ABSTRACT
We describe newly recovered cranial material of Bunostegos akokanensis, a pareiasaurian reptile known from the Upper Permian Moradi Formation of northern Niger. Bunostegos is highly autapomorphic, with diagnostic cranial features including two or three hemispherical bosses located above and between the external nares; laterally projecting supraorbital ‘horn’ formed by an enlarged postfrontal; large foramen present on ventral surface of postfrontal; and hemispherical supratemporal boss located at posterolateral corner of skull roof. We addressed the phylogenetic position of Bunostegos by incorporating it into a cladistic analysis of 29 parareptilian taxa (including all 21 currently valid pareiasaurs) and 127 cranial and postcranial characters. The results of this analysis place Bunostegos as more derived than middle Permian forms such as Bradysaurus and as the sister taxon to the clade including Deltavjatia plus Velosauria. Certain characters, such as the pattern of cranial ornamentation and the size and placement of the tabulars, appear to be more similar to more derived pareiasaurs such as Elginia from Scotland and Arganaceras from Morocco, but the most parsimonious tree topology indicates that these features were evolved independently in the Nigerien form. The lack of both dicynodont herbivores and Glossopteris, combined with the presence of a giant herbivorous captorhinid, indicates a markedly different community structure in the Permian of Niger compared with those for contemporaneous southern Pangean basins (i.e., Karoo, Luangwa, Ruhuhu). The endemic tetrapod fauna of Niger supports the theory that central Pangea was biogeographically isolated from the rest of the supercontinent by desert-like conditions during Late Permian times.
SUPPLEMENTAL DATA—Supplemental materials are available for this article for free at www.tandfonline.com/UJVP
INTRODUCTION
Pareiasaurs were a moderately diverse clade of parareptiles restricted to the middle and Upper Permian, but with a near-global distribution (Lee, Citation1997a). Historically, most of the group's diversity has come from the Beaufort Group of South Africa (10 of 21 named species; Lee, Citation1997a), but recent work has extended the geographic range of African pareiasaurs northward (Sidor et al., Citation2003; Jalil and Janvier, Citation2005). Sidor et al. (Citation2003) named Bunostegos akokanensis as the first pareiasaur from Niger on the basis of a weathered skull lacking lower jaws that was collected from the Upper Permian Moradi Formation in 2000. Despite the lack of surface detail, the holotype was clearly diagnosable as a new pareiasaur genus by virtue of its three enlarged supranarial bosses and paired supraorbital and supratemporal ‘horns.’
Additional fossils were collected from the Moradi Formation in 2003 and 2006 (). These include amphibian material described by Sidor et al. (Citation2005b) as the edopoid, Saharastega moradiensis, and the derived cochleosaurid, Nigerpeton ricqlesi (see also Damiani et al., Citation2006, Steyer et al., Citation2006). Reptilian fossils included juveniles and subadults of the large captorhinid Moradisaurus grandis (O’Keefe et al., Citation2005, Citation2006), as well as undescribed remains of a possible second, smaller captorhinid taxon (Sidor et al., Citation2005a). In addition, Smiley et al. (Citation2008) described the first therapsid from the formation as an indeterminate rubidgine gorgonopsid and noted that previous reports (Taquet, Citation1967, Citation1978; de Ricqlès and Taquet, Citation1982) suggesting the presence of a dicynodont were in error.
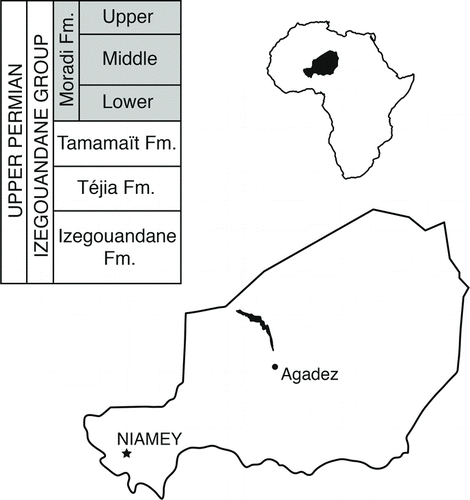
FIGURE 1 Geographic position of the study area and stratigraphy of the Permian Izegouandane Group of Niger. Filled area within country outline of Niger indicates mapped extent of the Izegouandane Group. Abbreviation: Fm., Formation.
Pareiasaur fossils were found to be relatively common in the Moradi Formation, with finds in 2003 and 2006 ranging from isolated elements to partially articulated skeletons with axial and appendicular material. The collected elements span a wide range of body sizes, suggesting the possibility that more than one pareiasaur species coexisted in the Moradi fauna. Here we describe cranial material referable to Bunostegos that provides critical information on the phylogenetic position of this remarkable taxon. Our cladistic analysis builds on that of Tsuji (Citation2006, Citation2010, in press) and results in phylogenetic support for an emerging picture of central Pangean endemism during Late Permian times (Sidor et al., Citation2005b; Tabor et al., Citation2011).
Institutional Abbreviations
BP, Bernard Price Institute for Palaeontological Research, University of the Witwatersrand, Johannesburg; MB, Museum für Naturkunde, Humboldt Universität zu Berlin, Berlin; MNHN, Muséum national d’Histoire naturelle, Paris; MNN, Musée National du Niger, Niamey; PIN, Paleontological Institute, Moscow; SMNS, Staatliches Museum für Naturkunde, Stuttgart.
BACKGROUND
Pareiasaurs are known exclusively from middle–Upper Permian rocks, with the bulk of the group's diversity concentrated in Africa. The most recent taxonomic revision recognizes eight genera from the Beaufort Group of South Africa (viz. Anthodon, Bradysaurus, Embrithosaurus, Nanoparia, Nochelesaurus, Pareiasaurus, Pareiasuchus, Pumiliopareia), but some of these genera are also known from Karoo-equivalent strata across southern Africa (Lee, Citation1997b). For example, Pareiasuchus is also known from the upper Madumabisa Mudstone Formation of Zambia (Lee et al., Citation1997), and Anthodon and Pareiasaurus are recorded from the Usili Formation of Tanzania (Sidor et al., Citation2010). Besides Bunostegos from Niger, other African pareiasaurs include indeterminate material from Malawi (Huene, Citation1944; Lee, Citation1997b) as well as the recently described Moroccan form, Arganaceras vacanti (Jalil and Janvier, Citation2005). It should be noted that Jalil and Janvier (Citation2005) recognized two distinct morphs of postcranial material from the same level of the Ikakern Formation as Arganaceras. However, they noted that none of this material could be unambiguously assigned to the named taxon (Jalil and Janvier, Citation2005).
The South American pareiasaur fossil record comes from the Rio do Rasto Formation of Brazil and is restricted to a few reasonably complete specimens. These bones were initially described as ‘Pareiasaurus’ americanus by Araújo (Citation1985), but this taxon was later transferred to a new genus, Provelosaurus, by Lee (Citation1997b) in order to keep the genus Pareiasaurus monophyletic. The latter assignment was upheld by Cisneros et al. (Citation2005).
Laurasian pareiasaurs are recorded from China (Sanchuan- saurus, Shansisaurus, and Shihtienfenia), Germany (Parasaurus), Russia (Deltavjatia, Scutosaurus, and Obirkovia), and Scotland (Elginia). Of these, the Russian forms are the best understood, each being known by several skulls and partial skeletons (Lee, Citation2000; Tsuji, Citation2010, in press). Elginia, a highly autapomorphic form known only from natural molds (Newton, Citation1893; Spencer and Lee, Citation2000), has attracted recent attention because of its unique cranial anatomy and superficial similarity to Arganaceras (Jalil and Janvier, Citation2005).
MATERIAL
Our description is based primarily on three specimens. The first, MNN MOR86, comprises a partial skull lacking its lower jaws. The second specimen, MNN MOR28, consists of a skull that is three-dimensionally preserved, but highly eroded on its dorsal surface. The third specimen, MNN MOR47, preserves the braincase including the sphenethmoid in left lateral view, along with part of the palate. Five additional specimens including identifiable cranial material are described where relevant.
The following specimens were studied for comparative purposes: MB R.939 (skull and anterior cervical vertebrae of Pareiasuchus peringueyi); MNHN ARG 518 (disarticulated cranial remains of Arganaceras vacanti); PIN 2212/6 (skull and lower jaw of a subadult Deltavjatia vjatkensis); and SMNS 58317 (skull and anterior skeleton of a subadult Deltavjatia vjatkensis).
SYSTEMATIC PALEONTOLOGY
Holotype
MNN MOR72, ventrally eroded skull lacking lower jaws.
Referred Material
MNN MOR25, eroded braincase and palate; MNN MOR28, weathered skull; MNN MOR35, fragmentary skull roof elements; MNN MOR37, partial skull including snout, interorbital region, and posterior margin of skull table; MNN MOR47, partial skull with braincase exposed in left lateral view and associated cervical vertebra; MNN MOR86, nearly complete skull, right scapulocoracoid, and two dorsal vertebrae; MNN MOR101, isolated left postfrontal, right scapulocoracoid, right innominate, and dorsal vertebra; MNN MOR104, isolated nasals, interorbital skull roof, right ulna, isolated neural spine, and isolated osteoderm.
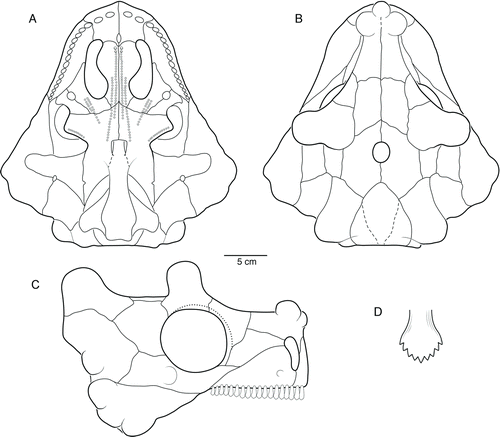
FIGURE 2 The skull of Bunostegos akokanensis (MNN MOR86). Photograph and interpretative drawing in A, dorsal and B, ventral views. Abbreviations: bo, basioccipital; ec, ectopterygoid; f, frontal; j, jugal; l, lacrimal; m, maxilla; nb, nasal boss; p, parietal; pal, palatine; pf, postfrontal; pm, premaxilla; po, postorbital; pop, paroccipital process of opisthotic; pp, postparietal; ppf, prepalatal foramen; prf, prefrontal; pt, pterygoid; q, quadrate; qj, quadratojugal; sq, squamosal; st, supratemporal; st h, supratemporal ‘horn’; t, tabular; v, vomer.
