ABSTRACT
Purpose
To investigate to what extent the OSDI can be utilized as a discriminative test for clinical findings.
Methods
One thousand and ninety patients with dry eye disease (DED) were consecutively included and examined for osmolarity, tear film break-up time (TFBUT), ocular protection index (OPI), ocular surface staining (OSS), Schirmer I test (ST), meibum expressibility (ME), meibum quality (MQ), and diagnosis of meibomian gland dysfunction (MGD). Receiver-operating characteristic curve (ROC) analysis considering optimum balanced sensitivity and specificity (close to 50%) was used for assessment.
Results
The present study on more than 1,000 patients indicates that the OSDI in the ROC curve analysis is a poor discriminator of pathological scores for TFBUT ≤ 5 (AUC = 0.553; p = .012) and ≤10 s (AUC = 0.608; p = .002), OSS ≥ 3 (AUC = 0.54; p = .043), ST ≤ 5 (AUC = 0.550; p = .032) and ≤10 mm/5 min (AUC = 0.544; p = .016), and ME ≥ 1 (AUC = 0.594; p = <0.001). Pathological scores for osmolarity >308 and >316 mOsm/L, OPI, OSS > 1, MQ, and MGD could not be discriminated by OSDI (p > .05).
Conclusion
Cut-off values for the OSDI can be defined to discriminate pathological TFBUT (≤5 and ≤10), OSS (≥3), ST (≤5 and ≤10) and ME, however, the discriminability was low. Our comprehensive study emphasises the importance of taking both symptoms and signs into account in DED management.
Introduction
A moist ocular surface, essential for normal sight, is maintained by the tear film in humans. Any alteration in the quality or quantity of tear film may interfere with its role in preventing dry eye disease (DED).Citation1 The term “dry eye” was first used in 1933 by Swedish ophthalmologist Henrik S.C. Sjögren. The 2017 International Dry Eye Workshop recently updated the definition of dry eye as a multifactorial disease, in which tear film hyperosmolarity and ocular surface inflammation play essential roles in the vicious cycle.Citation2
The stages of DED can range from mild, temporary ocular discomfort to chronic, severe pain with the deterioration of visual function. This disease can impose considerable cost by decreasing the quality of life and increasing the use of medical resources.Citation3,Citation4 The measurement of patient symptoms and signs is a critical aspect in the evaluation of DED. Several clinical tests are available for evaluating clinical signs such as osmolarity, tear film break-up time (TFBUT), ocular protection index (OPI), ocular surface dye staining (OSS), Schirmer I test (ST), meibum expressibility (ME), and meibum quality (MQ). Despite medical advances, limitations in using these techniques still remain. For example, the ST suffers from drawbacks of poor reproducibility, long testing time (i.e., 5 min), potential for evaporative loss, high variability, possibility of uneven absorption of tear by paper strip, and no well-defined cut-off value.Citation5–Citation9 In addition, the inherent variability of dry eye symptoms favours the use of questionnaires that record patient symptoms for diagnostic evaluations and follow-up examinations.Citation5 Among them, the ocular surface disease index (OSDI) is one of the most widely used survey instruments worldwide following its first introduction in 1997 by the Outcomes Research Group at Allergan Inc. (Irvine, CA). This 12-item questionnaire is used for assessment of symptoms related to DED and their effects on vision-related functioning in the past week.Citation10
The validity and reliability of OSDI to discriminate among the stages of DED (i.e., normal, mild to moderate and severe) was previously shown by Schiffman, et al.Citation11 A number of studies have used this symptom-based questionnaire for screening, diagnostic and evaluation purposes.Citation12–Citation16 Despite such examples, a consensus of clinical signs in DED patients may be more clinically relevant than solely relying on the OSDI.Citation17 Accordingly, several studies have reported either weak or no association between OSDI score and results from the commonly used clinical DED tests by correlation, regression and receiver-operating characteristic (ROC) curve analysis.Citation17–Citation20
The ROC curve analysis is a valuable tool to evaluate the accuracy of diagnostic tests and discriminative models.Citation21 Using possible diagnostic cut-off values, ROC curves graphically present the relationship between sensitivity and specificity of a test.Citation22 In the aforementioned studies, ROC curve analysis was performed using data collected from less than 100 participants to compare diagnostic performance between the signs and symptoms. To overcome this limitation a ten-fold larger cohort with extensive clinical parameters was used in the present study. Moreover, ROC correlations have never previously been published in a population living in Norway. All the participants in the current study were diagnosed with DED, whereas most other studies had a mixture of healthy subjects and patients with DED.
