Abstract
Previous experimental studies have shown that concentrated cigarette smoke particles (CSPs) deposit in the upper airways like much larger 6 to 7 μ m aerosols. Based on the frequent assumption that relative humidity (RH) in the lungs does not exceed approximately 99.5%, the hygroscopic growth of initially submicrometer CSPs is expected to be a relatively minor factor. However, the inhalation of mainstream smoke may result in humidity values ranging from sub-saturated through supersaturated conditions. The objective of this study is to evaluate the effect of condensation particle growth on the transport and deposition of CSPs in the upper respiratory tract under various RH and temperature conditions. To achieve this objective, a computational model of transport in the continuous phase surrounding a CSP was developed for a multicomponent aerosol consisting of water soluble and insoluble species. To evaluate the transport and deposition of dilute hygroscopic CSPs in the upper airways, a model of the human mouth-throat (MT) through approximately respiratory generation G6 was considered with four steady inhalation conditions. These inhalation conditions were representative of inhaled ambient cigarette smoke as well as warm and hot saturated smoke. Results indicate that RH conditions above 100% are possible in the upper respiratory tract during the inhalation of a warm or hot saturated airstream. For sub-saturated inhalation conditions, initial evaporation of the CSPs was observed followed by hygroscopic growth and diameter increases less than approximately 50%. In contrast, the inhalation of warm or hot saturated air resulted in significant particle growth in the MT and tracheobronchial regions. For the inhalation of warm saturated air 3°C above body temperature, initially 200 and 400 nm particles were observed to increase in size to above 3 μ m near the trachea inlet. The upper boundary inhalation condition of saturated 47°C air resulted in 7 to 8 μ m droplets entering the trachea. These results do not prove that the enhanced deposition of CSPs in the upper airways is only a result of condensational growth. However, this study does highlight condensational growth as a potentially significant mechanism in the deposition of smoke particles under saturated inhalation conditions.
INTRODUCTION
Relationships between cigarette smoke constituents and lung disease, respiratory and systemic cancer, and cardiovascular disease have been well established (CitationDoll and Hill 1950; CitationWynder and Graham 1950; WHO 2002; U.S. Department of Health and Human Services 2004; 2006). Considering bronchogenic carcinoma, a number of studies have reported evidence linking local sites of deposited cigarette smoke particles (CSPs) with disease formation (CitationBryson and Spencer 1951; CitationErmala and Holsti 1955; CitationMartonen 1986; CitationMartonen et al. 1987; CitationYang et al. 1989). For example, CitationYang et al. (1989) reported significantly elevated incidents of bronchogenic carcinoma at sites that coincided with previously observed CSP deposition from other studies (CitationBryson and Spencer 1951; CitationErmala and Holsti 1955; CitationMartonen et al. 1987). The review study of CitationMartonen (1986) also discussed a number of qualitative and quantitative comparisons between CSP deposition sites and cancer formation.
The study of CSP deposition in the respiratory airways was initiated nearly a century ago by CitationBaumberger (1923), who reported lung retention values in the range of 74 to 99%. However, after nearly a century of active research, there are still a number of unanswered questions related to the deposition of mainstream and sidestream smoke in the lungs. These questions persist largely due to the complexity of cigarette smoke transport, deposition, and absorption, which are characterized by a potentially dense interacting bolus of droplets, water vapor, and combustion gases moving through the variable and dynamic respiratory airways.
Considering CSPs, a commonly observed inconsistency occurs between the results of typical whole-lung deposition models and experimental observations (CitationIngebrethsen 1989; Martonen 1992; Phalen et al. 1994; Robinson and Yu 2001; Broday and Robinson 2003). The size range of CSPs has been well classified by a number of studies. Briefly, fresh cigarette smoke aerosols have mass median aerodynamic diameters in the range of 0.3 to 0.7 μ m (CitationKeith 1982; CitationDavies 1988; CitationPhalen et al. 1994; CitationBernstein 2004). For particles in this diameter range, traditional whole-lung deposition models typically calculate lung retention based on the mechanisms of sedimentation, impaction, and diffusion. Considering these three combined deposition mechanisms, there is a minimum in deposition efficiency for aerosols approximately 0.5 μ m in diameter. As a result, typical whole-lung models (CitationYeh and Schum 1980; ICRP 1994; NCRP 1997) predict the deposition of a monodisperse 0.5 μ m aerosol to range from 10 to 20% (CitationPhalen et al. 1994). CitationMuller et al. (1990) proposed a whole lung model of CSP deposition that included the effects of hygroscopic particle growth, coagulation, and breathing parameters. Results of this model indicated that CSPs would deposit at a rate of approximately 2% in the tracheobronchial (TB) region and 20% in the pulmonary region for a 0.3 μ m aerosol. In contrast to these models, experimental deposition values of dense cigarette smoke particles in the respiratory airways are significantly higher with regional deposition shifted toward the TB airways (CitationBaker and Dixon 2006).
In a summary of in vivo retention results, CitationBaker and Dixon (2006) reported that dense CSP deposition in the respiratory tract ranged from approximately 40–97%. These estimates are in relative agreement with the total lung retention estimates of CitationHinds et al. (1983), which indicated that dense CSP deposition is in the range of 22–75%. Based on clearance measurements, several studies have reported that dense CSP deposition occurs primarily in the TB region. CitationPritchard and Black (1984) reported that 54% of deposited dense CSPs were in the TB airways with the remaining 35% in the pulmonary region and 15% in the mouth-throat. From comparisons with other particle sizes, CitationPritchard and Black (1984) concluded that CSPs deposit in the respiratory airways like a much larger 6.5 μ m aerosol. Similarly, CitationHicks et al. (1986) reported that 45–81% of deposited smoke particles were in the TB region. In contrast to these results, experiments of CitationMcAughey et al. (1991) indicated that 35% of deposited smoke particles were in the TB airways with 65% in the pulmonary region. However, the TB deposition measurements of CitationMcAughey et al. (1991) remain one order of magnitude greater than the whole-lung predictions of CitationMuller et al. (1990) for this region.
In vitro models of dense CSPs in the extrathoracic and upper TB airways have been used to resolve relatively localized deposition characteristics. CitationErmala and Holsti (1955) evaluated deposition patterns of CSPs in plaster models of the extrathoracic and TB airways. This study reported high concentrations of particle deposition at well-defined sites in the mouth-throat and branching geometry. CitationMartonen et al. (1987) evaluated CSP deposition in respiratory models extending from the oral airway through respiratory generation G6. Deposition was observed to localize at carinal ridges and continue down posterior surfaces of the branches. CitationMartonen (1992) reported similarities in these deposition characteristics with local deposition data reported by CitationSchlesinger et al. (1982) for 6.7 μ m aerosols. CitationPhalen et al. (1994) considered dense sidestream cigarette smoke in TB cast models of adult and child airways. Based on comparisons to algebraic deposition models, CitationPhalen et al. (1994) reported that CSPs deposit similar to much larger 6–7 μ m aerosols in the TB region.
In addition to the traditional mechanisms of aerosol deposition, CSPs may be influenced by two types of phenomena, which can be classified as colligative and non-colligative effects. CitationPhalen et al. (1994) used the term “colligative effects” to describe the unique collective behavior of concentrated aerosols or clouds. These colligative mechanisms may include hydrodynamic interactions of highly concentrated aerosol sets or the trapping of dense gases (CitationMartonen 1992; CitationPhalen et al. 1994). Hydrodynamic interactions may reduce the drag on a collective body of particles by shielding the more centrally located internal elements. The net result is an increased settling velocity and a potential increase in the momentum of the aerosol cloud (CitationFuchs 1964; CitationHinds 1999). In the case of combustion aerosols, the particle cloud may also travel within a local concentration of a specific gas, like carbon monoxide (CitationDavies 1988; CitationPhalen et al. 1994). Non-colligative effects that have been evaluated as potentially important for cigarette smoke include coagulation (CitationRobinson and Yu 1999), particle charge (CitationStober 1984), and hygroscopic growth in the warm and humid respiratory airways (CitationRobinson and Yu 1998).
It is not entirely clear which of the transport mechanisms that may influence dense CSPs are most responsible for the elevated deposition rates observed in the TB region. CitationDavies (1988) suggested that hygroscopic growth in the humid respiratory airways may be responsible for the elevated TB deposition observed by CitationPritchard and Black (1984). In contrast, CitationStober (1984) concluded that electrical charge effects were more significant than hygroscopic growth in the deposition of CSPs. CitationHicks et al. (1986) found that submicrometer NaCl particles, which are known to be highly hygroscopic, deposit in the respiratory airways at values similar to dense CSPs. However, CitationHicks et al. (1986) also reported that exhaled NaCl particles had increased in size by a factor of 4.5 due to hygroscopic growth. In contrast, CSPs only increased in diameter by a factor of 1.7 at exhalation. As a result, CitationHicks et al. (1986) concluded that factors other than hygroscopic growth may be responsible for the elevated deposition rates of smoke aerosols. CitationMartonen (1992) considered a size increase of approximately 1.7 to be insufficient to explain the observed elevated deposition values of dense CSPs. Instead, CitationMartonen (1992) developed an analytic model to test the relative effect of hydrodynamic interactions in a cloud of dense CSPs. It was determined that hydrodynamic interactions of dense CSPs resulted in a cloud with the characteristics of a 25 μ m aerosol. CitationPhalen et al. (1994) evaluated a “characteristic motion” parameter suggested by CitationFuchs (1964) for a typical dense cloud of CSPs. This analysis indicated that hydrodynamic interactions have a significant effect on particle deposition. CitationRobinson and Yu (2001) developed a whole-lung model of dense CSP transport and deposition that included coagulation, hygroscopic growth, particle charge, and a theoretical approximation of cloud motion. Based on this model, the effects of coagulation, hygroscopic growth, and particle charge were estimated to increase total deposition by 16%. In contrast, cloud motion was shown to shift deposition to the tracheobronchial region and increase total deposition by 36%. CitationBroday and Robinson (2003) developed a unique model for CSP cloud motion that accounted for the concentration dependent hydrodynamic interactions of the surrounding aerosol on the mobility of individual particles. This approach was integrated into a whole-lung transport model for cigarette smoke and showed that a combination of cloud behavior, hygroscopic growth, and coagulation may explain elevated CSP deposition in the TB region.
