We have measured the fluorescence spectra of three different bacterial spores (B. globigii, B. cereus, and B. popillae) mixed with one of three different samples of domestic dust and using five excitation wavelengths. The data are analyzed with principal component analysis and clustered with partition around medoids method. We are able to correctly identify each of the samples, even with the dust involved. We also measured a separate preparation of B. globigii, ovalbumin, and corn smut fungal spores. All the B. globigii samples showed excellent clustering. The ovalbumin and corn smut spores showed weak or ambiguous clustering with B. cereus. While fluorescence combined with principal component analysis and clustering techniques shows strong promise to correctly identify bacterial spores, additional work is necessary to discriminate the fluorescence signals from other biological samples.
INTRODUCTION
Classical bacterial identification procedures include a morphological evaluation of the microorganism as well as tests for the organism's ability to grow in different media. These procedures generally require extensive time and human intervention. On the other hand, a number of unique instrumentation approaches, including fluorescence, have been applied to the detection and identification of bacteria (CitationCoburn et al. 1985; CitationRossi andWarner 1985; CitationReinisch et al. 1994; CitationSorrel et al. 1994; CitationSpector et al. 2000; CitationBronk and Reinisch 1993). We believe that the previous studies are encouraging and fluorescence spectroscopy has a strong potential to effectively detect and identify bacteria and bacterial spores in many different settings. Multiple excitation wavelengths and broad emission spectra with moderate wavelength resolution have been successful in identifying bacteria (CitationReinisch et al. 1994; CitationSorrel et al. 1994; Spector et al. 2002). Within the past ten years, additional studies of bacteria detection using multi-wavelength excitation and emission protocols have been published (CitationGray et al. 1998; CitationCheng et al. 1999; CitationHill et al. 1999; CitationLeblanc and Dufour 2002; CitationVasanthi et al. 2004). Each of the studies shows a varying degree of success. It is notable that some groups have succeeded to detect and identify biological aerosols, including bacterial spores, even with the inclusion of interferents ((CitationHairston et al. 1997; CitationEversole et al. 1999; CitationSeaver et al. 1999; CitationEversole 2001; CitationHybl et al. 2003; CitationSamuels et al. 2003; CitationScully et al. 2002). Thus, one must be able to work with high-dimensional spectroscopy.
One significant problem in high-dimensional spectroscopy is the partial or complete overlap of signals from the different constituents in multi-component samples, like bacteria and bacterial spores. Hence, the classification, detection and analysis of the sample from high-dimensional spectra are often difficult. The efficiency of classification algorithms decreases with the increased dimensionality of the data space. This fact, called Bellman's curse of dimensionality (CitationBellman 1961) imposes the necessity of pre-processing steps like principal component analysis (PCA) to the examination of the data.
PCA has been used for the analysis of UV/visible fluorescence data and is a powerful tool for the determination of correlations and classification from high-dimensional data (CitationSun et al. 1987; CitationSaltiel et al. 1994). While the experimental noise and other unknown dependencies of experimental conditions may add to the confusion when attempting to classify high-dimensional data, the PCA technique is based on the assumption that the variance of the spectral data may be used as a measure of spectral content (CitationJenson and Walty 1979). PCA is an exploratory multivariate statistical technique for examining relationships among several quantitative descriptors or variables (CitationBasilevsky et al. 1994; CitationEveritt and Dunn 1992). PCA produces a set of expression patterns known as principal components (PCs), and linear combinations of these patterns can be assembled to represent the behavior of all of the objects in a given data set.
There are many excellent books on PCA. For example, a hands-on how-to approach can be found in the textbook by CitationJackson (2003). This book provides the details about the computational aspects of PCA. Basically, the extraction of principal components amounts to a variance maximization by the rotation of the original variable space. For example, in a scatter plot we can think of the regression line as the original x-axis, rotated so that it approximates the regression line. This type of rotation is called variance maximizing because the goal of the rotation is to maximize the variability of the new variable, while minimizing the variance around the new variable.
