Abstract
Objective
Current monitoring methods of asthma, such as peak expiratory flow testing, have important limitations. The emergence of automated acoustic sound analysis, capturing cough, wheeze, and inhaler use, offers a promising avenue for improving asthma diagnosis and monitoring. This systematic review evaluated the validity of acoustic biomarkers in supporting the diagnosis of asthma and its monitoring.
Data sources
A search was performed using two databases (PubMed and Embase) for all relevant studies published before November 2023.
Study selection
27 studies were included for analysis. Eligible studies focused on acoustic signals as digital biomarkers in asthma, utilizing recording devices to register or analyze sound.
Results
Various respiratory acoustic signal types were analyzed, with cough and wheeze being predominant. Data collection methods included smartphones, custom sensors and digital stethoscopes. Across all studies, automated acoustic algorithms achieved average accuracy of cough and wheeze detection of 88.7% (range: 61.0 − 100.0%) with a median of 92.0%. The sensitivity of sound detection ranged from 54.0 to 100.0%, with a median of 90.3%; specificity ranged from 67.0 to 99.7%, with a median of 95.0%. Moreover, 70.4% (19/27) studies had a risk of bias identified.
Conclusions
This systematic review establishes the promising role of acoustic biomarkers, particularly cough and wheeze, in supporting the diagnosis of asthma and monitoring. The evidence suggests the potential for clinical integration of acoustic biomarkers, emphasizing the need for further validation in larger, clinically-diverse populations.
Introduction
In recent years, new methods of diagnosis and monitoring have arisen, including the use of digital biomarkers, which are characterized as data derived from digital devices (Citation1). This includes smartphones, sensors, wearables and implants, which record digital biomarkers that are objective, quantifiable and encompass both physiological and behavioral information (Citation2). As medical devices are more integrated into clinical practice, digital biomarkers have become a new tool for studying precision medicine.
Digital biomarkers allow for long-term, noninvasive, automatic, remote, continuous data collection and monitoring. For example, wristwatches collecting heart rate data to predict and notify users about abnormal heart rates (Citation3). Digital biomarkers have been identified and validated across different medical specialties for conditions such as schizophrenia (Citation3), Alzheimer’s disease (Citation4), neuromuscular disorders (Citation5) and congestive heart failure (Citation6).
Acoustic biomarkers are a subset of digital biomarkers which use sounds to identify a disease state or physiology. In cardiac monitoring, digital stethoscopes are now able to analyze heart sounds (Citation7,Citation8). Acoustic biomarkers have also been studied in gastrointestinal disease, where low-cost sensors can record and analyze bowel sounds to measure gastrointestinal motility (Citation9). Acoustic biomarkers have been assessed in orthopedics, where the sounds produced by joint articulation could be used to assess joint health (Citation10).
In the context of respiratory health, these acoustic biomarkers primarily involve the recording of respiratory sounds to analyze elements such as breathing patterns, coughs, wheezing and crackles (Citation11). Research into acoustic biomarkers in respiratory medicine increased during the COVID-19 pandemic, when they were used to predict COVID-19 diagnosis (Citation12–16). Since then, acoustic biomarkers have shown promise in monitoring and diagnosis in other respiratory diseases: COPD (Citation17–20), pneumonia (Citation21–23), sleep apnea (Citation24–26), as well as asthma, which received particular attention in recent years (Citation27–29). Remote monitoring of patients using acoustic technology in combination with artificial intelligence algorithms holds promise for continuous, remote patient monitoring (Citation30).
Asthma requires regular monitoring to achieve good control and prevent exacerbations by appropriately escalating treatment (Citation31). Despite the availability of effective treatments, optimal asthma control is seldom achieved (Citation32–38). Some acoustic respiratory devices are already commercially available for testing and the scientific community is likely to see a growing trend in development of these type of devices (Citation39). Automated acoustic monitoring could allow for long-term data collection about the patient’s asthma status, improve management and detect exacerbations early. For instance, a study of 89 asthmatics showed that 24-h cough frequency correlated with Asthma Control Questionnaire scores and FEV1 (Citation27). Similarly, longitudinal nocturnal cough monitoring using sound recording in a 29-day-long study in asthmatic patients was used to effectively predict asthma exacerbations (Citation28).
Acoustic monitoring has also been applied to wheeze analysis. In pediatric patients, breath sounds were recorded overnight continuously using a phono-pneumography sensor over the trachea. It was demonstrated that even with subjectively well-controlled asthma (based on asthma diary entries), children experience considerable amount of wheezing overnight, suggesting suboptimal management of asthma (Citation40). Furthermore, computerized analysis of wheeze sounds during tidal breathing correlated with asthma severity (Citation41).
To our knowledge, only specific reports and applications of acoustic biomarkers in asthma have been published, but no systematic review has been undertaken on the utility and application of the various aspects of acoustic biomarkers in asthma. This systematic review, therefore, aims to evaluate the validity of acoustic biomarkers in the diagnosis and monitoring of patients with asthma.