Based on the collective previous studies of CSPs, hygroscopic growth does not appear to be a significant mechanism responsible for elevated TB deposition. This conclusion is supported by in vivo and in vitro evidence. CitationHicks et al. (1986) reported that inhaled dense mainstream CSPs only increased in size by a factor of 1.7 at exhalation. However, size change during the initial inhalation phase could not be assessed in the in vivo experiments of CitationHicks et al. (1986). In addition, a number of in vitro studies have assessed the hygroscopic growth of individual CSPs. For example, CitationLi (1993) and CitationLi and Hopke (1993) considered the hygroscopic growth of mainstream and sidestream CSPs at a relative humidity (RH) of approximately 99.5%, which was assumed to represent lung conditions. CitationLi and Hopke (1993) reported an average growth ratio of 1.54 for mainstream CSPs with initial diameters ranging from 0.15–0.40 μ m. Sidestream CSPs were shown to grow by an average factor of 1.36 with initial diameters of 0.05–0.350 μ m. CitationIshizu et al. (1980) reported CSP growth ratios as a function of RH values below the saturated condition. Particle size was shown to increase dramatically due to hygroscopic growth as the RH approached 100%. Similarly, a number of other studies have reported that CSPs do not grow at RH values below 95% and reach an equilibrium growth ratio of 1.4 to 1.7 as the RH approaches 100% (CitationRobinson and Yu 1998; CitationRobinson and Yu 2001). However, the growth of CSPs at RH values significantly above 100% remains unclear.
In previous studies, it was often assumed that the maximum RH in the respiratory airways is approximately 99.5 to 100%. Based on this assumption, the reported growth ratios for CSPs appear insufficient to result in significant increases in TB deposition. However, it may be possible for RH values to exceed saturation conditions in the upper respiratory tract under certain conditions. For example, CitationFerron et al. (1984) and CitationSarangapani and Wexler (1996) have shown that supersaturation in the upper respiratory tract can be achieved during the inhalation of cool humid air, based on heat and mass transfer calculations. In a different scenario, supersaturated conditions may also be achieved in the upper respiratory tract through the direct inhalation of cigarette smoke. During active smoking, a potentially warm bolus of air, water vapor, combustion gases, and CSPs is inhaled. The temperature of this bolus may range from ambient conditions to above body temperature, depending on cigarette length, environment, inhalation rate, and the amount of dilution air provided by ventilation. One study reported that gases in the filter of an unventilated cigarette can reach 80°C (CitationWoodman et al. 1984). It is also reasonable that an inhaled bolus of cigarette smoke is saturated due to the combustion of organic material and cooling in the cigarette. This inhaled humid bolus will be further cooled in the mouth, resulting in potentially supersaturated conditions. Provided that RH values remain below approximately 400–500%, the supersaturated vapor will condense onto existing aerosols (i.e., heterogeneous nucleation) and airway walls (CitationHinds 1999). As a result, inhaled cigarette smoke may produce temporary supersaturated vapor conditions in the upper airways. Aerosols in this environment will continue to grow until the relative humidity is decreased below saturation conditions by dilution and absorption.
In summary, a number of studies have explored the relative roles of potential factors that may explain the elevated TB deposition of dense mainstream or sidestream CSPs. Based on the common assumption that RH in the lungs does not exceed 99.5%, hygroscopic growth is expected to be a relatively minor factor (CitationMartonen 1992; CitationPhalen et al. 1994; CitationRobinson and Yu 1998; CitationRobinson and Yu 2001). However, RH and temperature profiles in the mouth-throat and upper TB airways are transient functions of the inhaled conditions. The inhalation of a warm and humid bolus of cigarette smoke may result in supersaturated conditions and significant condensational growth of aerosol droplets.
The objective of this study is to consider the effect of condensation particle growth on the transport and deposition of CSPs in the upper respiratory tract under various RH and temperature conditions. To achieve this objective, a computational model of the transport surrounding a CSP is developed based on the approach of CitationFerron (1977), CitationRobinson and Yu (1998), and CitationLongest and Kleinstreuer (2005) for a multicomponent aerosol consisting of water soluble and insoluble species. The performance of this hygroscopic growth model is compared with the in vitro CSP data of CitationLi and Hopke (1993) and CitationIshizu et al. (1980). The multiple component hygroscopic growth model is then integrated into a CFD particle tracking routine. To evaluate transport and deposition of dilute hygroscopic CSPs in the upper TB airways, a model of the human mouth-throat through approximately respiratory generation G6 is considered with four steady inhalation conditions. These inhalation conditions are representative of inhaled unsaturated and saturated cigarette smoke. The associated temperature and RH fields in the airways are assessed and used to determine the evaporation, condensational growth, and deposition of the individual CSPs.
METHODS
Upper Airway Geometry
To evaluate the effects of condensational growth on hygroscopic cigarette smoke aerosols, realistic models of the mouth-throat (MT) and upper tracheobronchial (TB) regions were constructed based on the dimensions of an average adult male. The realistic MT geometry used in the upper airway model was originally developed by CitationXi and Longest (2007). This geometry was based on a dental impression cast of the mouth reported by CitationCheng et al. (1997b) joined with a medical image-based model of the pharynx and larynx. Further details of the MT geometry used in this study, including the construction procedure and critical dimensions, were previously described in the study of CitationXi and Longest (2007). The TB geometry used in this study was developed from a cast model of a middle age adult male. The MT and TB geometries were then smoothly connected in the region of the upper trachea (). The distance between the glottic aperture and main bifurcation was verified to be within reported average values (ICRP 1994). Further details of the computational TB model are described below.
FIG. 1 Surface model of the upper respiratory tract including the oral airway, larynx, and upper tracheobronchial region as viewed from the (a) front and (b) back. The surface model extends through respiratory generation G6 along some paths.
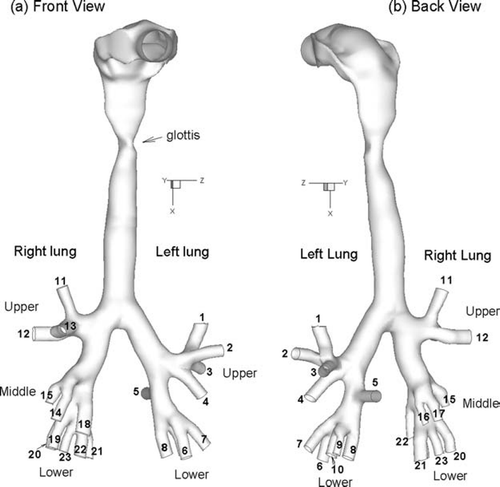
The TB geometry was based on a hollow cast model used in the in vitro deposition study of CitationCohen et al. (1990). The geometric parameters of the cast were in agreement with population means of an average adult male. The original cast used in the study of CitationCohen et al. (1990) was scanned by a multirow-detector helical CT scanner. The multi-slice CT images were then imported into MIMICS (Materialise, Ann Arbor MI) to convert the raw image data into a set of cross-sectional contours that define the solid geometry. Based on these contours, a surface geometry was manually constructed in Gambit 2.3 (Ansys, Inc.). Some distal bronchi were trimmed away due to a lack of resolution from the scan data. As a result, some distal branches in the range of generations G5 and G6 were not retained in the digital model. Most of the digital model paths extend from the trachea to generation G4 with some paths extending to generations G5 and G6. Twenty-three outlets and a total of 44 bronchi were retained in the final computational model. The surface geometry was then imported into ANSYS ICEM 10 (Ansys, Inc.) as an IGES file for meshing. To avoid excessive grid elements, some minor smoothing of the geometric surface was necessary, which resulted in diameter changes of less than 1%.
Flow Rates and Boundary Conditions
To assess the potential for condensational growth in the upper airways, a single steady inhalation flow rate of 34 L/min was considered. This inhalation flow rate provides a representative value that is consistent with the transient inhalation of mainstream (CitationFeng et al. 2006) and sidestream (ICRP 1994; CitationPhalen et al. 1994) cigarette smoke. The associated mean inlet and maximum laryngeal Reynolds numbers are 1,945 and 4,311, respectively. As a result, laminar through turbulent flow fields and a significant effect of turbulent particle dispersion are expected in the upper airway geometry. It is assumed that inhaled ambient air or mainstream smoke will enter the MT geometry with a relatively blunt velocity profile, which can be defined as
A puff of inhaled cigarette smoke is reported to contain approximately 70% ambient air, 17% gaseous species from combustion (including water vapor), 8% particulate matter (including condensed water), and 5% miscellaneous vapors (CitationJenkins et al. 1979; CitationBroday and Robinson 2003). In order to approximate the potential for condensational growth, inhaled components considered in this study include air, water vapor, and dilute concentrations of CSPs. The assumption of a dilute droplet concentration implies that the removal of water vapor from the airstream due to condensation is not included. The amount of water vapor in the inhaled air is based on representative relative humidity values, as described below. The assumption of a dilute droplet phase has been made in this study in order to approximate the maximum possible condensational growth for initially monodisperse particles and to eliminate the potential for dense aerosol effects. The inclusion of concentration density for actual mainstream (MS) cigarette smoke is reported to have two effects. First, concentrated MS cigarette smoke particles are reported to exhibit colligative behavior, which can significantly enhance deposition (CitationMartonen 1992; CitationPhalen et al. 1994; CitationRobinson and Yu 1999). The second expected effect of dense cigarette smoke is a limit to the amount of water vapor that is available for absorption on the particles. As a result, condensational growth may be rate limited due to the rapid removal of water vapor, as described in detail by CitationFinlay (1998) and CitationFinlay and Stapleton (1995). Therefore, the assumption of a dilute one-way coupled discrete phase is used in this study to isolate condensation effects and to approximate the maximum possible growth due to condensation for initially monodisperse particles. It is noted that approximating the maximum possible condensational growth in this study does not necessarily imply maximum potential deposition due to a complex interplay between the relevant transport mechanisms (CitationBroday and Georgopoulous 2001; CitationBroday and Robinson 2003).
