ABSTRACT
We here report on iguanians (both new and the previous record) from the earliest Eocene (MP 7) of the Dormaal locality in Belgium, from the time of the warmest global climates of the past 66 million years. Today iguanians are distributed mainly in the New World (Pleurodonta) and Old World (Acrodonta), having complicated biogeographic histories. Both lineages co-existed in Dormaal 56 Ma. Iguanians here document the presence of thermophilic faunas during greenhouse conditions in the northern mid-latitudes (above 50° north, the latitude of southern England). The complete maxilla of the agamid Tinosaurus europeocaenus is described and figured for the first time, being distinctive and furnishing a number of diagnostic characters. The dentary coronoid process of this species is also observed for the first time. Our morphological analysis supports the previous observation that Tinosaurus is similar to Leiolepis, but also differs from it by several distinguishing features. Some jaw character states present in T. europeocaenus are shared with the Indian T. indicus, Chinese T. doumuensis, and American Tinosaurus sp., but several differences among them are observed. Besides the well-known Geiseltaliellus, we here erect and describe a new pleurodontan taxon. The new taxon is represented by a maxilla with a unique and peculiar tooth crown morphology: the central cusp is bifurcated, markedly split into two distinct and well-separated “prongs.” This morphology likely indicates a high specialization on feeding sources. This might cause a higher extinction risk relative to generalists, because terrestrial ecosystems in Europe changed substantially during the Paleogene.
INTRODUCTION
Terrestrial ecosystems in Europe, but practically in the entire world, changed significantly during the Paleogene. The early Eocene is particularly interesting because the Eocene climate began with warming from the Paleocene–Eocene Thermal Maximum at 56 Ma. In fact, the warmest global climates of the past 66 Ma occurred during the early Eocene epoch (about 56–48 Ma; see e.g., Cramwinckel et al., Citation2018) when megathermal floral elements including palms were present even in Antarctica (e.g., Greenwood & Wing, Citation1995; Pross et al., Citation2012). The increase in temperatures during the early Eocene led to a rise in sea level, and many areas of Eurasia were submerged. Europe was an archipelago comprising various islands (e.g., Smith et al., Citation1994). However, the data regarding the early Eocene lizards are unfortunately scant. In Europe, the locality of Dormaal in Belgium represents one of the rare exceptions, serving as a window into the earliest Eocene (MP 7 reference level of the mammalian biochronological scale for the European Paleogene; BiochroM’97, Citation1997) “greenhouse world." Lizards from Dormaal were only briefly discussed by Hecht & Hoffstetter (Citation1962) and, moreover, the specimens were never figured by these authors. Beside this, other squamate specimens from Dormaal have been described and figured (Augé, Citation1990, Citation1992; Augé & Smith, Citation1997, Citation2002; Augé et al., Citation2022; Čerňanský et al., Citation2022; Folie et al., Citation2013; Sullivan et al., Citation2012).
One of the taxa are iguanian lizards which include two clades here, Acrodonta and Pleurodonta (Iguanidae sensu Torres-Carvajal et al., Citation2020). Both have very complicated biogeographic histories. Acrodonta (including Agamidae and Chamaeleonidae; sensu Estes et al., Citation1988) is an Old World clade today, although at least one lineage (Tinosaurus) is also documented from the Eocene of North America (Estes, Citation1983; Leidy Citation1872, Citation1873; Marsh, Citation1872; Smith, Citation2006, Citation2011). Today, Agamidae is a clade of lizards indigenous to continental Africa, Asia, Australia, and in a few southern, warm-temperate regions of Europe (Pough et al., Citation2004; Uetz et al., Citation2022). In contrast, Pleurodonta is primarily a New World group, with the exception of Madagascar and remote Pacific islands (Pough et al., Citation2004). During the Eocene and Oligocene, however, Pleurodonta (e.g., Corytophanidae) were also present in Europe (Augé, Citation1987, Citation1990; Augé & Pouit, Citation2012; Čerňanský et al., Citation2016; Estes, Citation1983; Kuhn, Citation1944; Rossmann, Citation2000, Smith, Citation2009a).
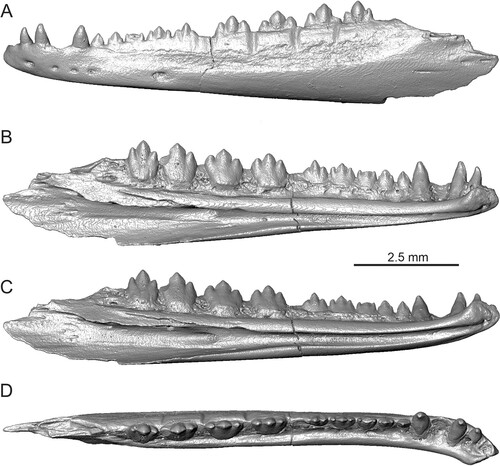
In Dormaal, both iguanians clades (Acrodonta and Pleurodonta) occurred together. The Dormaal fossils and those specimens reported from Silveirinha (MP 7; note, however, that this material is only very fragmentarily preserved) in Portugal (Rage & Augé, Citation2003) are the earliest Cenozoic representatives of these iconic lizards known in Europe. In regard to agamids, Tinosaurus europeocaenus was originally established by Augé & Smith (Citation1997; note that there are also other occurrences of this species in France: Avenay MP8 + 9, Mutigny MP8 + 9, Condé-en-Brie MP8 + 9, Prémontré MP 10; see Augé & Smith, Citation1997). The holotype of this taxon is represented by a fairly complete left dentary (). Further Dormaal material, although never been figured, consists of fragments of maxillae. Later, Augé et al. (Citation1997:fig. 7) figured a fragment of the maxilla posterior section of Tinosaurus sp. from Prémontré (MP 10, France; this specimen is also figured in Augé, Citation2005:fig. 43, and allocated there as T. europeocaenus). However, none of these jaws is complete. Thus, there is a lack of knowledge about the overall morphology of this element in this important European agamid as well as many details such as the anterior and posterior extremities. The oldest known unambiguous agamid record is Protodraco Wagner et al., Citation2021, from the lower Upper Cretaceous (99 Ma) amber deposits in Myanmar amber. Other acrodontan fossil lineages include Priscagaminae from the Cretaceous of China, Mongolia, and Uzbekistan (Borsuk-Białynicka & Moody, Citation1984; Gilmore, Citation1943; usually recovered on the stem of Acrodonta, see e.g., Gauthier et al., Citation2012). The origin of agamids is still debated and the center of their origin is suggested to be placed in either eastern Asia or in the Gondwana (Borsuk-Białynicka & Moody, Citation1984; Macey et al., Citation2000; Wagner et al., Citation2021). In this viewpoint, the Dormaal agamid (Augé & Smith, Citation1997) together with that reported from above-mentioned Silveirinha (cf. Tinosaurus in Rage & Augé, Citation2003) are important, because they form the first appearance of the clade in Europe (Augé Citation2007). Tinosaurus is suggested to be a newcomer here (Rage, Citation2013), showing that agamids were present in northwestern Europe already in the earliest Eocene, at least around 56 Ma. Together with different types of agamids known from the lower Eocene of Central Asia (Averianov & Danilov, Citation1996), these finds show rapid radiation and far geographic dispersal after the Cretaceous. The extent of their early Paleogene distribution is also highlighted by a find of a potential acrodont from the upper Paleocene of Morocco which could eventually belong to agamids (Augé & Rage, Citation2006) and especially the agamid from the lower–middle Eocene of Algeria (Rage et al., Citation2021).
FIGURE 1. Tinosaurus europeocaenus, the holotypic left dentary IRSNB R 202 in A, lateral; B, medial; C, ventromedial; and D, dorsal views.
Pleurodonts in Dormaal were previously documented only by one taxon—Geiseltaliellus (Augé, Citation1990, Citation2005, Citation2007). During the Eocene, the evidence of Pleurodonta in Europe is formed by this taxon known by several species (Augé, Citation1990, Citation2005; Kuhn, Citation1944; Smith, Citation2009a; MP 7–MP 29), Cadurciguana Augé, Citation1987 (MP 16–MP 19), Pseudolacerta De Stefano, Citation1903 (?MP 16–MP 19), and a nicely preserved, but undescribed, specimen of a polychrotine from Messel (see Smith et al., Citation2018; MP 11). The crown-group pleurodontans seem to be relatively much more diverse in the Americas as evidenced by several taxa such as Afairiguana avius, Babibasiliscus alxi, Suzanniwana patriciana, Orithyia oaklandi, and Aciprion formosum (Conrad, Citation2015; Smith, Citation2009b, Citation2011b). And, similar to Geiseltaliellus in Dormaal, these taxa document that a tropical (or subtropical) fauna was present during greenhouse conditions in the northern mid-latitudes (see Conrad, Citation2015).
We here report on the new material, including a fairly complete maxilla of the early Eocene agamid Tinosaurus europeocaenus, which possesses previously unknown structures. Besides this, new pleurodontan materials from the Dormaal locality are described, including Geiseltaliellus and a new taxon, showing a higher paleobiodiversity of this clade at the beginning of the Eocene.
Institutional Abbreviations—IRSNB R, Institut royal des Sciences naturelles de Belgique, Fossil Reptile collection, Brussels, Belgium; Vert, Institut royal des Sciences naturelles de Belgique, General collection of fossil vertebrates, Brussels, Belgium.
MATERIAL AND METHODS
Specimens Examined and Terminology
The studied material of fossils is housed in the Royal Belgian Institute of Natural Sciences in Brussels (Belgium), prefixed under individual Vert and IRSNB R numbers. The standard anatomical orientation system is used throughout this paper, and terminology describing individual bone structures is based on Rage & Augé (Citation2010) and Čerňanský (Citation2010, for acrodont lizards). Several extant agamid species are used for comparison (see Digimorph.org, Citation2002–2012; Evans, Citation2008; Georgalis et al., Citation2023; Holmes et al., Citation2010; Smith, Citation2011a; and coll. of A. Č. at the Department of Ecology, Comenius University in Bratislava, Slovakia).
X-ray Microtomography, Three-Dimensional Visualization, Photography, and Measurements
The fossil specimens were photographed using a Keyence VHX-7000 Series digital microscope and imaged on nano-computed tomography (CT) using the micro-CT facility at the Slovak Academy of Sciences in Banská Bystrica, using a Phoenix mikro-CTv|tome|x L240. The specimen IRSNB R 202 was imaged on a RX Solutions EasyTom 150 at the Royal Belgian Institute of Natural Sciences. The CT data sets were analysed using VG Studio Max 3.1. and Avizo 8.1. Some specimens were imaged with a FEI Quanta 200 environmental scanning electron microscope at the Royal Belgian Institute of Natural Sciences in Brussels.
GEOLOGICAL SETTING
The remains of the Dormaal iguanians were discovered at Dormaal stratotype, which is in the municipality of Zoutleeuw, in eastern Belgium. The Dormaal Sand Member consists of a series of thin and discontinuous layers of fluviatile pebbles, crossbedded lignitic and clayey sands with thin gray clay lenses pointing to rapidly changing deposition conditions in a fluviatile system (Smith & Smith, Citation1996; Steurbaut et al., Citation1999). It belongs to the lower part of the fluvio-lagoonal Tienen Formation that recorded the carbon isotope excursion of the PETM and contains abundant remains of terrestrial mammals, lizards, chelonians, crocodylians, and freshwater fish. The Dormaal fauna, which represents the reference level MP7 of the mammalian biochronological scale for the European Paleogene (BiochroM’97, Citation1997), has already yielded numerous mammal taxa, including the earliest modern placental mammals of Europe (Smith et al., Citation2006; Solé et al., Citation2014).