Methods
Inclusion criteria
Studies on the use of acoustic signals, such as cough or wheeze, as digital biomarkers in asthma were included. Studies used recording devices to register sound or performed analysis of prerecorded sounds. Studies tested the sound recording devices and compared them against manual sound annotation or other standards of care (such as clinician diagnosis or spirometry).
Exclusion criteria
Qualitative studies, animal studies, technical reports and non-English language studies were excluded. Studies relating to other respiratory diseases (such as COPD or COVID-19), studies including analysis based on other factors (such as patient-reported questionnaires) were excluded. In the first phase of abstract screening, technical reports were also excluded (engineering papers analyzing sound characteristics without testing in patient populations, as well as sound analysis protocols).
This systematic review was registered with the PROSPERO international database (PROSPERO ID: CRD42023473584) and followed the Preferred Reporting Items of Systematic Reviews and Meta-analyses guidelines (Citation42).
Literature search
A search was performed using two databases: PubMed and Embase. All studies in English up to 1st November 2023 were included. The search was conducted based on the following terms and their MeSH terms: “asthma”, “lung disease”, “cough”, “wheeze”, “breath”, “acoustic signal”, “sound” and “cough”. The full search strategy, registered on PROSPERO, and excluded terms are available in the Appendix.
Study selection
Titles and abstracts of search results were screened independently for eligibility by two authors. Full texts were obtained and evaluated. References of these full texts and relevant literature reviews were screened to identify any studies not captured by the original search strategy.
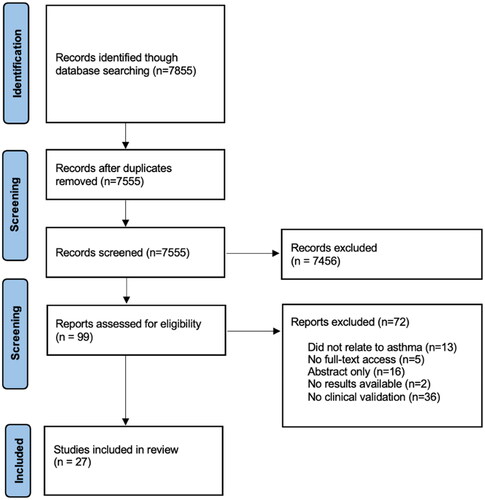
PRISMA diagram
Data abstraction
Data was abstracted from full-text papers into Microsoft Excel spreadsheets under the following data categories: Study Selection, Data Abstraction and Data Analysis, Study Characteristics, Patient characteristics, Data collection methods, Acoustic signals, Asthma identification, Risk of Bias Assessment. A full strategy for data abstraction is available in Appendix .
Risk of bias assessment
All studies included in the review were appraised using the QUADAS-2 tool for reviews to evaluate the risk of bias and applicability of studies (Citation44).
Statistical analysis
Statistical analysis and calculations were done using Microsoft Excel (Microsoft Corporation). Average accuracy has been calculated for all studies. For sensitivity and specificity, ranges and medians have been provided due to the limited availability of specific statistical details in studies (such as confidence intervals and sensitivity thresholds).
Outcomes
The primary outcome of the study was to assess the validity of automated acoustic sound analysis in the diagnosis and monitoring of asthma, including detection of acute exacerbations. The secondary outcome of the study was to identify the different types of acoustic signals collected and characterize the types of algorithms.
Results
Study selection
7855 Studies were identified in the initial abstract screening. Duplicates (n = 300) and studies not relevant to the study question were excluded (n = 7456). The majority of the latter were engineering studies which did not test the acoustic biomarkers on patients. 99 Studies were identified, of which 71 studies were excluded because of no full text access, no clinical validation or no mention of asthma. 19 Studies remained, and a further 8 were identified through reference screening as shown in , resulting in 27 studies for analysis.
Figure 1. Workflow representing phases of the systematic review process (PRISMA criteria (Citation43)).
Data abstraction and data analysis
describes the study details and patient characteristics. summarizes data collection methods, study results, conclusions and quality of the studies.
Table 1. Study details and patient population characteristics.
Table 2. Data collection methods, study results, conclusions and risk of bias (SN: sensitivity; SP: specificity; ACC: accuracy; NR: not reported).
Study characteristics
Of the 27 studies reviewed, the following acoustic signals were assessed: breathing (n = 7), coughing (n = 9), wheezing (n = 6), inhaler use (n = 3), speech (n = 1) and chest percussion sounds (n = 1). Studies took place in the following settings: at home (n = 4), clinic-based (n = 5), hospital (n = 11), research laboratory (n = 3) and non-reported (n = 2). The average study length was 41 days (range: 1 – 153).
Patient characteristics
The total number of tested patients was 2290, with an average of 88 and median of 56 patients (range: 3–585). 52% of patients were male, among data for which gender information was available.
Data collection methods
Acoustic data was collected using various electronic devices, most commonly smartphones (n = 12), custom microphones and sensors (n = 8), digital stethoscopes (electronic stethoscopes with a microphone) (n = 4) and other devices such as vibration sensors and digital inhalers with sensors (n = 3). Studies either monitored patients in real-time using body-worn sensors and devices (e.g. smartwatch), or used microphones (e.g. smartphone) to collect data for retrospective analysis (analyzing sound signals to detect trends and specific features).