Cigarette smoke and other hygroscopic aerosols may enter the mouth at a variety of temperature and relatively humidity conditions. For example, secondhand sidestream (SS) smoke is often inhaled at ambient conditions. Considering MS smoke, CitationKeith and Tesh (1965) reported the relative humidity (RH) of diluted MS smoke to be 60% at a temperature of 27°C. In contrast, CitationWoodman et al. (1984) reported that the temperature in a filter of an unventilated cigarette was a function of distance to the burning tip and could be as high as 80°C. The mean filter temperature reported by CitationWoodman et al. (1984) was approximately 47°C. Furthermore, it is expected that the combustion and cooling of organic compounds will create saturated water vapor conditions at the mouth inlet.
In this study, a range of inhaled relative humidity and temperature conditions was selected to characterize the potential for condensation droplet growth. These boundary conditions are used to determine the upper airway relative humidity and temperature fields by solving the appropriate conservation of mass and energy equations in the geometry. The spectrum of inhalation conditions include sub-saturated and saturated approximations. Specifically, four inhalation boundary conditions were considered, which are described in . The first condition (Case 1) represents the inhalation of cool ambient secondhand or sidestream (SS) smoke. In this sub-saturated case, initial evaporation of existing water from the particles is expected. Water evaporation from the airway walls will be required to raise the RH condition and induce hygroscopic growth. The second case represents the inhalation of warm ambient SS smoke with a RH of 60%. The third and fourth cases represent the inhalation of warm and hot saturated MS smoke, respectively. The inhalation of 40°C air and water vapor (Case 3) represents a condition that is only 3°C above body temperature. The inhalation of 47°C air and water vapor (Case 4) provides an upper temperature boundary condition to evaluate the maximum extent of condensational growth, which was selected as 10°C above body temperature. This temperature condition is the approximate midpoint filter temperature measured by CitationWoodman et al. (1984). With both of the saturated MS conditions, cooling in the MT and upper TB regions will result in supersaturated RH flow fields and accelerated condensational growth of the dilute droplets. As indicated above, the assumption of a dilute aerosol was made in this study for both MS and SS smoke to evaluate the maximum extent to which condensational growth can influence droplet transport and deposition.
TABLE 1 Initial temperature and relative humidity conditions at the mouth inlet ranging from sub-saturated to saturated conditions
In the body, the upper TB airway surfaces are lined with a thin layer of protective mucus that has an approximate thickness of 10–20 μ m (ICRP 1994). Considering airflow dynamics, the mucus lining was neglected in this study and a rigid wall assumption was applied. Aerosols were assumed to deposit at initial contact with the wall. Surface temperature of the airway model was assumed to be constant and equal to mean body conditions, i.e., 37°C. This approximate surface temperature represents an upper boundary value. Evaporative cooling of the airway surfaces may lower the surface temperature by a small amount. The reduced surface temperature may in turn increase the RH of the airstream. As a result, the assumption of a constant surface temperature provides a conservative estimate of airway relative humidity conditions. The mucus lining the airways was assumed to be isotonic with a surface relative humidity in the gas phase of 99.5%. This RH condition translates to a surface mass fraction of water vapor Y w,surf = 3.9 × 10− 2 for a surface temperature of 37°C.
Outlets of the TB geometry were extended approximately 10 diameters downstream such that the velocity was normal to the outlet planes (i.e., nearly developed flow profiles with no significant radial velocity component). The experimentally determined mass flow distribution of CitationCohen et al. (1990) was applied as the outflow boundary condition, which is provided in for outlets denoted in . CitationMa and Lutchen (2006) studied oscillating respiratory flows and showed that a mass flow outlet distribution provided a reasonable estimate in comparison to a significantly more complex downstream impedance model. These outlet conditions represent a first order approximation to the highly complex process of respiratory ventilation. Based on the applied boundary conditions, approximately 40% of the airflow enters the left lung and 60% enters the right, which is consistent with previous in vivo observations and whole-lung models (CitationHorsfield et al. 1971).
TABLE 2 Name, cross-sectional area, and flow ratio of the outlet bronchi
Continuous Phase Transport Equations
Based on Reynolds number conditions, laminar through fully turbulent flow is expected in the upper airway model. The transition of the flow regime from laminar to turbulent has been widely reported in the TB tree within normal physiological flow ranges (CitationPedley 1977). Moreover, CitationChan et al. (1980) indicated that the critical flow for the onset of turbulence was reduced by 1/3 to 1/4 if a respiratory cast was preceded by a larynx. To resolve these multiple flow regimes, the low Reynolds number (LRN) k-ω model was selected based on its ability to accurately predict pressure drop, velocity profiles, and shear stress for transitional and turbulent flows (CitationGhalichi et al. 1998). This model was demonstrated to accurately predict particle deposition profiles for transitional and turbulent flows in models of the oral airway (CitationXi and Longest 2007; CitationXi and Longest 2008) and multiple bifurcations (CitationLongest and Vinchurkar 2007b).
For laminar and turbulent flow, the Reynolds-averaged equations governing the conservation of mass and momentum are (CitationWilcox 1998)
The transport of water vapor in a turbulent flow field is governed by a convective-diffusive mass transfer relation (CitationBird et al. 1960)
To determine the temperature field in the upper airway model, the constant property thermal energy equation is expressed as
In this conservation of energy statement, C p is the constant specific heat, κ g is the gas conductivity, and Pr T is the turbulent Prandtl number, which is taken to be Pr T = 0.9. The enthalpy of each species is represented as h s , and the two species are air and water vapor. On the right-hand-side of Equation (Equation5), the first term represents conductive transport due to molecular and turbulent mechanisms while the second term accounts for energy transport due to species diffusion. This species diffusion term accounts for energy gain and loss from the flow field during condensation and evaporation, respectively.
Considering variable flow field properties, the binary diffusion coefficient of water vapor in air has been calculated from (CitationVargaftik 1975)
The temperature dependent saturation pressure of water vapor is determined from the Antoine equation (CitationGreen 1997)
The relative humidity of the mixture entering the upper airway model is dependent on the local temperature and mixture density. The local relative humidity will influence water vapor evaporation and condensation on the surface of droplets and on the walls of the geometry. Relative humidity of the ideal gas mixture can be expressed
The mass flux of water vapor at the wall depends on the local RH conditions. For near-wall RH values greater than 1, condensation onto the wall surface occurs. If the near-wall RH is less than one, then evaporation from the surface is assumed. The mass flux from the near-wall location to the surface is calculated as
Discrete Phase Transport Equations
The condensational growth of initially submicrometer CSPs in the respiratory tract is expected to result in a polydisperse distribution of aerosols ranging in size from approximately 200 nm up to potentially 10 μ m. Based on inlet conditions at the mouth and an inhalation flow rate of 34 L/min, the Stokes number (St = ρ p d p 2 C c U/18μ D) of these aerosols can range from approximately 1.5 × 10−5 to 1.8 × 10−2. Other characteristics of the aerosols considered included an assumed droplet density ρ p = 1.00 g/cm3, a density ratio α = ρ/ρ p ≈ 10−3, and a droplet or particle Reynolds number (Rep = ρ/u−v| d p/μ) below 1. Fresh MS smoke is reported to have a particle concentration density of approximately 3 × 109 part/cm3 (CitationPhalen et al. 1994). Analytical assessments by CitationPhalen et al. (1994) and CitationMartonen (1992) have indicated that this concentration density is sufficient to induce colligative effects and increase the deposition of the aerosol cloud. However, colligative effects are neglected in this study in order to isolate and highlight the potential role of condensational growth in deposition. Moreover, the effect of water vapor loss due to condensation onto the particles has been ignored. As a result, the effects of the droplets on the continuous phase have been neglected in this study, resulting in one-way coupled multiphase flow.
In the upper respiratory tract, the transport and deposition of submicrometer droplets are expected to be the result of inertial and diffusional effects. Droplets greater than approximately 1 μ m will deposit primarily by impaction and sedimentation. Turbulent fluctuations will also significantly affect the deposition of aerosols throughout the size range considered. To address this broad range of deposition mechanisms, a Lagrangian particle tracking method was employed (CitationLongest et al. 2004). The Lagrangian transport equations for particles ranging from 100 nm through 10 μ m can be expressed as
Here v i and u i are the components of the particle and local fluid velocity, respectively, and g i denotes gravity. The characteristic time required for a particle to respond to changes in fluid motion, or the particle relaxation time, is expressed as τ p = C c ρ p d p 2/18μ, where C c is the Cunningham correction factor for submicrometer aerosols. The pressure gradient or acceleration term for aerosols was neglected due to small values of the density ratio (CitationLongest et al. 2004). The drag factor f, which represents the ratio of the drag coefficient to Stokes drag, is based on the expression of CitationMorsi and Alexander (1972)
To model the effects of turbulent fluctuations on particle trajectories, a random walk method was employed (CitationGosman and Ioannides 1981; CitationCrowe et al. 1996; CitationMatida et al. 2000). This method assumes that the fluid velocity used in Equation (Equation10) is constant during the time that a particle spends in an eddy and is taken as
To determine the fluctuating component of the instantaneous velocity, u′ i is selected from a Gaussian distribution with a variance of 2k/3. The time that the particle spends in the eddy is the minimum of the eddy crossing time and the random eddy lifetime. The primary limitation of this eddy interaction model is that it does not account for reduced turbulent fluctuations in the wall-normal direction, which may result in an over-prediction of deposition (CitationKim et al. 1987; CitationMatida et al. 2000; CitationMatida et al. 2004). To better approximate turbulent effects on particle deposition, an anisotropic turbulence model has been applied in this study where the near-wall fluctuating velocity is calculated as (CitationWang and James 1999; CitationMatida et al. 2004)
The effect of Brownian motion on the trajectories of submicrometer particles has been included as a separate force per unit mass term at each time-step. This force has been calculated as
Droplet Evaporation and Condensation Model
A distinction can be made between droplet growth due to condensation and hygroscopic growth. Droplet growth due to condensation, or heterogeneous nucleation, is the general phenomena arising from RH values greater than 100% (CitationHinds 1999). A special case of condensational growth arises from droplets that contain a soluble component. Inclusion of the water soluble species reduces the water vapor pressure on the droplet surface, which allows condensation to take place at RH values below saturation conditions. Condensation onto a droplet at RH values below 100% due to a reduction in surface vapor pressure is referred to as hygroscopic growth in this study.