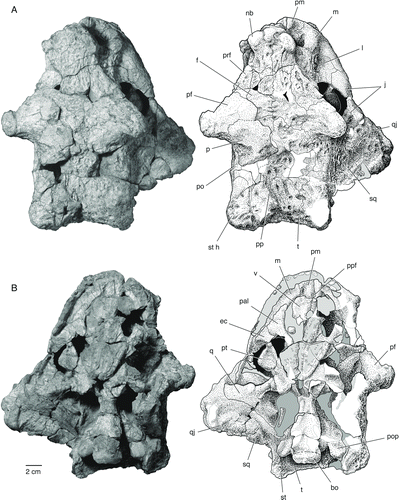
FIGURE 3 The skull of Bunostegos akokanensis (MNN MOR86). Photograph and interpretative drawing in right lateral view. Abbreviations: j, jugal; l, lacrimal; m, maxilla; mf, anterior maxillary foramen; n, nasal; pf, postfrontal; pm, premaxilla; po, postorbital; prf, prefrontal; qj, quadratojugal; sq, squamosal; st, supratemporal.
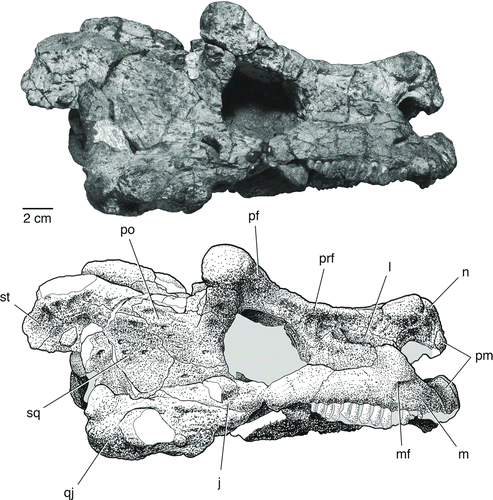
FIGURE 4 The skull of Bunostegos akokanensis (MNN MOR28). Photograph and interpretative drawing in ventral view. Abbreviations: bo, basioccipital; bt, basal tubera of basisphenoid; ch, choana; ec, ectopterygoid; m, maxilla; pal, palatine; pbs, parabasisphenoid; pt, pterygoid; q, quadrate; qj, quadratojugal; sub, suborbital foramen; v, vomer.
Locality and Horizon
The specimens described here were collected from several localities located approximately 20 km west of Arlit, Agadez Department, northern Niger (). The localities are within 1 km of each other and all fall within the upper one-third of the Moradi Formation. Detailed locality data are available to qualified researchers at the MNN or by contacting C.A.S. The Moradi Formation is typically considered to be Late Permian in age, although the data supporting such an assignment are not overwhelming (Taquet, Citation1972, Citation1976; Sidor et al., Citation2005b; Tabor et al., Citation2011).
Revised Diagnosis
Medium-sized pareiasaur with two or three hemispherical bosses located at the anterior end of the snout; nasal with posterolateral tab-like process articulating with the frontal; frontal and parietal lack central bosses; elongate, laterally projecting supraorbital ‘horn’ formed by postfrontal and overhanging orbit in dorsal view; large foramen present on ventral surface of postfrontal; hemispherical supratemporal boss located at posterolateral corner of skull roof; supraorbital ‘horn’ and supratemporal bosses with neck separating globular head from skull roof; postorbital extends more posteriorly than parietal on skull roof; pineal foramen equidistant from frontoparietal and parietal-postparietal sutures.
DESCRIPTION
Skull Roof
Unless noted, the following description is based on the most complete skull (MNN MOR86; , 3), with reference made to a referred skull (MNN MOR28; ) and the holotype (MNN MOR72) only when necessary. Despite the fact that sutures are often obscured by the dermal sculpturing and rugosity typical of pareiasaur crania, comparison of these three specimens in addition to other partial skulls (MNN MOR25, MNN MOR37, MNN MOR47, and MNN MOR104) permit a reasonably complete understanding of the skull ().
MNN MOR86 has been slightly crushed dorsoventrally and sheared so that the skull roof lies somewhat to the left of its natural position. In addition, much of the left half of the skull roof has been overridden the right side, thereby substantially decreasing the apparent total breadth. The bosses characteristic of the cranium of Bunostegos are broken and worn on most specimens, sometimes leaving only the base of each process. MNN MOR86 measures 23.7 cm from the anterior extent of the nasals to the posterior extent of the supratemporals, making it similar in size to the holotype (MNN MOR72 = ∼25 cm). Posterior to the orbits, the skull roof and the cheek region meet at a distinct angle, more closely resembling the shape of Bradysaurus than Pareiasaurus.
Premaxilla
The internarial bar, which is formed by the dorsal process of the left and right premaxillae, is mostly missing (). However, based on what is preserved, we believe that it was likely oriented vertically, as in most other pareiasaurs. Jalil and Janvier (Citation2005) coded Bunostegos as having a posterodorsally oriented dorsal process of the premaxilla (their character 26), but this is likely incorrect. This character was excluded from the present analysis because changing the outgroup taxa used in the analysis rendered the character uninformative. Above the round external naris, a small portion of each premaxilla is preserved overlapping the corresponding nasal. The premaxilla-nasal contact is positioned anteriorly and dorsally, so that the premaxilla forms the anterior margin of the external naris and the nasal forms its dorsal margin.
A prepalatal foramen is visible between the premaxillae and vomers in ventral view and is a feature typical of pareiasaurs (). In MNN MOR86, this midline foramen is largely positioned between the premaxillae, with very little contribution by the vomers. However, the remaining sutures between the premaxilla, vomer, and maxilla are not clearly visible in MNN MOR86 (, 3). Bunostegos appears to have two, or possibly three, teeth in the premaxilla. Lee et al. (Citation1997) reported two premaxillary teeth in Pareiasuchus nasicornis (BP/1/3653), but then figured three teeth in the same paper. To further confuse the matter, Lee (Citation1997a, Citation1997b) figured other pareiasaurs as having three premaxillary teeth, whereas Boonstra (Citation1934:11, 21) stated that all pareiasaurs possess two premaxillary teeth. Specimens of Deltavjatia, in which the premaxilla-maxilla suture is clear, (SMNS 58317) bear two premaxillary teeth (Tsuji, Citation2010, in press).
Septomaxilla
This element is rarely reported in the description of pareiasaur skulls, with Boonstra (Citation1934:12) noting its presence only in Anthodon. A small plate of bone is suspended by matrix in the left external naris of MNN MOR86. However, we suspect that this element is a displaced piece of vomer and not the septomaxilla. The loss of the septomaxilla is probably characteristic of pareiasaurs, with its recognition in Anthodon a misinterpretation.
Maxilla
The maxilla forms most of the lateral surface of the snout, contacting the premaxilla beneath the external naris and the lacrimal along its dorsal edge (). The maxilla contributes to the ventral and posterior margins of the external narial opening, and posteriorly contacts the jugal along a posteroventrally trending suture. Posteriorly, the maxilla extends along the ventral margin of the skull to a point just behind the anterior margin of the orbit, where it forms a near-vertical suture with the jugal. Thus, as preserved, the maxilla fails to contact the quadratojugal in lateral aspect, although the relevant bones are eroded in this region (). Maxilla-quadratojugal contact is typically considered to be a synapomorphy of pareiasaurs (Lee, Citation1997a), and a suture between the two elements in Bunostegos is best seen in ventral view, where the anterior process of the quadratojugal contacts the posterior-most extension of the maxillary alveolar ridge (). In lateral view, the suture is generally obscured by the ventral extent of the jugal.
The external surface of the maxilla is smooth, lacking the prominent boss or ‘horn’ seen in Elginia or some specimens of Pareiasaurus and Scutosaurus. However, the posterior border of the nostril is marked by a very thick ridge of bone located immediately below the most anterior part of the lacrimal-maxilla contact. This feature is not observable in the holotype (MNN MOR72) because of erosion, but appears similar to the condition described for Bradysaurus baini (Lee, Citation1997a:207). A single, large anterior lateral maxillary foramen (sensu Laurin and Reisz, Citation1995) is present just below this thickened maxillary ridge () and probably transmitted fibers of the maxillary branch of the trigeminal nerve in life.
In ventral view, the maxilla forms a distinct shelf medial to the tooth row, the anterior portion of which forms the anterolateral border of the choana (). Posteriorly, along the choana, the palatine broadly underlaps the medial surface of the maxilla. The curvature of the lateral boundary of the internal choana, formed by the maxilla and palatine, resembles that found in other pareiasaurs (Lee, Citation1997a:).