SYSTEMATIC PALEONTOLOGY
SQUAMATA Oppel, Citation1811
IGUANIA Cope, Citation1864
ACRODONTA Cope, Citation1864
AGAMIDAE Spix, Citation1825
TINOSAURUS Marsh, Citation1872
TINOSAURUS EUROPEOCAENUS Augé and Smith, Citation1997
(, , )
FIGURE 2. Tinosaurus europeocaenus. A–F, right maxilla IRSNB R 457 in A, lateral view; B, medial view with detail of teeth; C, ventral view; D, dorsal view; E, anteromedial view; and F, dorsoposteromedial view. G–J, left maxilla IRSNB R 458 in G, lateral; H, medial; I, ventral; and J, dorsal views.
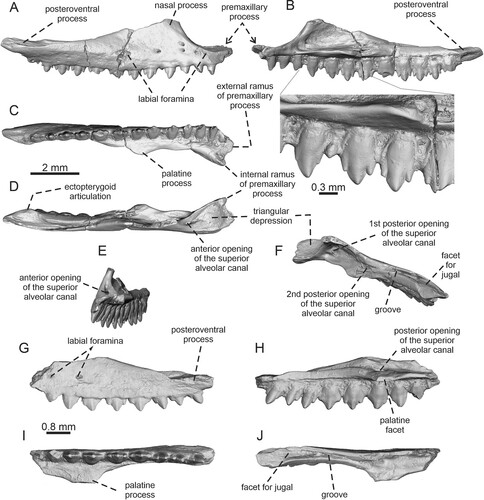
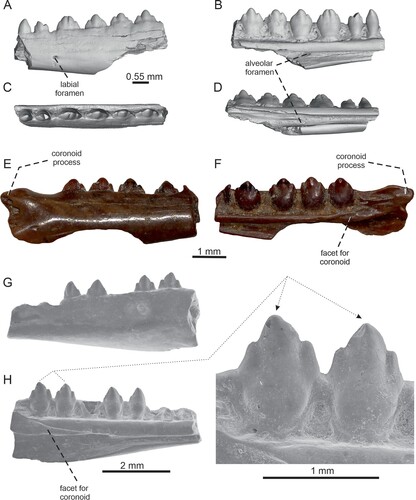
FIGURE 3. Tinosaurus europeocaenus. A–D, left dentary IRSNB R 459 in A, lateral; B, medial; C, dorsal; D, ventromedial views. E–F, right dentary IRSNB R 460 in E, lateral; and F, medial views. G–H, left dentary IRSNB R 461 in G, lateral; and H, medial views with detail of teeth.
Holotype—A left dentary IRSNB R 202.
Revised Diagnosis—A small species of Tinosaurus that differs from other extinct species in the following combination of features: (1) small size: the anteroposterior length of maxilla is 9.4 mm, length of the holotypic dentary is 10.9 mm (in Tinosaurus indicus, the maximum preserved jaw length is more or less double the size, i.e., 23 mm); (2) the tricuspidity is strongly developed, the mesial and distal cusps are prominent, distinctly separated from the central cusp (contra Tinosaurus stenodon and T. indicus); (3) a less steeply inclined posterior edge of the nasal process of maxilla (contra a more steeply inclined posterior edge of the process in T. indicus); (4) the first four maxillary teeth are pleurodont (as Tinosaurus doumuensis, contra T. indicus); (5) the pleurodont teeth in maxilla are not separated from the acrodont series by a gap (contra T. indicus and T. doumuensis); (6) the first five teeth in dentary are pleurodont (contra four in T. indicus); and (7) the coronoid process of the dentary is short and its posterior tip is not markedly dorsally elevated—it does not reach the level of the tooth apices (contra T. indicus and Pseudotinosaurus asiaticus).
Material Examined—One right maxilla IRSNB R 457, one left maxilla IRSNB R 458, three left dentaries IRSNB R 459, IRSNB R 461, IRSNB R 462 and two right dentaries IRSNB R 460, Vert-16786-274.
Locality and Horizon—Dormaal, Flemish Brabant, eastern Belgium, Dormaal Member, Tienen Formation, Landen Group, earliest Eocene (MP 7).
Description
Maxilla—Two maxillae are preserved in a good condition (). The description is mainly based on the fairly complete right maxilla IRSNB R 457. The left jaw element is represented by the incompletely preserved specimen IRSNB R 458, which is a posterior portion of the maxilla. Note, however, that the posteriormost end is broken off. The maxilla is anteroposteriorly elongated (the maximum anteroposterior length is 9.4 mm) and relatively strong with a slight medial curvature at its anterior end. It consists of two major portions: the dental portion bearing the marginal dentition and the dorsally extending nasal process. The external surface of the bone is smooth, with the exception of the ventral (supralabial) region, where five labial foramina in a row are present (). One additional foramen is located dorsal to the third one (counted from anterior). All foramina are located only in the anterior half of the element (more precisely at the base of the nasal process of the maxilla; therefore, only two labial foramina are preserved in IRSNB R 458, see ). The ventral and posteroventral narial margin is preserved. It forms the anterior border of the nasal process, which rises here dorsally. The anteromedial edge of the nasal process is markedly thickened. The length of the ventral portion of the nasal process is approximately 1/3 of the length of the entire dental portion. The nasal process of the maxilla forms an almost perpendicular wall, although note that its dorsal preserved region slightly turns medially. However, the dorsalmost portion of the nasal process is broken off. No lacrimal indentation or trace of contact with the lacrimal and the prefrontal is visible on the medial surface of the nasal process. The posterior edge of the nasal process gradually declines posteriorly, being less steeply inclined. The anterior extremity of the dental portion of the maxilla is divided into a robust, dorsoventrally flat external ramus of the premaxillary process and more medially oriented and slightly more dorsally developed internal ramus (). The internal ramus is smaller, but better defined. It is also somewhat mediolaterally compressed rather than flat. An oval, but only very shallow notch is present between these rami. Because of the presence of these rami, the whole anterior region gradually widens anteriorly in dorsal view. In this view, this area has an appearance of a more or less right triangle (), where the right angle is at the tip of the medial ramus. However, the posterior region of this ‘triangular' area is not flat, but it gradually rises dorsally. The posteromedial margin of this area is bordered by a sharp medial ridge. The ridge runs posterolaterally from the medial ramus and further, it rises dorsally. Here, it forms the anteromedial margin of the nasal process. The lateral margin of this area is formed by a well-visible edge. On the posterior side of this area, more precisely at the anterior base of the nasal process, a large elliptical anterior opening of the superior alveolar canal is located (). The alveolar canal continues posteriorly inside the bone and CT reveals that the canal is divided inside. Due to this, the first, larger posterior opening of this canal is located close to the anterior one. It is visible inside the shallow depression formed by the nasal process, the medial ridge running from the medial ramus and the supradental shelf (). The foramen is located in the ventral section of this depression, at the level between the fourth and fifth tooth positions (counted from anterior). Two smaller, second posterior foramina are located at the level of the palatine process, or slightly posterior (the level around the seventh–eighth tooth positions; note, however, that this area is damaged and it is unclear whether both openings were exposed when this region was complete; ). A posterior foramen is also preserved in IRSNB R 458, where this superior alveolar foramen is located at the level of the maximum medial expansion of the palatine process of the maxilla (at the level of sixth preserved tooth position counted from posterior; ). The foramen is located in a depression here. In the medial view, a prominent medial horizontal expansion (the supradental shelf) is visible. It runs from the root of the medial ramus of the premaxillary process. In IRSNB R 457, the expansion protrudes medially around the level of the seventh tooth position (counted from anterior). Here, it forms the palatine process of the maxilla, although the palatine facet itself is rather short. Posterior to the palatine process, the expansion diminishes and merges gradually with the bone. The horizontal expansion completely fades away at the level of the 10th tooth position (counted from anterior); such a condtion of the shelf is possible to see also in IRSNB R 458 at a level of the fifth tooth position (counted from posterior). The dental portion of the complete maxilla bears a single row of 13 tooth positions (12 teeth are still attached, nine with complete crowns and three with more or less broken crowns); eight teeth are preserved in IRSNB R 458. The posteroventral process gradually narrows posteriorly, forming a long and narrow portion. In the posteriormost portion, there is a short section without dentition. On the dorsal surface, the posteroventral process of the maxilla bears a well-demarked articulation area for the jugal, although it is unclear how far anteriorly the jugal was extended (see Remarks). The long anterior section of this region is concave, forming a deep longitudinal furrow (slit sensu Smith, Citation2011a) which is well-visible in dorsal view (). In this section, two small foramina are located at the floor of this groove: the anterior at the level of the fourth tooth position (counted from posterior) and the posterior, slightly larger, at the level between the second and third tooth positions (counted from the posterior area). The groove (or slit) reaches anteriorly the level of the sixth tooth position (counted from the posterior area; the same condition is present in IRSNB R 458) and it does not reach the nasal process. The posterior section at the level of the last and penultimate tooth positions, which clearly forms the articulation area, is wider and convex—in contrast to its anterior section; the same convex condition can be seen in the posterior section of the left maxilla fragment of Tinosaurus sp. described by Smith (Citation2011) from North America. This convex articulation area is well-visible in both dorsal and lateral views. Posterior to the tooth row, this area is slightly expanded medially, forming here the ectopterygoid articulation ().
Dentary—Five dentary fragments are available in the material. The specimen IRSNB R 459 represents a fragment of the left dentary which bears six teeth (). The external surface is smooth, the preserved portion is pierced by labial foramina in the ventral half of the dentary, but only one is preserved. It is small and rounded. In medial view, Meckel’s groove is fully open, but narrow. The alveolar foramen is preserved, separated from Meckel’s groove by the intramandibular septum. The septum has no free ventral margin. Meckel’s groove is roofed by a straight supraalveolar ridge which forms a weakly medially expanded shelf. However, the dental groove appears to be almost absent—the tooth bases reach very close to the ridge (the distance may increase in the posterior section of the tooth row). In the specimen IRSNB R 462, a small, narrow, anteroposteriorly elongated and nearly horizontal symphysis is preserved as well (). The specimen IRSNB R 460 represents the posteriormost section of the right dentary, including the coronoid process (). The coronoid facet is well visible on the medial side and reaches the level between the penultimate and the last tooth position in this specimen (), but at the level of the third tooth position (counted from posterior) in IRSNB R 461 () and Vert-16786-274. It should be noted that in lateral view (), the posteroventral section is slightly weathered. However, the overall shape of the process appears to be preserved. The coronoid process is short, triangular, but well-pointed. Although the root portion of the process is slightly bulged dorsally, the whole process is posteriorly directed and its posterior tip is not dorsally elevated. The ventrally located portion of the dentary reached further posteriorly.
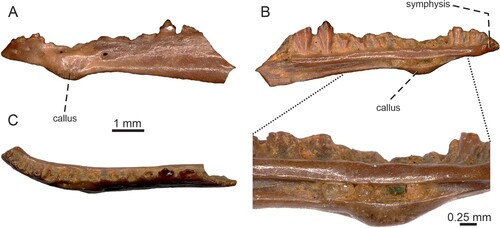
FIGURE 4. Tinosaurus europeocaenus, left dentary IRSNB R 462 showing a bony callus in A, lateral; B, medial with a detail of the callus; and C, dorsal views.