The evaporation and condensation model employed for CSPs is based on previous approximations for salts (CitationFerron 1977; CitationFerron et al. 1988; CitationHinds 1999), CSPs (CitationLi and Hopke 1993; CitationRobinson and Yu 1998), and multicomponent evaporating liquid droplets in the respiratory tract (CitationLongest and Kleinstreuer 2005). In this model, CSPs are assumed to consist of liquid water, water soluble components, and non-soluble components. The dissolution of water soluble components creates the potential for hygroscopic growth at RH values less than 100%. The associated reduction in surface vapor pressure will also increase the rate of condensational growth at RH values greater than 100%. In this volatilization model, only water vapor is assumed to evaporate and condense at the droplet surface. As a result, the remaining soluble and non-soluble components are assumed to be non-volatile. The adequacy of these assumptions will be assessed based on comparisons to existing in vitro data for CSP hygroscopic growth.
Conservation of energy for an immersed droplet, indicated by the subscript d, under rapid mixing model (RMM) conditions can be expressed (CitationLongest and Kleinstreuer 2005)
Conservation of mass for an immersed droplet based on the evaporating flux can be expressed as
For a semi-empirical RMM solution, the area-averaged heat flux is evaluated from
In this expression, Kn is the Knudsen number, defined as Kn = 2λ/d p , where λ is the mean free path of air. The factor α T is an accommodation coefficient, which is assumed to be unity (CitationHinds 1999; CitationFinlay 2001).
Considering mass transfer, the area-averaged mass flux is
The concentration of water vapor on the droplet surface can be approximated using Raoult's law for dilute fractions of soluble components. Cigarette smoke particles have been reported to contain up to 25% liquid water by mass (CitationNorman 1977) and to have a mass ratio of soluble to insoluble components of approximately 60% (CitationLi and Hopke 1993) (). As a result, condensed species in CSPs are not dilute on a mass basis. However, the molecular weight of CSPs can be approximated as 450 kg/kmol (). The associated water mole fraction of CSPs is then 93%. Therefore, Raoult's law provides a reasonable approximation for the surface concentration of water vapor on the droplet surface and can be expressed as
In the above expression, P v,sat (T d ) is the temperature dependent saturation pressure of water vapor, calculated from Equation (Equation7). The mole fraction of liquid water (X w ) is based on the soluble component and expressed as
TABLE 3 Initial properties of cigarette smoke particles (CSPs) for mainstream (MS) and sidestream (SS) conditions
The non-dimensional Nusselt and Sherwood numbers employed in Equations (Equation16) and (Equation18) for droplet surface heat and mass transfer are based on the empirically derived expressions of CitationClift et al. (1978)
Numerical Method
To solve the governing mass and momentum conservation equations in each of the cases considered, the CFD package Fluent 6 was employed. User-supplied Fortran and C programs were used for the calculation of initial flow and particle profiles, particle evaporation and condensation, near-wall anisotropic turbulence approximations, near-wall particle interpolation (CitationLongest and Xi 2007), Brownian motion (CitationLongest and Xi 2007), and deposition enhancement factors. All transport equations were discretized to be at least second order accurate in space. For the convective terms, a second order upwind scheme was used to interpolate values from cell centers to nodes. The diffusion terms were discretized using central differences. A segregated implicit solver was employed to evaluate the resulting linear system of equations. This solver uses the Gauss-Seidel method in conjunction with an algebraic multigrid approach. The SIMPLEC algorithm was employed to evaluate pressure-velocity coupling. The outer iteration procedure was stopped when the global mass residual had been reduced from its original value by five orders of magnitude and when the residual-reduction-rates for both mass and momentum were sufficiently small. To ensure that a converged solution had been reached, residual and reduction rate factors were decreased by an order of magnitude and the results were compared. The stricter convergence criteria produced a negligible effect on both velocity and particle deposition fields. To improve accuracy, cgs units were employed, and all calculations were performed in double precision.
For the upper airway model, a tetrahedral grid with near-wall wedge shaped elements was generated using ANSYS ICEM 10 software. A sufficiently dense mesh was applied in the near-wall region throughout the geometry so that the y+ values of the LRN k-ω model were maintained on the order of approximately 1 or less at the first grid point above the wall. Considering grid convergence, meshes consisting of approximately 650,000, 980,000, and 1,400,000 cells were tested. Negligible variations in the parameters of interest, i.e., the velocity field, particle condensational growth, and local particle deposition efficiencies, were observed between the two finest meshes considered. As a result, the final mesh of the upper airway model contained approximately 980,000 cells.
Particle trajectories were calculated within the steady flow fields of interest as a post-processing step. The integration scheme employed to solve Equation (Equation10) was based on the Runge-Kutta method with a minimum of 20 integration steps in each control volume. An error control routine was also employed to actively adapt the particle time-step and maintain sufficient accuracy bounds (CitationLongest et al. 2004). Doubling the number of integration steps within each control volume had a negligible (less than 1%) effect on cumulative particle deposition and growth values.
Deposition Factors
The deposition fraction (DF) is defined as the ratio of particles depositing within a region to the number of particles entering the geometry. The aerosol deposition efficiency (DE) is defined as the ratio of particles depositing within a region to the particles entering that region. The sum of local or sectional DF values gives the total or cumulative DF for an entire region. Localized deposition can be presented in terms of a deposition enhancement factor, which quantifies local deposition as a ratio of the total or regional deposition. A deposition enhancement factor (DEF), similar to the enhancement factor suggested by CitationBalashazy et al. (1999), for local region j can be defined as
Deposition Model Testing
Previous studies have validated the Lagrangian tracking approach for the deposition of micrometer particles in the realistic MT geometry (CitationXi and Longest 2007) and bifurcating airway models (CitationLongest and Oldham 2006; CitationLongest et al. 2006; CitationLongest and Vinchurkar 2007b). Furthermore, CitationLongest and Xi (2007) showed that Brownian motion and near-wall interpolation modifications to the particle tracking code of Fluent 6 are necessary to approximate the deposition of submicrometer particles in a curved tube and MT geometry. In the current study, results of the Lagrangian tracking algorithm are compared with existing experimental results for the deposition of submicrometer aerosols in the MT and TB regions. Validations of the condensational growth model applied to individual droplets are presented in the next section. The deposition of submicrometer particles in the MT geometry based on Lagrangian particle tracking is compared with the in vitro results of CitationCheng et al. (1993; Citation1997a) in . The numerical MT geometry used in this comparison is based on the in vitro model and was implemented in the upper airway geometry shown in . For flow rates of 4 and 10 L/min, predictions of the CFD model are observed to match the in vitro conditions to a high degree across a range of submicrometer particle sizes.
FIG. 2 Comparison of submicrometer particle deposition with existing experimental results for the (a) mouth-throat (MT) geometry, and (b) tracheobronchial (TB) region.
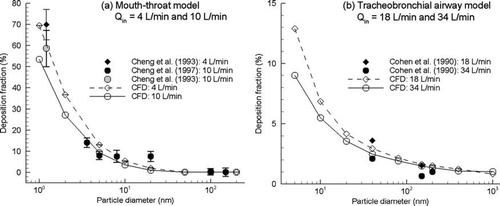
The deposition of submicrometer particles in the TB geometry is compared to the in vitro results of CitationCohen et al. (1990) in . For this comparison, the numerical TB geometry including the larynx is a reproduction of the experimental model. Dilute submicrometer aerosols consistent with CSPs are considered at inhalation flow rates of 18 and 34 L/min. It is observed that the Lagrangian tracking model agrees with the in vitro results for both of the flow rate conditions considered. The use of user-defined BM and near-wall interpolation routines, as described by CitationLongest and Xi (2007), and near-wall anisotropic corrections were necessary to achieve agreement with the experimental data.
RESULTS
Hygroscopic Growth of Individual Particles
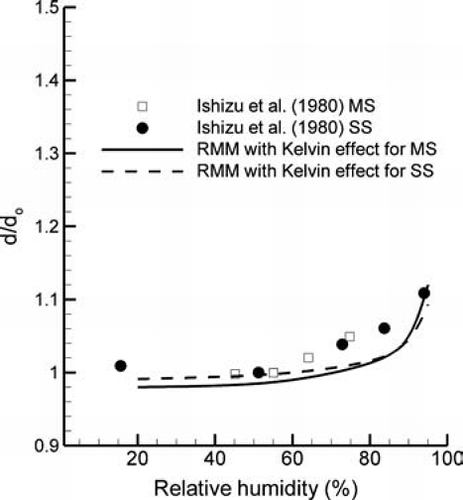
Results of the condensational growth model for CSPs have been compared with the experimental data sets of CitationLi and Hopke (1993) and CitationIshizu et al. (1980). Ratios of predicted particle diameter change (d/d o ) across a range of initial particle sizes (d o ) are shown in comparison with the experiential data of CitationLi and Hopke (1993) in for a RH of 99.5%. The properties used for MS and SS CSPs are given in . For both MS and SS particles, predictions of the rapid mixing model (RMM) appear to match the trend of the experimental data with increasing growth ratios for larger initial particle sizes. However, predictions at a RH of 99.5% are approximately 12% to less than 1% below the experimental values. The result of neglecting the Kelvin effect on hygroscopic growth is also shown in . As expected, including the Kelvin effect reduces the total growth ratio of smaller particles. As the particle increases in size, the competing influences of the Kelvin effect, hygroscopic properties, and dilution reach an equilibrium resulting in the observed steady state particle diameters. The non-continuum factors described by Equation (Equation17) affect the rate of hygroscopic growth but do not influence the final equilibrium particle size.
FIG. 3 Equilibrium diameter change vs. initial particle size (d o ) due to hygroscopic growth for mainstream (MS) and sidestream (SS) smoke particles at relative humidity (RH) values of (a) 99.5% and (b) 99.75%.