Herein we assess to what extent the OSDI self-report questionnaire can be utilized as a discriminative test for obtaining pathological osmolarity, TFBUT, OPI, ST, OSS, ME, MQ, and MGD scores using ROC curve analyses in a Norwegian cohort of 1090 DED patients.
Materials and methods
Patients
A total of 1090 patients (average age: 53 ± 16, range: 8–95, female: 797, and male: 295) with DED of different aetiologies were consecutively recruited into the study at the Norwegian Dry Eye Clinic between 2013 and 2016. Contact lens users and patients with pre-existing ocular pathologies were not excluded from the study. The patients first completed the ocular surface disease index (OSDI) questionnaire (Allergan Inc., Irvine, CA).Citation11 They then underwent a comprehensive ophthalmological examination.
The data from the questionnaires and clinical findings were made anonymous by a single ophthalmologist and included in the Norwegian Dry Eye Clinic databank. All examinations were carried out at the same clinic. The use of the data for the study has been reviewed by the Regional Committee for Medical & Health Research Ethics, Section C, South East Norway (REC). REC found the research project “Evaluation of data from the Norwegian Dry Eye Clinic” to be outside the remit of the Act on Medical and Health Research (2008) and therefore can be implemented without its approval. A REC letter of exemption has been provided. Prior to data collection, informed consent was obtained from all participants. All procedures performed in this study were in compliance with the Declaration of Helsinki.
Ophthalmological examination
All patients were examined by one ophthalmologist and an assistant within office hours from 9:00 AM to 4:00 PM by the following stepwise tests: tear film osmolarity measurement using a TearLab Osmolarity System (TearLab Corp, San Diego, CA), tear quality evaluation using TFBUT after instillation of 5 µl of 2% fluorescein sodium, calculation of OPI as the ratio of the TFBUT divided by the blink intervalCitation23, OSS using fluorescein and lissamine green (Oxford Grading Scheme: 0–15; range of corneal staining: 0–5)Citation24, and ST (without anaesthesia) for 5 min. ME was recorded based on the number of secreted glands of the lower eyelid viewed at the slit lamp when light pressure was applied by cotton tips on the central five meibomian glands (0 = all five glands expressible; 1 = three to four glands expressible; 2 = one to two glands expressible; and 3 = no gland expressible). For MQ, the central eight glands of the lower eyelid were scored on a scale of 0 to 3 for each gland (0 = clear; 1 = cloudy; 2 = cloudy with debris (granular); and 3 = thick, like toothpaste). The sum of the central eight glands was then calculated (total score range, 0–24). The diagnosis of meibomian gland dysfunction (MGD) was based on the suggestions by the international workshop on MGD.Citation25
Pathological findings (clinical signs) used in the present study were: Osmolarity >308 and >316 mOsm/LCitation26,Citation27, TFBUT ≤5 and ≤10 secondsCitation27,Citation28, OPI < 1, OSS >1 and ≥3, ST ≤5 and ≤10 mmCitation5,Citation27,Citation29, ME ≥ 1, MQ > 1, and MGD >. 0Citation30,Citation31 Only 520 subjects were examined for tear film osmolarity measurement in comparison with other tests (n = 1090).
Statistical analysis
Data from the right eye were used for statistical analysis. The ability of the OSDI questionnaire to discriminate pathological scores was analysed by receiver-operating characteristic (ROC) curve analysis with SPSS version 21 (SPSS Inc., Chicago, IL, USA). Plotting sensitivity (true positive rate) as a function of the 1-specificity (false positive rate) for defined cut-off points of a parameter created an area under the curve (AUC) that measures how well a parameter can distinguish between diagnostic groups. The AUC closer to 1 indicates better performance of the test while AUC of 0.05 shows no discrimination. The significant difference between these two AUC values is determined by calculated P value. This study considered optimum balanced sensitivity and specificity being values close to 50%.