Dentition—The tooth implantation is acrodont (the teeth are attached to the crest of the jawbone; see Jenkins et al., Citation2017), but heterodont. Note, however, that the lingual walls of the teeth are distinctly taller than the labial walls along the entire tooth row (typical condition present in agamids). Thus, a subacrodont (subpleurodont, sensu Averianov & Danilov, Citation1996) condition would be more precise in this case. Moreover, a trace of the pleurodont condition (sensu Moody, Citation1980) in the anterior region is present (the first four teeth). In agamids, these teeth are secondarily pleurodont, i.e., a full acrodont set of teeth is laid down, and then the anterior ones are replaced by pleurodont teeth. The tooth replacement is restricted to the anterior pleurodont teeth only (see Cooper et al., Citation1970; Robinson, Citation1976; Smith et al., Citation2011a). Note that in acrodontan iguanians, the earliest fossils had pleurodont teeth mesially while their distal teeth were implanted through an intermediary stage between pleurodonty and acrodonty (Simões et al., Citation2015). This confirms that pleurodonty likely was the plesiomorphic condition among Lepidosauria (Evans, Citation1984; Jenkins et al., Citation2017), whereas the acrodonty evolved independently in various lineages (Jenkins et al., Citation2017; Simões et al., Citation2015; Whiteside, Citation1986). Unfortunately, the anteriormost tooth in IRSNB R 457 is missing and for this reason, it cannot be proved for sure whether it formed a caniniform tooth as often present in agamids (Moody, Citation1980) or not. But based on the size of the preserved anteriormost tooth loci, it can be estimated that even if present, it was probably not markedly large. The teeth, especially the ones located in the anterior region, have a pointed appearance (the same is true for the dentary IRSNB R 462 described below). They increase in robustness posteriorly but it is worth noting that the teeth located in the posterior half are almost the same. The penultimate and last teeth are smaller in IRSNB R 457, whereas on IRSNB R 458, the last three posterior teeth are smaller, narrower, and more posteriorly inclined. The teeth located in the posterior two-thirds of the tooth row (in a complete maxilla), are strongly tricuspid (tricuspidity starts from the fifth tooth counted from anterior). The central cusp is dominant, being about twice the size of its mesial and distal accessory cusps. The teeth are compressed mediolaterally. The sizes of the interdental gaps are small in the posterior two-thirds of the tooth row, distinctly wider in the anterior first third.
Dentary Pathology—The specimen IRSNB R 462 (), which represents a left dentary, shows signs of injury and healing. It bears a large bony callus located in the ventral section of the dentary, at the level of the fifth–seventh tooth positions. The bone here is slightly swollen mediolaterally as well. The definitive bony callus in IRSNB R 462 most likely represents a bony material (the cartilaginous callus is resorbed and calcified) after osteomyelitis that was formed as a connecting bridge across a bone fracture during repair. Although such injury (fractured jaw) was serious and the animal certainly suffered, it shows that the fracture healing process of the dentary was succesful and the animal clearly survived this trauma.
Remarks—The groove (or furrow) in maxilla which is connected or partly forms the jugal articulation (at least an anterior portion of the suborbital process of jugal might be likely wedged here) is called a slit by Smith (Citation2011). Smith observed this feature at least in some specimens of Leiolepis (the sole extant member of Leiolepidinae) and stated that it receives neither the edge of the jugal nor the ectopterygoid. According to Smith (Citation2011), its presence is presumably related to a yet undetermined feature of the connective tissue. This groove is clearly well-developed in Tinosaurus europeocaenus as it is present in both available specimens in which this portion is preserved. In regard to Leiolepis, however, it remains unclear how far anteriorly the jugal extended in Tinosaurus europeocaenus (see Discussion). The specimen IRSNB R 457 (best-preserved maxilla) described and illustrated here () is likely the specimen which was previously mentioned and briefly discussed by Hecht and Hoffstetter (Citation1962:4).
The dentaries described here have very similar dentition in regard to the tooth morphology as the above-described maxillae. However, they appear to differ from the holotypic dentary IRSNB R 202 () described by Augé and Smith (Citation1997) by one feature: the dental groove is almost absent (except IRSNB R 460, see ) and therefore the tooth bases reach very close to the ridge (for the detailed description of the holotype, see Augé, Citation2005; Augé & Smith, Citation1997). This is similar to the condition present in Pseudotinosaurus (Tinosaurus) asiaticus (see Gilmore, Citation1943). It should be noted, however, that the dental groove is large in the type of Tinosaurus stenodon (Estes, Citation1983). In Tinosaurus indicus, the development of the dental groove can slightly vary in available specimens and can be less prominent (see Prasad & Bajpai, Citation2008; Rana et al., Citation2013; Smith et al., Citation2016). It seems possible that a similar variability could be present in T. europeocaenus from Dormaal as well. Therefore, until this character is better understood, we prefer to provisionally refer the new dentaries to the species T. europeocaenus than to create a new species.
The specimen IRSNB R 460 represents the posteriormost section of the right dentary. This section is partly damaged in the holotypic dentary described by Augé and Smith (Citation1997). Thus, it helps to observe the morphology of the posterior end of the dentary, especially the coronoid process. The coronoid facet of the dentary reaches the level between the penultimate and the last posterior tooth position, whereas it reaches the level of the penultimate tooth position in the holotypic dentary (as in Calotes emma, Leiolepis belliana). In IRSNB R 461, it reaches to the level of the third tooth position. This is probably an individual or ontogenetic difference. In Tinosaurus doumuensis, the anteromedial process of the coronoid reaches the level of the third tooth (counted from posterior; Dong et al., Citation2016).
PLEURODONTA Cope, Citation1864 (sensu Frost et al., Citation2001)
FAMILY INDET.
BIFURCODENTODON gen. nov.
Type Species—Bifurcodentodon ragei sp. nov.
Diagnosis—As for Bifurcodentodon ragei, the only known species.
Etymology—The genus is named in recognition of its atypical, bifurcated tooth crowns.
BIFURCODENTODON RAGEI, gen. et sp. nov.
()
FIGURE 5. Bifurcodentodon ragei gen. et sp. nov., the holotypic left maxilla IRSNB R 463. A, lateral view with detail of teeth; B, medial view with detail of teeth; C, dorsal view; D, ventral view; E, dorsoposteromedial view with the detail of foramina set in a gutter; F, anteromedial view.
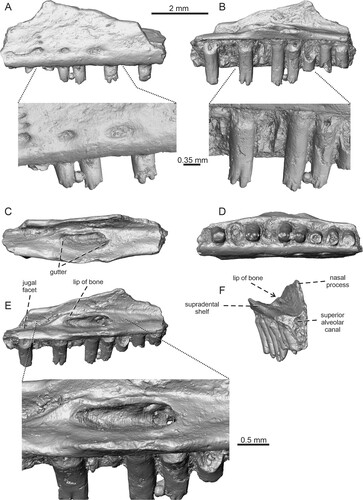
Holotype—IRSNB R 463, incomplete left maxilla.
Diagnosis—A small-sized lizard differing from all fossil and extant taxa of Pleurodonta by a unique and complex tooth morphology: the central cusp is bifurcated, markedly split into two distinct and well-separated portions. Besides this central region, tooth crowns possess small additional mesial and distal cusps, forming well-defined sharp bulges. Besides this feature, this taxon is characterized by the following combination of characters: (1) a dorsomedially projecting lip of bone on the lateral margin of the gutter in which the superior alveolar foramen is set; and (2) rugose nasal process.
Etymology—ragei; in honour of French paleoherpetologist Jean-Claude Rage (1 March 1943 Lyon – 30 March 2018 Paris) for his valuable contributions to vertebrate paleontology and particularly to squamate evolution.
Type Locality and Horizon—Dormaal, Flemish Brabant, eastern Belgium, Dormaal Member, Tienen Formation, Landen Group, earliest Eocene (MP 7).
Description
Maxilla—The holotype represents a fragment of the central portion of a left maxilla (). It bears 10 tooth positions – four teeth are still attached, whereas three other teeth with tooth crowns being completely broken off are present too. The supradental shelf is well developed and straight. The portion located at the level of the superior alveolar foramen is dorsomedially expanded, forming a palatine articulation. The area formed by the dorsal surface of the shelf is concave; a shallow depression is present here. The superior alveolar foramen is set in a gutter (sensu Smith, Citation2006). The superior alveolar nerve penetrates the maxilla in several places in the anterior region of the lateral wall and anterior end inside this gutter. The gutter is developed on the dorsal surface of the palatal shelf of the maxilla. It spreads over the area from the level of fourth to seventh tooth position (counted from anterior), being bordered (or framed) dorsolaterally and partly posteriorly by a well-developed, sharp lip of bone (this margin slightly protrudes dorsally above the adjacent lateral area). Thus, there is a narrow additional groove between this lip and the laterally located nasal process. A deeper, wedge-shaped facet for the jugal is located posterior to this. The floor of this area is penetrated by three additional foramina. The nasal process is only partly preserved, the anteroventral orbital margin is visible. In anterior direction, the nasal process gradually increases dorsally. The lateral surface of the bone is pierced by a ventrally located series of large, more or less elliptical labial foramina (four are preserved). The first and second ones (counted from anterior) are connected on the surface by a narrow groove. Two additional small foramina are present dorsally above the second labial foramen (counted from anterior). The rest of the surface possesses weakly developed irregular rugosities.
Dentition—The dentition is pleurodont. The teeth have small interdental gaps. They are robust and relatively high, exceeding the jaw parapet by one half of their height. The tooth crowns have very unusual, unique and complex morphology. The central dominant cusp distinctly split, being bifurcated into two well-separated portions. Both portions are slightly distally oriented rather than having only a ventral orientation. In these two “prongs,” this condition is more prominent in the distal one. Besides this central region, tooth crowns possess small mesial and distal accessory cusps, forming well-expanded sharp bulges. Thus, four cusps are present in total. Although a complete tooth crown is preserved only in the seventh tooth (counted from anterior), the tooth crowns of others (fourth and fifth in particular) support the presence of the same type of morphology. The tooth necks are rounded in horizontal sections, whereas the crowns are slightly mediolaterally compressed. In ventral view, the tooth necks become broader towards the base. They are gradually mediolaterally expanded, but the tooth base itself is slightly compressed. The resorption pits are located at the bases of some teeth.
Remarks—The presence of a pleurodont dentition, where the tooth crowns possess mesial and distal accessory cusps (see Smith, Citation2009a; although note that they evolved independently in other squamate groups) in combination with a gutter for the superior alveolar foramen (see e.g., Smith, Citation2006) allow us to allocate Bifurcodentodon ragei gen. et sp. nov. to Iguania, more precisely to Pleurodonta.
FIGURE 6. Geiseltaliellus sp., premaxilla IRSNB R 294 in A, anterior; B, posterior; and C, dorsal views.
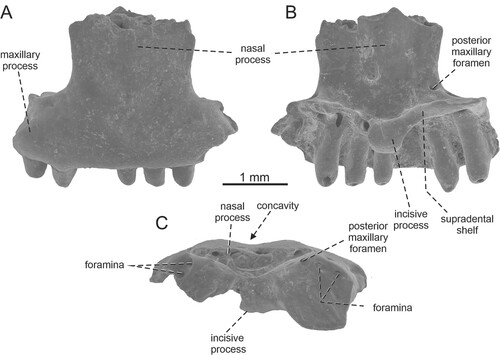
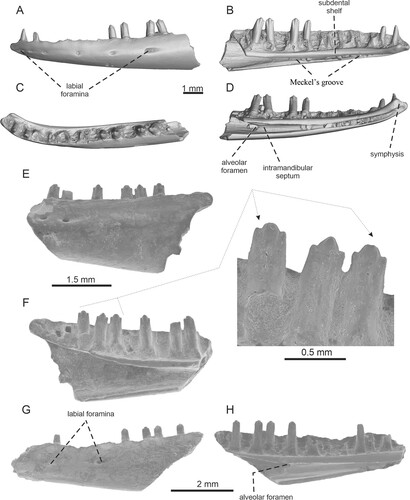
FIGURE 7. Geiseltaliellus sp. A–D, left dentary IRSNB R 464 in A, lateral; B, medial; C, dorsal; D, ventromedial views. E–F, left dentary IRSNB R 465. E, lateral view; F, medial view with detail of teeth. G–H, left dentary IRSNB R 466 in G, lateral and H, medial views.
Material Examined—Premaxilla IRSNB R 294, four left dentaries IRSNB R 464, IRSNB R 465, IRSNB R 466, Vert-20681-82, two right dentaries Vert-16786-54, Vert-20681-87.