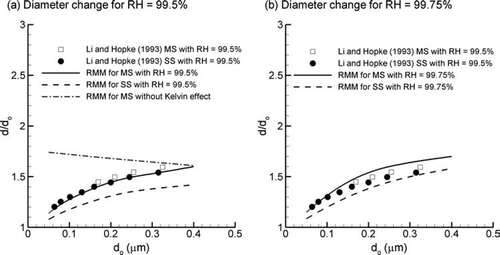
The experimental results of CitationLi and Hopke (1993) at a RH of 99.5% are compared with the numerical results with a RH of 99.75% in . As shown in the figure, a minor increase in the RH of 0.25% results in an improved agreement between the experimental and numerical data for both MS and SS particles across a wide range of initial sizes. The results of CitationLi and Hopke (1993) do not report experimental errors in the RH measurements. However, these measurements are expected to become less precise in the range of 100%. It is expected that a 0.25% increase in RH is within the bounds of experimental error for RH values near saturation conditions. The condensational growth model appears to provide a reasonable estimate of the size increase of CSPs. Furthermore, these results indicate that a relatively minor change in the RH conditions (i.e., 0.25%) can have a noticeable impact on the hygroscopic growth of CSPs.
To further evaluate the effect of RH on the condensational growth of CSPs, numerical results are compared with the experimental data of CitationIshizu et al. (1980) in . Based on the experimental data for MS and SS particles, relatively little growth is observed for RH values below 60%. In contrast, equilibrium particle growth ratios increase exponentially as the RH approaches 100%. Results of the numerical RMM appear to match the experimental data to a high degree across the range of RH values considered (). Furthermore, relatively little difference is observed in the growth ratios of MS and SS particles for both the experimental results and model predictions.
The time histories of CSP size change for condensation and evaporation across a range of RH conditions are shown in and , respectively. The initial particle size used in these simulations was 400 nm. Considering hygroscopic size change at RH values less than 100%, a majority of the observed growth occurs within the first 0.005 s (). Furthermore, size change is observed to increase non-linearly as the RH increases above 99.5%. For a RH of 101%, an equilibrium particle size is not attained and condensation particle growth will continue indefinitely. Considering the 0.02 second period shown in , the diameter growth ratio for a RH of 101% is observed to be approximately 4. The mean particle residence time through the MT and TB geometries is approximately one order of magnitude greater than the 0.02 s time scale shown in . As a result, significant particle size change is expected for relatively minor supersaturation conditions.
FIG. 5 Time history of CSP size change as a function of RH for (a) condensational growth and (b) evaporation.
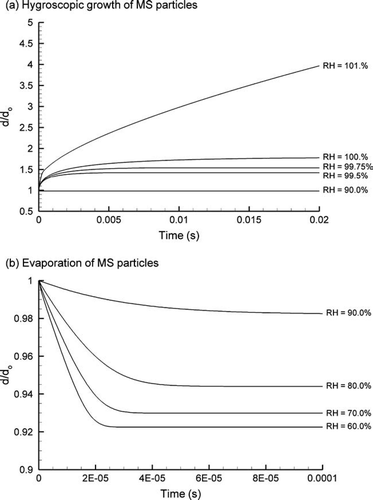
An evaluation of CSPs at RH values below saturation conditions is shown in . These predictions are based on the evaporation of water vapor with the assumption that the droplet initially contains 25% liquid water. In comparison with condensational growth, evaporation at sub-saturated conditions appears to be a more rapid process. Relatively little evaporation is observed at a RH of 90%. However, for RH values of 80% and below, the droplets evaporate very quickly to a steady state condition, i.e., within 4 × 10−5 seconds. It is noted that some liquid water remains in the droplet due to the attraction of the soluble components for all sub-saturated RH conditions considered.
Temperature and Relative Humidity Conditions in the Upper Airways
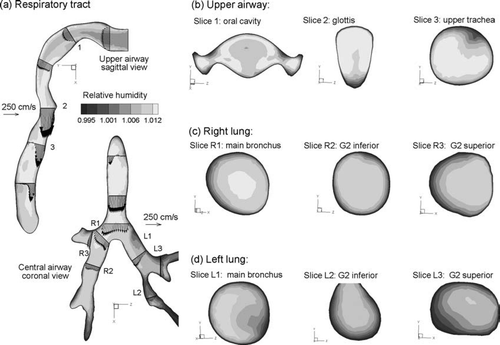
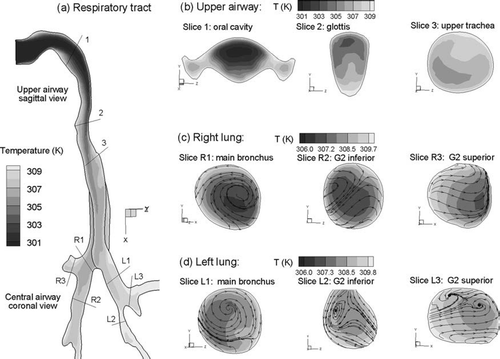
Steady state temperature fields in the upper airway model for sub-saturated Case 2 conditions (Tinlet = 27°C and RHinlet = 60%) are shown in . Considering the midplane view of the MT and TB regions (), mean temperatures in the distal region are observed to approach the constant wall temperature of 37°C (310 K). The temperature is above 307 K at the first carina and typically above 309 K in the second bifurcations. This steady progression of temperature increase is further illustrated in the cross-sectional slices of , , . Specifically, temperatures approach 310 K in the lateral low flow regions of the oral cavity shown in Slice 1 (). Enhanced convection in the posterior glottic region results in a lower temperature in comparison to the anterior glottic region (Slice 2). In both the right and left lungs, progressively increasing temperature profiles are observed after each bifurcation ( and ). To illustrate the effects of secondary velocity motion on temperature profiles, two-dimensional streamtraces are shown in the TB cross-sectional slices. These streamtraces are observed to be highly non-uniform as a result of the complex vortical flow patterns, which enhance the transport of thermal energy and water vapor.
FIG. 6 Steady state temperature profiles for inhalation Case 2 (Tinlet = 27°C, RHinlet = 60%): (a) in the midplane, and at selected cross-sectional slices of the (b) mouth-throat, (c) right lung, and (d) left lung.
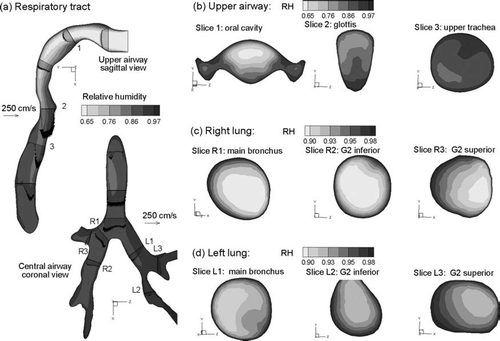
The relative humidity conditions associated with the temperature profiles for inhalation Case 2 (Tinlet = 27°C and RHinlet = 60%) are shown in . Based on and , the growth of CSPs is expected for RH approximately greater than 90%. Considering the midplane slices of the MT and TB regions (), RH profiles greater than 90% are observed near the wall and just downstream of the main bronchi. Cross-sectional patterns of RH appear similar to the corresponding temperature profiles for Case 2 conditions (, , vs. , , ). Interestingly, RH values appear significantly lower in the right lung compared with the left. This asymmetrical result is attributed to an increased flow distribution to the right lung (60%) and the associated decrease in fluid residence time available for heat transfer from the wall ().
FIG. 7 Steady state RH profiles for inhalation Case 2 (Tinlet = 27°C, RHinlet = 60%): (a) in the midplane, and at selected cross-sectional slices of the (b) mouth-throat, (c) right lung, and (d) left lung.
Temperature conditions for Case 3 (Tinlet = 40°C and RHinlet = 100%) are shown in . For this inhalation condition, initially warm saturated air is cooled due to the lower wall temperature of 37°C (310 K). Rapid mixing in the MT and trachea significantly reduces the airstream temperature to approximately 311 K near the first carina (). Further temperature reductions are observed in the main and lobar bronchi as the mean temperature approaches the wall boundary condition of 310 K. Cross-sectional patterns of temperature profiles in the MT and TB regions are similar to those observed in Case 2, with the regions of high and low temperature reversed due to the cooling effect of Case 3 (, , vs. , , ). However, closer inspection of the cross-sectional temperature patterns reveals some differences between Cases 2 and 3. For example, secondary motion appears to skew the temperature profiles at Slices R1 and R2 for Case 2 more than with Case 3. Considering secondary streamlines, significant differences are observed between the vortical flow patterns of Case 2 (, ) and Case 3 (, ). As a result, it appears that a temperature difference in inhalation conditions can significantly impact the secondary flow field characteristics in the upper TB region.
FIG. 8 Steady state temperature profiles for inhalation Case 3 (Tinlet = 40°C, RHinlet = 100%): (a) in the midplane, and at selected cross-sectional slices of the (b) mouth-throat, (c) right lung, and (d) left lung.
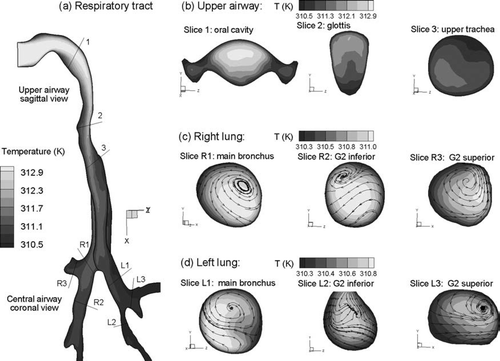
Steady state relative humidity conditions for Case 3 (Tinlet = 40°C and RHinlet = 100%) are shown in . The mid-plane slice of the MT region shows an inhaled profile of saturated air (). Cooling in the MT is then observed to increase the RH to supersaturated conditions greater than 101%. This level of supersaturation is observed to persist through a major portion of the trachea. However, absorption of the supersaturated water vapor onto airway walls acts to reduce the RH values in the deeper TB region. Reduced supersaturated conditions below 101% are observed near the first carina and persist through the main bronchi. Sub-saturated conditions are not observed until the more distal bifurcations. Cross-sectional slices provide further details regarding the RH conditions throughout the MT and TB regions (, , ). In the MT, a significant portion of the cross-sectional flow field is observed to have RH values greater than 101% (). Supersaturated conditions are then observed to persist through a majority of the flow field into the main and lobar bronchi of the left and right lungs ( and ).