After the line on which the variance maximum is found, there remains some variability around this line. In PCA, after the first factor has been extracted, that is, after the first line has been drawn through the data, another line is found that maximizes the remaining variability. In this manner, consecutive factors are extracted. Each consecutive factor is defined to maximize the variability that is not captured by the preceding factors, therefore the factors are orthogonal to each other.
When using PCA as a data reduction method, one must ask, how many PCs are necessary to extract? Note that consecutive PCs account for less and less variability. The decision of when to stop extracting factors depends on when there is little random variability left. The decision is arbitrary; however, various guidelines have been developed.
The eigenvalues of the PCs are a measure of how much variance each successive factor extracts. More than 95% of the variance is normally extracted in the first three or four factors. So, many analysis schemes retain only factors with eigenvalues greater than one. This criterion, proposed by CitationKaiser (1960) is probably the one most widely used. On the other hand, a more modern study of PCA has shown that no predetermined subset of the PCs can arbitrarily give the best description of the data (CitationYeung and Ruzzo 2001). One must judiciously select the right subset of PCs. In the analysis presented here, we use only the first two PCs as they account form most of the variance and give an adequate separation. This happens to follow Kaiser's rule. We find the results are essentially unchanged when additional PCs are retained.
After the PCs are found, they need to be grouped according to the sample. This grouping is termed clustering. We are referring to a clustering of the principal components and not of the bacterial spores. Clustering techniques have been applied to a wide variety of research problems (CitationBonnet 2000; CitationEisen et al. 1998). Cluster classification is a method for displaying the similarities and dissimilarities between pairs of objects (samples) in a set. There are many cluster techniques. Criteria usually used are the split of classes for separation, the diameter of the partitions for homogeneity and the sum of (squared) distances between elements in different classes (CitationHansen and Jaumard 1997).
One of the problems in cluster analysis is the objective assessment of the validity of clusters found by a clustering algorithm. This can be studied by re-analysis of a modified version of the data set. The modified data can be obtained by changing the values of the variables or weighting some of the objects (CitationGordon and De Cata 1988; CitationJolliffe et al. 1988; CitationChen and Milligan 1996). The final stage of classification is the description of valid clusters, in order to allow the efficient summarization of data and assignment of new objects to these clusters (CitationKaufman and Rousseeuw 1990). In this study we modified the data by considering other excitation wavelengths and varying the range of emission wavelengths used in the PCA.
We are encouraged in our study by the report of CitationLeblanc and Dufour (2002). They were able to successfully identify suspensions of bacteria using four excitation wavelengths and PCA. They made the broad claim that in addition to the species, the strain of the bacteria could be identified. There are several significant differences between their work and the work presented here. They worked with aqueous suspensions of bacteria and this work uses dry spores. They did not use interferents, however, this study does. They used only four excitation wavelengths and this study uses five.
The objective of identifying Bacillus spores is, of course, complicated. The spores can be mixed with dust or dirt (interferents). The spores can be produced by many different procedures and grown with different media. Additionally, the spores might or might not be washed. In this study, we only consider the interferents. We investigate whether the identification of Bacillus spores is retained or destroyed by including domestic dust with our samples of spores. We also look at the consequences of measuring samples that are not in our library of known fluorescence profiles. It is an important question to ask what happens when something never measured before is added to the unknowns.
MATERIAL AND METHODS
The selection of Bacillus spores used for this study was based on previous work (CitationBronk and Reinisch 1993) and the characteristics and availability of the spores. The spores that simulate pathogens were used to assess the feasibility of 2D spectroscopy that could detect and identify spores posing an immediate threat. The Bacillus spores used for this experiment were furnished as a generous gift from the United States Army Soldier and Biological Chemical Command (SBCCOM). None of the details of spore production methods were made available to us, except that none of the spores were treated with any special flow agent or anti-clumping compounds. All the spore samples were measured as they were provided and in a dry state.