Assuming that there are two premaxillary teeth, the remains of at least 13 teeth are present in the right maxilla of MNN MOR86, with spaces for two more. Although most of the teeth are incompletely preserved, the seventh maxillary tooth retains four cusps along the apex of its crown. We suspect that the five central-most cusps are missing, so our reconstruction shows a total of nine cusps (). Unfortunately, the presence of an internal cingulum cannot be determined. Cingula are typically present on the lingual surface of maxillary teeth of pareiasaurs found in younger strata such as Pareiasuchus and Deltavjatia, but are absent in older pareiasaurs such as Bradysaurus and Embrithosaurus (Lee, Citation1997b). The maxillary teeth of Bunostegos are oriented vertically, and do not angle outwards as in Scutosaurus (Bystrov, Citation1957; Lee, Citation1995), anteroventrally as in Arganaceras (Jalil and Janvier, Citation2005), or inwards as purported in Bradysaurus baini and Pareiasuchus nasicornis (Lee, Citation1997b). The two teeth present in the premaxilla and the 15 reconstructed in the maxilla indicate that Bunostegos possessed at least 17 alveoli in its upper jaw. The number of upper jaw alveoli in Bunostegos is therefore intermediate between that of Deltavjatia, Pareiasuchus, and Pareiasaurus, which have 14 or 15 alveoli, and Bradysaurus and Embrithosaurus (and presumably Nochelesaurus), which have 18–20 alveoli in the upper jaw (Lee, Citation1995). As with most Paleozoic tetrapods, pareiasaurs undoubtedly increased the number of marginal teeth through ontogeny. For example, a juvenile Deltavjatia (PIN 2212/6) has only 11 upper jaw alveoli. However, because skull size and inferred phylogenetic position have a distinct relationship within pareiasaurs (viz. derived pareiasaurs are smaller and have fewer teeth), whether tooth count is simply a function of skull size rather than having a taxonomic or phylogenetic meaning is an open question.
Lacrimal
The right lacrimal is complete and lacks any indication of a central boss (). As in other pareiasaurs, it contacts the nasal, maxilla, and prefrontal. Anteriorly, the lacrimal forms only a small portion of the margin of the external naris, but posteriorly it makes up a substantial part of the orbital margin. The lateral surface of the lacrimal is very rugose anteriorly, but this texture fades posteriorly and ventrally, where a shallow fossa is formed in front of the orbit. There is one larger, ventrally situated, and three smaller, more dorsally situated lacrimal foramina. These lie on the posterior edge of the lacrimal, inside the anterior edge of the orbit.
Nasal
As in the holotype, three semicircular bosses adorn the anterior portion of the snout above the external nares (). One large knob is present on the anterolateral aspect of each nasal and between these lies the base of a third, median boss. A small foramen is present immediately ventral to each lateral knob and above the aperture of the external naris. This foramen is readily observable on isolated nasals pertaining to a juvenile individual (MNN MOR104). Pareiasuchus peringueyi and P. nasicornis both possess a boss on the nasal that overlies the nostril (Boonstra, Citation1934; Lee, Citation1997b; Lee et al., Citation1997), and the base of a median nasal boss was illustrated in Elginia (Newton, Citation1893) and described in a single specimen of Bradysaurus (Boonstra, Citation1934). In P. nasicornis, these bosses have been interpreted as discrete ossifications, distinct from the nasal elements (Lee et al., Citation1997), but there is no evidence that this is the case in Bunostegos. A well-formed nasal boss is present in Arganaceras, but it is clear that this, too, is an elaboration of the nasal and not a discrete ossification (C.A.S., pers. observ.). Isolated nasals of juvenile Bunostegos () show no indication of a median boss, suggesting individual, sexual, or ontogenetic variation in this feature.
The nasal forms the dorsal margin of the external naris, above its contact with the lacrimal and premaxilla. The dorsal surface of the nasal is rugose, which obscures recognition of the midline, internasal suture. Posteriorly, the nasal-frontal suture can be made out but it is not possible to discern whether the dorsolateral tab-like process of the posterior nasal, visible on the holotype, is present. The breadth of the nasals in Bunostegos gives the snout an appearance that is broader than tall, similar to Pareiasuchus nasicornis (Lee et al., Citation1997), whereas in Arganaceras and Elginia the snout appears taller than wide (Jalil and Janvier, Citation2005).
Prefrontal
The right prefrontal is more complete than the left. It is clearly divided into dorsal and lateral faces by a thickened ridge of bone that extends anteriorly from the anterodorsal part of the orbit to the lateral nasal boss. On its lateral surface, the prefrontal-lacrimal suture runs anteriorly and slightly dorsally from the orbit to a position just posterior to the nasal boss (). Because a small part of the right orbital rim is missing, the frontal-prefrontal suture is visible in cross-section. It is difficult to follow this suture dorsally on the skull roof, but from what can be made out, the prefrontal is restricted laterally (). As in other pareiasaurs, the prefrontal contacts the postfrontal to exclude the frontal from contributing to the dorsal margin of the orbit. We suspect that Bunostegos either lacked a central boss on the prefrontal, which would be unusual among pareiasaurs, or, like Arganaceras, possessed a greatly reduced one. Unfortunately, the appropriate section of the orbital margin is missing on both sides of MNN MOR86 and is not available on any of the other referred skulls. The ventral process at the anterior edge of the prefrontal forms a solid connection with the palate, running just posterior to the lacrimal. This suture can best be seen in MNN MOR72, because this area of the palate is missing in MNN MOR86.
Frontal
The dorsal surface of the frontal is rugose, but lacks a central boss (). In this character Bunostegos more closely resembles the condition seen in Arganaceras, Elginia, and Pareiasaurus. It contacts the nasal, prefrontal, postfrontal, and parietal along sutures that correspond to the pattern seen in other pareiasaurs. The element is relatively short in pareiasaurs compared with their outgroups, the nycteroleters, in which the frontal is a long, slim bone, more than four times as long as wide (Tsuji et al., Citation2012). The frontal-parietal suture is roughly perpendicular to the midline sutures of the frontals and parietals. This is slightly different than the corresponding contact in the holotype (Sidor et al., Citation2003:) in which the suture extends slightly anteriorly before proceeding directly lateral to the postfrontal. However, this difference may be due to the fact that the external surface of the holotype is severely weathered, such that the observed sutures are all ‘deep’ to some degree.
Postfrontal
MNN MOR86, MOR101, and MOR104 all display the diagnostic postfrontal morphology of Bunostegos; this element is very large and extends laterally to take the form of a prominent, rounded ‘horn’ over the orbit. In contrast to other pareiasaurs, the postfrontal in Bunostegos forms nearly the entire dorsal orbital border to obscure the orbit completely when viewed from above. On all specimens where this area is accessible, a foramen can be seen to penetrate the ventral surface of the postfrontal (). We suspect that this foramen carried an enriched blood supply to the supraorbital ‘horn,’ which suggests that it was covered by a keratinous sheath in life. A corresponding, albeit smaller, foramen is present in Arganaceras. Interestingly, although the skull of the Moroccan pareiasaur is substantially larger than Bunostegos, its supraorbital horn is smaller, probably requiring a relatively smaller blood supply. Among pareiasaurs, an enlarged postfrontal ‘horn,’ or elongate postfrontal boss, is also present in Elginia (Lee, Citation2000). A subadult Deltavjatia (SMNS 58317) has a correspondingly smaller boss, yet it is still clearly distinguishable from the surrounding dermal sculpturing.
Sutural contacts of the postfrontal are best seen on the right side in MNN MOR86 (, 3) and agree with those found in the holotype (MNN MOR72) and MNN MOR104. In MNN MOR86, the right supraorbital ‘horn’ has been deformed upwards, so that it does not extend as far laterally as in the holotype. Despite this deformation, this bone retains a shallow transversely oriented trough on its underside that continued laterally under the main projection of the ‘horn.’ The left postfrontal and postorbital of MNN MOR86 have been translated medially. This movement is most apparent posteriorly, where the latter elements override the left frontal and parietal ().
Postorbital
Considerably more information about the postorbital in Bunostegos can be added to the description of Sidor et al. (Citation2003). On the right side, the postorbital can be seen to form the dorsal half of posterior margin of the orbit (). Above it, the enlarged postfrontal forms the dorsal orbital margin. The postorbital-postfrontal contact can be seen along the posterior base of the right supraorbital ‘horn,’ until a triple junction with the parietal is formed medially. The postorbital is sutured to the jugal ventrally, the latter forming the ventral half of the orbital margin. In frontal section, the postorbital is a curved plate forming a small horizontal contribution to the skull roof and a larger vertical portion of the cheek. Posteriorly, the postorbital contacts the squamosal and the supratemporal, and appears very long in dorsal view (). Bunostegos is unique among pareiasaurs in that the postorbital extends farther posteriorly than the parietal.
Jugal
Aside from a small gap in the suborbital bar, nearly all of the right jugal is present and well preserved. In lateral view, the jugal has a prominent anterior process that contacts the maxilla and lacrimal at a level slightly in front of the orbit, and it has a well-developed ventral process projecting below the level of the tooth row (). Posteroventral to the orbit, the jugal forms a low-lying boss just dorsal of the anterior part of the jugal-quadratojugal suture. Contacts between the jugal and quadratojugal, the jugal and squamosal, and the jugal and postorbital are visible and correspond to the pattern commonly seen in pareiasaurs.
Squamosal
The squamosal is a large, rectangular element forming the rear of the cheek above the quadratojugal in most pareiasaurs. This element is mostly preserved on the right side in MNN MOR86 (). The external surface of the squamosal bears small pits and relatively low furrows. The squamosal-jugal contact is complete and angled dorsally and slightly anteriorly to intersection of these two bones and the postorbital. Above this, the squamosal contacts the postorbital along a dorsally and slightly posteriorly oriented suture. The contact between the squamosal and supratemporal begins along the posterior margin of the skull, just beneath the base of the supratemporal ‘horn.’ This suture passes slightly upwards anteriorly, before arcing ventrally along the postorbital. As in several other pareiasaur genera, a small boss is present on the squamosal along its posterior edge (e.g., Jalil and Janvier, Citation2005:).
Quadratojugal
This element is entirely missing from the holotype and is incompletely preserved in MNN MOR86. Nonetheless, some details of its anatomy in Bunostegos are now possible to evaluate. As in other pareiasaurs, the quadratojugal is a large element that forms the ventral margin of the skull behind the orbit, with an anterior process that extends along the ventral edge of the jugal to contact the maxilla. Anteriorly, the external surface of the quadratojugal is relatively unornamented where it contacts the jugal. The remains of at least two bosses are present on the ventral and posterior margins of this element (). These appear to have been approximately equal in size to the nasal knobs and slightly larger than those of the jugal or squamosal.