Particle Transport, Growth, and Deposition
The effects of evaporation and condensation on inhaled monodisperse 200 nm particles for the four inhalation conditions are illustrated in . The aerosol considered was assumed to be MS smoke particles with initial hygroscopic conditions as shown in . For the initially sub-saturated conditions of Case 1, the particles are observed to quickly evaporate to below 170 nm in some cases (). As the RH of the airstream increases due to mass transfer from the airway walls, some hygroscopic growth occurs near the outlets for Case 1 conditions. Particles are observed to reach approximately 220 nm prior to exiting the geometry (). Considering the sub-saturated conditions of Case 2, evaporation is again observed to initially occur in the upper MT region (). However, increased RH values associated with mass transfer from the walls result in some particle growth in the trachea and main bronchi. Exiting particles are observed to be approximately 230 nm or greater at the outlets for Case 2 conditions. As illustrated in and , RH values below 100% can produce the relatively minor hygroscopic growth ratios observed in .
FIG. 10 Droplet trajectories colored according to transient size for inhalation conditions (a) Case 1, (b) Case 2, (c) Case 3, and (d) Case 4.
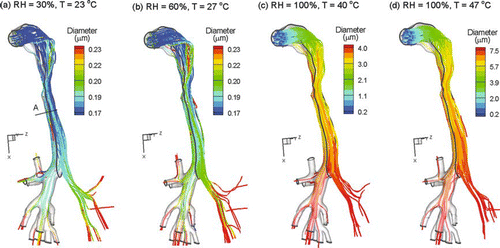
For the supersaturated conditions of Cases 3 and 4, significant condensational growth of the particles is observed (). Considering Case 3, condensational growth in the MT region results in a particle size increase to approximately 2.5 μ m (). Particles under these conditions continue to grow and reach approximately 4 μ m near the outlets. Even more dramatic particle growth is observed under Case 4 conditions (). With this upper boundary of inhaled temperature, growth up to 4 μ m is observed in the MT region. Due to enhanced particle deposition, fewer trajectories exit the respiratory model. Continued condensational growth results in particles larger than 7 μ m at the TB outlets.
Size distributions of initially submicrometer particles entering the upper trachea, denoted by Slice A in , are shown in . These size distributions are also tabulated in based on count median aerodynamic diameter (CMAD) and mass median aerodynamic diameter (MMAD). Hygroscopic particle properties are based on the MS conditions for CSPs shown in . Condensational growth for initially 200 nm particles is shown in and for sub-saturated and saturated inhalation conditions, respectively. For 200 nm particles inhaled under Case 1 conditions, the CSPs have nearly all decreased in size due to evaporation at Slice A, resulting in a distribution with a MMAD of 189 nm ( and ). Similar results are observed for Case 2 conditions, with a MMAD of 197 nm ( and ).
FIG. 11 Particle size distribution presented as mass fraction per micrometer for initially 200 nm particles under (a) sub-saturated and (b) saturated conditions; as well as 400 nm particles under (c) sub-saturated and (d) saturated conditions.
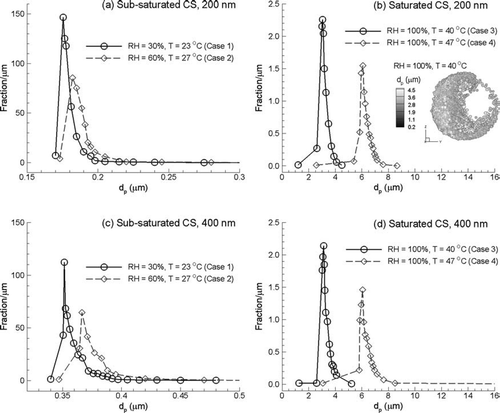
TABLE 4 Particle size statistics with evaporation and condensational growth in the upper respiratory airway sampled at Slice A
Considering saturated inhalation conditions and 200 nm aerosols, a significant amount of condensational growth is observed at Slice A (). For Case 3 inhalation conditions, the particle distribution in the trachea has a MMAD of approximately 3.6 μ m ( and ). The enhanced humidity conditions of Case 4 result in a MMAD of 7.2 μ m entering the trachea ( and ). Cross-sectional locations and particle sizes as a result of condensational growth at Slice A are also shown in for Case 3 conditions and an initially 200 nm aerosol.
Distributions of diameters as a result of condensational growth for initially 400 nm particles are shown in and , and reported in . For sub-saturated conditions, 400 nm CSPs are shown to decrease in diameter at Slice A as a result of evaporation (). The associated MMADs for Cases 1 and 2 are 370 nm and 387 nm, respectively. In contrast, significant growth is again observed for the initially submicrometer particles under saturated inhalation conditions (). For Case 3 inhalation conditions, 400 nm particles result in an increased size distribution with a MMAD of 4.0 μ m at Slice A ( and ). Case 4 inhalation conditions result in a MMAD of 7.4 μ m ( and ).
Comparisons between results for 200 and 400 nm particles can be made to highlight the effect of the initial particle size on evaporation and condensation. For sub-saturated conditions, both particle sizes evaporate by less than 10% for Case 1 and less than 5% for Case 2 conditions at Slice A (). As a result, particle sizes in the trachea do not change significantly for sub-saturated conditions. In contrast, particle sizes at Slice A under supersaturated conditions are relatively independent of the initial particle size. For Case 3 inhalation conditions, the MMADs for initially 200 and 400 nm aerosols differ by approximately 10% (3.6 vs. 4.0 μ m). For Case 4, the MMADs for the two initial particle sizes differ by less than 5% (7.2 vs. 7.4 μ m). As a result, it appears that the initial particle size has a negligible effect on the final diameter in the case of significant condensational growth for an initially monodisperse aerosol. In contrast, the results of clearly show that the degree of saturation, which is determined by the inhalation and wall surface conditions, has the largest impact on aerosol size growth.
Regional and local deposition characteristics under Case 3 inhalation conditions (Tinlet = 40°C and RHinlet = 100%) at 34 L/min are shown in . The deposition of 200 nm particles without condensational growth is presented in . Deposition fractions in the MT and upper TB geometries are 0.52 and 1.82%, respectively, resulting in a total DF of 2.34% (). Including hygroscopic particle growth under Case 3 conditions is observed to result in MT and TB deposition fractions of 0.73 and 2.68%, respectively (). As a result, hygroscopic growth for Case 3 conditions increases the regional and total deposition fractions by a factor of approximately 1.5.
FIG. 12 Deposition under Case 3 inhalation conditions for initially 200 nm particles (a) without condensational growth and (b) with condensational growth. The associated deposition enhancement factor (DEF) is shown (c) without condensational growth and (d) with condensational growth.
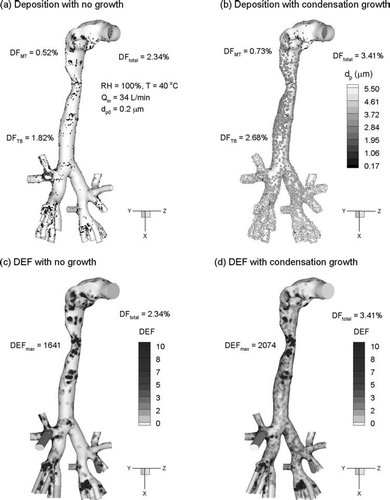
The effects of condensational growth on local deposition enhancements are shown in and . For 200 nm particles and no growth, significant hot spots of deposition are observed in the trachea as well as the second and third bifurcations (). However, relatively little deposition enhancement is observed at the main carina. The maximum deposition enhancement factor (DEF) without growth is 1,641, which represents the fraction of local deposition compared with total deposition. For 200 nm particles and condensational growth under Case 3 conditions, local deposition enhancement is observed to increase significantly throughout the geometry (). As a result of increased particle size and deposition by impaction, a hot spot in deposition is observed at the main carina. Compared to the results without growth, condensation produces elevated localizations of deposition at the second and third bifurcations. Furthermore, condensational growth is observed to increase the maximum DEF by approximately 30% compared to conditions without growth.
Cumulative deposition fractions in the x-direction () through the MT and upper TB geometries are shown in . These deposition fractions are based on 200 nm particles with and without condensational growth for Case 3 and Case 4 inhalation conditions in and , respectively. Results are also presented for a constant particle diameter that is representative of the growth observed with each inhalation case. For Cases 3 and 4, these representative constant particle diameters are 3 and 7 μ m, respectively. Considering Case 3 conditions, condensation growth is shown to first affect the deposition of 200 nm particles in the region of the larynx. Downstream of the larynx, the cumulative deposition fraction curves continue to separate, indicating continued growth and deposition. Near the outlet, total deposition for condensational growth is observed to approach the total deposition of the constant 3 μ m aerosol under Case 3 conditions ().
FIG. 13 Cumulative particle deposition fraction (DF) as a percentage along the x-axis of the geometry for (a) Case 3 and (b) Case 4 inhalation conditions.
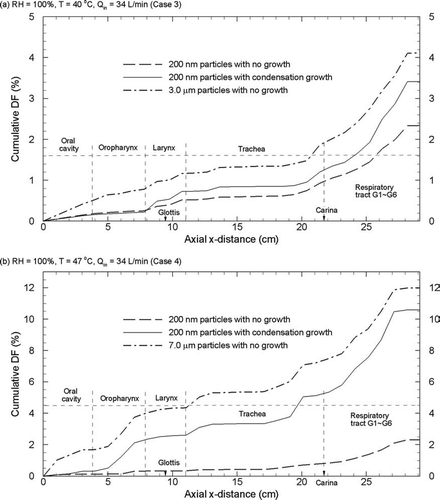
As expected, hygroscopic growth under Case 4 conditions results in a significant increase in cumulative deposition throughout the geometry (). Condensational growth is observed to initially enhance the deposition of 200 nm particles in the oropharynx region. Thereafter, deposition of the condensation aerosols appears to be consistent with, but approximately 2% below, the deposition of a constant diameter 7 μ m aerosol. The resulting total deposition for the 200 nm condensation aerosol and constant 7 μ m particles is approximately 10–12%. However, the trend in deposition is sharply increasing at the outlet of the upper TB geometry considered in this study. As a result, it appears reasonable that a total rate of deposition in the full 16 generations of the TB geometry may be greater than 50% due to condensational growth effects.