Bacterial Spore Identification
The rapid detection and identification of bacteria and bacterial spores have many applications. One of the more recent applications is for the detection and identification of bacterial spores that might be used in bioterrorism. One would like to have a method to quickly collect and identify the potential danger from an unknown powder spilling from an opened letter. If multi-wavelength excitation/emission spectra are collected to assist in the identification of the powder, a simple but powerful data reduction method must be used.
The objective of this work is to apply PCA for dimensional reduction of fluorescence data and then to use cluster analysis to isolate the most discriminatory building blocks from the fluorescence information and to classify bacteria on the basis of their resemblances of 2D fingerprinting. In this study we are interested in the clustering and classification of microorganisms based on the criteria mentioned above, that is: To find the separation and diameter of the clusters formed by known and unknown samples so as to get a method for rapid identification of biological pathogens.
If the spores were to be distributed in an act of bioterrorism, the collection of the spores for analysis would most likely involve the collection of dust and other particles in addition to the spores. We have, therefore, added domestic dust to our samples.
A simple, but effective, method of collecting the spilled powder or spores for analysis is to use a piece of tape. Spilled powder could be picked up with the sticky side of tape. The tape could then be put into a device where the powder is analyzed.
Tape Fluorescence
Examining the fluorescence properties of many tapes and glues, we found that 3M Super Strength Mailing Tape (3M, St. Paul, Minnesota, USA) provided a minimal amount of fluorescence and the peaks are away from the spore fluorescence peaks (see ). The tape is cut into 1 × 3 cm pieces and is held at 45 degrees in a 1 cm quartz cuvette. The fluorescence is measured on a Hitachi 4500 spectrofluorometer (Tokyo, Japan) with 5 nm bandwidth (both excitation and emission) and scanning at 1200 nm per min. Detection is made with a photomultiplier running in a current mode (non-photon counting). The spectra are measured with excitations at 280, 310, 340, 370, and 400 nm. We selected these wavelengths because these show reasonable fluorescence signals from bacteria and bacterial spores and wanted to arbitrarily limit the excitation to five wavelengths. The emission is measured every 0.2 nm from 50 nm longer than the excitation wavelength to 150 nm longer than the excitation wavelength.
FIG. 1 Fluorescence fingerprints of the tape substrate used. The fluorescence signal is divided into 15 equally spaced contour lines. The maximum fluorescence is shaded darkest. The emission is measured from 50 nm longer than the excitation wavelength to 150 nm longer than the excitation wavelength. Excitation wavelengths used are 280, 310, 340, 370, and 400 nm.
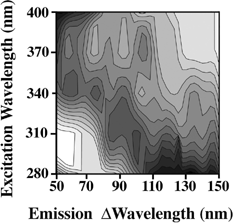
We note that we are using a very small number of excitation wavelengths and a very narrow range of emission wavelengths. This narrow range of measurements is used because any system devised to monitor for pathogens will probably use a limited data set to make the measurements and analysis fast and less expensive.
The data are visualized with Mathematica (Wolfram, Urbana, Illinois, USA). Mathematica is used to smooth the data and interpolate between the excitation wavelengths and draw contours. Fifteen equally spaced contours are drawn between the maximum and minimum fluorescence signals. The positive peaks are shaded dark. The absolute fluorescence intensity is not recorded for any of the data sets, since the amount of dust or spores on the tape would change this value and the amount of dust or spores collected in an actual bioterrorism attack is highly variable.
Spore Fluorescence
Nine pieces of tape are used to collect domestic dust from three different locations in a house (living room, bathroom, and bedroom). We call these samples: dust A, B, and C, respectively. The tape with dust from each of the three locations is lightly coated with one each of three different Bacillus spores (Bacillus globigii—BG; Bacillus cereus—BC; Bacillus popillae—BP). We estimate that there is about 10 times (by mass) more dust than spores on the tape. We note that both the dust and spores are inhomogeneously scattered on the tape. There are nine samples with each combination of dust and bacterial spore. The fluorescence of each dust and spore combination is then measured. These nine samples are used as the training set.