Locality and Horizon—Dormaal, Flemish Brabant, eastern Belgium, Dormaal Member, Tienen Formation, Landen Group, earliest Eocene (MP 7).
Description
Premaxilla—The premaxilla is unpaired, small, but robustly built (). It is a triradiate, T-shaped element, consists of a ventral toothbearing portion and the posterodorsally directed nasal process. The dental portion bears seven tooth positions (five teeth are still attached). The external surface is smooth. Only the base of the nasal process is preserved. This anteroventral portion is very broad, occupying the area equal to the five-tooth position. It slightly widens dorsally, however, the rest of the process is broken off. It is roughly triangular in cross section, but distinctly flat. The anterior surface of the process is weakly convex, although it possesses a weak median concavity near its base. The maxillary processes are well developed, but short (relative to the width of the nasal process). On their dorsolateral edges, they bear a wedge-shaped facet for the external ramus of the premaxillary process of the maxilla on each side. The anterior premaxillary foramina are absent, although the right side of the nasal process appears to bear a tiny elliptical depression. A posterior premaxillary foramen (sensu Bahl, Citation1937) pierces the posterior surface of the process on the right side, just medial to the lateral edge. An additional pair of foramina is present on each side, being located in a shallow depression. They pierce the bone ventrolateral to the main posterior premaxillary foramen—the dorsal one is located around the contact of the nasal and maxillary process, whereas the ventral one lies on the dorsal side of the maxillary process, posterior to the dorsal edge of this process. In the internal side, the supradental shelf is V-shaped. Its lateral sides expand posterolaterally (note, however, that the left side is damaged). In the mid-region, the supradental shelf protrudes into a short, but well-developed incisive process. It projects anteroventrally, being slightly bilobed (mainly in its dorsal region, where the groove is developed).
Dentary—The description of the dentary is based on the several dentary fragments (). The dentary is an anteroposteriorly elongated element. The anterior region is rounded in dorsal view, and strongly curved medially. It has a C-shape in its transverse section. In the preserved portion of the dentary IRSNB R 464, the jaw parapet supports 15 tooth positions (six teeth are still attached). Meckel’s groove is fully open, being very narrow (except for the posteriormost region), but deep. It gradually widens posteriorly. In the mid-region, the ventral margin slightly rises dorsomedially, forming a small lamina, i.e., the so-called inframeckelian lip (sensu Smith Citation2009b). This septum is very close to the subdental shelf, so the opening of Meckel’s groove is very narrow. In the posterior region, the rounded alveolar foramen is located at the level of the 15th tooth position (counted from anterior). Anterior to this foramen, the intramandibular septum forms the ventromedial wall of the alveolar canal. The foramen is also well visible in the specimen IRSNB R 466. The specimen IRSNB R 465 represents the posteriormost section of the dentary. It shows that Meckel’s groove in this region becomes significantly wider (in contrast to the anteriorly located area). It is due to the fact that the posterior dorsal portion which bears teeth is significantly elevated dorsally being ended by a short and blunt coronoid process. Here, the dentary attains considerable height. The facet for the anteromedial process of coronoid appears to be present (note, however, that this region is partly damaged). It reaches the level of the third tooth position (counted from posterior). The thin subdental shelf is almost straight, except for the dorsally bent part in the posterior region as previously mentioned. Anteriorly, the shelf continues into a small symphysis which is an anteroposteriorly elongate, and weakly concave (i.e., dorsoventrally depressed in the mid-region; ventrally, at its sagittal midpoint, the symphysis is actually pierced by Meckel’s groove). A facet for the splenial can be observed in the ventral margin of the several specimens. It appears to extend anteriorly to the level of around the 11th tooth position (counted from anterior).
The external surface is smooth and pierced by six labial foramina. Their size gradually increases posteriorly; the largest is the sixth foramen. This last preserved foramen is drop-shaped and the elongated part is the posterior one (in other words, this last foramen is accompanied by a weak posterior short groove). The anterior first three foramina are much closer to each other, whereas the posterior three ones are widely spaced. The specimen IRSNB R 465 shows that the last posterior foramen is located at the level of the ninth tooth position (counted from posterior), whereas the further posterior portion of the dentary is smooth. The last preserved foramen in IRSNB R 465 is, however, not accompanied by posterior grooves; it is not drop-shaped. Only a hardly observed, indistinct shallow depression is present there. This shows individual variability among Dormaal specimens. The facet for the anterolateral process of the coronoid cannot be observed on the external dorsal surface of IRSNB R 465.
Dentition—The implantation is pleurodont. The teeth have rather small interdental gaps. Those on the premaxilla decrease in size medially, thus the smallest tooth is the central one. In dentary, teeth are relatively high and surpass the jaw parapet by around one half of their height. The overall size of the teeth gradually increases posteriorly, thus the anterior teeth are pointed and smaller than posterior ones. In the posterior region, teeth are more robust. Here, they are strongly tricuspid, with a dominant central cusp and mesial and distal accessory cusps of equal size, which are well separated from the main one. The tricuspidity starts at the level of the 12th tooth (counted from anterior), although note that the incipient condition is already visible at the 11th one (note that the 10th tooth is not preserved and is unknown). The tooth necks become broader towards the base in dorsal view—they are gradually mediolaterally expanded, but the tooth bases themselves are slightly compressed. In the posterior teeth, the tooth crown is slightly medially and distally expanded due to a presence of cusps in comparison to a tooth neck. The resorption pits are located at the bases of some teeth.
Remarks—The specimen IRSNB R 294 (premaxilla) described here is the specimen which was previously figured and described by Augé (Citation2005:fig. 19).
DISCUSSION
Modern iguanians are widely distributed and their greatest species richness occurs in the tropics. These lizards have complicated biogeographic histories (see Introduction). The biogeography of the group, with acrodontans occurring in the Old World and pleurodontans occurring only in the Americas today (except Opluridae from Madagascar and Brachylophus from Fiji), clearly reflects their basal phylogenetic dichotomy (Frost & Etheridge, Citation1989). The Dormaal locality clearly shows the co-existence of both Pleurodonta and Acrodonta in Europe during the earliest Eocene. This appears to be supported by fragments reported from Silveirinha (Portugal, MP 7; Rage & Augé, Citation2003). The co-existence of these two clades can be seen in some younger localities, such as Prémontré (France, MP 10; see Augé, Citation2003; Augé et al., Citation1997). In Dormaal, Pleurodonta appear to be slightly more diverse, represented at least by two different taxa. Today, Acrodonta and Pleurodonta occur together only in Madagascar, where a highly characteristic and morphologically specialized clade of acrodont iguanians—chameleons, can be found as well (see Čerňanský et al., Citation2020; Tolley & Herrel, Citation2013; Uetz et al., Citation2022).
Iguanians in Dormaal, together with previously described gekkotans (Čerňanský et al., Citation2022), represent rather thermophilic taxa during greenhouse conditions in the northern mid-latitudes (above 50° north, the latitude of southern England). Understanding the Eocene is relevant for present global climate change, including sea level change, as well as the expansion of distribution of thermophilic taxa, including parasites that cause serious infectious diseases such as malaria (Alimi et al., Citation2015; Huey et al., Citation2009; Smith et al., Citation1994). Although interpretation of such global climate reflects the classical view based on the implicit assumption that thermal physiology of diverse lizard clades is highly conserved, the evidence that some organisms are able to respond to climatic changes over short timescales exists (e.g., Bradshaw & Holzapfel, Citation2006). The data also include the Puerto Rican iguanian lizard Anolis cristatellus (Leal & Gunderson, Citation2012). In regard to the Paleogene, however, all data (Cramwinckel et al., Citation2018), including various fossil organisms and their paleodistributions, indicate a much warmer global climate during the early–middle Eocene (see Introduction). For example, turtles and alligators are documented even in northern Canada, in the regions well above the Arctic Circle (Eberle et al., Citation2014; Estes & Hutchinson, Citation1980). Although it seems that Eocene Arctic alligators probably had a somewhat greater climatic window than that implied by today’s crocodilian distribution (Markwick, Citation1994), inhabiting evidently cooler winters (0–3.5°C) and summers (19–20°C), their occurrence in the fossil record is a reliable climate proxy for above-freezing temperatures (Eberle et al., Citation2010). Thus, together with other fossils, they form a critical paleontological contribution indicating that this region of the Arctic had been mild, temperate, and ice-free during the early–middle Eocene (Eberle et al., Citation2014; Estes & Hutchinson, Citation1980). This is therefore consistent with that fact and it is important to point out that lizard groups that are today mainly tropical (e.g., Corytophanidae) were present in higher latitudes in both North America and Europe.
Acrodontans in Dormaal—New Data on Tinosaurus europeocaenus
The acrodontans in Dormaal are represented by Tinosaurus europeocaenus Augé & Smith (Citation1997). The genus Tinosaurus was established to accommodate Tinosaurus stenodon, based upon a dentary fragment from the Eocene of the Bridger Formation of Wyoming, U.S.A. (Marsh, Citation1872). But later, materials allocated to the genus have been described from several localities around the world; Tinosaurus appears to be globally widespread during the Paleogene. Besides T. europeocaenus, T. postremus is described based on a fragment of the left dentary from the latest Paleocene–earliest Eocene of Kazakhstan (Averianov Citation2001), isloated teeth referred as Tinosaurus sp. are described from the lower–middle Eocene of Pakistan (Rage, Citation1987), T. doumuensis is described from the middle Paleocene of Anhui, China (Dong et al., Citation2016; Hou, Citation1974), T. lushihensis from the upper Eocene of Henan, China (Dong, Citation1965) and T. yuanquensis from the middle Eocene of Shanxi, China (Li, Citation1991). From India, T. indicus is described from the lower Eocene of Gujarat (Prasad & Bajpai, Citation2008; Rana et al., Citation2013; Smith et al., Citation2016). Gilmore (Citation1943) described a right dentary fragment from the middle Eocene of Shara Murun area as a new species, T. asiaticus. Later, a new generic name, Pseudotinosaurus, was erected for this material by Alifanov (Citation1991). This lineage most likely survived in Mongolia until the Oligocene (Čerňanský & Augé, Citation2019). Thus, Tinosaurus is the most species-rich acrodontan lizard known in the fossil record. The problem of Tinosaurus is, however, that tricuspid teeth of a similar form are probably present in some 200 living species of Agamidae, more precisely members of Draconinae and Leiolepis Cuvier, Citation1829 (Smith et al., Citation2011). According to Smith (Citation2011a) the polyphyly of “Tinosaurus” cannot be excluded (see also Smith et al., Citation2011). It seems to be a wastebin taxon (Estes, Citation1983; Smith et al., Citation2011). This agamid genus as a whole, with all its currently referred species, certainly requires a detailed revision in regard of its clear apomorphic features. More complete specimens are therefore crucial.
The almost complete maxilla of Tinosaurus europeocaenus described here is distinctive and furnishes a number of useful, previously unknown, specific characters. Thus, it can help the better understanding of the European species. However, T. europeocaenus is outside the continent where the type material of the Tinosaurus type species was described. Moreover, the observations based on the Dormaal material cannot necessarily apply generally to all other species of Tinosaurus. Some species are represented solely by incomplete dentary fragments, which allocation to Tinosaurus, as mentioned previously, is based primarily on the basis of tricuspid, acrodont cheek teeth and geological age (Estes, Citation1983). Note that T. doumuensis is problematic. Indeed, a re-examination of T. lushihensis (this taxon is represented by the posterior portion of the maxilla exposed in medial view) by Dong et al. (Citation2016) did not find any obvious differences between this species and T. doumuensis. Moreover, the uncertainty of the presence of clearly tricuspid teeth in T. doumuensis brings further doubt on its attribution and even its validity (Dong et al., Citation2016). In any case, herein we retain the original attribution—as Dong et al. (Citation2016) did in their paper—and we include the material of this species in our comparison below. Based on the morphology of Dormaal maxillae described here, the following character states can be observed for T. europeocaenus as well as data of the dentary bone (both here and previously described):
The maxilla is rather anteroposteriorly long and narrow contra robust in Uromastyx and Pogona vitticeps. Extant Uromastyx species are mainly herbivorous, which is correlated with shorter and deeper jaws than those of insectivorous forms (Herrel et al., Citation1999).