Acoustic signals
Overall, 73,750 acoustic signals were analyzed across all studies, with average of 3352 and median of 781 (range: 45–30,304).
Asthma identification
Across the 27 studies, the algorithms for automated asthma detection achieved an average accuracy of 88.7% (range: 61.0 − 100.0%) with a median of 92.0%. The sensitivity of asthma detection ranged from 54.0 to 100.0% with a median of 90.3%; specificity ranged from 67.0 to 99.7%, with a median of 95.0%.
Risk of bias assessment
All studies have been reviewed for risk of bias and applicability using the QUADAS-2 tool. 70.4% (19 out of 27) studies have risk identified in at least one domain in the QUADS-2 tool. Full results are detailed in . For both the “Risk of Bias” and “Applicability Concerns” categories, “Index Test” was the area with most studies demonstrating risk of bias (10 studies were marked as “high risk” of bias in both categories for “Index Test”).
Table 3. QUADAS-2 risk of bias and applicability of studies.
Cough monitoring and predicting asthma exacerbations
Most studies in the review analyzed various forms of cough detection in order to diagnose asthma or predict asthma exacerbations. Smartphone-recorded nocturnal cough detected periods of uncontrolled asthma (Citation28). Nocturnal cough and sleep quality predicted asthma exacerbations with 70.0–75.0% accuracy, 75.0–88.0% sensitivity and 57.0–72.0% specificity (Citation28). Other studies used an algorithm to analyze overnight recordings, which predicted asthma exacerbations without the need for peak-flow monitoring or spirometry (Citation45,Citation46). Similarly, a study on 94 adult patients with physician-diagnosed asthma recorded cough sounds overnight with a mobile phone for 30 days. Coughs were recorded in 53% of nights and were 4.5 times more frequent in asthmatics (Citation29). A clinic-based study on an automated cough sound analytic system for the identification of asthma in children demonstrated 97.0% agreement between the automated analyzer and the clinical asthma diagnosis (Citation47).
Wheeze detection
Several studies in the review focused on wheeze detection as a way of monitoring and predicting exacerbations, with various approaches, such as using custom wearable sensors (Citation48). A proof-of-concept study described a low-cost, flexible aluminum foil sensor, which was noninvasively attached to the human chest to detect wheezing. The device underwent limited testing on 4 participants, however, a precision measure of variability (gage repeatability) reached 92.1% (Citation48). An integrated smartphone application system (which collected physiological, environmental and patient-reported data) demonstrated up to 96.0% accuracy in detecting patients’ wheezing from sound recording and accurately classifying them as wheezing or normal breathing (Citation49). Digital stethoscopes are also used in asthma acoustic monitoring. Artificial intelligence (AI) was applied to neural networks for polyphonic sound detection to detect patterns in the recorded sounds, including any pathology in breathing cycles. 522 Auscultatory sounds from 50 pediatric asthmatic patients demonstrated that AI-powered digital stethoscopes can improve the accuracy of auscultatory examinations (13% improvement in sensitivity of wheeze detection by the algorithm in comparison to clinicians) (Citation50). Another custom-made, hand-held device with a wheeze recognition algorithm identified wheezing with 96.6% sensitivity and 98.5% specificity (Citation51).
Asthma severity
Studies recorded wheeze sounds at several positions on the chest- acoustic frequency features were analyzed, and there were significant differences between asthma severity levels, showing that the technology was able to classify patients according to severity level (Citation41,Citation52).
Inhaler use
By recording inhalation sounds during inhaler use, the patient’s inhalation flow profile can be estimated. Inhalation technique could be assessed by estimating inhalation flow profiles and other measures, such as inspiratory capacity, with 90.89% accuracy of estimating peak expiratory flow (Citation53). In two studies, an algorithm based on inhalation sounds was developed to automatically assesses adherence to a dry powder inhaler by detecting the use of the inhaler automatically over a 3 month period (Citation54,Citation55).
Discussion
The aim of the systematic review was to assess the validity of acoustic biomarkers in the diagnosis and monitoring of asthma. Studies of acoustic biomarkers in asthma showed high average accuracy (88.7%) in the detection of asthma. Several methods of acoustic data collection have been identified, with cough and wheezing being present in most discussed studies as the main acoustic measures collected and analyzed by algorithms.
Standard asthma questionnaires rely on subjective reporting of a patient’s symptoms and have the potential to be replaced by long-term monitoring of acoustic biomarkers, which shows promise in monitoring asthma control. Moreover, the technology presented in the studies are accessible and almost fully automated, which allows for long-term remote monitoring. Many models integrate the use of AI-guided algorithms, which are trained on acoustic databases, which further facilitates decision making (Citation12,Citation13,Citation56,Citation57). Use of acoustic biomarkers can help to reduce some patient-related issues with asthma management, such as poor adherence or poor inhaler technique.