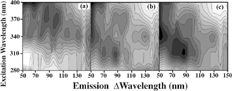
We show in the fluorescence emission/excitation spectra that are measured for dust A and the three different bacterial spores on dust A. In addition, we measure the fluorescence signals from three other samples. We measure the fluorescence signals from a sample of BG spores prepared from an outside source. The outside source is Merck Chemicals (St. Louis, Missouri, USA Lot D-1-1732-6). We also measure corn smut, a fungal spore (Lenoir, North Carolina, Lot 8553-10), and ovalbumin (egg white and a growth medium ingredient) (Sigma, St. Louis, Missouri, USA). These fluorescence spectra are shown in .
FIG. 2 Fluorescence fingerprints of the (a) dust A; (b) BC and dust A; (c) BG and dust A; (d) BP and dust A. The fluorescence signal is divided into 15 equally spaced contour lines. The maximum fluorescence is shaded darkest. The emission is measured from 50 nm longer than the excitation wavelength to 150 nm longer than the excitation wavelength. Excitation wavelengths used are 280, 310, 340, 370, and 400 nm.
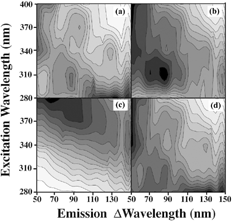
FIG. 3 Fluorescence fingerprints of the (a) Merck BG; (b) corn smut fungal spores; (c) ovalbumin. The fluorescence signal is divided into 15 equally spaced contour lines. The maximum fluorescence is shaded darkest. The emission is measured from 50 nm longer than the excitation wavelength to 150 nm longer than the excitation wavelength. Excitation wavelengths are 280, 310, 340, 370, and 400 nm.
We note that our method of drawing contour lines effectively normalizes all the spectra to the maximum fluorescence. However, none of the fluorescence spectra were normalized before being submitted to PCA. PCA and PAM cluster classification were carried out using the R software package (GNU project, Version 2, Boston, Massachusetts, USA http://www.gnu.org. It is part of the standard PCA protocols to make the diagonal elements of the correlation matrix equal to one. Thus, there is a normalization-like process that is part of PCA. We have used the PCA package as supplied.
Principal Component Analysis
In a we show the two-dimensional PCA plot (component 1 versus component 2) of three samples of BG, BC, and BP. We also considered smoothing the data. We used a 20-node spline to smooth the 500-point emission spectrum from each excitation wavelength, as this would give smoothing that is approximately equal to the bandwidth of the monochromators. The smoothing created almost no change to the PCA.
FIG. 4 (a) PCA plot of three samples of BG (open squares), BC (open circles), BP (crosses) (9 measurements). Each sample is mixed with dust as explained in the text. Principal component 1 versus component 2 is plotted. Plots of the PCs of same three samples of BG, BC, BP (9 measurements) with the added sample of (b) Merck BG (solid diamond); (c) corn smut (solid diamond); and (d) ovalbumin (solid diamond).
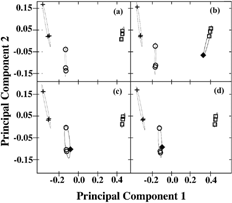
Each sample is measured as a dry spore on tape with a background of domestic dust from three different locations (living room, bathroom, and bedroom). Even though these samples contain a mixture of materials, one can see by the grouping of the data that the spore fluorescence signal is consistent and therefore dominates in the analysis.