The small size—the maximum anteroposterior length of maxilla is 9.4 mm, length of the holotypic dentary is 10.9 mm, whereas in Tinosaurus indicus, the maximum preserved jaw length is 23 mm.
The tricuspidity is strongly developed: the mesial and distal cusps are prominent and distinctly separated from the central cusp, whereas in T. indicus (see Prasad & Bajpai, Citation2008) and the holotypic dentary of Tinosaurus stenodon (see Estes, Citation1983), mesial and distal cusps are poorly differentiated.
Five labial foramina are present in the anterior half of the maxilla (more precisely at the level of the nasal process of the maxilla). Five are located in a row and one additional foramen (as it is in the case of IRSNB R 457) can be located dorsal to them. A row of 5–6 labial foramina is present in T. indicus (Rana et al., Citation2013). Four labial foramina are preserved in available specimens of Leiolepis (see Digimorph.org).
The length of the ventral portion of the nasal process is approximately one-third of the length of the entire dental portion in T. europeocaenus. Comparable size is present in T. doumuensis and although the maxilla of T. indicus described by Rana et al. (Citation2013) is incomplete, a similar pattern can be estimated. Among living agamids, this feature is present in Leiolepis and Bronchocela jubata, but the anteropoteriorly short nasal process is present in e.g., Uromastyx (for maxilla of U. benti, see Holmes et al., Citation2010:fig. 6), Draco, Agama agama (for A. mossambica, see Smith Citation2011a:fig. 4), and Physignathus cocincinus.
The anteromedial edge of the nasal process of the maxilla is markedly thickened. This is present in e.g., T. indicus and in extant Leiolepis belliana, Bronchocela cristatela, Pogona vitticeps, and Physignathus cocincinus.
No lacrimal indentation or trace of contact with the lacrimal on the medial surface of the nasal process. The same condition can be observed in T. indicus, Leiolepis, and Uromastyx but also in Pleurodonta and it is most likely a plesiomorphic character state (Rana et al., Citation2013). In contrast, it is present in Draco quinquefasciatus.
A less steeply inclined posterior edge of the nasal process in T. europeocaenus (it appears to be similar to that in T. doumuensis, see Estes, Citation1983:fig. 10e). Note, however, that according to Dong et al. (Citation2016) the process is broken in the holotype of the latter species and it is difficult to observe its general shape, whereas a more steeply inclined posterior edge of the process is present in T. indicus (Rana et al., Citation2013).
The posteroventral process is not stepped (maxillary reentrant on the jugal sensu Smith, Citation2011a), but gradually narrows posteriorly as in all other species (T. doumuensis, T. indicus, and Tinosaurus sp. (PTRM 19340 described by Smith [Citation2011a] from the upper Eocene Chadron Formation of North Dakota, U.S.A., representing a fragment of the left maxilla). Among extant agamids, Tinosaurus shares this character state uniquely with Leiolepis (see also Smith, Citation2011a); contra Agama agama, Uromastyx, Physignathus cocincinus, Pogona vitticeps, Diploderma (Japalura) polygonatum, Bronchocela jubata, Draco volans, and D. quinquefasciatus.
The presence of a longitudinal groove (slit sensu Smith, Citation2011a) on the dorsal surface of the supradental shelf, posterior to the nasal process. Among living agamids, this feature is present only in Leiolepis and the absence is considered plesiomorphic (Smith, Citation2011a). However, the groove was not found in the maxilla fragments described by Smith (Citation2011a) as Tinosaurus sp. from North America.
The number of maxillary teeth is around 13 (11 are present in Leiolepis triploida, L. belliana, 14 are present in Bronchocela jubata, 15 in D. volans, 17 in Agama agama, 18 in D. quinquefasciatus, 19 in Uromastyx). It should be noted, however, that tooth number in virtually all lizards is quite variable and also likely to be size related, so these numbers should not be interpreted as absolutes.
The first four maxillary teeth are pleurodont. The same condition is present in T. doumuensis and in extant Hydrosaurus amboinensis (see Smith et al., Citation2011). In T. indicus, the first two anterior teeth are conical, pleurodont (the second tooth is likely much larger than the first one, being inclined posteriorly).
The pleurodont teeth are not separated from the acrodont series by a gap. In contrast, a diastema is present in T. indicus (Rana et al., Citation2013) and a short gap is observed in T. doumuensis too (Dong et al., Citation2016).
The first maxillary tooth is not preserved in the specimen IRSNB R 457, but based on the size of the preserved loci, it can be estimated that even if a caniniform tooth is present in some forms, it was not markedly large (contra Draco and especially Leiolepis in which a caniniform anterior tooth is extremely large).
Tricuspidity in maxilla starts from the fifth tooth (counted from anterior). Posterior teeth are strongly tricuspid. Within agamid lizards, tricuspid teeth are present in Leiolepis and Draconinae (Smith et al., Citation2011; Rana et al., Citation2013).
The holotypic dentary bears 14 tooth positions – the first five teeth (see Augé & Smith, Citation1997) were pleurodont (contra T. indicus, which has four, see Prasad & Bajpai, Citation2008). Tricuspidity starts from the seventh tooth (counted from anterior).
The symphysis is small, nearly horizontal (see also Augé & Smith, Citation1997). The same condition is present in T. indicus (see Rana et al., Citation2013). Among extant taxa, a horizontal symphysis is present in Draco and Hydrosaurus (Evans et al., Citation2002:fig. 10) whereas in many other agamid lizards, including Leiolepis, the symphysis enlarges and becomes subvertical (Rana et al., Citation2013, see Digimorph.org).
The coronoid facet of the dentary reaches the level of around the penultimate tooth position as in Calotes emma and Leiolepis belliana; contra Agama agama (the coronoid process reaches much further anteriorly—the level of sixth tooth position).
The coronoid process of the dentary is well defined, pointed, but short and its posterior tip is not markedly dorsally elevated (it does not reach the level of the tooth apices). The ventrally located portion of the dentary reaches much further posteriorly (contra Uromastyx, Leiolepis, Draco, Agama). The posterior direction of the coronoid process in T. europeocaenus seems to be different from the specimen of T. indicus described by Smith et al. (Citation2016:fig. 9) from the lower Eocene of Vastan mine, India. In the Indian specimen, the coronoid process of the dentary is robust and dorsally elevated, overreaching the level of tooth apices. The same condition can be seen also in Pseudotinosaurus (Tinosaurus) asiaticus (Gilmore, Citation1943:fig. 5). The shape of the posterior margin of the dentary of T. doumuensis is indistinct and thus unknown (Dong et al., Citation2016). Unfortunately, the type material of T. stenodon is formed only by a dentary fragment.
From the morphological analysis above, we can support the previous observations (e.g., Rana et al., Citation2013; Smith, Citation2011a; Smith et al., Citation2011) that Tinosaurus is similar to Leiolepis in many aspects, but also differs from it by several features (see above). Leiolepis and Uromastyx are remnants of one of the most ancient clades within Acrodonta (Gauthier et al., Citation2012; Moody, Citation1980; Reeder et al., Citation2015; Zheng & Wiens, Citation2016). Some maxillary character states present in the European T. europeocaenus are shared with Indian T. indicus, Chinese T. doumuensis, and American Tinosaurus sp. (described by Smith, Citation2011a), but several differences among these species are observed (the same is true for the dentary e.g., the differences in the shape and elevation of the coronoid process). In many species currently referred to Tinosaurus, the morphology of the maxilla is either completely unknown or only partly known. Among the taxa, in which the maxilla can be more or less observed, the overall morphology of T. europeocaenus maxilla appears to resemble at the most that in T. doumuensis. Although the illustration and redescription of the holotype IVPP V 4453 is provided by Dong et al. (Citation2016:fig. 3), this problematic Chinese taxon still needs revision using CT methods. This might help to observe characters in detail which are discussed here and shed light on the problem with its validity (see Dong et al., Citation2016).
In Europe, Tinosaurus was progressively less abundant during later stages of the Eocene and the youngest record is known from the middle Eocene (MP 13; Duffaud & Rage, Citation1997). Later in the Oligocene (MP 22), other agamid immigrants appeared on this continent, e.g., Uromastyx europaeus (see Augé, Citation2005; Rage, Citation2013; Rage & Augé, Citation2015). In summary, Tinosaurus likely represents a paraphyletic assemblage. There is evidence that the North American species that typifies the genus Tinosaurus seems to have affinities with the agamid lineage of leiolepidines or draconines; we cannot be fully sure that the European species truly belongs to the North American genus (although this is very likely). If Tinosaurus europeocaenus is indeed a member of the North American genus Tinosaurus, then it represents another shared faunal element between Europe and North America, with congeneric species occurring in both these continents during the Eocene (e.g., the lizard Saniwa: Augé et al., Citation2022; the turtle Axestemys: Georgalis & Joyce, Citation2017) as well as many other non-congeneric but still very closely related taxa in both continents (e.g., charinaine snakes; Smith & Scanferla, Citation2021). These imply faunal exchanges between North America and Europe during the Paleocene–Eocene Thermal Maximum (PETM), but nevertheless the coeval Asian reptile fossil record is rather poor to afford definite conclusions.
Pleurodontans in Dormaal: Two Taxa Present
Geiseltaliellus in Dormaal, the Earliest Record in Europe—All known species of Geiseltaliellus are from Europe. The occurrence of this taxon in Dormaal was already reported by Augé (Citation1990, Citation2005, Citation2007). In his book, Augé (Citation2005) figured and described a premaxilla (fig. 19; the specimen IRSNB R 294 described here, see ) and allocated this specimen as Geiseltaliellus sp., whereas maxillae and dentaries were described as G. longicaudus. Later, however, Smith (Citation2009a) described a new species, G. maarius, currently known from the middle Messel Formation, middle Eocene (MP 11). In regard to premaxilla, the Dormaal specimen differs from G. grisolli, G. longicaudus, and G. maarius by having much broader nasal process of the premaxilla. In fact, although the distal portion of the process is unknown, the ventral portion appears to be much broader than in all other known species. This deserves a comment. One could tend to suggest that it is too broad for Geiseltaliellus. However, it should be noted that a broad nasal process is fairly characteristic of crown Corytophaninae (including fossil Orithyia Smith, Citation2011b) and of Suzanniwana Smith, Citation2009b, which might be closely related to Geiseltaliellus (see Smith, Citation2009a, Citationb). It seems to be possible that a broad nasal process is a plesiomorphic feature, a primitive feature still present when the Geiseltaliellus lineage appeared in Europe. In later forms, its width became reduced. In stratigraphically slightly younger specimens, e.g., from Prémontré (MP 10), we can recognize such a trend of a narrower nasal process of premaxilla (for Prémontré, see Augé, Citation2003:fig. 2).
In regard to dentaries, our material described here differs from G. grisolli Augé, Citation2005 in having much narrower and slightly more rounded subdental shelf anteriorly on the dentary. The Dormaal dentary material resembles that of G. longicaudus. However, all differences in regard to dentary (in contrast to premaxilla) are very small if compared with G. maarius as well, e.g., the facet for the anteromedial process of coronoid reaches the level of the penultimate tooth position (counted from posterior) in the Messel taxon. Thus, in fact, dentary fragments described here do not display enough of the diagnostic features necessary for rigorous alpha taxonomy. For this reason, we allocated the Dormaal material described here only as Geiseltaliellus sp.