Parietal
The right parietal is relatively complete, although a portion of its posterior edge is broken. Deformation of the left postorbital-postfrontal complex has overrun the dorsal surface of the parietal and obscured most of the margin of the parietal foramen. The position of the parietal foramen can be best seen in the holotype (Sidor et al., Citation2003:), where it is situated about halfway along the interparietal suture. In all other pareiasaurs, the foramen is closer to the frontoparietal suture; the atypical condition in Bunostegos is likely due to the large size and anterior extent of the postparietal. As in the holotype (Sidor et al., Citation2003:), the parietal contacts the frontal anteriorly, the postfrontal anterolaterally, and the postorbital laterally (). The posterior contacts of the parietal are more difficult to make out, but we suspect that the condition depicted by Sidor et al. (Citation2003) is essentially correct: the parietal contacts the supratemporal laterally, with the median postparietal interposed along the interparietal suture behind the parietal foramen.
Postparietal
This element was depicted by Sidor et al. (2003:) as a small, rectangular bone positioned entirely on the skull table between the parietals and supratemporals. This arrangement mirrors that proposed by Lee (Citation1997a; Spencer and Lee, Citation2000) for Elginia, although Jalil and Janvier (Citation2005) suggested that the conditions in Elginia and Bunostegos might not be homologous. We can tentatively identify the posterior border of the postparietal, where it presumably contacts the enlarged tabular, but the lateral extent of the postparietal is unclear (). Unfortunately, MNN MOR86 provides little new information on the morphology of the postparietal. In ventral view, the contact between the tabular and the overlying supratemporal can be determined, but the suture between the postparietal and the former cannot be determined. Targeted preparation of other specimens (e.g., MNN MOR37) has also failed to shed light on this anatomy.
Supratemporal
We follow Lee (Citation1995, Citation1997a, 1997b; Lee et al., Citation1997) in considering the large element of the posterolateral skull roof to be the supratemporal (contra Hartmann-Weinberg, Citation1933; Boonstra, Citation1934; Bystrov, Citation1957). Among pareiasaurs, Elginia has the best-developed projection on the supratemporal, where it takes the form of a long, gently curved ‘horn’ (Newton, Citation1893; Spencer and Lee, Citation2000). A similar feature occurs in Arganaceras (Jalil and Janvier, Citation2005:) and Bunostegos, although the ‘horn’ in the latter ends in a rounded, globular swelling. In the holotype of Bunostegos, these ‘horns’ are well developed and project laterally from the skull roof (Sidor et al., Citation2003:). By contrast, in MNN MOR86, they are somewhat eroded but clearly did not project as far laterally as those of the holotype (). It is therefore possible that the holotypic skull is somewhat flattened dorsoventrally. The region of the left supratemporal ‘horn’ in MNN MOR86 has been deformed so that the normally laterally facing part of the supratemporal faces ventrally.
Contacts between the supratemporal and the squamosal, postorbital, and parietal can be made out (, 3). The suture between the supratemporal and tabular is oriented differently in dorsal than in ventral view. Dorsally, the suture is broadly concave and is positioned approximately halfway between the lateral edge of the skull table and the midline. The nature of the contact between the supratemporal and the postparietal cannot be distinguished in its entirety; it appears, however, that the large tabulars exclude contact between the postparietal and the supratemporal (). On the ventral surface, the tabulars broadly underlap the supratemporal, so the supratemporal appears much thinner in ventral view ().
Tabular
Lee (Citation1997a) discussed the presence of a skull element interposed between the postparietal and supratemporal in post-Embrithosaurus pareiasaurs, and commented on its various interpretation as a tabular (Wild, Citation1985), a cervical osteoderm incorporated into the skull roof (Brink, Citation1955), or as a neomorphic ossification. Jalil and Janvier (Citation2005) supported the last proposal in Arganaceras, on the basis of its firm connection to the adjoining bones. Most recently, Tsuji (Citation2006, Citation2010) has provided evidence that this element is homologous to the tabular in parareptiles such as Macroleter and Nyctiphruretus. We adopt Tsuji's (2006, 2010) interpretation.
Sidor et al. (Citation2003) found some evidence for the presence of an enlarged ‘supernumerary element’ (i.e., tabular) along the posterior margin of the skull in Bunostegos, although its anterior sutural configuration was poorly understood. In MNN MOR86, crushing of the left supratemporal region towards the midline has obscured much of the relevant area. However, as noted above, a suture is visible between the more lateral, horn-bearing supratemporal and the more medial element, here interpreted as the tabular. In MNN MOR86, the area is well preserved but lacks obvious sutures on its dorsal surface. Preparation of the ventral surface the posterior skull roof of MNN MOR86 has revealed an extensive underlap of the tabular under the supratemporal, with the former element appearing to be quite extensive on the ventral surface ().
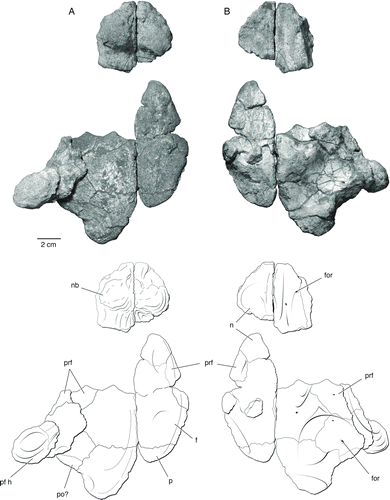
FIGURE 7 The braincase of Bunostegos akokanensis (MNN MOR47). Photograph and interpretative drawing in left lateral view. Abbreviations: bo, basioccipital; cp, cultriform process; ex, exoccipital; f, frontal; fo, foramen ovale; jf, jugular foramen; pop, paroccipital process; pro, prootic; pt, pterygoid; sph, sphenethmoid; II, III, IV, foramen for cranial nerves; XII, foramina for hypoglossal nerve.
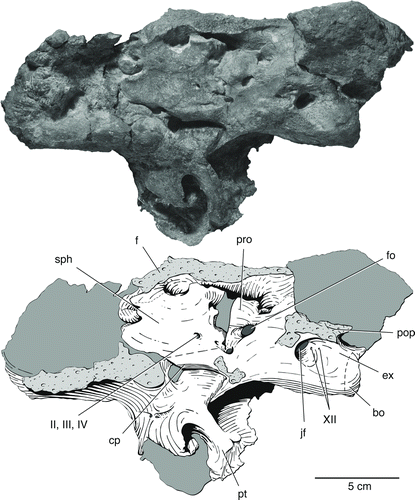
FIGURE 8 The palate and braincase of Bunostegos akokanensis (MNN MOR25). Photograph and interpretative drawing in ventral view. Abbreviations: bo, basioccipital; can, neurovascular canal; cp, cultriform process; pal, palatine; pbs, parabasisphenoid; pt, pterygoid; q, quadrate; qj, quadratojugal; tub, quadrate tubercle; v, vomer.
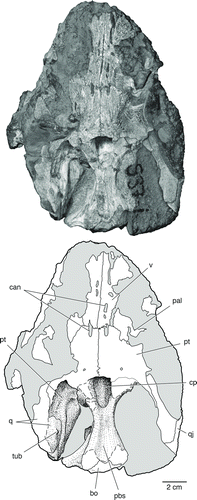
Palate and Braincase
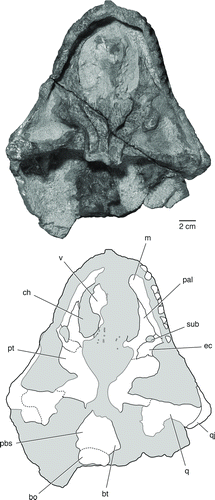
The right half of the palate is mostly preserved, although a substantial piece is missing in the region of the palatine and pterygoid (). In addition, some portions of the palate remain unprepared, such as the dentigerous parts of the vomer and pterygoid. Nevertheless, much additional morphological detail is available when compared with the severely eroded palate of the holotype. The lateral exposure of the braincase in MNN MOR47 allows a rare lateral view of the pareiasaurian braincase (). As in most (adult) specimens, however, the braincase elements can be fused together, making it difficult to delimit the individual elements.
Vomer
The morphology of the ventral surface of the vomer is mostly obscured by adhering matrix (). However, the holotypic specimen shows that the vomer of Bunostegos is laterally expanded near its midpoint (the ‘alar flange’ of Damiani and Modesto, Citation2001), contributing to the ‘C’-shaped outline of the choanae in ventral view (Sidor et al., Citation2003:). Viewed through the incomplete left naris, the dorsal laminae of both vomers are exposed in cross-section. Damiani and Modesto (Citation2001) noted that the dentition of the pareiasaurian vomer can be divided into an anterior row of larger teeth and paired posterior rows of smaller teeth. This pattern is present in MNN MOR86, with the roots of at least four anterior teeth (∼2 mm diameter) visible on the left side. Large teeth are also partially exposed in MNN MOR28, although they are still largely embedded in matrix (). More posteriorly, the roots of substantially smaller teeth (<1 mm diameter) form two subparallel rows. These rows are present on the preserved left vomer and continue onto the corresponding pterygoid. Anteriorly, the vomer contacts the premaxilla, where it forms a limited contribution to the prepalatal foramen, and posteriorly it contacts the pterygoid medially and the palatine laterally. Although highly weathered, the basic outline of the posterior contacts of the vomer are exposed in MNN MOR25 (). The vomer in MNN MOR25 also shows infillings that we interpret as the remains of neurovascular canals that extended anteroposteriorly within the vomer and pterygoid.
Palatine
Only the anterior portion of the right palatine is preserved (). It underlaps the palatal portion of the maxilla and forms the posterior half of the lateral margin of the right choana. Posteriorly, the palatine sutures to the ectopterygoid, with a comparatively small posterior palatal foramen in between. The posterior palatal (or suborbital) foramen is more clearly exposed in MNN MOR28, where it is larger and enclosed between the palatine and ectopterygoid close to the triple junction of these two elements with the maxilla (). Most of the body of the palatine, where it contacts the pterygoid, is missing or obscured by matrix, but the posterolaterally oriented palatine-pterygoid suture can be seen in MNN MOR25 (). There are two parallel rows of denticles visible on the ventral surface of the palatine that continue onto the pterygoid, although they are largely covered with matrix ().