In b we show the plot from the PCA of the same 9 measurements of BG, BC, and BP with the BG prepared from an outside source, Merck Chemicals. We show the plot from PCA of the same 9 measurements with corn smut (a fungal spore) added (C), and ovalbumin (egg white and growth medium ingredient) added (D). For plotting the PCs, we use the first two principal components determined from the excitation wavelengths of 280, 310, 340, 370, and 400 nm.
The PCA does not change significantly if the data are smoothed. It also remains relatively constant if we increase in emission data from 50 nm longer than the excitation wavelength to 250 nm longer than the excitation wavelength. We can also make small changes in the excitation wavelengths selected and the results remain the same.
If major changes are made, such as using only excitation wavelengths longer than 400 nm, the results are affected. At these wavelengths there is very little fluorescence signal from the spores and the dust and signal noise create spurious results with the PCA. Likewise, if the emission wavelengths include wavelengths closer to the excitation wavelengths, then elastic scattering from the sample dominates the fluorescence signal and the PCA is adversely affected. While the PCA is reasonably robust to measurement parameters, the results can be invalidated by a careless selection of measurement parameters.
Cluster Analysis: Partition Around Medoids (PAM)
After PCA is used to reduce the dimensionality of the data, we must determine if we are still able to group the measurements correctly. Ideally, we would like to employ an analysis that would group or cluster the Merck BG with our other BG measurements and have the corn smut and ovalbumin not cluster with any of our three bacterial spore measurements.
There are several ways to cluster data. We considered partitioning algorithms with these data. The algorithms partition a data set of n objects into k clusters. The number of clusters, k, must be given. Two common methods of clustering are k-means and k-medoids (more commonly called Partition Around Medoids or PAM) (CitationKaufman and Rousseeuw 1990). We will first discuss how k-means works. The k-means arbitrarily divides the n objects into k clusters. The centroid of each cluster is found. The centroid is the geometrical centre of all the objects in the cluster. The distances needed to find the centre can be calculated as Euclidian , where x and y are the distances on the principal component plot. The distances can be calculated in higher dimensions if more than two principal components are used. After this initial arbitrary grouping, the distance between each object and each centroid is computed. The objects are then regrouped around the closest centroid. A new centroid is calculated and the process iterates. Thus, the objects are grouped with a minimum distance between the objects and the centroid of the group.
PAM is very similar to k-means. The only difference is that medoids are used instead of centroids. A medoid is the object closest to the centroid. Centroids are a position in space. A medoid is an object that happens to be close to the centroid. The medoids are used, instead of centroids, typically when the number of objects in a group is small. While the k-means method of clustering is generally considered very efficient, in that the number of iterations required is small, the method often terminates at a local minimum instead of finding the global minimum. Also, k-means does not handle outliers or very noisy data. PAM is much more effective with noisy data or outliers. PAM also works well for small data sets.
The PAM program in R also plots the silhouettes of the cluster strength. The cluster strength is defined as
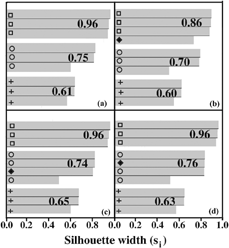
The silhouettes of the clusters shown in are given in . Each shaded bar is associated with an object in . The corresponding symbols are given. The number at the end of each cluster is the average Si for that cluster. In b, the Merck BG clusters well with the other BG samples. In c and d, the ovalbumin and corn smut cluster with the BC. This is an incorrect clustering.
FIG. 5 (a) Silhouette plot of the strength of clustering using PAM with three samples of BG (open squares), BC (open circles), BP (crosses) (9 measurements) as shown in . Silhouette plot of the strength of clustering using PAM with the same three samples of BG, BC, BP (9 measurements) with the added sample of (b) Merck BG (solid diamond); (c) corn smut (solid diamond); and (d) ovalbumin (solid diamond). The cluster strength (Si) is explained in the text.