Paleobiodiversity and Risk of Extinction in Specialists vs. Generalists—In regard to Dormaal, pleurodontans were much more diverse in Europe during the earliest Eocene than previously thought. Besides Geiseltaliellus mentioned above, a new taxon is described here. This group in Dormaal further documents the subtropical fauna present during greenhouse conditions in the northern mid-latitudes approximately 56 Ma. However, whereas Geiseltaliellus is known from several younger Eocene localities in Europe (e.g., Smith, Citation2009a) and seems to survive through the Oligocene (Augé & Pouit, Citation2012; Čerňanský et al., Citation2016), Bifurcodentodon gen. nov. was very likely not so successful. Although more complete specimens of Bifurcodentodon gen. nov. are needed to understand all aspects of its paleoecology, its peculiar tooth crown morphology seems to indicate a high specialization on feeding sources and preferred habitats. Reptiles have diverse tooth shapes, from simple unicuspid to complex multicuspid teeth, reflecting functional adaptation to a variety of diets and eating styles. The problem is that although squamates seem to be ideal subjects for investigating relationships between diet and dental patterns, studies exploring patterns between tooth shape and diet are remarkably rare for squamates (Christensen & Melstrom, Citation2021). Therefore, caution is needed here to interpret the particular diet of Bifurcodentodon ragei gen. et sp. nov. For example, although Amblyrhynchus cristatus and Iguana iguana exhibit a tendency on dental complexity in regard to herbivory, there is not necessarily a strong general correlation on this. Ctenosaura, which is generally insectivorous as juvenile and herbivorous as adult, decreases dental complexity during ontogeny (Christensen & Melstrom, Citation2021). In general, the effect of specialization causes a higher extinction risk and one of the key factors is indeed the degree of habitat and/or diet specialization (Owens & Bennett, Citation2000; Purvis et al., Citation2000). Animals can avoid competition with others through specialization on certain diets or habitat types, but this also leads to an increasing of the dependence on specific resources (Begon et al., Citation1996). But when these resources decline, specialists are likely to suffer more than generalists because generalists can use other available resources (Harcourt et al., Citation2002; Hopkins et al., Citation2002 Wilson et al., Citation1999). A correlation between habitat specialization and extinction risk has been found in various animals, including reptiles (see Foufopoulos & Ives, Citation1999). It seems to be plausible that the global climatic change and the reorganization across the later stages might have caused the extinction of the Bifurcodentodon gen. nov. The extinction is documented in several lizard lineages (Rage, Citation2013), whereas others, e.g., lacertids, have been sucessful. Although the warmest global climates of the past 66 Ma occurred during the early Eocene epoch (about 56–48 Ma), decreasing CO2 was the main cause of a cooling trend that began 50 million years ago (Hansen et al., Citation2008; Zachos et al., Citation2008). Then, a distinct mean annual temperature and precipitation drop started during the late Eocene and early Oligocene (Burchardt, Citation1978; Cavelier et al., Citation1981; Janis, Citation1993; Prothero & Berggren, Citation1992; Rage, Citation2013; Rea et al., Citation1990). It turns out that reptiles inhabiting forests are more threatened than those in arid habitats (Cox et al., Citation2022). The most significant cooling was at 33.5 Ma, around the Eocene/Oligocene boundary, and is commonly correlated with the so called “Grande Coupure” extinction event; during that time, it is known that several lineages of squamates became extinct (Augé, Citation2005; Georgalis et al., Citation2021; Rage, Citation2013; Rage & Augé, Citation1993). This event is also characterized by changes in vegetation from Eocene dense forests to Oligocene more open environment (Prothero & Heaton, Citation1996). Later, members of pleurodontans were likely pushed toward the equator as that area became more suitable and the higher latitudes cooled.
Identifying the factors that influence the extinction risk of organisms is crucial in conservation biology because they help elucidate the nature of the extinction process. Lizards, as ectothermic vertebrates, are known to be strongly affected by climatic conditions and changes (e.g., Markwick, Citation2002). In general, climate change will have a major impact on biodiversity by increasing the extinction risk of many species or changing their distributions (Pounds et al., Citation1999). Thus, the importance of the Eocene is magnified for understanding future global climate change and identifying endangered species to help their preservation.
AVAILABILITY OF MATERIALS AND DATA
All specimens are cataloged and accessible in the fossil reptile collection of the Institut royal des Sciences naturelles de Belgique, Brussels. Digital surface models of the figured fossil specimens IRSNB R 202, IRSNB R 457-459, IRSNB R 463-464 are available on Morphosource and Virtual Collections:
IRSNB R 202: https://www.morphosource.org/concern/media/000494757?locale=en
IRSNB R 457: https://www.morphosource.org/concern/media/000494918?locale=en
RSNB R 458: https://www.morphosource.org/concern/media/000494923?locale=en
IRSNB R 459: https://www.morphosource.org/concern/media/000494933?locale=en
IRSNB R 463: https://www.morphosource.org/concern/media/000494743?locale=en
IRSNB R 464: https://www.morphosource.org/concern/media/000494751?locale=en
ACKNOWLEDGMENTS
We thank K. T. Smith (Senckenberg Research Institute, Germany) for his helpful advice. L. Despontin and C. Locatelli (Royal Belgian Institute of Natural Sciences, Brussels) respectively did the SEM pictures and the CT-scan of IRSNB R 202. For critically reading the manuscript, we thank H.-D. Sues (Editor), G. Georgalis (Polish Academy of Sciences, Poland) and J. D. Daza (Sam Houston State University, U.S.A.). This work was supported by SYNTHESYS project BE-TAF-8234 (to A. Č.) funded by the European Commission (http://www.synthesys.info/), the Scientific Grant Agency of the Ministry of Education of Slovak Republic and Slovak Academy of Sciences, Grant Nr. 1/0191/21 (to A. Č.), and Belspo BRAIN project BR/121/A3/PALEURAFRICA (to T. S.) funded by the Belgian Science Policy Office.
LITERATURE CITED
- Alifanov, V. R. (1991). A revision of Tinosaurus asiaticus Gilmor [sic] (Agamidae). Paleontologicheskiy Zhurnal, 3, 115–119. [in Russian]
- Alimi, T. O., Fuller D. O., Qualls W. A., Herrera S. V., Arevalo-Herrera M., Quinones M. L., Lacerda M. V., & Beier J. C. (2015). Predicting potential ranges of primary malaria vectors and malaria in northern South America based on projected changes in climate, land cover and human population. Parasites & Vectors, 8, 431.
- Augé, M. L. (1987). Confirmation de la présence d’Iguanidae (Reptilia, Lacertilia) dans l’Éocène européen. Comptes Rendus de l’Académie des Sciences, Series II, 305, 633–636.
- Augé, M. L. (1990). La faune de lézards et d’amphisbènes (Reptilia, Squamata) du gisement de Dormaal (Belgique, Eocène inférieur). Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre, 60, 161–173.
- Augé, M. L. (1992). Campinosaurus woutersi n.g. n.sp., Anguimorphe nouveau (Lacertilia) de l’Éocène inférieur de Dormaal (Belgique). Une relique éocène des Dorsetisauridae du Jurassique terminal/Crétacé basal? Comptes rendus de l'Académie des Sciences, 315, 885–889.
- Augé, M. L. (2003). La faune de Lacertilia (Reptilia, Squamata) de l’Éocène inférieur de Prémontré (Bassin de Paris, France). Geodiversitas, 25, 539–574.
- Augé, M. (2005). Evolution des lézards du Paléogène en Europe. Mémoires du Muséum national d’Histoire naturelle, Paris, 192, 1–369.
- Augé, M. L. (2007). Past and present distribution of iguanid lizards. Arquivos do Museu Nacional, Rio de Janeiro, 65, 403–416.
- Augé, M. L., Duffaud, S., Lapparent de Broin, F., Rage, J.-C., & Vasse, D. (1997). Les amphibiens et les reptiles de Prémontré (Cuisien,Bassin parisien): une herpétofaune de référence pour l'Eocène inférieur. Géologie de la France, 1, 23–33.
- Augé, M.L., Folie, A., Smith, R., Phélizon, A., Gigase, P., & Smith, T. (2022). Revision of the oldest varanid, Saniwa orsmaelensis Dollo, 1923, from the earliest Eocene of northwest Europe. Comptes Rendus Palevol, 21, 511–529.
- Augé, M. L., & Pouit, D. (2012). Presence of iguanid lizards in the European Oligocene Lazarus taxa and fossil abundance. Bulletin de la Societe Geologique de France, 183, 653–660.
- Augé, M. L., & Rage, J.-C. (2006). Herpetofaunas from the upper Paleocene and lower Eocene of Morocco. Annales de Paléontologie, 92, 235–253.
- Augé, M. L., & Smith, R. (1997). Les Agamidae (Reptilia, Squamata) du Paléogène d’Europe occidentale. Belgian Journal of Zoology, 127, 123–138.
- Augé, M. L., & Smith, R. (2002). Nouveaux Lacertidae (Reptilia, Squamata) de l’Eocène inférieur européen. Belgian Journal of Zoology, 131, 3–15.
- Averianov, A. (2001). A new species of Tinosaurus from the Paleocene of Kazakhstan (Squamata: Agamidae). Zoosystematica Rossica, 9, 459–460.
- Averianov, A., & Danilov, I. (1996). Agamid lizards (Reptilia, Sauria, Agamidae) from the Early Eocene of Kyrgyzstan. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte, 12, 739–750.
- Bahl, K. N. (1937). Skull of Varanus monitor (Linn.). Records of the Indian Museum, 39, 133–174.
- Begon, M. E., Harper, J. L., & Townsend, C. R. (1996). Ecology. individuals, populations and communities. 3rd edition. Blackwell Science, Oxford, United Kingdom, 1068 pp.
- Biochro M. (1997). Synthèses et tableaux de correlation. In J.-P. Aguilar, S. Legendre & J. Michaux (Eds.), Actes du Congrès BiochroM’97 Montpellier. Mémoires et Travaux de l’Ecole Pratique des Hautes Etudes, Institut de Montpellier, 21, pp. 769–805.
- Borsuk-Bialynicka, M., & Moody, S. M. (1984). Priscagaminae, a new subfamily of the Agamidae (Sauria) from the Late Cretaceous of the Gobi Desert. Acta Palaeontologica Polonica, 29, 51–81.
- Bradshaw, W. E., & Holzapfel, C. M. (2006). Evolutionary responses to rapid climate change. Science, 312, 1477–1478.
- Burchardt, B. (1978). Oxygen isotope paleotemperatures from the Tertiary period in the North Sea area. Nature, 275, 121–123.
- Cavelier, C., Chateauneuf, J. J., Pomerol, Ch., Rabussier, D., Renard, M., & Vergnaud-Grazzini, C. (1981). The geological events at the Eocene/Oligocene boundary. Palaeogeography Palaeoclimatology Palaeoecology, 36, 223–248.
- Čerňanský, A. (2010). A revision of chamaeleonids from the Lower Miocene of the Czech Republic with description of a new species of Chamaeleo (Squamata, Chamaeleonidae). Geobios, 43, 605–613.
- Čerňanský, A., & Augé, M. L. (2019). The Oligocene and Miocene fossil lizards (Reptilia, Squamata) of Central Mongolia. Geodiversitas, 41, 811–839.
- Čerňanský, A., Daza, J. D., Smith, R., Bauer, A. M., Smith, T., & Folie, A. (2022). A new gecko from the earliest Eocene of Dormaal, Belgium - a thermophilic element of the “greenhouse world”. Royal Society Open Science, 9, 220429.
- Čerňanský, A., Herrel, A., Kibii, J. M., Anderson, C. V., Boistel, R., & Lehmann, T. (2020). The only complete articulated early Miocene chameleon skull (Rusinga Island, Kenya) suggests an African origin for Madagascar’s endemic chameleons. Scientific Reports, 10, 109.