Ectopterygoid
A small portion of the right ectopterygoid is preserved (). As in other pareiasaurs, it articulates with the maxilla laterally and with the palatine anteriorly. In addition, a portion of its posteromedial contact with the pterygoid is preserved. The posterior edge of the ectopterygoid also underlaps the anterior process of the jugal.
Pterygoid
The body of the pterygoid is mostly preserved, but remains covered in a thin layer of red matrix. Nonetheless, four rows of small teeth can be seen on each side (). As in other pareiasaurs, the medial two rows are parallel, oriented parasagitally, and pass anteriorly to continue on the corresponding vomer. The lateral two rows are oriented anterolaterally. The majority of pareiasaurs, except perhaps Elginia, have a single row of prominent denticles on the posterior edge of the transverse flange of the pterygoid (Lee, Citation1997a). Unfortunately, no specimen of Bunostegos preserves the relevant section. Although the transverse flange is not complete in any specimen, it is clear that it was reduced and directed more anterolaterally than laterally and approached the cheek, as in the nycteroleters such as Macroleter (Tsuji, Citation2006). It is also clear that the transverse flange in Bunostegos, as in most pareiasaurs, did not extend as far ventrally as in their nycteroleter relatives.
Posteromedially, the pterygoid forms an immobile sutural connection with the basipterygoid process of the basisphenoid (), as occurs in all adequately known pareiasaurs (Lee, Citation1997a). Sidor et al. (Citation2003) indicated that the interpterygoid vacuity of Bunostegos was present and ‘U’-shaped. However, this coding resulted from a misinterpretation of Lee's (1997a:character 18) distinction between character states. Although the new material does not help address this issue, the morphology of the holotype of Bunostegos remains most similar to that of Bradysaurus and Deltavjatia: the posterior margin of the pterygoids is oriented almost transversely in front of the basisphenoid (, 4, 7; see also Newton, Citation1893:pl. 39). Although much of the ventral surface of the pterygoids is eroded, MNN MOR25 most clearly demonstrates this condition (). We have reworded this character (number 21) in our analysis.
Only the right quadrate ramus of the pterygoid is preserved. Its articulation with the quadrate is eroded, but as observed by Sidor et al. (Citation2003) in the holotype, the quadrate was positioned posteriorly when compared with most other pareiasaurs. In this regard, Bunostegos is most similar to Pareiasuchus nasicornis (Lee et al., Citation1997). A conspicuous subhorizontal ridge also extends along the medial face of the quadrate ramus of the pterygoid (), attenuating in prominence posteriorly. Based on its location, we suspect that this ridge served to limit dorsal movement of the quadrate, but the distribution and significance of this feature among pareiasaurs is unclear. The quadrate process of the pterygoid extends posteriorly almost to the level of the paroccipital process.
Quadrate
This element is best preserved on the right side of MNN MOR86, although additional morphology can be discerned in MNN MOR25 and MOR28. In MNN MOR86, the articular condyles are complete and the dorsal ramus is in place (). The articular condyle is wider than long, and features two relatively flat articular surfaces separated by a slight depression. In MNN MOR86, the condyle is slightly posterolaterally directed (), but in MNN MOR28, which has suffered less distortion, the condyles are directed laterally (). All of the available specimens agree that jaw articulation was well anterior to the occipital condyle in Bunostegos. A well-defined tubercle with a pointed tip is present on the medial aspect of quadrate dorsal ramus. This tubercle is best preserved in MNN MOR25 and projects medially and slightly posteriorly into the cranioquadrate space (). Based on its shape and position, we suspect that the tubercle was a soft tissue attachment point related to the stapes.
The dorsal process of the quadrate extends anteriorly as far as the level of the beginning of the transverse flange of the pterygoid, as a tall flange of bone applied to the lateral surface of the pterygoid. The element continues posteriorly, following the quadrate ramus of the pterygoid, and is applied to the medial side of the squamosal. The holotypic specimen (MNN MOR72) shows this area in cross-section, and although the poor preservation of the specimen makes this area difficult to interpret, the quadrate can be seen to extend posteriorly to the level of the paroccipital process. This interpretation differs from that illustrated by Sidor et al. (2003:), which did not show the full posterior extent of the quadrate. The quadrate foramen can be seen in MNN MOR86 at the junction of the quadrate, quadratojugal, and squamosal ().
Opisthotic and Prootic
The braincase of pareiasaurs is typically highly ossified, and even in juveniles the suture between the prootic and the opisthotic is difficult to distinguish (Tsuji, Citation2010). The opisthotic can be seen arising from the parabasisphenoid anterior to the exoccipital, where it forms the anterior margin of the jugular foramen (). More anteriorly, the opisthotic forms the caudal edge of the foramen ovalis. In MNN MOR47, the distance between the jugular foramen and the foramen ovalis is longer than is portrayed for most other pareiasaurs such as Pareiasuchus nasicornis (Lee et al., Citation1997:) and Pareiasaurus serridens (Boonstra, Citation1934:), although some of this may be due to distortion of MNN MOR47. The opisthotic forms the ventral and the majority of the lateral portion of the paroccipital process that connects the braincase to the skull roof, with the prootic forming the remainder. The opisthotic alone sutures to the squamosal and supratemporal in a broad, anteroposteriorly expanded process. The paroccipital process is ‘U’-shaped in occipital view, directed straight laterally before curving upwards to the dorsolateral corner of the skull roof.
The prootic forms the anterior part of the foramen ovalis and has a posterolateral flange that forms the anterior edge of the paroccipital process. Anteriorly, the prootic forms the entirety of the dorsum sellae, which overlies the cultriform process of the parabasisphenoid.
Basisphenoid and Parasphenoid
The basisphenoid and parasphenoid are at least partially preserved in several specimens (, 4, 7, 8), but typically fused indistinguishably. In ventral view, the cultriform process is short and broad, almost as wide as the interpterygoid vacuity, with a broad midline ridge along its ventral surface (). The anterior edge of this process is oriented transversely. The basipterygoid process of the basisphenoid is well preserved in MNN MOR86 and MOR25. It is oval in outline, with its long axis oriented anterodorsally-posteroventrally. Its ventral surface is smooth and featureless, lacking the tubercles seen in Pareiasaurus or Scutosaurus (Lee, Citation1997a).
Despite the dorsal displacement experienced by the braincase in this specimen, MNN MOR28 best preserves the ventral surface of the basisphenoid and demonstrates the presence of broad, expanded basal tubera (). These were mostly eroded away in MNN MOR47 () and slightly damaged in MNN MOR86 (). The basal tubera are positioned posteriorly, close to the occipital condyle, a condition most similar to Bradysaurus and differing from taxa such as Anthodon where the tubera are located midway between the occipital condyle and the basipterygoid processes (Lee Citation1997a). Lee (Citation1997a) proposed a distinction in the anatomy of the basisphenoid, where basal pareiasaurs such as Bradysaurus and Nochelesaurus retain a primitive ‘waisting’ of the basisphenoid, and more-derived pareiasaurs show no significant transverse constriction. The anatomy of Bunostegos is difficult to assign to either configuration, but in general it appears that the length and relative narrowness of the basisphenoid is most similar to that depicted for Bradysaurus (Lee, Citation1997b:).
MNN MOR47 shows the cultriform process to cradle the base of the sphenethmoid (). In lateral view, the prootic can be seen to suture along the dorsal margin of the basisphenoid, with the anterior portion of the fenestra ovalis visible.
Sphenethmoid
The exposure of the braincase in lateral view in MMN MOR47 affords a rare view of a pareiasaur sphenethmoid (), although portions of this element can also be seen in MNN MOR86. Lee (Citation1995) interpreted this midline braincase element as a pleurosphenoid, whereas deBraga and Rieppel (Citation1997) argued for its recognition as a sphenethmoid, which we adopt here. Boonstra (Citation1934:18) provided a description of this element for Pareiasaurus serridens and Watson (Citation1914:) discussed it in a specimen that was subsequently attributed to Embrithosaurus by Lee (Citation1997a). An incomplete sphenethmoid of Pareiasuchus was figured by Haughton (Citation1929:fig. 23). The sphenethmoid is a trapezoidal element in lateral view that arises from the cultriform process and contacts the underside of the frontals. In sagittal section the element appears as a ‘V,’ with two plates of bone that diverge near their dorsal edge. As proposed by Boonstra (Citation1934), a foramen near the posterior edge of the sphenethmoid likely transmitted cranial nerves II, III, and IV in life ().
Basioccipital
As noted by Sidor et al. (Citation2003), the basioccipital forms most of the occipital condyle in Bunostegos, although the limits of the adjacent exoccipitals are difficult to make out. The suture between the basisphenoid and basioccipital is visible in the holotype, although a break along what appears to be the suture confounds its interpretation. Among the available specimens, the eroded ventral surface of MNN MOR25 partially shows the path of this suture (). In contrast with the condition depicted for Pareiasuchus nasicornis (Lee et al., Citation1997:), the basisphenoid-basioccipital suture is concave anteriorly at its midpoint. The ventral surface of the basioccipital is smooth, lacking the median tubercle described for Arganaceras and Scutosaurus (Jalil and Janvier, Citation2005) as well as Pareiasuchus nasicornis (Lee et al., Citation1997).