DISCUSSION
Fluorescence is a valuable tool in probing different materials. The surrounding material influences the emissions from intrinsic fluorophores. Subtle differences in the emission spectra can, therefore, be used to identify the environment of the fluorophore. In fact the 2D fingerprint offers some preliminary information for identification of species.
PCA is an effective and convenient way to reduce the high-dimensional fluorescence data. The classification of samples using cluster analysis is easily and quickly evaluated from the PCs. Yet, there are obvious limitations. We selected only 5 excitation wavelengths for the PCA. We chose excitation wavelengths where the fluorescence from the spores is large. We also chose a 100 nm range of emission wavelengths to sample the typical 50–100 nm wide fluorescence peaks. Since our dry samples reflect stray light into the detection system, we started our emission wavelength collection at 50 nm longer than the excitation wavelength.
Even with the small number of samples, it is clear that both PCA and PAM clustering separate and distinguish among the three different bacterial spores (BG, BC, and BP). Even when one of the spores is produced from a different source, it is still appropriately classified.
When samples are used that are not bacterial spores, the PCA and PAM cluster analysis attempted to cluster these samples with the BC. Since PAM must be given the number of clusters to use, PAM would apportion all the samples into one of three clusters. If a sample really did not belong in a particular cluster, then this would be indicated in the silhouette plot, with the strength (Si) considerably lower than the other strengths.
More work needs to be done with the interferents. Although the spores represented only about 10% of the material on the tape, the spore fluorescence was still greater than the dust fluorescence. This is helpful in detecting a small number of spores in a very dusty environment. However, it also brings up the question of how much dust can be added to a spore sample before the PCA is not able to identify the spore. Some of our simulations suggest that the PCA works as long as the spore fluorescence intensity is greater than the dust fluorescence intensity. We are currently trying to determine how that changes with the absolute spore number and the difference between the spore and dust fluorescence. We are also working on a way to convert fluorescence to relative amounts of spores and dust. Obviously, the dust concentration must be much greater than the spore concentration before the identification is obfuscated.
CONCLUSIONS
The aim of our clustering analysis is to provide objective classification of the known and unknown samples; objective in the sense that the analysis of the same set of organisms by the same sequence of numerical methods produces the same classification. We are able to show that fluorescence is a valuable tool in probing bacterial spores. The 2D fingerprint offers some preliminary information for identification of species. PCA is a powerful means for the mining of low-dimensional information from high-dimensional fluorescence data. PAM was found to be useful for the interpretation of results of clustering. Although we are able to correctly identify bacterial spore samples and samples mixed with dust, samples that are not bacterial spores may be misclassified.
Acknowledgments
We thank NovaSol for funding this work. We are most grateful to Christchurch School of Medicine and Health Sciences, New Zealand and Dr. Tony Kettle for allowing us to use their spectrofluorometer.
REFERENCES
- Basilevsky , A. 1994 . Statistical Factor Analysis and Related Methods, Theory and Applications. , New York : John Wiley & Sons .
- Bellman , R. 1961 . Adaptive Control Processes: A Guided Tour , Princeton , NJ : Princeton University Press .
- Bonnet , N. 2000 . Artificial Intelligence and Pattern Recognition Techniques in Microscope Image Processing and Analysis . Advances in Imaging and Electron Physics , 114 : 1 – 77 . [CSA]
- Bronk , B. V. and Reinisch , L. 1993 . Variability of Steady State Bacterial Fluorescence with Respect to Growth Conditions . Appl. Spect. , 47 : 436 – 440 . [CSA] [CROSSREF]
- Chen , R. and Milligan , G. M. 1996 . Measuring the Influence of Individual Data Points in a Cluster Analysis . J. Classification , 13 : 315 – 335 . [CSA] [CROSSREF]
- Cheng , Y. S. , Barr , E. B. , Fan , B. J. , Hargis , P. J. , Rader , D. J. , O'Hern , T. J. , Torczynski , J. R. , Tisone , G. C. , Preppernau , B. L. , Young , S. A. and Radloff , R. J. 1999 . Detection of Bioaerosols Using Multivavelength UV Fluorescence Spectroscopy . Aerosol Sci. Technol. , 30 : 186 – 201 . [CSA] [CROSSREF]
- Coburn , J. T. , Lytle , F. E. and Huber , D. M. 1985 . Identification of Bacterial Pathogens by Laser Excited Fluorescence . Anal. Chem. , 57 : 1669 – 1673 . [CSA] [CROSSREF]
- Eisen , M. B. , Spellman , P. T. , Brown , P. O. and Botstein , D. 1998 . Cluster Analysis and Display of Genome-Wide Expression Patterns . Proc. Nat. Acad. Sci. USA , 95 : 14863 – 14868 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Everitt , B. S. and Dunn , G. 1992 . Applied Multivariate Data Analysis. , New York : Oxford University Press .