DISCUSSION
The primary implication of this study is that condensational growth may play a more significant role than previously considered in the deposition of CSPs. A number of in vivo and in vitro studies have reported that CSPs deposit in the upper TB airways like a much larger 6–7 μ m aerosol (CitationSchlesinger et al. 1982; CitationPritchard and Black 1984; CitationMartonen 1992; CitationPhalen et al. 1994). Furthermore, CitationBlack and Pritchard (1984) showed that CSPs deposit in the TB region like a 6.5 μ m aerosol and in the pulmonary region like a 0.3 μ m aerosol. However, it has been widely established that the maximum sub-saturated growth ratio of CSPs is on the order of 1.4–1.7 (CitationIshizu et al. 1980; CitationMartonen 1992; CitationLi and Hopke 1993; CitationRobinson and Yu 1998). Based on this relatively minor size change, it was concluded that hygroscopic growth could not be responsible for significant enhancements in deposition (CitationIngebrethsen 1989; CitationMartonen 1992; CitationPhalen et al. 1994; CitationRobinson and Yu 1998). The current study suggests that during the inhalation of MS smoke, the RH may exceed 100%. As a result, significant particle growth in the supersaturated upper airways was found to be possible. Interestingly, for the relatively mild inhalation temperature of 3°C above average body conditions (Case 3), initially 200 and 400 nm particles were observed to increase in size to above 3 μ m near the trachea inlet. (). Even more striking is the fact that the upper boundary inhalation temperature conditions of Case 4 resulted in a 7–8 μ m particle entering the trachea. Clearly, these results do not prove that the enhanced deposition of CSPs is only a result of condensational growth. However, this study does highlight condensational growth as a potentially significant mechanism in the deposition of smoke particles under initially supersaturated conditions.
Comparisons of the current upper airway deposition data can be made to the in vitro deposition data of CitationPhalen et al. (1994). This experimental study considered the deposition of concentrated sidestream smoke delivered at steady state to a model pharynx and three generation TB geometry. In the experiment, water vapor was present in the concentrated smoke due to combustion; however, the walls of the model geometry were not pre-wetted. Considering the adult case used by CitationPhalen et al. (1994), deposition in the MT and TB regions were 5.5 and 5.7%, respectively, resulting in a total deposition fraction of 11.2%. The deposition results for 200 nm particle and no condensation in the current study are 0.52% in the MT geometry and 1.82% in the TB region. In contrast, Case 4 deposition results with an initially 200 nm particle size and condensation in the MT and TB regions are 2.5% and 8%, respectively. The resulting predicted total deposition of 10.5% for Case 4 conditions is within 10% of the total deposition reported by CitationPhalen et al. (1994). This comparison is relatively good, considering that the MT and TB geometries were significantly different between the study of CitationPhalen et al. (1994) and the current model.
The results of this study support a hypothesis that the enhanced deposition of MS smoke particles may be largely influenced by condensational growth in a supersaturated environment. This theory requires that warm or hot smoke is inhaled at saturation or near saturation conditions. The current results illustrate that warm and hot saturated air can result in supersaturated conditions through the upper airway model to approximately generation G3–G6. Furthermore, results of this study also show that significant growth is possible in a relatively mild supersaturated environment, e.g., 101% for Case 3 conditions. Due to wall absorption, the RH fields appear to approach the mucus surface condition of 99.5% near the geometry outlets, i.e., approximately generations G3–G6. Because the condensed micrometer droplets are primarily composed of water, some evaporation may then occur in the deep lung, where RH conditions are expected to be 99.5%. To verify the potential for particle evaporation in the deep lung, an additional simulation was performed for a 200 nm CSP that had increased in size to 7 μ m due to water condensation. It was found that the initially 200 nm condensed droplet could evaporate from 7 μ m to below 1 μ m in less than 2 seconds at a RH of 99.5%. This significant evaporation is possible because of the large amount of water present in the 7 μ m droplet, which results in negligible hygroscopic effects until the droplet diameter falls below approximately 1 μ m. Continued evaporation will result in an equilibrium diameter that is greater than the initial size of 200 nm and similar to the growth ratios shown in . A time period of 2 seconds is consistent with a single inhalation cycle, or one half of a full breathing cycle.
As a result of the above observations, it appears possible that condensational growth could be responsible for MS smoke particles depositing like 3–8 μ m aerosols in the upper TB region, as observed by CitationBlack and Pritchard (1984) and CitationPhalen et al. (1994). Evaporation in the deeper lung at a sub-saturated RH of 99.5% could then result in submicrometer aerosols, which is consistent with the deposition findings of CitationBlack and Pritchard (1984). Nevertheless, there is currently no experimental evidence that directly proves significant condensational growth of CSPs in the upper respiratory airways, as reported in the current study.
The hypothesis of significant condensational growth of CSPs in the upper airways and subsequent evaporation in the lower airways does not contradict the in vivo observations of CitationHicks et al. (1986). The study of CitationHicks et al. (1986) reported that inhaled dense mainstream CSPs only increased in size by a factor of 1.7 based on measurements made at exhalation. However, CitationHicks et al. (1986) were not able to assess size change during inhalation as the CSPs passed through the upper airways. It is reasonable that significant size change did occur during the initial inhalation of CSPs in the experiment of CitationHicks et al. (1986). Partial evaporation in the deeper lung and during exhalation, as described above, could then explain the relatively small growth ratios that were measured in the experiment.
While the proposed condensational growth hypothesis may be possible, there are currently a number of problems with this theory. First, there may be an insufficient amount of water vapor in cigarette smoke to account for the significant growth of a concentrated aerosol. As a result, condensational growth may deplete the RH in the upper airway and limit the amount of subsequent size increase, which has been described for general aerosols by CitationFinlay and Stapleton (1995) and CitationFinlay (1998). This continuous phase limiting effect will be especially significant for dense smoke aerosols. In addition, if significant condensational growth is possible, it is not clear why larger particles are not observed in impactor testing (CitationPhalen et al. 1994). Finally, the upper boundary on inhalation temperature of 47°C is too high for current ventilated cigarettes. Case 4 conditions may be more representative of previously used unventilated or unfiltered cigarettes, which were employed in the studies of CitationBlack and Pritchard (1984), CitationHicks et al. (1986), and CitationPhalen et al. (1994). It is not clear why the filter temperature recorded by CitationWoodman et al. (1984) reached conditions as high as 80°C.
A primary simplification that was made in the current study was the assumption of dilute aerosol effects. Convincing studies by CitationMartonen (1992)and CitationPhalen et al. (1994) have elegantly shown that colligative effects are significant for concentrated CSPs. More recent studies by CitationRobinson and Yu (2001) and CitationBroday and Robinson (2003) have included colligative effects in whole lung deposition models. In particular, the whole-lung transport model of CitationBroday and Robinson (2003) included the concentration dependent effect of aerosol hydrodynamic interactions on individual particles and clearly illustrated a resulting increase in the upper TB deposition of CSPs, as observed experimentally. Furthermore, collective hydrodynamic interactions are known to affect the settling of dense aerosols (CitationFuchs 1964; CitationHinds 1999). Therefore, it seems most likely that collective hydrodynamic interactions can significantly influence the inertial deposition of dense CSPs.
In addition to the assumption of a dilute one-way coupled aerosol, other simplifications of the current study include the consideration of initially monodisperse particles, use of the droplet RMM model, and the specification of a constant airway surface temperature. For a polydisperse aerosol, smaller particles may grow at a slower rate than larger particles due to the Kelvin effect. The resulting competition for the available water vapor may further slow the growth of smaller aerosols and may even induce evaporation (CitationBroday and Georgopoulous 2001). The polydisperse nature of CSPs may also influence deposition as a function of size dependent growth (CitationBroday and Georgopoulous 2001) and aerosol concentration (CitationBroday and Robinson 2003). Considering condensation, significant aerosol growth may create a concentration gradient of species within the droplet with increased liquid water at the surface. As a result, the RMM approach may over predict the rate of condensational growth for significant size increases and RH values close to 100%. Finally, a single wall temperature (37°C) was assumed for the airway surface extending from the MT to the TB region. Other studies have reported and implemented an increase in airway wall surface temperature from the oral cavity (34°C) to body temperature conditions (37°C) around the third bifurcation (CitationFerron et al. 1984; CitationMcFadden et al. 1985; CitationKaufman and Farahmand 2006). The inclusion of a reduced wall surface temperature in the model will increase the predicted RH values and may further increase the expected growth of CSPs in the MT.
Considering the existing evidence as a whole, it appears that condensational growth may play a more significant role in the deposition of CSPs than previously considered. It is striking that the condensation model predicted CSPs to reach a diameter of 3–8 μ m at the trachea inlet, which is consistent with the observations of previous experimental studies. However, these results do not support or disprove the existing evidence of colligative effects for CSPs. Therefore, a complete theory on the enhanced deposition of CSPs remains unclear. An important conclusion that can be drawn from this study is that future deposition experiments should carefully monitor and report RH and temperature conditions for CSP aerosols.
Based on the current numerical findings, future experimental studies are needed to better evaluate the relation of both condensational growth and colligative effects on the enhanced deposition of CSPs. These studies should carefully control RH conditions and measure particle sizes nearly instantaneously, perhaps with real-time laser diffraction measurements. Experiments designed to isolate condensational growth effects from hydrodynamic interactions would be especially valuable. A computational fluid dynamics model of colligative effects is also needed.
In conclusion, the current results show that supersaturated RH conditions can create a significant increase in the size of CSPs in the upper airways. It is interesting that the saturated cases considered in this study resulted in 3–8 μ m particles entering the trachea and depositing in the TB region, which is consistent with previous experimental deposition data. However, colligative effects as a result of hydrodynamic particle interactions were not included in this study and are expected to further enhance deposition. As a result, the transport and deposition of concentrated CSPs may be a function of both colligative and condensation growth mechanisms. Future studies are needed to isolate the specific effects of these deposition mechanisms for CSPs and other hygroscopic combustion and pharmaceutical aerosols.