- Čerňanský, A., Klembara, J., & Müller, J. (2016). The new rare record of the late Oligocene lizards and amphisbaenians from Germany and its impact on our knowledge of the European terminal Palaeogene. Palaeobiodiversity and Palaeoenvironments, 96, 559–587.
- Christensen, K., & Melstrom K. M. (2021). Quantitative analyses of squamate dentition demonstrate novel morphological patterns. PLoS ONE, 16(9), e0257427.
- Conrad, J. L. (2015). A New Eocene Casquehead Lizard (Reptilia, Corytophanidae) from North America. PLoS ONE, 10, e0127900.
- Cooper, J. S., Poole, D. F. G., & Lawson, R. (1970). The dentition of agamid lizards with special reference to tooth replacement. Journal of Zoology, 162, 85–98
- Cope, E. D. (1864). On the characters of the higher groups of Reptilia, Squamata and especially of the Diploglossa. Proceedings Academy of Natural Sciences of Philadelphia, 16, 224–231.
- Cox, N., Young, B. E., Bowles, P., Fernandez, M., Marin, J., Rapacciuolo, G., Böhm, M., Brooks, T. M., Hedges, S. B., Hilton-Taylor, C., Hoffmann, M., Jenkins, R. K. B., Tognelli, M. F., Alexander, G. J., Allison, A., Ananjeva, N. B., Auliya, M., Avila, L. J., Chapple, D. G., Cisneros-Heredia, D. F., Cogger, H. G., Colli, G. R., de Silva, A., Eisemberg, C. C., Els, J., Fong, A. G., Grant, T. D., Hitchmough, R. A., Iskandar, D. T., Kidera, N., Martins, M., Meiri, S., Mitchell, N. J., Molur, S., Nogueira, C. C., Ortiz, J. C., Penner, J., Rhodin, A. G. J., Rivas, G. A., Rödel, M.-O., Roll, U., Sanders, K. L., Santos-Barrera, G., Shea, G. M., Spawls, S., Stuart, B. L., Tolley, K. A., Trape, J.-F., Vidal, M. A., Wagner, P., Wallace, B. P., & Xie, Y. (2022). A global reptile assessment highlights shared conservation needs of tetrapods. Nature, 605, 285–290.
- Cramwinckel, M. J., Huber, M., Kocken, I. J., Agnini, C., Bijl, P. K., Bohaty, S. M., Frieling, J., Goldner, A., Hilgen, F. J., Kip, E. L., Peterse, F., Van der Ploeg, R., Röhl, U., Schouten, S., & Sluijs, A. (2018). Synchronous tropical and polar temperature evolution in the Eocene. Nature, 559, 382–386.
- Cuvier, G. J. L. N. F. D. (1829). Le Regne Animal Distribué, d'apres son Organisation, pur servir de base à l'Histoire naturelle des Animaux et d'introduction à l'Anatomie Comparé. Nouvelle Edition [second edition]. Vol. 2. Les Reptiles. Déterville, Paris, i-xvi, p. 1–406.
- De Stefano, G. (1903). I sauri del Quercy appartenenti alla collezione Rossignol. Atti della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano, 42, 382–418.
- Digimorph.org. (2002–2012). Digital morphology: a national science foundation digital library at the University of Texas at Austin [internet]. Austin, TX: The High Resolution X-ray Computed Tomography Facility at the University of Texas at Austin. Available at: http://www.digimorph.org/. Accessed 2022.
- Dong, Z. M. (1965). A new species of Tinosaurus from Lushih, Honan. Vertebrata PalAsiatica, 9, 79–82. [in Chinese with English summary]
- Dong, L. P., Evans, S. E., & Wang, Y. (2016). Taxonomic revision of lizards from the Paleocene deposits of the Qianshan Basin, Anhui, China. Vertebrata PalAsiatica, 54, 243–268.
- Duffaud, S., & Rage, J.-C. (1997). Les remplissages karstiques polyphasés (Eocène, Oligocène, Pliocène) de Saint-Maximin (Phosphorites du Gard) et leur apport à la connaissance des faunes européennes notamment pour l'Eocène moyen (MP13). 2.- Systématique: Amphibiens et reptiles. In J. P. Aguilar, & J. Michaux (Eds.), BiochroM’97. Mémoires et travaux de l'Institut de Montpellier de l'Ecole Pratique Hautes Etudes, 21, 729–735.
- Eberle, J. J., Fricke, H. C., Humphrey, J. D., Hackett, L., Newbrey, M. G., & Hutchison, J. H. (2010). Seasonal variability in Arctic temperatures during early Eocene time. Earth and Planetary Science Letters, 296, 481–486.
- Eberle, J. J., Gottfried, M. D., Hutchison, J. H., & Brochu, C. A. (2014). First Record of Eocene Bony Fishes and Crocodyliforms from Canada’s Western Arctic. PloS ONE, 9(5), e96079
- Estes, R. (1983). Sauria Terrestria, Amphisbaenia. In P. Wellnhofer (Ed.), Encyclopedia of Paleoherpetology, Part 10a. Gustav Fischer Verlag, Stuttgart - New York, 249 pp.
- Estes, R., de Queiroz, K., & Gauthier, J. A. (1988). Phylogenetic relationships within Squamata. In R. Estes, & G. K. Pregill (Eds.), Phylogenetic Relationships of the Lizard Families. Stanford University Press, Stanford, pp. 119–281.
- Estes, R., & Hutchison, J. H. (1980). Eocene lower vertebrates from Ellesmere Island, Canadian Arctic Archipelago. Palaeogeography, Palaeoclimatology, Palaeoecology, 30, 325–347.
- Evans, S. E. (1984). The classification of the Lepidosauria. Zoological Journal of the Linnean Society, 82, 87–100.
- Evans, S. E. (2008). The skull of lizards and tuatara. In C. Gans, A. S. Gaunt, & K. Adler (Eds.), Biology of the Reptilia, Volume 20, Morphology H: the skull of Lepidosauria. Society for the Study of Amphibians and Reptiles, Ithaca, New York, pp. 1–347.
- Evans, S. E., Prasad, G. V. R., & Manhas, B. K. (2002). Fossil lizards from the Jurassic Kota Formation of India. Journal of Vertebrate Paleontology, 22, 299–312.
- Fitzinger, L. J. (1843). Systema reptilium Fasciculus primus Amblyglossae. Braumüller et Seidel Bibliopolas, Vindobonae. p. vi + 106.
- Folie, A., Smith, R., & Smith, T. (2013). New amphisbaenian lizards from the Early Paleogene of Europe and their implications for the early evolution of modern amphisbaenians. Geologica Belgica, 16, 227–235.
- Foufopoulos, J., & Ives, A. R. (1999). Reptile extinctions on land-bridge islands: life-history attributes and vulnerability to extinction. The American Naturalist, 153, 1–25.
- Frost, D. R., & Etheridge, R. (1989). A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata). Miscellaneous publication - University of Kansas, Museum of Natural History, 81, 1–65.
- Frost, D. R., Etheridge, R., Janies, D., & Titus, T. A. (2001). Total evidence, sequence alignment, evolution of polychrotid lizards, and a reclassification of the Iguania (Squamata: Iguania). American Museum Novitates, 3343, 1–38.
- Gauthier, J. A., Kearney, M., Maisano, J. A., Rieppel, O., & Behlke, A. D. (2012). Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bulletin of the Peabody Museum of Natural History, 53, 3–308.
- Georgalis, G. L., Čerňanský, A., Göktaş, F., Alpagut, B., Şarbak, A., & Mayda S. (2023). The antiquity of Asian chameleons—first potential Chamaeleonidae and associated squamate fauna from the Lower and Middle Miocene of Anatolia. Journal of Vertebrate Paleontology, e2160644.
- Georgalis, G. L., & Joyce, W. G. (2017). A review of the fossil record of Old World turtles of the clade Pan-Trionychidae. Bulletin of the Peabody Museum of Natural History, 58, 115–208.
- Georgalis, G.L., Rabi, M., & Smith, K.T. (2021). Taxonomic revision of the snakes of the genera Palaeopython and Paleryx (Serpentes, Constrictores) from the Paleogene of Europe. Swiss Journal of Palaeontology, 140 (18), 1–140.
- Gilmore, C. W. (1943). Fossil lizards of Mongolia. Bulletin of the American Museum of Natural History, 81, 361–384.
- Greenwood, D. R., & Wing, S. L. (1995). Eocene continental climates and latitudinal temperature gradients. Geology, 23, 1044–1048.
- Hansen, J., Sato, M., Kharecha, P., Beerling, D., Berner, R., Masson-Delmotte, V., Pagani, M., Raymo, M., Royer, D. L., & Zachos, J. C. (2008). Target atmospheric CO2: Where should humanity aim? The Open Atmospheric Science Journal, 2, 217–231.
- Harcourt, A. H., Coppeto, S. A., & Parks, S. A. (2002). Rarity, specialization and extinction in primates. Journal of Biogeography, 29, 445–456.
- Hecht, M. K., & Hoffstetter, R. (1962). Note préliminaire sur les Amphibiens et les Squamates du Landénien supérieur et du Tongrien de Belgique. Bulletin de l ‘Institut royal des Sciences naturelles de Belgique, 38, 1–30.
- Herrel, A., Aerts, P., Fret, J., & De Vree, F. (1999). Morphology of the feeding system in agamid lizards: ecological correlates. The Anatomical Record, 254, 496–507.
- Holmes, R. B., Murray, A. M., Chatrath, P., Attia, Y. S., & Simons, E.L. (2010). Agamid lizard (Agamidae: Uromastycinae) from the lower Oligocene of Egypt. Historical Biology, 22, 215–223.
- Hopkins, G. W., Thacker, J. I., Dixon, A. F. G., Waring, P., & Telfer, M. G. (2002). Identifying rarity in insects: the importance of host plant range. Biological Conservation, 105, 293–307.
- Hou, L. H. (1974). Paleocene lizards from Anhui, China. Vertebrata PalAsiatica, 12, 193–202. [in Chinese with English summary].
- Huey, R. B., Deutsch, C. A., Tewksbury, J. J., Vitt, L. J., Hertz, P. E., Alvarez Pérez, H. J., & Garland, Jr T. (2009). Why tropical forest lizards are vulnerable to climate warming. Proceedings of the Royal Society B: Biological Sciences, 276, 1939–1948.
- Janis, Ch. M. (1993). Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annual Review of Ecology and Systematics, 24, 467–500.
- Jenkins, K. M., Jones, M. E. H., Zikmund, T., Boyde, A., & Daza, J. D. (2017). A review of tooth implantation among rhynchocephalians (Lepidosauria). Journal of Herpetology, 51, 300–306.
- Kuhn, O. (1944). Weitere Lacertilier, insbesondere Iguanidae aus dem Eozän des Geiseltales. Palaeontologische Zeitschrift, 23, 360–366.
- Leal, M., & Gunderson, A. R. (2012). Rapid change in the thermal tolerance of a tropical lizard. The American Naturalist, 180, 815–822.
- Leidy, J. (1872). Remarks on fossils from Wyoming. Proceedings of the Academy of Natural Sciences of Philadelphia, 1872, 277.
- Leidy, J. (1873). Contributions to the extinct vertebrate fauna of the Western Territories. Annual report. United States Geological and Geographical Survey of the Territories, 6, 14–358.
- Li, J. L. (1991). Fossil reptiles from Zhaili Member, Hedi Formation, Yuanqu, Shanxi. Vertebrata PalAsiatica, 29, 276–285. [in Chinese with English summary]
- Macey, J. R., II Schulte, J. A., Larson, A., Ananjeva, N. B., Wang, Y., Pethiyagoda, R., Rastegar-Pouyani, N., & Papenfuss, T. J. (2000). Evaluating trans-Tethys migration: an example using acrodont lizard phylogenetics. Systematic Biology, 49, 233–256.
- Markwick, P. J. (1994). “Equability”, continentality, and Tertiary “climate”: the crocodilian perspective. Geology, 22, 613–616.