Similarly to studies in COVID-1913 (Citation58), studies of acoustic biomarkers in asthma demonstrated high specificity and sensitivity in distinguishing coughs and wheezing from asthmatic and non-asthmatic patients, which raises the potential for automated support in reaching a clinical decision of asthma diagnosis using passive sound recording (Citation49). Other studies, such as ones using acoustic features of cough in COPD, show promise in predicting exacerbations. For instance, a respiratory sensor (which recorded respiratory sounds) was able to predict 78.8% of COPD exacerbations on average 5 days earlier than when the patient sought medical attention (Citation17). Detecting asthma exacerbations was, however, described in only one of the reviewed studies, where monitoring nocturnal cough predicted asthma exacerbations with 70–75% accuracy (Citation28). Other studies analyzing the usefulness of acoustic biomarkers in respiratory medicine drew similar findings to this review.
Our results are similar to other studies of acoustic biomarkers in respiratory medicine. It is possible to diagnose COVID-19 with high sensitivity and specificity (98.5 and 94.2%) using cough sound analysis, whereas findings in asthma reached 97% (Citation13,Citation47). Several studies in our review utilized digital stethoscopes with good outcomes, similar to other applications of this technology other areas of medicine, such as cardiology. Real-time monitoring of heart disease using similar technology achieved 97 and 88% accuracy respectively for classifying abnormal and normal heart sounds in early detection of cardiac disease (Citation59). Digital stethoscopes have similar limitations across fields of medicine, including the lack of randomized trials comparing them to gold-standard diagnostic techniques.
Another promising area of research described was the use acoustic biomarkers in assessing inhaler adherence and technique. Use of digital inhalers, which lack the ability to measure acoustic biomarkers, has led to improved inhaler adherence and reduced exacerbation rates, as outlined in a recent review of 20 studies of digital inhalers in asthma and COPD (Citation60). The added benefit of acoustic biomarkers is that they can be used to evaluate inhaler technique, such as the study included in our review which estimated inhalation flow with 90.9% accuracy. A similar study in patients with various airways diseases (including COPD) showed that audio signal analysis estimated peak inspiratory flow rate through an inhaler mouthpiece with 88.2% accuracy. Furthermore, audio analysis detected inhaler technique errors at a similar rate compared to a clinical reviewer checklist for inhaler technique (85% for the audio analysis, compared to 90% for the checklist assessment) (Citation61). Research is needed to assess if the use of acoustic biomarkers to evaluate inhaler technique results in improved asthma outcomes or sustained improvements in inhaler techniques.
The evolution of digital health data analytics is an opportunity for the optimization of outcomes in patients with respiratory disease. New literature suggests the potential of smartphone-based diagnostic tools that use audio recordings to monitor symptoms (Citation62). It minimizes the limitations of patient-driven monitoring and leads to early escalation to a clinician when symptoms persist or deteriorate despite best medical management (Citation63). Additionally, it is a way to ease the significant burden of respiratory disease on healthcare systems by introducing cost-effective solutions which reduce labor intensive management strategies. In many cases, most importantly, it also increases patient engagement in their own medication adherence and self-monitoring (Citation64).
This systematic review has important limitations. Many studies chosen for the review focused on the technical validation of algorithms (which analyzed prerecorded sound patterns) with limited testing in real-time and in real-word situations. There was little to no mention of statistical power calculations in the studies included. The external validity of the results is limited by the relatively small sample sizes in the included studies (average of 88 patients per study). Data extraction was very heterogenous, due to limited methodological detail, such as lack of patient recruitment information. Studies in the review were also found through screening of medical databases only. Future studies should also screen engineering and computing study databases, in order to find missing datasets of acoustic biomarkers with larger sample sizes.
70.4% (19/27) of the included studies were identified as having a risk of bias using the QUADAS-2 tool, of which the most common area was the Index Test, where 10 studies had high risk of bias in the “Risk of Bias” and “Applicability Concerns” categories. A pre-specified index test threshold was not explicitly stated in some of the studies, possibly resulting in an overestimation of performance of some algorithms. This also resulted in limited comparison between studies, as they lacked standardization. Data analysis could not be performed uniformly on all studies in the review due to unavailability of sensitivity or specificity thresholds in some of the studies analyzed. Therefore, a range of results are provided in this review instead of average results and confidence intervals, which could be improved in future studies by explicitly stating performance thresholds for algorithms, enabling meta-analysis studies.
There is a need for a multidisciplinary approach where clinicians provide input into algorithm development, and engineers offer technical advice. Focus needs to be put on real-word applications of algorithms to check the reproducibility of those solutions. Large acoustic datasets should be validated clinically, so that gold-standard comparators can be used in future study design, as seen in COVID-19 acoustic testing (Citation16).
Conclusions
Acoustic biomarkers are a promising new tool in the diagnosis and monitoring of asthma. However, trials of their use in asthma have important limitations. Therefore, randomized-control trials of acoustic biomarkers in asthma diagnosis and monitoring are warranted, before they can be used widely in clinical practice.
Acknowledgments
Many thanks to Dr. Paul Pffefer and Zofia Przypaśniak for their advice and help with project conceptualization and advice. The research did not receive any grant or form of funding from public, commercial or nonprofit sectors.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Vasudevan S, Saha A, Tarver ME, Patel B. Digital biomarkers: convergence of digital health technologies and biomarkers. NPJ Digit Med 2022;5(1):36. doi:10.1038/s41746-022-00583-z.