- Eversole , J. D. , Cary , W. K. Jr. , Scotto , C. S. , Pierson , R. , Spence , M. and Campillo , A. J. 2001 . Continuous Bioaerosol Monitoring using UV Excitation Fluorescence: Outdoor Test Results . Field Anal. Chem. Technol. , 15 : 205 – 212 . [CSA] [CROSSREF]
- Eversole , J. D. , Hardgrove , J. J. , Cary , W. K. Jr. , Choulas , D. P. and Seaver , M. 1999 . Continuous, Rapid Biological Aerosol Detection with use of UV Fluorescence: Outdoor Test Results . Field Anal. Chem. Technol. , 3 : 249 – 259 . [CSA] [CROSSREF]
- Gordon , A. D. and De Cata . 1988 . Stability and Influence in Sum of Squares Clustering . Metron. , 46 : 347 – 360 . [CSA]
- Gray , P. C. , Shokair , I. R. , Rosenthal , S. E. , Tisone , G. C. , Wagner , J. S. , Rigdon , L. D. , Siragusa , G. R. and Heinen , R. J. 1998 . Distinguishabilty of Biological Material by use of Ultraviolet Multispectral Fluorescence . Applied Optics , 37 : 6037 – 6041 . [CSA]
- Hairston , P. P. , Ho , J. and Quant , F. R. 1997 . Design of an Instrument for Real Time Detection of Aerosols Using Simultaneous Measurements of Particle of Aerodynamic Size and Intrinsic Fluorescence . J. Aerosol Sci. , 28 : 471 – 482 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Hansen , P. and Jaumard , B. 1997 . Cluster Analysis and Mathematical Programming . Mathematical Programming , 79 : 191 – 215 . [CSA] [CROSSREF]
- Hill , S. C. , Pinnick , R. G. , Niles , S. , Pan , Y. L. , Holler , S. , Chang , R. K. , Bottiger , J. , Chen , B. T. , Orr , C. S. and Feather , G. 1999 . Real-time Measurement of Fluorescence Spectra from Single Airborne Particles . Field Anal. Chem. Technol. , 3 : 221 – 239 . [CSA] [CROSSREF]
- Hybl , J. D. , Lithgow , G. A. and Buckley , S. G. 2003 . Laser-Induced Breakdown Spectroscopy Detection and Classification of Biological Aerosols . Applied Spectroscopy , 57 : 1207 – 1215 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Jackson , J. E. 2003 . A User's Guide to Principal Components. , New York : John Wiley & Sons .
- Jenson , S. K. and Walty , F. A. 1979 . Principal Components Analysis and Canonical Analysis in Remote Sensing . Proc. Annual. Am. Soc. Photogrammetry , : 79 – 143 . [CSA]
- Jolliffe , I. T. , Jones , B. and Morgan , B. J. T. 1988 . “ Stability and Influence in Cluster Analysis ” . In Data Analysis and informatics , Edited by: Diday , E. pp. 507 – 514 . Amsterdam : North-Holland .