- Markwick, P. J. (2002). Integrating the present and past records of climate, biodiversity and biogeography: implications for palaeoecology and palaeoclimatology. In J. A. Crame, & A. W. Owen (Eds.), Palaeobiogeography and Biodiversity Change: the Ordovician and Mesozoic-Cenozoic Radiations. Geological Society, London, Special Publications, 194, 179–199.
- Marsh, O. C. (1872). Preliminary description of new Tertiary reptiles. American Journal of Science, 4, 298–309.
- Moody, S. (1980). Phylogenetic and historical biogeographical relationships of the genera in the family Agamidae (Reptilia: Lacertilia) [Ph.D. dissertation]. University of Michigan, Ann Arbor, Michigan, 373 pp.
- Oppel, M. (1811). Die Ordnungen, Familien und Gattungen der Reptilien als Prodrom einer Naturgeschichte derselben. J. Lindauer, München, 86 pp.
- Owens, I. P. F., & Bennett, P. M. (2000). Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proceedings of the National Academy of Sciences of the United States of America, 97, 12144–12148.
- Pough, F. H., Andrews, R. M., Cadle, J. E., Crump, M. L., Savitzky, A. H., & Wells, K. D. (2004). Herpetology (3rd edition). Upper Saddle River: Pearson, 736 pp.
- Pounds, J. A., Fogden, M. P. L., & Campbell, J. H. (1999). Biological responses to climate change on a tropical mountain. Nature, 398, 611–615.
- Prasad, G. V. R., & Bajpai, S. (2008). Agamid lizards from the early Eocene of Western India: oldest Cenozoic lizards from South Asia. Palaeontologica Electronica, 11, 1.4A.
- Pross, J., Contreras, L., Bijl, P. K., Greenwood, D. R., Bohaty, S. M., Schouten, S., Bendle, J. A., Röhl, U., Tauxe, L., Raine, J. I., Huck, C. E., van de Flierdt, T., Jamieson, S. S. R., Stickley, C. E., van de Schootbrugge, B., Escutia, C., Brinkhuis, H., & Integrated Ocean Drilling Program Expedition 318 Scientists. (2012). Persistent near-tropical warmth on the Antarctic continent during the early Eocene epoch. Nature, 488, 73–77.
- Prothero, D. R., & Berggren, W. A. (1992). Eocene-Oligocene Climatic and Biotic Evolution. Princeton University Press, Princeton, 582 pp.
- Prothero, D. R., & Heaton, T. H. (1996). Faunal stability during the early Oligocene climatic crash. Palaeogeography, Palaeoclimatology, Palaeoecology, 127, 257–283.
- Purvis, A., Gittleman, J. L., Cowlishaw, G., & Mace, G. M. (2000). Predicting extinction risk in declining species. Proceedings of the Royal Society of London Series B, 267, 1947–1952.
- Rage, J.-C. (1987). Lower vertebrates from the early-middle Eocene Kuldana Formation of Kohat (Pakistan): Squamata. Contributions from the Museum of Paleontology, the University of Michigan, 27, 187–193.
- Rage, J.-C. (2013). Mesozoic and Cenozoic squamates of Europe. In J. D. Gardner, & R. L. Nydam (Eds.), Mesozoic and Cenozoic lissamphibian and squamate assemblages of Laurasia. Palaeobiodiversity and Palaeoenvironments, 93, 517–534.
- Rage, J.-C., Adaci, M., Bensalah, M., Mahboubi, M., Marivaux, L., Mebrouk, F., & Tabuce, R. (2021). Latest Early-early Middle Eocene deposits of Algeria (Glib Zegdou, HGL50), yield the richest and most diverse fauna of amphibians and squamate reptiles from the Palaeogene of Africa. Palaeovertebrata, 44, 32.
- Rage, J. C., & Augé, M. L. (1993). Squamates from the Cenozoic of the western part of Europe. A review. Revue de Paléobiologie, 7, 199–216.
- Rage, J.-C., & Augé, M. L. (2003). Amphibians and squamate reptiles from the lower Eocene of Silveirinha (Portugal). Ciências da Terra (UNL), 15, 103–116.
- Rage, J.-C., & Augé, M. L. (2010). Squamate reptiles from the middle Eocene of Lissieu (France). A landmark in the middle Eocene of Europe. Geobios, 43, 253–268.
- Rage, J.-C., & Augé M. L. (2015). Valbro: A new site of vertebrates from the early Oligocene (MP 22) of France (Quercy). III – Amphibians and squamates. Annales de Paleontologie, 101, 29–41.
- Rana, R. S., Augé, M. L., Folie, A., Rose, K. D., Kumar, K., Singh, L., Sahni, A., & Smith, T. (2013). High diversity of acrodontan lizards in the Early Eocene Vastan Lignite Mine of India. Geologica Belgica, 16, 290–301.
- Rea, D. K., Zachos, J. C., Owen, R. M., & Gingerich, P. D. (1990). Global change at the Paleocene-Eocene boundary: climatic and evolutionary consequences of tectonic events. Palaeogeograophy, Palaeoclimatology, Palaeoecology, 79, 117–128.
- Reeder, T. W., Townsend, T. M., Mulcahy, D. G., Noonan, B. P., Wood, P. L. Jr., Sites, J. W. Jr., & Wiens, J. J. (2015). Integrated Analyses Resolve Conflicts over Squamate Reptile Phylogeny and Reveal Unexpected Placements for Fossil Taxa. PLoS ONE, 10, e0118199.
- Robinson, P. L. (1976). How Sphenodon and Uromastyx grow their teeth and use them. In A. d’A. Bellairs, & C. B. Cox (Eds.), Morphology and Biology of Reptiles (Linnean Society Symposium Series Number 3). Academic Press, London, pp. 43–64.
- Rossmann, T. (2000). Osteologische Beschreibung von Geiseltaliellus longicaudus Kuhn, 1944 (Squamata: Iguanoidea) aus dem Mittleren Eozän der Fossillagerstätten Geiseltal und Grube Messel (Deutschland), mit einer Revision der Gattung Geiseltaliellus. Palaeontographica A, 258, 117–158.
- Simões, T. R., Wilner, E., Caldwell, M. W., Weinschütz, L. C., & Kellner, A. W. A. (2015). A stem acrodontan lizard in the cretaceous of Brazil revises early lizard evolution in Gondwana. Nature Communications, 6, 8149.
- Smith, A. G., Smith, D. G., & Funnell, B. M. (1994). Atlas of Mesozoic and Cenozoic coastlines. Cambridge University Press, 109 pp.
- Smith, K. T. (2006). A diverse new assemblage of late Eocene wquamates (Reptilia) from the Caadron Formation of North Dakota, U.S.A. Palaeontologia Electronica, 9, 5A:44.
- Smith, K. T. (2009a). Eocene lizards of the clade Geiseltaliellus from Messel and Geiseltal, Germany, and the early radiation of Iguanidae (Squamata: Iguania). Bulletin Yale Peabody Museum of Natural History, 50, 219–306.
- Smith, K. T. (2009b). A new lizard assemblage from the earliest Eocene (zone Wa0) of the Bighorn Basin, Wyoming, USA: biogeography during the warmest interval of the Cenozoic. Journal of Systematic Palaeontology, 7, 299–358.
- Smith, K. T. (2011a). On the phylogenetic affinity of the extinct acrodontan lizard Tinosaurus. Bonner Zoologische Monographien, 57, 9–28.
- Smith, K. T. (2011b). The Evolution of Mid-latitude Faunas during the Eocene: Late Eocene Lizards of the Medicine Pole Hills Reconsidered. Bulletin of the Peabody Museum of Natural History, 52, 3–105.
- Smith, K. T., Čerňanský, A., Scanferla, A., & Schaal, S. (2018). Lizards and snakes: warmth-loving sunbathers. In K. T, Smith, S. F. K. Schaal, & J. Habersetzer (Eds.), Messel, An Ancient Greenhouse Ecosystem. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main, pp. 122–147.
- Smith, K. T., & Scanferla, A. (2021). A nearly complete skeleton of the oldest definitive erycine boid (Messel, Germany). Geodiversitas, 43, 1–24.
- Smith, K. T., Schaal, S., Sun, W., & Li, C. T. (2011). Acrodont iguanians (Squamata) from the middle Eocene of the Huadian Basin of Jilin Province, China, with a critique of the taxon “Tinosaurus”. Vertebrata PalAsiatica, 49, 69–84.
- Smith, T., Kumar, K., Rana, R. S., Folie, A., Solé, F., Noiret, C., Steeman, T., Sahni A., & Rose, K. D. (2016). New early Eocene vertebrate assemblage from western India reveals a mixed fauna of European and Gondwana affinities. Geoscience Frontiers, 7, 969–1001.
- Smith, T., Rose, K. D., & Gingerich, P. D. (2006). Rapid Asia-Europe-North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene-Eocene Thermal Maximum. Proceedings of the National Academy of Sciences of the United States of America, 103, 11223–11227.
- Smith, T., & Smith, R. (1996). Synthèse des données actuelles sur les vertébrés de la transition Paléocène-Eocène de Dormaal (Belgique). Bulletin de la Société belge de Géologie, 104, 119–131.
- Solé, F., Smith, R., Coillot, T., De Bast, E., & Smith, T. (2014). Dental and tarsal anatomy of ‘Miacis’ latouri and a phylogenetic analysis of the earliest carnivoraforms (Mammalia, Carnivoramorpha). Journal of Vertebrate Paleontology, 31, 1–21.
- Spix, J.B. von. (1825). Animalia nova sive species nova lacertarum quas in itinere per Brasiliam annis MDCCCXVII–MDCCCXX jussu et auspicius Maximiliani Josephi I Bavariae Regis suscepto collegit et descripsit Dr. J.B. de Spix. Lipsiae: Monachii [Munich], T.O. Weigel, F.S. Hübschmanni, 26 pp.
- Steurbaut, E., De Coninck, J., Roche, E., & Smith, T. (1999). The Dormaal Sands and the Paleocene/Eocene boundary in Belgium. Bulletin de la Société Géologique de France, 170, 217–227.
- Sullivan, R. M., Augé, M. L., Wille, E., & Smith, R. (2012). A new glyptosaurine lizard from the earliest Eocene of Dormaal, Belgium. Bulletin de la Société géologique de France, 183, 629–635.
- Tolley, K. A., & Herrel, A. (2013). The Biology of Chameleons. University of California Press, Berkeley and Los Angeles, 288 pp.
- Torres-Carvajal, O., de Queiroz, K., & Schulte, J. A. II. (2020). “Iguanidae.” in Phylonyms: A Companion to the PhyloCode. In K. de Queiroz, P. D. Cantino, & J. A. Gauthier (Eds.), the Chemical Rubber Company (CRC) Press, Boca Raton, Florida, pp. 1159–1164.
- Uetz, P., Freed, P., & Hošek, J. (2022). The Reptile Database. http://www.reptile-database.org. [accessed February 2022]
- Wagner, P., Stanley, E. L., Daza, J. D., & Bauer, A. M. (2021). A new agamid lizard in mid-Cretaceous amber from northern Myanmar. Cretaceous Research, 124, 104813.
- Whiteside, D. I. (1986). The head skeleton of the Rhaetian sphenodontid Diphydontosaurus avonis gen. et sp. nov. and the modernizing of a living fossil. Philosophical Transactions of the Royal Society B: Biological Sciences, 312, 379–430.
- Wilson, J. D., Morris, A. J., Arroyo, B. E., Clark, S. C., & Bradbury, R. B. (1999). A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agriculture Ecosystems & Environment, 75, 13–30.
- Zachos, J., Dickens G., & Zeebe, R. (2008). An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature, 451, 279–283.
- Zheng, Y., & Wiens, J. J. (2016). Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Molecular Phylogenetics and Evolution, 94, 537–547.