- Califf RM. Biomarker definitions and their applications. Exp Biol Med 2018;243(3):213–221. doi:10.1177/1535370217750088.
- Fraccaro P, Beukenhorst A, Sperrin M, Harper S, Palmier-Claus J, Lewis S, Van der Veer SN, Peek N. Digital biomarkers from geolocation data in bipolar disorder and schizophrenia: a systematic review. J Am Med Inform Assoc 2019;26(11):1412–1420. doi:10.1093/jamia/ocz043.
- Piau A, Wild K, Mattek N, Kaye J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: systematic review. J Med Internet Res 2019;21(8):e12785. doi:10.2196/12785.
- Youn BY, Ko Y, Moon S, Lee J, Ko SG, Kim JY. Digital biomarkers for neuromuscular disorders: a systematic scoping review. Diagnostics 2021;11(7):1275–1279. doi:10.3390/diagnostics11071275.
- Juen J, Cheng Q, Schatz B. A natural walking monitor for pulmonary patients using mobile phones. IEEE J Biomed Health Inform 2015;19(4):1399–1405. doi:10.1109/JBHI.2015.2427511.
- Watsjold B, Ilgen J, Monteiro S, Sibbald M, Goldberger ZD, Thompson WR, Norman G. Do you hear what you see? Utilizing phonocardiography to enhance proficiency in cardiac auscultation. Perspect Med Educ 2021;10(3):148–154. doi:10.1007/s40037-020-00646-5.
- Durand LG, Pibarot P. Review: most recent advancements in digital signal processing of the phonocardiogram. Crit Rev Biomed Eng 2017;45(1–6):453–509. doi:10.1615/CritRevBiomedEng.v45.i1-6.170.
- Du X, Allwood G, Webberley KM, Osseiran A, Marshall BJ. Bowel sounds identification and migrating motor complex detection with low-cost piezoelectric acoustic sensing device. Sensors 2018;18(12):4240. doi:10.3390/s18124240.
- Whittingslow DC, Jeong HK, Ganti VG, Kirkpatrick NJ, Kogler GF, Inan OT. Acoustic emissions as a non-invasive biomarker of the structural health of the knee. Ann Biomed Eng 2020;48(1):225–235. doi:10.1007/s10439-019-02333-x.
- Gabaldon-Figueira JC, Brew J, Doré DH, Umashankar N, Chaccour J, Orrillo V, Tsang LY, Blavia I, Fernández-Montero A, Bartolomé J, et al. Digital acoustic surveillance for early detection of respiratory disease outbreaks in Spain: a protocol for an observational study. BMJ Open 2021;11(7):e051278. doi:10.1136/bmjopen-2021-051278.
- Imran A, Posokhova I, Qureshi HN, Masood U, Riaz MS, Ali K, John CN, Hussain MI, Nabeel M. AI4COVID-19: AI enabled preliminary diagnosis for COVID-19 from cough samples via an app. Inform Med Unlocked 2020;20:100378. doi:10.1016/j.imu.2020.100378.
- Laguarta J, Hueto F, Subirana B. COVID-19 artificial intelligence diagnosis using only cough recordings. IEEE Open J Eng Med Biol 2020;1:275–281. doi:10.1109/OJEMB.2020.3026928.
- Pinkas G, Karny Y, Malachi A, Barkai G, Bachar G, Aharonson V. SARS-CoV-2 detection from voice. IEEE Open J Eng Med Biol 2020;1:268–274. doi:10.1109/OJEMB.2020.3026468.
- Andreu-Perez J, Perez-Espinosa H, Timonet E, Kiani M, Giron-Perez MI, Benitez-Trinidad AB, Jarchi D, Rosales-Perez A, Gatzoulis N, Reyes-Galaviz OF, et al. A generic deep learning based cough analysis system from clinically validated samples for point-of-need Covid-19 test and severity levels. IEEE Trans Serv Comput 2022;15(3):1220–1232. doi:10.1109/TSC.2021.3061402.
- Santosh KC, Rasmussen N, Mamun M, Aryal S. A systematic review on cough sound analysis for Covid-19 diagnosis and screening: is my cough sound COVID-19? PeerJ Comput Sci 2022;8:e958. doi:10.7717/peerj-cs.958.
- Fernandez-Granero MA, Sanchez-Morillo D, Leon-Jimenez A. Computerised analysis of telemonitored respiratory sounds for predicting acute exacerbations of COPD. Sensors 2015;15(10):26978–26996. doi:10.3390/s151026978.
- Boeselt T, Kroenig J, Lueders T-S, Koehler N, Beutel B, Hildebrandt O, Koehler U, Conradt R. Acoustic monitoring of night-time respiratory symptoms in 14 patients with exacerbated COPD over a 3-week period. Int J Chron Obstruct Pulmon Dis 2022;17:2977–2986. doi:10.2147/COPD.S377069.
- Crooks MG, den Brinker A, Hayman Y, Williamson JD, Innes A, Wright CE, Hill P, Morice AH. Continuous cough monitoring using ambient sound recording during convalescence from a COPD exacerbation. Lung 2017;195(3):289–294. doi:10.1007/s00408-017-9996-2.