Results
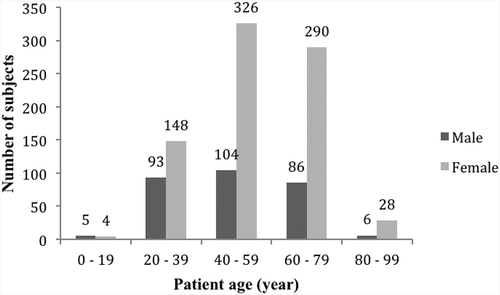
The patient demographics (average age: 53 ± 16, range: 8–95) are shown in . The number of females referred to the clinic for DED tests was higher than males in all age groups, except 0–19. Age group 40–59 presented the highest number of patients (n = 430), irrespective of sex, whereas the lowest number belonged to 80–99 (n = 34) and 0–19 (n = 9), respectively.
Figure 1. Subjects demographics: Bar graph showing the age and gender distribution among 1090 subjects tested for dry eye disease.
The results of ROC curve analysis to determine the optimum balanced sensitivity and specificity of OSDI for discriminating pathological scores are presented in and . With TFBUT ≤ 5 s as the classification variable, the optimum balanced sensitivity (57) and specificity (43) were determined by the OSDI at 30.3 as cut-off value with the area under the curve (AUC) of 0.553 ± 0.021 (p = .012). For TFBUT ≤ 10 s, the optimum balanced sensitivity (69%) and specificity (31%) were determined by 22.8 as OSDI cut-off value (AUC = 0.608 ± 0.034; p = .002). With ME, the optimum balanced sensitivity (56%) and specificity (44%) were determined by 31.5 as OSDI cut-off value (AUC = 0.594 ± 0.021; p < .001). The calculated AUC for TFBUT ≤ 10 and ME were discriminated by OSDI with higher AUC values than the other classification variables, although the values fell into a poor category of discriminators (0.6–0.7).
Table 1. Summary of ROC curve parameters and distribution of clinical tests.
Figure 2. ROC curve is displayed to determine the optimum balanced sensitivity and specificity of OSDI to predict pathological osmolarity, TFBUT, OPI, ST, OSS, ME, MQ and MGD. Abbreviations: tear film break-up time (TFBUT), ocular protection index (OPI), Schirmer I test (ST), ocular surface staining (OSS, Oxford Grading Scheme), meibum expressibility (ME), meibum quality (MQ), meibomian gland disease (MGD).
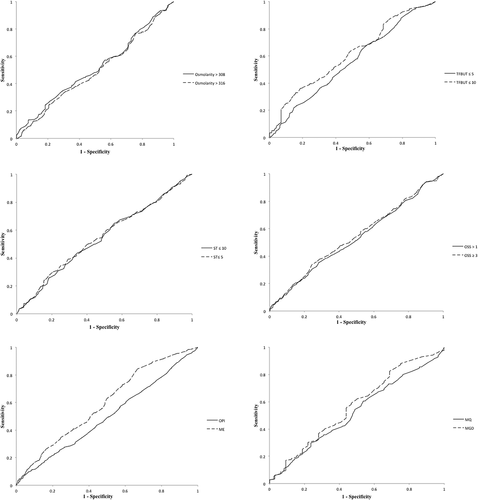
Optimum balanced sensitivity (53%) and specificity (47%) for discriminating ST ≤ 5 score were obtained by 35.7 as OSDI cut-off value (AUC = 0.550 ± 0.024; p = .032). With ST ≤ 10 mm/5 min, the optimum balanced sensitivity (52%) and specificity (48%) were obtained by 35.2 as OSDI cut-off value (AUC = 0.544 ± 0.018; p = .016). Considering OSS (≥3), OSDI with cut-off value of 22.9 gave rise to an optimally balanced sensitivity (68%) and specificity (32%) (AUC = 0.541 ± 0.021; p = .043). Although the discriminative ability of OSDI for ST ≤5 and ≤10 mm/5 min and OSS ≥ 3 was significant in this study, the performance was poor (AUC 0.5–0.6).Citation21
Pathological scores for osmolarity >308 and >316 mOsm/L, OPI, OSS > 1, MQ and MGD could not be discriminated by OSDI in the ROC curve analysis (p > .05) ().