Exoccipital
The lateral aspect of the exoccipital is well exposed in MNN MOR47 (), although sutures delimiting it from the surrounding elements are difficult to discern. The element extends dorsolaterally from the basioccipital, and the lateral process underlies the opisthotic where the latter begins to form the paroccipital process. The exoccipital has a medial process, but none of the available specimens makes it clear if it met its counterpart at the midline, thereby excluding the supraoccipital from the foramen magnum. A series of small foramina can be seen on the ventrolateral edge of the exoccipital, close to where it fuses with the basioccipital, for the hypoglossal nerve, similar to the condition illustrated for P. nasicornis (Lee et al., Citation1997:) and Deltavjatia (Tsuji, Citation2010:fig. 4.15). Dorsally, the exoccipital fuses indistinguishably with the supraoccipital. Lee (Citation1995) considered the possession of a lateral flange on the exoccipital a diagnostic character of pareiasaurs, but more recently this feature was demonstrated in Macroleter (Tsuji, Citation2006), and perhaps it may be a feature of all pareiasauromorphs.
DISCUSSION
Previous Analyses of Pareiasaur Phylogeny
The phylogenetic relationships of pareiasaurs have been the subject of some cladistic work, but usually in the broader context of turtle origins or amniote phylogeny (Gauthier et al., Citation1988; Lee, Citation1993, Citation1995, Citation1997b; Laurin and Reisz, Citation1995; deBraga and Rieppel, Citation1997; Hill, Citation2005; Tsuji and Müller, Citation2009; Lyson et al., Citation2010). Of these, Lee (Citation1997b) has provided the only detailed analysis of pareiasaur interrelationships, with 19 pareiasaur terminal taxa scored for 128 characters. This data matrix was revised and updated by Jalil and Janvier (Citation2005), who included Arganaceras vacanti as well as two types of unnamed pareiasaur postcrania from the Ikakern Formation of Morocco. In one of their analyses, Jalil and Janvier (Citation2005) also included Bunostegos, with codings based on the published description of Sidor et al. (Citation2003). However, as with Lee (Citation1997b), all of Jalil and Janvier's (2005) analyses included turtles as an ingroup and a long list of parareptile outgroups (viz. Millerettidae, Nycteroleteridae, Nyctiphruretidae, Owenetta, Barasaurus, Procolophonidae, Lanthosuchidae, and Sclerosaurus).
Tsuji and Müller (Citation2008) rescored the German pareiasaur Parasaurus and included it in a modified version of the Jalil and Janvier (Citation2005) matrix. Tsuji and Müller (Citation2008) eliminated turtles from the ingroup and included three other parareptiles (Macroleter poezicus, Millerettidae, and Procolophonidae), using Millerettidae and Procolophonidae as outgroups. In this analysis, Bunostegos remained relatively basally positioned as in Jalil and Janvier (Citation2005). Tsuji (Citation2010, in press) further modified the Jalil and Janvier (Citation2005) and Tsuji and Müller (Citation2008) matrices, also including all nycteroleter parareptiles in the analysis, but did not include a comprehensive reevaluation of Bunostegos.
Current Phylogenetic Analysis
We prefer to restrict our analysis to the interrelationships of pareiasaurs and their closest relatives, because some of Lee's (1997b) outgroups are of doubtful appropriateness (e.g., Sclerosaurus has been identified as a derived procolophonid, not the sister taxon to Pareiasauria; see deBraga, Citation2003; Cisneros et al., Citation2005; Tsuji, Citation2006; Sues and Reisz, Citation2008) and turtles are now considered to fall outside of Parareptilia (Zardoya and Meyer, Citation1998; Rieppel and Reisz, Citation1999; Rieppel, Citation2000; Hill, Citation2005), although some debate remains (Lyson et al., Citation2010). The monophyly of traditional pareiasaurs has never been doubted seriously; Lee (Citation1997b) suggested that the clade could be diagnosed by 37 synapomorphies, whereas deBraga and Rieppel (Citation1997) suggested that it was diagnosed by 26 synapomorphies. Pareiasaurs are clearly distinctive fossils, and probably would have been similarly distinctive in life (). More recent analyses have shown that certain nycteroleter taxa form the closest outgroups to pareiasaurs (Tsuji, Citation2006; Tsuji and Müller, Citation2009; Tsuji et al., Citation2012), but pareiasaur ingroup relationships remain little modified from the results of Lee's (1997b) initial work.
Presented in Appendix 1 is a summary of the changes made to the character list and character matrix presented in CitationTsuji (in press), which combines selected characters from the study of Jalil and Janvier (Citation2005) and Lee (Citation1997a), as well as new characters developed in the course of our work on Bunostegos. Appendix 2 includes our data matrix, with 127 characters coded for 21 pareiasaur taxa, six nycteroleters, as well as ‘Owenetta’ kitchingorum and Millerettidae. The matrix in Appendix 2 was subjected to a traditional search with 5000 random addition sequences and 1000 trees per replication using the Tree Bisection Reconnection (TBR) algorithm in TNT (Goloboff et al., Citation2008). The default collapsing rule in TNT was used, which eliminates branches for which the minimum possible length is zero. All of the characters were left unordered and unweighted. Millerettidae was the sole outgroup. A bootstrap analysis was run using 5000 replicates, and the Bremer decay indices were also determined, both using TNT (Goloboff et al., Citation2008). Forty-five minimum length trees of 213 steps were recovered, with a strict consensus yielding a relatively well-resolved topology, although the support values for most of the nodes are not particularly high (; see online Supplementary Data). In all trees, Bunostegos is recovered as the sister taxon to a clade including all other non-Tapinocephalus and Pristerognathus assemblage zone pareiasaurs. Velosauria, a group of derived pareiasaurs defined by Lee (Citation1994) as the ancestor of Therischia and Pumiliopareiasauria and all its descendants, is recovered in this analysis.
FIGURE 10 Cladistic relationships of Bunostegos akokanensis within Pareiasauria. A strict consensus of the 45 most parsimonious topologies recovered from a TNT analysis. Bremer decay values above one and bootstrap values >50% are listed above and below, respectively, each well-supported branch.
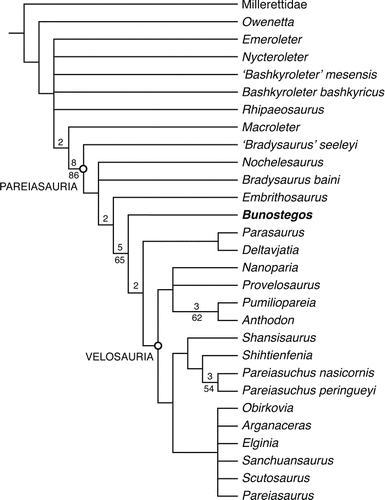
In their initial description, Sidor et al. (Citation2003) noted that Bunostegos possessed features of both primitive and derived pareiasaurs. The present analysis suggests that, at least on basis of its cranial anatomy, Bunostegos is best considered a relatively basal pareiasaur. This position can be attributed to several specific codings. In particular, the shape (character 54) and high number of marginal teeth (character 51), the shape of the anterior edge of the interpterygoid vacuity (character 21), and the shape and orientation of the paroccipital process (character 6). We were unable to determine with confidence the sutural contacts of the postparietal and supratemporal, which has been considered a key feature linking Elginia, Arganaceras, and possibly Bunostegos (Jalil and Janvier, Citation2005). As such, character 33 was coded as unknown (Appendix 2) for Bunostegos. However, when this character was rescored to the state present in Elginia and Arganaceras and the analysis rerun, the resulting strict consensus tree does not change (tree length 213 steps; consistency index = 0.751; retention index = 0.877), which reinforces the interpretation that this character is convergent.
Age of the Moradi Formation
The Moradi Formation has been considered Late Permian in age (Taquet, Citation1972; de Ricqlés and Taquet, Citation1982; Sidor et al., Citation2005b; Smiley et al., Citation2008). This age assignment is based primarily on biostratigraphic correlations with other, better-known Permian faunas, but such correlations are not without conflict. Taquet (Citation1972) indicated a Late Permian age based on the presence of a pareiasaur and a captorhinid (Moradisaurus) that was similar to the Russian taxon Gecatogomphius. However, Bunostegos is arguably more similar to middle Permian forms, and Gecatagomphius is now considered to be from the middle Permian of Russia. As outlined by Sidor et al. (Citation2005b), the temnospondyls of the Moradi Formation are members of clades that were primarily known from the Carboniferous. The only evidence supporting a Late Permian (as opposed to middle Permian) age for the fauna is the presence of a large, presumably derived, gorgonopsid (Smiley et al., Citation2008). The phylogenetic position of Bunostegos recovered here is equivocal, because this taxon fits between middle Permian and Late Permian pareiasaurs. The absence of biostratigraphically useful dicynodonts in the Moradi Formation makes it particularly difficult to tie the Moradi vertebrate fauna to other Pangean assemblages.
Biogeographic Implications
Lee (Citation1997b) suggested that pareiasaurs had their origin in Gondwana, based on the early appearance of Bradysaurus, Embrithosaurus, and Nochelesaurus in the middle Permian of South Africa. However, the remainder of his pareiasaur cladogram was claimed to have little biogeographic structure (Lee, Citation1997b:291–292). More recently, Tsuji (Citation2006) found Macroleter poezicus, a parareptile from Russia, to be the closest relative of Pareiasauria within Parareptilia. Subsequent analyses have supported the hypothesis that the nycteroleters are either the monophyletic sister taxon of, or grade into, pareiasaurs (Tsuji, Citation2010; Tsuji et al., Citation2012). Most nycteroleters are from the middle Permian of Russia, and therefore Laurasian, and although a member of the group has been identified from the Chickasha Formation in Oklahoma (Reisz and Laurin, Citation2001), another specimen has been recovered from the Tapinocephalus Assemblage Zone in South Africa (Cisneros and Tsuji, Citation2009). We suggest that no biogeographic scenario for the origin of pareiasaurs can be definitively supported at present. However, given the current fossil record, we agree with Lee (Citation1997b) that pareiasaurs probably radiated in southern Pangea, as all of the middle Permian members of the group have been recovered from southern African rocks.