- Kaiser , H. F. 1960 . The Application of Electronic Computers to Factor Analysis . Educational and Psychological Measurement , 20 : 141 – 151 . [CSA]
- Kaufman , L. and Rousseeuw , P. J. 1990 . Finding Groups in Data. An Introduction to Cluster Analysis , New York : Wiley–Interscience .
- Leblanc , L. and Dufour , E. 2002 . Monitoring the Identity of Bacteria Using Their Intrinsic Fluorescence . FEMS Microbiology Letters , 211 : 147 – 153 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Reinisch , L. , Tribble , J. , Werkhaven , J. A. and Ossoff , R. H. 1994 . Non–Invasive Optical Diagnosis of Bacteria Causing Otitis Media . Laryngoscope , 104 : 264 – 268 . [PUBMED] [INFOTRIEVE] [CSA]
- Rossi , T. M. and Warner , I. M. 1985 . “ Bacterial Identification Using Fluorescence Spectroscopy ” . In Rapid Detection and Identification of Microorganisms , Edited by: Nelson , W. H. pp. 1 – 50 . Weinheim , , Germany : Verlag Chemie .
- Saltiel , J. , Sears , D. F. , Choi , Y. O. , Sun , Y. P. and Eaker , D. W. 1994 . Fluorescence, Fluorescence-Excitation, and Ultraviolet Absorption Spectra of Trans-1-(2-naphthyl)-2-phenylethene Conformers . J. Phys. Chem. , 98 : 35 – 46 . [CSA] [CROSSREF]
- Samuels , A. C. , DeLucia , F. C. Jr. , McNesby , K. L. and Miziolek , A. W. 2003 . Laser-Induced Breakdown Spectroscopy of Bacterial Spores, Molds, Pollens and Protein: Initial Studies of Discrimination Potential . Applied Optics , 42 : 6205 – 6209 . [PUBMED] [INFOTRIEVE] [CSA]
- Scully , M. O. , Kattawar , G. W. , Lucht , R. P. , Opatrny , T. , Pilloff , H. , Rebane , A. , Sokolov , A. V. and Zubairy , M. S. 2002 . FAST CARS: Engineering a Laser Spectroscopic Technique for Rapid Identification of Bacterial Spores . Proc. Nat. Acad. Sci. USA , 99 : 10994 – 11001 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Seaver , M. , Eversole , J. D. , Hardgrove , J. J. , Cary , W. K. Jr. and Roselle , D. C. 1999 . Size and Fluorescence Measurements for Field Detection of Bioaerosols . Aerosol Sci. Technol. , 30 : 174 – 185 . [CSA] [CROSSREF]
- Sorrel , M. J. , Tribble , J. , Reinisch , L. , Werkhaven , J. A. and Ossoff , R. H. 1994 . Bacteria Identification of Otitis Media with Fluorescence Spectroscopy . Lasers Surg. Med. , 14 : 155 – 163 . [CSA]
- Spector , B. C. , Reinisch , L. , Smith , D. and Werkhaven , J. A. 2000 . Non-invasive Fluorescent Identification of Bacteria Causing Acute Otitis Media in a Chinchilla Model . Laryngoscope , 110 : 1119 – 1123 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Sun , Y. P. , Sears , D. F. and Saltiel , J. 1987 . Three-Component Self-Modeling Technique Applied to Luminescence Spectra . Anal. Chem. , 59 : 2515 – 2519 . [CSA] [CROSSREF]
- Vasanthi , S. , Huston , A. L. , Scotto , C. and Eversole , J. D. 2004 . Multiple UV Wavelength Excitation and Fluorescence of Bio Aerosols . Optics Express , 12 : 4457 – 4466 . [CSA] [CROSSREF]
- Yeung , K. Y. and Ruzzo , W. L. 2001 . Principal Component Analysis for Clustering Gene Expression Data . Bioinformatics , 17 : 763 – 774 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]