transported to litter with lower nutrient content under conditions of high water availability, lignin in litter mixtures usually decays faster under these conditions. There are two possible explanations for the weakened antagonistic interactions. First, the facilitative effect of increased water availability is strong enough to override the negative effects of secondary compounds. Similarly, antagonistic interactions of secondary compounds might be eliminated by the activity of soil fauna (Jiang et al. Citation2013). Second, the improved quality (decreased ratio of lignin:N) of mixed litter at high water availability might offset the inhibitory effects of secondary compounds. The lignin:N ratio was lower at higher water availability, which might result from rapid loss of lignin. This result is consistent with observations by Rosemond et al. (Citation2010) that nutrient enrichment reduced the litter C:N ratio and that the antagonistic interactions disappeared.
Acknowledgments
This study was financially supported by the National Key Technology R&D Program (2014BAC09B03) and International Science & Technology Cooperation Program of China (2012DFB30030). The authors gratefully acknowledge Dr Hou Zhiyong and Li Xu for creating the mesocosms, and Chen Xinsheng, Li Feng, and Xie Yonghong for suggestions for writing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aerts R, Chapin FS. 2000. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res. 30:1–67.
- Berglund SL, Ågren GI. 2012. When will litter mixtures decompose faster or slower than individual litters? A model for two litters. Oikos. 121:1112–1120.
- Briones MJI, Ineson P. 1996. Decomposition of eucalyptus leaves in litter mixtures. Soil Biol Biochem. 28:1381–1388.
- Chen XS, Deng ZM, Xie YH, Li F, Hou ZY, Li X, Li YF. 2014. Effects of sediment burial disturbance on the vegetative propagation of Phalaris arundinacea with different shoot statuses. Aquat Ecol. 48:409–416.
- Clein JS, Schimel JP. 1994. Reduction in microbial activity in birch litter due to repeated drying and rewetting events. Soil Biol Biochem. 26:403–406.
- Duan JC, Wang SP, Zhang ZH, Xu GP, Luo CY, Chang XF, Zhu XX, Cui SJ, Zhao XQ, Wang WY, Du MY. 2013. Non-additive effect of species diversity and temperature sensitivity of mixed litter decomposition in the alpine meadow on Tibetan Plateau. Soil Biol Biochem. 57:841–847.
- Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S. 2010. Diversity meets decomposition. Trends Ecol Evol. 25:372–380.
- Gingerich RT, Merovich W, Anderson JT. 2014. Influence of environmental parameters on litter decomposition in wetlands in West Virginia, USA. J Freshw Ecol. 29:535–549.
- Gonçalves AL, Canhoto C. 2009. Decomposition of eucalypt and alder mixtures: responses to variation in evenness. Fund Appl Limnol. 173:293–303.
- Graça MA, Bälocher F, Gessner MO, editors. 2005. Methods to study litter decomposition: a practical Guide. New York (NY): Springer-Verlag New York Inc. Press; p. 53–121.
- Hättenschwiler S, Tiunov AV, Scheu S. 2005. Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst. 36:191–218.
- Hoorens B, Aerts R, Stroetenga M. 2003. Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia. 137:578–586.
- Jiang YF, Yin XQ, Wang FB. 2013. The influence of litter mixing on decomposition and soil fauna assemblages in a Pinus koraiensis mixed broad-leaved forest of the Changbai Mountains, China. Eur J Soil Biol. 55:28–39.
- Langhans SD, Tiegs SD, Gessner MO, Tockner K. 2008. Leaf-decomposition heterogeneity across a riverine floodplain mosaic. Aquat Sci. 70:337–346.
- Li DJ, Peng SL, Chen BM. 2013. The effects of leaf litter evenness on decomposition depend on which plant functional group is dominant. Plant Soil. 365:255–266.
- Liu P, Huang JH, Han XG, Sun OJ, Zhou ZY. 2006. Differential responses of litter decomposition to increased soil nutrients and water between two contrasting grassland plant species of Inner Mongolia, China. Appl Soil Ecol. 34:266–275.
- Liu P, Sun OJ, Huang JH, Li LH, Han XG. 2007. Nonadditive effects of litter mixtures on decomposition and correlation with initial litter N and P concentrations in grassland plant species of northern China. Biol Fert Soils. 44:211–216.
- Olson JS. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology. 44:322–331.
- Osono T, Ono Y, Takeda H. 2003. Fungal ingrowth on forest floor and decomposing needle litter of Chamaecyparis obtusa in relation to resource availability and moisture condition. Soil Biol Biochem. 35:1423–1431.
- Pan Y, Xie YH, Deng ZM, Tang Y, Pan DD. 2014. High water level impedes the adaptation of Polygonum hydropiper to deep burial: responses of biomass allocation and root morphology. Sci Rep UK. 4:e5612.
- Parveen I, Wilson T, Donnison IS, Cookson AR, Hauck B, Threadgill MD. 2013. Potential sources of high value chemicals from leaves, stems and flowers of Miscanthus sinensis ‘Goliath’ and Miscanthus sacchariflorus. Phytochemistry. 92:160–167.
- Rosemond AD, Swan CM, Kominoski SJ, Dye SE. 2010. Non-additive effects of litter mixing are suppressed in a nutrient-enriched stream. Oikos. 119:326–336.
- Schimel JP, Hättenschwiler S. 2007. Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem. 39:1428–1436.
- Schweitzer JA, Bailey JK, Hart SC, Whitham TG. 2005. Nonadditive effects of mixing cottonwood genotypes on litter decomposition and nutrient dynamics. Ecology. 86:2834–2840.
- Sylvain C, Jean W, Olaf B, Damien B, Stephan H. 2011. Litter composition rather than plant presence affects decomposition of tropical litter mixtures. Plant Soil. 343:273–286.
- Taylor BR, Mallaley C, Cairns JF. 2007. Limited evidence that mixing leaf litter accelerates decomposition or increases diversity of decomposers in streams of eastern Canada. Hydrobiologia. 592:405–422.
- Tiunov AV. 2009. Particle size alters litter diversity effects on decomposition. Soil Biol Biochem. 41:176–178.
- Xu GP, Hu YG, Wang SP, Zhang ZH, Chang XF, Duan JC, Luo CY, Chao ZG, Su AL, Lin QY, et al. 2010. Effects of litter quality and climate change along an elevation gradient on litter mass loss in an alpine meadow ecosystem on the Tibetan Plateau. Plant Ecol. 209:257–268.