Sidor et al. (Citation2005b) argued that the composition of the Moradi Formation tetrapod fauna was distinctive and tied its endemic nature to the arid conditions inferred for central Pangea (Gibbs et al., Citation2002; Rees et al., Citation2002). More recent analyses of Moradi paleosols by Tabor et al. (Citation2011) have confirmed that deposition of the Moradi Formation occurred under semiarid to hyperarid conditions. According to the climate hypothesis, Moradi tetrapods owe some of their distinctive qualities (e.g., highly autapomorphic morphology, long ghost lineages) to a long period of isolation from the remainder of tetrapod faunas on Pangea. For example, the temnospondyls Nigerpeton and Saharastega are most closely related to taxa known from the Permo-Carboniferous of Euramerica. Although less pronounced, the phylogenetic position and anatomy of Bunostegos mirrors that of the Moradi temnospondyls; Bunostegos clusters with relatively early diverging members of Pareiasauria, but it is also highly autapomorphic, particularly with regards to its cranial sculpture, which appears to share convergent characters with more derived pareiasaurs such as Elginia and Arganaceras.
Among middle or Upper Permian tetrapod assemblages, the Moradi Formation is closest in composition to that of the Ikakern Formation of Morocco. Both formations have at least one pareiasaur (Bunostegos, Arganaceras), a large captorhinid with multiple toothrows (Moradisaurus, unnamed taxon; Jalil and Dutuit, Citation1996), and amphibians belonging to groups that typically occur in the Permo-Carboniferous of North America (Nigerpeton, Diplocaulus; Dutuit, Citation1988). Steyer and Jalil (Citation2009) recently noted the presence of the first temnospondyl from the Ikakern Formation, noting that it likely belonged to a non-stereospondyl or non-euskelian (sensu Yates and Warren, Citation2000). Given these similarities, we tentatively suggest that the tetrapod assemblages of the Moradi and Ikakern formations represent a faunal province distinct from that of southern Pangea (e.g., Karoo-type assemblages).
Other Pareiasaurs from the Moradi Formation
Sidor et al. (Citation2003) noted that a pareiasaur skull from the Moradi Formation was figured by Taquet (Citation1976:pl. 1). This skull, which appears similar to Bunostegos in its possession of supraorbital bosses, was never named or described, and is now missing from the MNHN (P. Taquet, pers. comm., 2008).
Additional pareiasaur material collected in 2003 and 2006 is undergoing preparation. Fortunately, several specimens discussed here include both cranial and postcranial elements, which should permit much of the postcranial skeleton of Bunostegos to be identified. A preliminary examination of our Moradi collection suggests that there is nothing definitive to establish greater than one pareiasaur taxon in the fauna. However, it is important to recognize that pareiasaur genera are known to co-occur in the same formation in South Africa and in China, leaving open the possibility that Bunostegos coexisted with a second pareiasaur in the Permian of Niger.
CONCLUSIONS
Fossils referable to Bunostegos akokanensis permit an improved understanding of the cranial morphology and phylogenetic position of this taxon among pareiasaurs. Phylogenetically, Bunostegos sits between early diverging taxa such as Bradysaurus and Embrithosaurus and the derived velosaurs (e.g., Anthodon, Pareiasuchus, Scutosaurus). However, Bunostegos is also highly autapomorphic, with at least eight unique features identified in this work. The presence of Bunostegos at a low paleolatitude adds support to the theory that the tetrapod fauna of central Pangea was isolated by xeric conditions during the Late Permian (Sidor et al., Citation2005b; Tabor et al., Citation2011).
APPENDIX 1. Modifications to character list of Tsuji (in press).
Tsuji (in press) constructed the most recent cladistic data matrix for pareiasauromorphs, which included 125 characters (73 cranial, 52 postcranial) in 29 taxa. This matrix was a revision of the matrix of Jalil and Janvier (Citation2005), which was itself a revision of Lee (Citation1997b). We redefined one character from Tsuji (in press), added two new characters, and substantially rescored one taxon, Bunostegos akokanensis.
Redefined Character
| 1. | Interpterygoid vacuity anterior shape: ‘V’-shaped (0); ‘U’-shaped (1); transversely oriented (2). | ||||
New Characters
| 1. | Postfrontal ‘horn’: absent (0); present (1). | ||||
| 2. | Central boss on frontal: absent (0); present (1). | ||||
APPENDIX 2 Data matrix used in the cladistic analysis of pareiasauromorph interrelationships (). This matrix corresponds to that of Tsuji (in press), but incorporating the revisions noted in Appendix 1. ‘?’ denotes missing data. ‘A’ denotes polymorphism for 0, 1, and 2. ‘B’ denotes polymorphism for 0 and 1. : , Bashkyroleter; , Bradysaurus; , ‘Owenetta’; , Pareiasuchus.
Tsuji et al Supplementary Data
Download Zip (5.7 KB)ACKNOWLEDGMENTS
We thank D. Blackburn, D. Chaney, A. Dindine, B. Gado, H. Larsson, C. Looy, T. Lyman, A. Maga, R. O’Keefe, D. Sindy, T. Smiley, B. Tahirou, and S. Thomas for assistance in the field, and the personnel of the U.S. Embassy in Niamey for support. Additionally, we acknowledge J. Alexander, B. Crowley, J. Groenke, V. Heisey, and A. Huttenlocker for their excellent preparation of the material reported in this paper. For the illustrations in –, we thank J. Swales. We are grateful to M. Boulay for permission to feature his restoration. Field work in 2003 was supported by National Geographic Society grant 7258–02 (to C.A.S.). Field work in 2006 and continuing research has been supported by NSF EAR-0617718 (to C.A.S.) and EAR-0617250 (to N.J.T.).
Handling editor: Sean Modesto.
LITERATURE CITED
- Araújo , D. C. F. 1985 . Sobre Pareiasaurus americanus sp. nov., do Permiano Superior do Rio Grande do Sul, Brasil. I—diagnose específica . Anais da Academia Brasileira de Ciencias , 57 : 63 – 66 .
- Boonstra , L. D. 1934 . Pareiasaurian studies. Part IX.—the cranial osteology . Annals of the South African Museum , 31 : 1 – 38 .
- Brink , A. S. 1955 . On Nanoparia Broom . Palaeontologia africana , 3 : 57 – 63 .
- Bystrov , A. P. 1957 . [The pareiasaur skull] . Trudy Paleontologischeskogo Instituta AN SSSR , 68 : 3 – 18 . [Russian]
- Cisneros , J. C. and Tsuji , L. A. 2009 . Nycteroleter affinities of a Permian parareptile from the South African Karoo Basin . Acta Palaeontologica Polonica , 54 : 165 – 169 .
- Cisneros , J. C. , Abdala , F. and Malabarba , M. C. 2005 . Pareiasaurids from the Rio do Rasto Formation, southern Brazil: biostratigraphic implications for Permian faunas of the Paraná Basin . Revista Brasileira de Paleontologia , 8 : 13 – 24 .
- Damiani , R. J. and Modesto , S. P. 2001 . The morphology of the pareiasaurian vomer . Neues Jahrbuch für Geologie und Paläontologie, Monatshefte , 2000 : 423 – 434 .
- Damiani , R. , Sidor , C. A. , Steyer , J. S. , Smith , R. M. H. , Larsson , H. C. E. , Maga , A. and Ide , O. 2006 . The vertebrate fauna of the Upper Permian of Niger. V. The primitive temnospondyl Saharastega moradiensis . Journal of Vertebrate Paleontology , 26 : 559 – 572 .
- deBraga , M. 2003 . The postcranial skeleton, phylogenetic position, and probable lifestyle of the Early Triassic reptile Procolophon trigoniceps . Canadian Journal of Earth Sciences , 40 : 527 – 556 .
- deBraga , M. and Rieppel , O. 1997 . Reptile phylogeny and the interrelationships of turtles . Zoological Journal of the Linnean Society , 120 : 281 – 354 .
- Dutuit , J. M. 1988 . Diplocaulus minimus n. sp. (Amphibia: Nectridea), lépospondyle de la formation d’Argana dans l’Atlas occidental marocain . Comptes Rendus de l’Académie des Sciences, Paris , 307 : 851 – 854 .
- Gauthier , J. , Kluge , A. and Rowe , T. 1988 . Amniote phylogeny and the importance of fossils . Cladistics , 4 : 105 – 209 .
- Gibbs , M. T. , Rees , P. M. , Kutzbach , J. E. , Ziegler , A. M. , Behling , P. J. and Rowley , D. B. 2002 . Simulations of Permian climate and comparisons with climate-sensitive sediments . Journal of Geology , 110 : 33 – 55 .
- Goloboff , P. A. , Farris , J. S. and Nixon , K. C. 2008 . TNT, a free program for phylogenetic analysis . Cladistics , 24 : 774 – 786 .
- Hartmann-Weinberg , A. P. 1933 . Die evolution der Pareiasauriden . Trudy Paleontologischeskogo Instituta AN SSSR , 3 : 3 – 66 .
- Haughton , S. H. 1929 . Pareiasaurian studies. Part II.—notes on some pareiasaurian brain-cases . Annals of the South African Museum , 28 : 88 – 96 .
- Hill , R. V. 2005 . Integration of morphological data sets for phylogenetic analysis of Amniota: the importance of integumentary characters and increased taxonomic sampling . Systematic Biology , 58 : 530 – 547 .
- Huene , F. von. 1944 . Pareiasaurierreste aus dem Ruhuhu-Gebiet . Paläontologisches Zeitschrifte , 23 : 386 – 410 .
- Jalil , N.-E. and Dutuit , J. M. 1996 . Permian captorhinid reptiles from the Argana Formation, Morocco . Palaeontology , 39 : 907 – 918 .
- Jalil , N.-E. and Janvier , P. 2005 . Les pareiasaures (Amniota, Parareptilia) du Permien supérieur du Bassin d’Argana, Maroc . Geodiversitas , 27 : 35 – 132 .
- Laurin , M. and Reisz , R. R. 1995 . A reevaluation of early amniote phylogeny . Zoological Journal of the Linnean Society , 113 : 165 – 223 .