Discussion
The present study on more than 1,000 patients indicates that the OSDI in ROC curve analysis is a poor discriminator of pathological scores for TFBUT (≤5 and ≤10 s), OSS (≥3), ST (≤5 and ≤10 mm/5 min) and ME. It cannot discriminate osmolarity (>308 and >316 mOsm/L), OPI, OSS (>1), MQ and MGD. Similar to our study, poor diagnostic performance of OSDI was shown by Fenga, et al.Citation19 for TFBUT, OSS, and MGD. Moreover, the association between symptoms and signs was weak or absent in most previous studies using correlation and regression analyses.Citation17–Citation20 However, the findings of a study by Pult et al.Citation32 examining the relationship of several clinical tests with DED symptoms differed from those of previous reports. These authors reported that non-invasive tear film break-up time (NIBUT) and TMH were significantly, but moderately, related to OSDI with excellent (AUC 0.895, p < .001) and acceptable (AUC 0.715, p = .013) discrimination power, respectively. NIBUT involves the application of a regular grid-like pattern, which is projected onto the surface of the tear film. Any discontinuity of such regular pattern indicates disruption of the tear film.Citation33 NIBUT was developed to overcome potential limitations of TFBUT, such as reflex tearing stimulation by fluorescein and possible changes in tear film properties.Citation34 It should be noted that NIBUT cannot be directly compared with TFBUT due to poor correlation between them.Citation35,Citation36 In general, the results from different studies suggest that OSDI cannot be the only tool used to predict and/or discriminate clinically evident DED.
A possible explanation for the differences between our findings and those of the published studies may relate to differences in study population. In our study, we included 1,090 patients with DED of different aetiologies, whereas Pult et al.Citation32 considered 47 healthy non-contact lens users, and Fenga, et al.Citation19 recruited 64 video display terminal operators working at screens for at least 20 h a week. Differences in inclusion criteria (i.e., subject’s status of health/disease) make it difficult to compare these investigations. In addition, there are considerable differences in sample size. The large sample size is a key strength of the current study, as the sample size is critical in increasing statistical robustness in ROC curve analysis.Citation37
Among classification variables used in this study, TFBUT ≤ 10 s and ME despite having higher calculated AUC values could poorly be discriminated by OSDI because they fell into a poor category of discriminators (0.6–0.7). TFBUT, introduced in 1969, is used to measure the relative stability of the precorneal tear film.Citation38,Citation39 It is a quick and simple test and therefore popular for diagnosing DED. Several international diagnostic criteria have assigned it as a gold standard.Citation40–Citation42 TFBUT was previously shown to be associated with OSDICitation16 and other DED tests.Citation43–Citation45 ME is used to assess the MG function.Citation46 Korb and Blackie revealed a correlation between ME and DED symptoms in 133 symptomatic and asymptomatic subjects.Citation46 However, Yeotikar, et al.Citation20 found no association between the OSDI and ME in 185 subjects with no pre-existing ocular and systemic abnormalities. The latter study linked the progressive MG loss to age concomitant with reduced quality and quantity of the meibum produced.