- Windmon A, Minakshi M, Bharti P, Chellappan S, Johansson M, Jenkins BA, Athilingam PR. TussisWatch: A smart-phone system to identify cough episodes as early symptoms of chronic obstructive pulmonary disease and congestive heart failure. IEEE J Biomed Health Inform 2019;23(4):1566–1573. doi:10.1109/JBHI.2018.2872038.
- Mor R, Kushnir I, Meyer JJ, Ekstein J, Ben-Dov I. Breath sound distribution images of patients with pneumonia and pleural effusion. Respir Care 2007;52(12):1753–1760.
- Rao A, Ruiz J, Bao C, Roy S. Tabla: a proof-of-concept auscultatory percussion device for low-cost pneumonia detection. Sensors 2018;18(8):2689. doi:10.3390/s18082689.
- Boesch M, Rassouli F, Baty F, Schwärzler A, Widmer S, Tinschert P, Shih I, Cleres D, Barata F, Fleisch E, et al. Smartphone-based cough monitoring as a near real-time digital pneumonia biomarker. ERJ Open Res 2023;9(3):00518-2022. doi:10.1183/23120541.00518-2022.
- Kim T, Kim JW, Lee K. Detection of sleep disordered breathing severity using acoustic biomarker and machine learning techniques. Biomed Eng Online 2018;17(1):16. doi:10.1186/s12938-018-0448-x.
- Rosenwein T, Dafna E, Tarasiuk A, Zigel Y. Breath-by-breath detection of apneic events for OSA severity estimation using non-contact audio recordings. Conf Proc IEEE Eng Med Biol Soc 2015;2015:7688–7691.
- Cheng S, Wang C, Yue K, Li R, Shen F, Shuai W, Li W, Dai L. Automated sleep apnea detection in snoring signal using long short-term memory neural networks. Biomed Signal Process Control 2022;71:103238. doi:10.1016/j.bspc.2021.103238.
- Marsden PA, Satia I, Ibrahim B, Woodcock A, Yates L, Donnelly I, Jolly L, Thomson NC, Fowler SJ, Smith JA, et al. Objective cough frequency, airway inflammation, and disease control in asthma. Chest 2016;149(6):1460–1466. doi:10.1016/j.chest.2016.02.676.
- Tinschert P, Rassouli F, Barata F, Steurer-Stey C, Fleisch E, Puhan MA, Kowatsch T, Brutsche MH. Nocturnal cough and sleep quality to assess asthma control and predict attacks. J Asthma Aller 2020;13:669–678. doi:10.2147/jaa.s278155.
- Rassouli F, Tinschert P, Barata F, Steurer-Stey C, Fleisch E, Puhan MA, Baty F, Kowatsch T, Brutsche MH. Characteristics of asthma-related nocturnal cough: a potential new digital biomarker. J Asthma Aller 2020;13:649–657. doi:10.2147/JAA.S278119.
- Kraman SS, Pasterkamp H, Wodicka GR. Smart devices are poised to revolutionize the usefulness of respiratory sounds. Chest 2023;163(6):1519–1528. doi:10.1016/j.chest.2023.01.024.
- Mukherjee M, Stoddart A, Gupta RP, Nwaru BI, Farr A, Heaven M, Fitzsimmons D, Bandyopadhyay A, Aftab C, Simpson CR, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med 2016;14(1):113. doi:10.1186/s12916-016-0657-8.
- Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, Cullinan P, Custovic A, Ducharme FM, Fahy JV, et al. After asthma: redefining airways diseases. Lancet 2018;391(10118):350–400. doi:10.1016/S0140-6736(17)30879-6.
- Demoly P, Paggiaro P, Plaza V, Bolge SC, Kannan H, Sohier B, Adamek L. Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur Respir Rev 2009;18(112):105–112. doi:10.1183/09059180.00001209.
- Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev 2017;4(4):CD012226. doi:10.1002/14651858.CD012226.pub2.
- Chongmelaxme B, Chaiyakunapruk N, Dilokthornsakul P. Incorporating adherence in cost-effectiveness analyses of asthma: a systematic review. J Med Econ 2019;22(6):554–566. doi:10.1080/13696998.2019.1572014.
- Anam K, Al-Jumaily AA. Active exoskeleton control systems: state of the art. Procedia Eng 2012;41:988–994. doi:10.1016/j.proeng.2012.07.273.
- Bårnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care 2015;60(3):455–468. doi:10.4187/respcare.03200.
- Armeftis C, Gratziou C, Siafakas N, Katsaounou P, Pana ZD, Bakakos P. An update on asthma diagnosis. J Asthma 2023;60(12):2104–2110. doi:10.1080/02770903.2023.2228911.
- Tinschert P, Groh M. Resmonics I acoustic artificial intelligence for respiratory health and infection prevention. Resmonics [last accessed 29 February 2024]. https://www.resmonics.ai/.