- Lee , M. S. Y. 1993 . The origin of the turtle body plan: bridging a famous morphological gap . Science , 261 : 1716 – 1720 .
- Lee , M. S. Y. 1994 . Evolutionary morphology of pareiasaurs , 499 Cambridge , , U.K : University of Cambridge . Ph.D. Dissertation
- Lee , M. S. Y. 1995 . Historical burden in systematics and the interrelationships of ‘parareptiles.’ . Biological Reviews , 70 : 459 – 547 .
- Lee , M. S. Y. 1997a . A taxonomic revision of pareiasaurian reptiles: implications for Permian terrestrial palaeoecology . Modern Geology , 21 : 231 – 298 .
- Lee , M. S. Y. 1997b . Pareiasaur phylogeny and origin of turtles . Zoological Journal of the Linnean Society , 120 : 197 – 280 .
- Lee , M. S. Y. 2000 . “ The Russian pareiasaurs ” . In The Age of Dinosaurs in Russia and Mongolia , Edited by: Benton , M. J. , Shishkin , M. A. , Unwin , D. M. and Kurochkin , E. N. 71 – 85 . New York : Cambridge University Press .
- Lee , M. S. Y. , Gow , C. E. and Kitching , J. W. 1997 . Anatomy and relationships of the pareiasaur Pareiasuchus nasicornis from the Upper Permian of Zambia . Palaeontology , 40 : 307 – 335 .
- Lyson , T. R. , Bever , G. S. , Bhullar , B. -A. S. , Joyce , W. G. and Gauthier , J. A. 2010 . Transitional fossils and the origin of turtles . Biology Letters , 6 : 830 – 833 .
- Newton , E. T. 1893 . On some new reptiles from the Elgin Sandstones . Philosophical Transactions of the Royal Society of London, Series B , 184 : 431 – 503 .
- O’Keefe , F. R. , Sidor , C. A. , Larsson , H. C. E. , Maga , A. and Ide , O. 2005 . The vertebrate fauna of the Upper Permian of Niger—III, morphology and ontogeny of the hindlimb of Moradisaurus grandis (Reptilia, Captorhinidae) . Journal of Vertebrate Paleontology , 25 : 309 – 319 .
- O’Keefe , F. R. , Sidor , C. A. , Larsson , H. C. E. , Maga , A. and Ide , O. 2006 . Evolution and homology of the astragalus in early amniotes: new fossils, new perspectives . Journal of Morphology , 267 : 415 – 425 .
- Rees , P. M. , Ziegler , A. M. , Gibbs , M. T. , Kutzbach , J. E. , Behling , P. J. and Rowley , D. B. 2002 . Permian phytogeographic patterns and climate data/model comparisons . Journal of Geology , 110 : 1 – 31 .
- Reisz , R. R. and Laurin , M. 2001 . The reptile Macroleter: first vertebrate evidence for correlation of Upper Permian continental strata of North America and Russia . Geological Society of America Bulletin , 113 : 1229 – 1233 .
- Ricqlès , A. de and Taquet , P. 1982 . La faune de vertébrés du Permien Supérieur du Niger. I. Le captorhinomorphe Moradisaurus grandis (Reptilia, Cotylosauria) . Annales de Paléontologie (Vert.-Invert.) , 68 : 33 – 106 .
- Rieppel , O. 2000 . Turtles as diapsid reptiles . Zoologica Scripta , 29 : 199 – 212 .
- Rieppel , O. and Reisz , R. R. 1999 . The origin and early evolution of turtles . Annual Review of Ecology and Systematics , 30 : 1 – 22 .
- Seeley , H. G. 1888 . Researches on the structure, organisation, and classification of the fossil Reptilia.—II. On Pareiasaurus bombidens (Owen), and the significance of its affinities to amphibians, reptiles, and mammals . Philosophical Transactions of the Royal Society of London, Series B , 179 : 59 – 109 .
- Sidor , C. A. , Blackburn , D. C. and Gado , B. 2003 . The vertebrate fauna of the Upper Permian of Niger—II, preliminary description of a new pareiasaur . Palaeontologia africana , 39 : 45 – 52 .
- Sidor , C. A. , Larsson , H. C. E. , O’Keefe , F. R. , Blackburn , D. C. and Smith , R. M. H. 2005a . Upper Permian captorhinid and pareiasaur reptiles from Niger: new data and interpretation . Journal of Vertebrate Paleontology , 25 ( 3, Supplement ) : 115A
- Sidor , C. A. , Angielczyk , K. D. , Weide , D. M. , Smith , R. M. H. , Nesbitt , S. J. and Tsuji , L. A. 2010 . Tetrapod fauna of the lowermost Usili Formation (Songea Group, Ruhuhu Basin) of southern Tanzania, with a new burnetiid record . Journal of Vertebrate Paleontology , 30 : 696 – 703 .
- Sidor , C. A. , O’Keefe , F. R. , Damiani , R. , Steyer , J. S. , Smith , R. M. H. , Larsson , H. C. E. , Sereno , P. C. , Ide , O. and Maga , A. 2005b . Permian tetrapods from the Sahara show climate-controlled endemism in Pangaea . Nature , 343 : 886 – 889 .
- Smiley , T. M. , Sidor , C. A. , Ide , O. and Maga , A. 2008 . Vertebrate fauna of the Upper Permian of Niger. VI. First evidence of a gorgonopsian therapsid . Journal of Vertebrate Paleontology , 28 : 543 – 547 .
- Spencer , P. S. and Lee , M. S. Y. 2000 . A juvenile Elginia and early growth in pareiasaurs . Journal of Paleontology , 74 : 1191 – 1195 .
- Steyer , J. S. and Jalil , N.-E. 2009 . First evidence of a temnospondyl in the Late Permian of the Argana Basin, Morocco . Special Papers in Palaeontology , 81 : 155 – 160 .
- Steyer , J. S. , Damiani , R. , Sidor , C. A. , O’Keefe , F. R. , Larsson , H. C. E. , Maga , A. and Ide , O. 2006 . The vertebrate fauna of the Upper Permian of Niger. IV. Nigerpeton ricqlesi (Temnospondyli: Cochleosauridae), and the edopoid colonization of Gondwana . Journal of Vertebrate Paleontology , 26 : 18 – 28 .
- Sues , H.-D. and Reisz , R. R. 2008 . Anatomy and phylogenetic relationships of Sclerosaurus armatus (Amniota: Parareptilia) from the Buntsandstein (Triassic) of Europe . Journal of Vertebrate Paleontology , 28 : 1031 – 1042 .
- Tabor , N. J. , Smith , R. M. H. , Steyer , J. S. , Sidor , C. A. and Poulsen , C. J. 2011 . The Permian Moradi Formation of northern Niger: paleosol morphology, petrography and mineralogy . Palaeogeography, Palaeoclimatology, Palaeoecology , 299 : 200 – 213 .
- Taquet , P. 1967 . “ Découvertes paléontologiques récentes dans le Nord du Niger ” . In Problèmes actuels de Paléontologie—Évolution des Vertébrés , Edited by: Anonymous . 415 – 418 . Paris : Centre National de la Rechererche Scientifique .
- Taquet , P. 1972 . Un exemple de datation et de corrélation stratigraphique basé sur les Captorhinomorphes (Reptiles cotylosauriens) . Mémoires du Bureau de Recherches Géologiques et Minières , 77 : 407 – 409 .
- Taquet , P. 1976 . Géologie et paléontologie du gisement de Gadoufaoua (Aptien du Niger) , 191 Paris : Cahiers de Paléontologie .
- Taquet , P. 1978 . Niger et Gondwana . Annales de la Société Géologique du Nord , 97 : 337 – 341 .
- Tsuji , L. A. 2006 . Cranial anatomy and phylogenetic affinities of the Permian parareptile Macroleter poezicus . Journal of Vertebrate Paleontology , 26 : 849 – 865 .
- Tsuji , L. A. 2010 . Evolution, morphology and paleobiology of the Pareiasauria and their relatives (Amniota: Parareptilia) , 220 Berlin , , Germany : Humboldt Universität zu Berlin . Ph.D. dissertation
- Tsuji , L. A. In press . Anatomy, cranial ontogeny, and phylogenetic relationships of the pareiasaur Deltavjatia rossicus from the Late Permian of central Russia . Earth and Environmental Science Transactions of the Royal Society of Edinburgh
- Tsuji , L. A. and Müller , J. 2008 . A re-evaluation of Parasaurus geinitzi, the first named pareiasaur (Amniota, Parareptilia) . Canadian Journal of Earth Sciences , 45 : 1111 – 1121 .
- Tsuji , L. A. and Müller , J. 2009 . Assembling the history of the Parareptilia: phylogeny, diversification, and a new definition of the clade . Fossil Record , 12 : 71 – 81 .
- Tsuji , L. A. , Müller , J. and Reisz , R. R. 2012 . Anatomy of Emeroleter levis and the phylogeny of the nycteroleter parareptiles . Journal of Vertebrate Paleontology , 32 : 45 – 67 .
- Watson , D. M. S. 1914 . On the skull of a pareiasaurian reptile, and on the relationship of that type . Proceedings of the Zoological Society of London , 1914 : 155 – 180 .
- Wild , R. 1985 . Ein schädelrest von Parasaurus geinitzi H. v. Meyer (Reptilia; Cotylosauria) aus dem Kupferschiefer (Permian) von Richelsdorf (Hessen) . Geologische Blätter für Nordost-Bayern (Gedenkschrift B. v. Freyberg) , 34/35 : 897 – 920 .
- Yates , A. M. and Warren , A. 2000 . The phylogeny of the ‘higher’ temnospondyls (Vertebrata: Choanata) and its implications for the monophyly and origins of the Stereospondyli . Zoological Journal of the Linnean Society , 128 : 77 – 121 .
- Zardoya , R. and Meyer , A. 1998 . Complete mitochondrial genome suggests diapsid affinities of turtles . Proceedings of the National Academy of Sciences of the United States of America , 95 : 14226 – 14231 .