Our study showed how the choice of cut-off value for classifying a parameter (i.e. OSS >1 and ≥3) affected the results of ROC curve analysis. The lack of well-defined cut-off values for the available DED tests is one of the many challenging tasks for ophthalmologists and eye care practitioners. This disadvantage is caused mainly by the absence of dichotomous separation between non-DED and DED subjects, and by the presence of factors influencing the degree of disease manifestation, such as environmental conditions.Citation47
Another confounding variable comprises the words used in symptom-based questionnaires, which are sometimes misunderstood by participants, leading to bias. In the case of OSDI, 12 questions are divided into three categories: vision-related function (six questions), ocular symptoms (three questions), and environmental triggers (three questions). The choice of words for describing symptoms in each category is suggested to reflect individual and regional differences in language rather than specific qualities.Citation48,Citation49 Knottnerus and TugwellCitation50 suggested that the performance of cross-cultural adaptation of questionnaires could improve the assessment of symptoms. For example, Santo, et al.Citation51 reported a successful culturally adapted Brazilian–Portuguese version of OSDI for monitoring DED in Brazilian patients. However, further studies, which take differences among languages into account, are required. Additional limitations associated with the OSDI questionnaire include (1) absence of DED symptoms, such as foreign body sensation and tearing, (2) failure to assess effects on patients’ daily living and (3) a lack of measurement related to severity in responses. In addition, the OSDI questionnaire is time-consuming for the patient and the health-care professional with regard to scoring.Citation52 Moreover, it is not unidimensional, as a study by Dougherty, et al.Citation10 revealed the inability of OSDI in meeting the standard of unidimensionality after applying principal component analysis. The authors indicated that its current scoring system is not ideal. However, it could be optimized using Rasch analysis.
Previous studies have demonstrated that clinical tests are unable to fully diagnose DED.Citation9,Citation47,Citation53 As our study indicates that the application of the OSDI cannot reliably discriminate any of our clinical tests, it is evident that both recording of symptoms and clinical signs should be taken into account while deciding treatment strategies, until other more comprehensive questionnaires and/or clinical parameters emerge. Further studies are warranted to explore how well other questionnaires, such as McMonnies Questionnaire and Symptom Assessment in Dry Eye (SANDE), perform in discriminating clinical signs. Discovering biomarkers in the tear film that correlate with symptoms better than do the currently used clinical parameters, is another intriguing avenue for research to improve diagnosis of dry eye disease. Inflammatory biomarkers including cytokines and chemokines (e.g., TNF-a, IL-6, IL-17a, and IL-8)Citation54–Citation61 and matrix metalloproteinase-9, an enzyme involved in tissue remodeling and inflammatory processCitation62-Citation64 have been investigated in tear samples. In addition, several attempts have been made to propose other biomarkers such as lipid metabolism products (e.g., arachidonic acid, prostaglandin E2, eicosapentaenoic acid and docosahexaenoic acid),Citation65,Citation66 and proteins (e.g., α-1 acid glycoprotein1, S100 A9/calgranulin B and α-1 acid glycoprotein1).Citation67,Citation68 Despite growing body of literature, the methods of sample collection, preparation, storage, and data processing need to be standardized using defined guidelines for better comparison of data among studies.Citation69
Conclusions
Using ROC curve analysis, our study shows that the cut-off values for the OSDI questionnaire can be defined to discriminate TFBUT (≤5 and ≤10), OSS (≥3), ST (≤5 and ≤10), and ME scores. OSDI score, however, is not a good discriminative test for these clinical parameters in spite of the large sample size. Moreover, OSDI score could not be used to discriminate pathological osmolarity (>308 and >316), OPI, OSS (>1), MQ, and MGD scores. Thus, OSDI cannot be the only tool used to discriminate clinically evident DED; both symptoms and signs should be taken into account.
Acknowledgments
This work was supported by the Oslo University Hospital, Norway and the Norwegian Dry Eye Clinic, Oslo, Norway.
Disclosure Statement
We declare the authors have no competing interests as defined by Current Eye Research, or other interests that might be perceived to influence the interpretation of the article. The authors alone are responsible for the content and writing of the paper.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (10.1080/02713683.2022.2109830)
Additional information
Funding
References
- Rahman MQ, Chuah KS, Macdonald ECA, Trusler JPM, Ramaesh K. The effect of pH, dilution, and temperature on the viscosity of ocular lubricants[mdash]shift in rheological parameters and potential clinical significance. Eye. 2012;26(12):1579–84. doi:10.1038/eye.2012.211.
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo C-K, Liu Z, Nelson JD, Nichols JJ. Tsubota K and others. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83. doi:10.1016/j.jtos.2017.05.008.
- Nelson JD, Helms H, Fiscella R, Southwell Y, Hirsch JD. A new look at dry eye disease and its treatment. Adv Ther. 2000;17:84–93.