- Boner AL, Piacentini GL, Peroni DG, Irving CS, Goldstein D, Gavriely N, Godfrey S. Children with nocturnal asthma wheeze intermittently during sleep. J Asthma 2010;47(3):290–294. doi:10.3109/02770900903497188.
- Nabi FG, Sundaraj K, Lam CK. Asthma severity identification from pulmonary acoustic signal for computerized decision support system. J Pak Med Assoc 2021;71(1(A):41–46. doi:10.47391/JPMA.156.
- Wieczorek K, Anath S, Velazquez-Pimentel D. PROSPERO registration [last accessed 13 January 2024]. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=473584.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339(jul21 1):b2700–b2700. doi:10.1136/bmj.b2700.
- University of Bristol. QUADAS-2 [last accessed 21 February 2024]. https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/.
- Porter P, Brisbane J, Abeyratne U, Bear N, Claxton S. A smartphone-based algorithm comprising cough analysis and patient-reported symptoms identifies acute exacerbations of asthma: a prospective, double blind, diagnostic accuracy study. J Asthma 2023;60(2):368–376. doi:10.1080/02770903.2022.2051546.
- Barata F, Tinschert P, Rassouli F, Steurer-Stey C, Fleisch E, Puhan MA, Brutsche M, Kotz D, Kowatsch T. Automatic recognition, segmentation, and sex assignment of nocturnal asthmatic coughs and cough epochs in smartphone audio recordings: observational field study. J Med Internet Res 2020;22(7):e18082. doi:10.2196/18082.
- Porter P, Abeyratne U, Swarnkar V, Tan J, Ng T-W, Brisbane JM, Speldewinde D, Choveaux J, Sharan R, Kosasih K, et al. A prospective multicentre study testing the diagnostic accuracy of an automated cough sound centred analytic system for the identification of common respiratory disorders in children. Respir Res 2019;20(1):81. doi:10.1186/s12931-019-1046-6.
- Khan SM, Qaiser N, Shaikh SF, Hussain MM. Design analysis and human tests of foil-based wheezing monitoring system for asthma detection. IEEE Trans Electron Dev 2020;67(1):249–257. doi:10.1109/TED.2019.2951580.
- Ra HK, Salekin A, Yoon HJ, et al. AsthmaGuide: an asthma monitoring and advice ecosystem. In 2016 IEEE Wireless Health (WH); 2016: 1–8. doi:10.1109/WH.2016.7764567.
- Grzywalski T, Piecuch M, Szajek M, Bręborowicz A, Hafke-Dys H, Kociński J, Pastusiak A, Belluzzo R. Practical implementation of artificial intelligence algorithms in pulmonary auscultation examination. Eur J Pediatr 2019;178(6):883–890. doi:10.1007/s00431-019-03363-2.
- Habukawa C, Ohgami N, Arai T, Makata H, Tomikawa M, Fujino T, Manabe T, Ogihara Y, Ohtani K, Shirao K, et al. Wheeze recognition algorithm for remote medical care device in children: validation study. JMIR Pediatr Parent 2021;4(2):e28865. doi:10.2196/28865.
- Nabi FG, Sundaraj K, Lam CK, Palaniappan R. Characterization and classification of asthmatic wheeze sounds according to severity level using spectral integrated features. Comput Biol Med 2019;104:52–61. doi:10.1016/j.compbiomed.2018.10.035.
- Taylor TE, Lacalle Muls H, Costello RW, Reilly RB. Estimation of inhalation flow profile using audio-based methods to assess inhaler medication adherence. PLOS One 2018;13(1):e0191330. doi:10.1371/journal.pone.0191330.
- Holmes MS, D'arcy S, Costello RW, Reilly RB. Acoustic analysis of inhaler sounds from community-dwelling asthmatic patients for automatic assessment of adherence. IEEE J Transl Eng Health Med 2014;2:2700210–2700210. doi:10.1109/JTEHM.2014.2310480.
- Holmes MS, Le Menn M, D’Arcy S. Automatic identification and accurate temporal detection of inhalations in asthma inhaler recordings. Conf Proc IEEE Eng Med Biol Soc 2012;2012:2595–2598.
- Kothalawala DM, Kadalayil L, Weiss VBN, Kyyaly MA, Arshad SH, Holloway JW, Rezwan FI. Prediction models for childhood asthma: a systematic review. Pediatr Allergy Immunol 2020;31(6):616–627. doi:10.1111/pai.13247.
- Yu G, Li Z, Li S, Liu J, Sun M, Liu X, Sun F, Zheng J, Li Y, Yu Y, et al. The role of artificial intelligence in identifying asthma in pediatric inpatient setting. Ann Transl Med 2020;8(21):1367–1367. doi:10.21037/atm-20-2501a.
- Huang Y, Meng S, Zhang Y, Wu S, Zhang Y, Zhang Y, Ye Y, Wei Q, Zhao N, Jiang J, et al. The respiratory sound features of COVID-19 patients fill gaps between clinical data and screening methods. bioRxiv (published online April 10, 2020). doi:10.1101/2020.04.07.20051060.
- Chowdhury MEH, Khandakar A, Alzoubi K, Mansoor S, M Tahir A, Reaz MBI, Al-Emadi N. Real-time smart-digital stethoscope system for heart diseases monitoring. Sensors 2019;19(12):2781. doi:10.3390/s19122781.