- Fd PT, Fernandes RS, Bernardes TF, Bonfioli AA, Carneiro Soares EJ. Dry eye disease. Semin Ophthalmol. 2010;25(3):84–93. doi:10.3109/08820538.2010.488568.
- Bron AJ, Abelson MB, Ousler G, Pearce E, Tomlinson A, Yokoi N, Smith JA, Begley C, Caffery B, Nichols K. Methodologies to diagnose and monitor dry eye disease. Ocul Surf. 2007;5:108–52.
- Nelson P. A shorter Schirmer tear test. Optom Mon. 1982;73:568–69.
- Cho P, Yap M. Schirmer test. II. A clinical study of its repeatability. Optom Vis Sci. 1993;70:157–59.
- Saleh T, McDermott B, Bates A, Ewings P. Phenol red thread test vs Schirmer‘s test: a comparative study. Eye. 2006;20(8):913–15. doi:10.1038/sj.eye.6702052.
- Senchyna M, Wax MB. Quantitative assessment of tear production: a review of methods and utility in dry eye drug discovery. J Ocul Biol Dis Infor. 2008;1(1):1–6. doi:10.1007/s12177-008-9006-2.
- Dougherty BE, Nichols JJ, Nichols KK. Rasch analysis of the ocular surface disease index (OSDI). Invest Ophthalmol Vis Sci. 2011;52(12):8630–35. doi:10.1167/iovs.11-8027.
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthal. 2000;118:615–21.
- Li M, Gong L, Chapin WJ, Zhu M. Assessment of vision-related quality of life in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5722–27. doi:10.1167/iovs.11-9094.
- Ünlü C, Güney E, Bis A, Akçalı G, Erdoğan G, Bayramlar H. Comparison of ocular-surface disease index questionnaire, tearfilm break-up time, and Schirmer tests for the evaluation of the tearfilm in computer users with and without dry-eye symptomatology. Clin Ophthalmol. 2012;6:1303–06. doi:10.2147/OPTH.S33588.
- Rossi GCM, Tinelli C, Pasinetti GM, Milano G, Bianchi PE. Dry eye syndrome-related quality of life in glaucoma patients. Eur J Ophthalmol. 2009;19:572–79.
- Portello JK, Rosenfield M, Bababekova Y, Estrada JM, Leon A. Computer-related visual symptoms in office workers. Ophthalmic Physiol Opt. 2012;32:375–82. doi:10.1111/j.1475-1313.2012.00925.x.
- García-Catalán M, Jerez-Olivera E, Benítez-Del-Castillo-Sánchez J. Dry eye and quality of life. Arch Soc Esp Oftalmol. 2009;84:451–58.
- Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, Geerling G, Figueiredo F, Lemp MA. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161–66. doi:10.1111/aos.12012.
- Onwubiko SN, Eze BI, Udeh NN, Onwasigwe EN, Umeh RE. Dry eye disease: concordance between the diagnostic tests in african eyes. Eye Cont Lens. 2015;42(6). www.ingentaconnect.com/content/wk/ecl/2016/00000042/00000006/art00012.
- Fenga C, Aragona P, Di NC, Spinella R. Comparison of ocular surface disease index and tear osmolarity as markers of ocular surface dysfunction in video terminal display workers. Am J Ophthalmol. 2014;158(1):41–48. e2. doi:10.1016/j.ajo.2014.03.007.
- Yeotikar NS, Zhu H, Markoulli M, Nichols KK, Naduvilath T, Papas EB. Functional and morphologic changes of meibomian glands in an asymptomatic adult population functional and morphologic changes of meibomian glands. Invest Ophthalmol Vis Sci. 2016;57(10):3996–4007. doi:10.1167/iovs.15-18467.
- Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–57. doi:10.1161/CIRCULATIONAHA.105.594929.
- Beck JR, Shultz EK. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch Pathol Lab Med. 1986;110:13–20.
- Ousler III GW, Hagberg KW, Schindelar M, Welch D, Abelson MB. The ocular protection index. Cornea. 2008;27(5):509–13. doi:10.1097/ICO.0b013e31816583f6.
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50.
- Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O‘Brien T, Rolando M, Tsubota K, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–64. doi:10.1167/iovs.10-6997g.
- Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47(10):4309–15. doi:10.1167/iovs.05-1504.
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–74. doi:10.1016/j.jtos.2017.05.001.
- Kim KT, Kim J-H, Kong YT, Chae JB, Hyung S. Reliability of a new modified tear breakup time method: dry tear breakup time. Graefes Arch Clin Exp Ophthalmol. 2015;253(8):1355–61. doi:10.1007/s00417-015-3080-5.
- Danjo Y. Diagnostic usefulness and cutoff value of Schirmer’s I test in the Japanese diagnostic criteria of dry eye. Graefes Arch Clin Exp Ophthalmol. 1997;235:761–66.
- Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, Foulks GN. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–37. doi:10.1167/iovs.10-6997b.
- Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, Yee R, Yokoi N, Arita R, Dogru M. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–49. doi:10.1167/iovs.10-6997f.
- Pult H, Purslow C, Murphy PJ. The relationship between clinical signs and dry eye symptoms. Eye. 2011;25(4):502–10. doi:10.1038/eye.2010.228.
- Sweeney DF, Millar TJ, Raju SR. Tear film stability: a review. Exp Eye Res. 2013;117:28–38. doi:10.1016/j.exer.2013.08.010.
- Luce DA Method and apparatus for tear film measurement. Google Patents; 2010.
- Nichols JJ, Nichols KK, Puent B, Saracino M, Mitchell GL. Evaluation of tear film interference patterns and measures of tear break-up time. Optom Vis Sci. 2002;79:363–69.
- Cho P, Douthwaite W. The relation between invasive and noninvasive tear break-up time. Optom Vis Sci. 1995;72:17–22.
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiol. 1982;143(1):29–36. doi:10.1148/radiology.143.1.7063747.
- Norn M. Desiccation of the precorneal film. Acta Ophthalmol. 1969;47(4):865–80. doi:10.1111/j.1755-3768.1969.tb03711.x.
- Lee JH, Kee CW. The significance of tear film break-up time in the diagnosis of dry eye syndrome. Korean J Ophthalmol. 1988;2(2):69–71. doi:10.3341/kjo.1988.2.2.69.
- Jiang Y, Ye H, Xu J, Lu Y. Noninvasive keratograph assessment of tear film break-up time and location in patients with age-related cataracts and dry eye syndrome. J Int Med Res. 2014;42(2):494–502. doi:10.1177/0300060513504701.
- Abelson MB, Ousler GW, Nally LA, Welch D, Krenzer K. Alternative reference values for tear film break up time in normal and dry eye populations. Lacrimal Gland Tear Film Dry Eye Syndromes. 2002;3:1121–25. Springer.
- Vitali C, Moutsopoulos HM, Bombardieri S. The european community study group on diagnostic criteria for Sjögren‘s syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjögren‘s syndrome. Ann Rheum Dis. 1994;53:637–47.
- Alves M, Reinach PS, Paula JS, e Cruz AAV, Bachette L, Faustino J, Aranha FP, Vigorito A, de Souza CA, Rocha EM. Comparison of diagnostic tests in distinct well-defined conditions related to dry eye disease. PLos One. 2014;9(5):e97921. doi:10.1371/journal.pone.0097921.
- Lin P-Y, Cheng C-Y, Hsu W-M, Tsai S-Y, Lin M-W, Liu J-H CP. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: the Shihpai eye study. Invest Ophthalmol Vis Sci. 2005;46(5):1593–98. doi:10.1167/iovs.04-0864.
- Begley CG, Caffery B, Chalmers RL, Mitchell GL. Use of the dry eye questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficient dry eye. Cornea. 2002;21:664–70.
- Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–47. doi:10.1097/ICO.0b013e3181814cff.
- Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, Goto E. The challenge of dry eye diagnosis. Clin Ophthalmol. 2008;2:31–55.
- Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf. 2009;7:199–211.