- Chan AHY, Pleasants RA, Dhand R, Tilley SL, Schworer SA, Costello RW, Merchant R. Digital inhalers for asthma or chronic obstructive pulmonary disease: a scientific perspective. Pulm Ther 2021;7(2):345–376. doi:10.1007/s41030-021-00167-4.
- Taylor TE, Zigel Y, Egan C, Hughes F, Costello RW, Reilly RB. Objective assessment of patient inhaler user technique using an audio-based classification approach. Sci Rep 2018;8(1):2164. doi:10.1038/s41598-018-20523-w.
- Larson EC, Lee T, Liu S, Rosenfeld M, Patel SN. Accurate and privacy preserving cough sensing using a low-cost microphone. In proceedings of the 13th International Conference on Ubiquitous Computing. UbiComp’11. Association for Computing Machinery; 2011: 375–384. doi:10.1145/2030112.2030163.
- Barata F, Kipfer K, Weber M, Tinschert P, Fleisch E, Kowatsch T. Towards device-agnostic mobile cough detection with convolutional neural networks. 2019 IEEE International Conference on Healthcare Informatics (ICHI) (published online 2019). doi:10.1109/ICHI.2019.8904554.
- Zia A, Brassart A, Thomas S, Ye F, Stephenson JJ, Mullins CD, Jones CA. Patient-centric structural determinants of adherence rates among asthma populations: exploring the potential of patient activation and encouragement tool TRUSTR to improve adherence. J Health Econ Outcomes Res 2020;7(2):111–122. doi:10.36469/jheor.2020.13607.
Appendix A
Full search strategy used in the systematic review.
#1 Asthma [MH]
#2 Lung Diseases, Obstructive [MH:NOEXP]
#3 Anti-asthmatic agents [MH]
#4 Asthm
#5 Bronchospas*
#6 Bronchoconstrict*
#7 Bronch* and (constrict*)
#8 Cough*
#9 Wheez*
#10 Breath*
#11 (bronchial* or (respiratory) or (airway*) or (lung*)) and (hypersensitiv* or (hyperreactiv*) or (allerg*))
#12 OR/1-11
#10 Acoustic signal [MH]
#11 Sound signal [MH]
#12 Sound recording [MH]
#13 Cough detection [MH]
#14 (Breath* and (sound*)) and (detect* or record* or analys* [tiab] or monitor* [tiab] or evaluat* [tiab])
#15 (Wheez* [tiab]) and (detect* [tiab] or record* [tiab] or analys* [tiab] or monitor* [tiab] or evaluat* [tiab])
#16 (Cough* [tiab]) and (detect* [tiab] or record* [tiab] or analys* [tiab] or monitor* [tiab] or evaluat* [tiab])
#17 OR/10-16
#18 Animals[mh] not (humans[mh])
#19 editorial[publication type]
#20 review[publication type] not (systematic review [publication type])
#21 Letter [publication type]
#22 case report
#23 report of a case
#24 a case of
#25 (mice or (mouse) or (rat) or (animal*) or (pig))
#26 ((COPD) or (chronic bronchitis) or (emphysema) or (chronic obstructive pulmonary disease) or (cancer) or (bronchiectasis) or (fibrosis) or (surgery) or (operation)) not (asthma)
#27 OR/18-26
#28 #12 and #17
#29 #28 NOT #27
SHORT VERSION:
(Asthma [MH]) or (Lung Diseases, Obstructive [MH:NOEXP]) or (Anti-asthmatic agents [MH]) or (Asthma [tiab]) or (Bronchospas* [tiab]) or (Bronchoconstrict* [tiab]) or (Bronch* [tiab] and (constrict* [tiab])) or ((bronchial* [tiab] or (respiratory[tiab]) or (airway* [tiab]) or (lung* [tiab])) and (hypersensitiv* [tiab] or (hyperreactiv* [tiab]) or (allerg* [tiab])))
AND
(Acoustic signal [MH]) or (Sound signal [MH]) or (Sound recording [MH]) or (Cough detection [MH]) or ((Breath* [tiab] and (sound* [tiab])) and (detect* [tiab] or record* [tiab] or analys* [tiab] or monitor* [tiab] or evaluat* [tiab])) or ((Wheez* [tiab]) and (detect* [tiab] or record* [tiab] or analys* [tiab] or monitor* [tiab] or evaluat* [tiab])) or ((Cough* [tiab]) and (detect* [tiab] or record* [tiab] or analys* [tiab] or monitor* [tiab] or evaluat* [tiab]))
NOT
(Animals[mh] not (humans[mh])) or (editorial[publication type]) or (review[publication type] not (systematic review [publication type])) or (Letter [publication type]) or (“case report”) or (“report of a case”) or (“a case of”) or ((mice or (mouse) or (rat) or (animal*) or (pig))) or (((COPD) or (chronic bronchitis) or (emphysema) or (chronic obstructive pulmonary disease) or (cancer) or (bronchiectasis) or (fibrosis) or (surgery) or (operation)) not (asthma))