- Vroman DT, Sandoval HP, Fernández de Castro LE, Kasper TJ, Holzer MP, Solomon KD. Effect of hinge location on corneal sensation and dry eye after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2005;31(10):1881–87. doi:10.1016/j.jcrs.2005.03.074.
- Knottnerus JA, Tugwell P. Inter-population differences in measuring: appreciation by adaptation. J Clin Epidemiol. 2015;68(4):357–59. doi:10.1016/j.jclinepi.2015.02.004.
- Santo RM, Ribeiro-Ferreira F, Alves MR, Epstein J, Novaes P. Enhancing the cross-cultural adaptation and validation process: linguistic and psychometric testing of the Brazilian–Portuguese version of a self-report measure for dry eye. J Clin Epidemiol. 2015;68(4):370–78. doi:10.1016/j.jclinepi.2014.07.009.
- Grubbs JR Jr, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014;33(2):215. doi:10.1097/ICO.0000000000000038.
- Zeev MS-B, Miller DD, Latkany R. Diagnosis of dry eye disease and emerging technologies. Clin Ophthalmol. 2014;8:581–90. doi:10.2147/OPTH.S45444.
- Yoon K-C, Park C-S, You I-C, Choi H-J, Lee K-H, Im S-K, Park H-Y, Pflugfelder SC. Expression of CXCL9,-10,-11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51(2):643–50. doi:10.1167/iovs.09-3425.
- Choi W, Li Z, Oh H-J, Im S-K, Lee S-H, Park S-H, You I-C, Yoon K-C. Expression of CCR5 and its ligands CCL3,-4, and-5 in the tear film and ocular surface of patients with dry eye disease. Curr Eye Res. 2012;37(1):12–17. doi:10.3109/02713683.2011.622852.
- Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52(10):7725–30. doi:10.1167/iovs.11-7266.
- Meadows JF, Dionne K, Nichols KK. Differential profiling of T-cell cytokines as measured by protein microarray across dry eye subgroups. Cornea. 2016;35(3):329–35. doi:10.1097/ICO.0000000000000721.
- Yoon K-C, Jeong I-Y, Park Y-G, Yang S-Y. Interleukin-6 and tumor necrosis factor-α levels in tears of patients with dry eye syndrome. Cornea. 2007;26(4):431–37. doi:10.1097/ICO.0b013e31803dcda2.
- Lam H, Bleiden L, De Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205. e1. doi:10.1016/j.ajo.2008.08.032.
- Ji YW, Mittal SK, Hwang HS, Chang E-J, Lee JH, Seo Y, Yeo A, Noh H, Lee HS, Chauhan SK. Lacrimal gland–derived IL-22 regulates IL-17-mediated ocular mucosal inflammation. Mucosal Immunol. 2017;10(5):1202. doi:10.1038/mi.2016.119.
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023–27. doi:10.1097/ICO.0b013e3181a16578.
- Acera A, Vecino E, Duran JA. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2013;54(13):8285–91. doi:10.1167/iovs.13-12235.
- Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmol. 2016;123(11):2300–08. doi:10.1016/j.ophtha.2016.07.028.
- Sambursky R, Davitt III WF, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014;33(8):812–18. doi:10.1097/ICO.0000000000000175.
- Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A. ω-3 tear film lipids correlate with clinical measures of dry eye. Invest Ophthalmol Vis Sci. 2016;57(6):2472–78. doi:10.1167/iovs.16-19131.
- Kenchegowda S, He J, Bazan H. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins Leukotrienes Essent Fatty Acids. 2013;88(1):27–31. doi:10.1016/j.plefa.2012.03.010.
- Zhou L, Beuerman RW. Quantitative proteomic analysis of N-linked glycoproteins in human tear fluid. Mass spectrometry of glycoproteins. Springer; 2013. p. 297–306.
- Tong L, Zhou L, Beuerman RW, Zhao SZ, Li XR. Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br J Ophthalmol. 2011;95(6):848–52. doi:10.1136/bjo.2010.185256.
- Tamhane M, Cabrera-Ghayouri S, Abelian G, Viswanath V. Review of biomarkers in ocular matrices: challenges and opportunities. Pharm Res. 2019;36(3):40. doi:10.1007/s11095-019-2599-2.