Acknowledgments
This work was sponsored by Philip Morris USA. The authors thank Dr. Michael Oldham for helpful discussions and for reviewing the manuscript. Dr. Beverly Cohen is gratefully acknowledged for providing the tracheobronchial lung cast. Dr. Karen A. Kurdziel and James McCumiskey, VCU Department of Radiology and Molecular Imaging Center, are gratefully acknowledged for providing initial CT template data and for scanning the lung cast.
Notes
*R (right); L (left); apx (apical); inf (inferior); ant (anterior); post (posterior); med (medial); mid (middle); lat (lateral); up (upper); low (lower).
‡The distribution of flow rates are from measurements in a human tracheobronchial cast (CitationCohen et al., 1990) that was identical with the computational model.
REFERENCES
- Allen , M. D. and Ratable , O. G. 1985 . Slip Correction Measurements of Spherical Solid Aerosol Particles in an Improved Millikan Apparatus . Aerosol Sci. Technol. , 4 : 269 – 286 .
- Baker , R. R. and Dixon , M. 2006 . The Retention of Tobacco Smoke Constituents in the Human Respiratory Tract . Inhal. Toxicol. , 17 : 255 – 294 .
- Balashazy , I. , Hofmann , W. and Heistracher , T. 1999 . Computation of Local Enhancement Factors for the Quantification of Particle Deposition Patterns in Airway Bifurcations . J. Aerosol Sci. , 30 : 185 – 203 .
- Balashazy , I. , Hofmann , W. and Heistracher , T. 2003 . Local Particle Deposition Patterns May Play a Key Role in the Development of Lung Cancer . Translat. Physiol. , 94 : 1719 – 1725 .
- Baumberger , J. P. 1923 . The Amount of Smoke Produced From Tobacco and Its Absorption in Smoking As Determined By Electrical Precipitation . J. Pharmacol. Exp. Therapy , 21 : 47 – 57 .
- Bernstein , G. M. 2004 . A Review of the Influence of Particle Size, Puff Volume, and Inhalation Pattern on the Deposition of Cigarette Smoke Particles in the Respiratory Tract . Inhal. Toxicol. , 16 : 675 – 689 .
- Bird , R. B. , Steward , W. E. and Lightfoot , E. N. 1960 . Transport Phenomena , New York : John Wiley & Sons .
- Black , A. and Pritchard , J. N. 1984 . A Comparison of the Regional Deposition and Short–Term Clearance of Tar Particulate Material from Cigarette Smoke, With That of 2.5 Micrometer Polystyrene Microspheres . J. Aerosol Sci. , 15 ( 3 ) : 224 – 227 .
- Broday , D. M. and Georgopoulous , G. 2001 . Growth and Deposition of Hygroscopic Particulate Matter in the Human Lung . Aerosol Sci. Technol. , 34 : 144 – 159 .
- Broday , D. M. and Robinson , R. 2003 . Application of Cloud Dynamics to Dosimetry of Cigarette Smoke Particles in the Lungs . Aerosol Sci. Technol. , 37 : 510 – 527 .
- Bryson , C. C. and Spencer , H. 1951 . Carcinoma of the Bronchus . Quarterly J. Medicine , 78 : 173 – 186 .
- Chan , T. L. , Schreck , R. M. and Lippmann , M. 1980 . Effect of the Laryngeal Jet on Particle Deposition in the Human Trachea and Upper Bronchial Airways . J. Aerosol Sci. , 11 : 447 – 459 .
- Cheng , K. H. , Cheng , Y. S. , Yeh , H. C. and Swift , D. L. 1997a . An Experimental Method for Measuring Aerosol Deposition Efficiency in the Human Oral Airway . Am. Ind. Hyg. Assoc. J. , 58 : 207 – 213 .
- Cheng , K. H. , Cheng , Y. S. , Yeh , H. C. and Swift , D. L. 1997b . Measurements of Airway Dimensions and Calculation of Mass Transfer Characteristics of the Human Oral Passage . J. Biomechan. Engineer. , 119 : 476 – 482 .
- Cheng , Y. S. , Su , Y. F. , Yeh , H. C. and Swift , D. L. 1993 . Deposition of Thoron Progeny in Human Head Airways . Aerosol Sci. Technol. , 18 : 359 – 375 .
- Clift , R. , Grace , J. R. and Weber , M. E. 1978 . Bubbles, Drops, and Particles , New York : Academic Press .
- Cohen , B. S. , Sussman , R. G. and Lippmann , M. 1990 . Ultrafine Particle Deposition in a Human Tracheobronchial Cast . Aerosol Sci. Technol. , 12 : 1082 – 1093 .
- Crowe , C. T. , Troutt , T. R. and Chung , J. N. 1996 . Numerical Models for Two-Phase Turbulent Flows . Ann. Rev. Fluid Mech. , 28 : 11 – 43 .
- Davies , C. N. 1988 . Cigarette Smoke: Generation and Properties of the Aerosol . J. Aerosol Sci. , 19 : 463 – 469 .
- Doll , R. and Hill , A. B. 1950 . Smoking and Carcinoma of the Lung: Preliminary Report . BMJ , 2 : 739 – 748 .
- Ermala , P. and Holsti , L. R. 1955 . Distribution and Absorption of Tobacco Tar in the Organs of the Respiratory Tract . Cancer , 8 : 673 – 678 .
- Feng , S. , Plunkett , S. E. , Lam , K. , Kapur , S. , Mahammad , R. , Jin , Y. , Zimmermann , M. , Mendes , P. , Kinser , R. and Roethig , J. 2006 . A New Method for Estimating the Retention of Selected Smoke Constituents in the Respiratory Tract of Smokers During Cigarette Smoking . Inhal. Toxicol. , 19 : 169 – 179 .
- Ferron , G. A. 1977 . The Size of Soluble Aerosol Particles as a Function of the Humidity of the Air: Application to the Human Respiratory Tract . J. Aerosol Sci. , 3 : 251 – 267 .
- Ferron , G. A. , Haider , B. and Kreyling , W. G. 1984 . Conditions for Measuring Supersaturation in the Human Lung Using Aerosols . J. Aerosol Sci. , 15 : 211 – 215 .
- Ferron , G. A. , Kreyling , W. G. and Haider , B. 1988 . Inhalation of Salt Aerosol Particles—II. Growth and Deposition in the Human Respiratory Tract . J. Aerosol Sci. , 19 ( 5 ) : 611 – 631 .
- Finlay , W. H. 1998 . Estimating the Type of Hygroscopic Behavior Exhibited by Aqueous Droplets . J. Aerosol Med. , 11 ( 4 ) : 221 – 229 .
- Finlay , W. H. 2001 . The Mechanics of Inhaled Pharmaceutical Aerosols , San Diego : Academic Press .
- Finlay , W. H. and Stapleton , K. W. 1995 . The Effect on Regional Lung Deposition of Coupled Heat and Mass-Transfer Between Hygroscopic Droplets and Their Surrounding Phase . J. Aerosol Sci. , 26 ( 4 ) : 655 – 670 .
- Fuchs , N. A. 1964 . The Mechanics of Aerosols , Oxford : Pergamon .
- Fuchs , N. A. and Sutugin , A. G. 1970 . Highly Dispersed Aerosols , Ann Arbor : Ann Arbor Science Publ. .
- Ghalichi , F. , Deng , X. , Champlain , A. D. , Douville , Y. , King , M. and Guidoin , R. 1998 . Low Reynolds Number Turbulence Modeling of Blood Flow in Arterial Stenoses . Biorheology , 35 ( 4&5 ) : 281 – 294 .
- Gosman , A. D. and Ioannides , E. 1981 . Aspects of Computer Simulation of Liquid-Fueled Combustors . J. Energy , 7 : 482 – 490 .
- Green , D. W. 1997 . Perry's Chemical Engineers' Handbook , New York : Mcgraw-Hill .
- Hicks , J. F. , Prichard , J. N. , Black , A. and Megaw , W. J. 1986 . “ Experimental Evaluation of Aerosol Growth in the Human Respiratory Tract ” . In Aerosols: Formation and Reactivity , 244 – 247 . Oxford : Pergamon Press .
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles , New York : John Wiley and Sons .
- Hinds , W. C. , First , M. W. , Huber , G. L. and Shea , J. W. 1983 . A Method for Measuring Respiratory Deposition of Cigarette Smoke During Smoking . Am. Ind. Hyg. Assoc. J. , 44 : 113 – 118 .
- Horsfield , K. , Dart , G. , Olson , D. E. , Filley , G. F. and Cumming , G. 1971 . Models of the Human Bronchial Tree . J. Applied Physiol. , 31 : 207 – 217 .
- ICRP . 1994 . Human Respiratory Tract Model for Radiological Protection , New York : Elsevier Science Ltd. .
- Ingebrethsen , B. J. 1989 . “ The Physical Properties of Mainstream Cigarette Smoke and their Relationship to Deposition in the Respiratory Tract ” . In Extrapolation of Dosimetric Relationships for Inhaled Particles and Gases , Edited by: Crapo , J. D. , Smolko , E. D. , Miller , F. J. , Graham , J. A. and Hayes , A. W. San Diego : Academic Press .
- Ishizu , Y. , Ohta , K. and Okada , T. 1980 . The Effect of Moisture on the Growth of Cigarette Smoke Particles . Beitr. Zur Tabakforschung , 10 : 161 – 168 .
- Jenkins , R. W. , Francis , R. W. , Flachsbart , H. and Stober , W. 1979 . Chemical Variability of Mainstream Cigarette Smoke as a Function of Aerodynamic Particle Size . J. Aerosol Science , 10 : 355 – 362 .
- Kaufman , J. W. and Farahmand , K. 2006 . In Vivo Measurements of Human Oral Cavity Heat and Water Vapor Transport . Respir. Physiol. Neurobiol. , 150 : 261 – 277 .
- Keith , C. H. 1982 . Particle Size Studies on Tobacco Smoke . Beitr. Zur Tabakforschung , 11 ( 3 ) : 123 – 131 .
- Keith , C. H. and Tesh , P. G. 1965 . Measurement of the Total Smoke Issuing from A Burning Cigarette . Tobacco Sci. , 9 : 61 – 64 .
- Kim , J. , Moin , P. and Moser , R. D. 1987 . Turbulence Statistics in Fully Developed Channel Flow at Low Reynolds Number . J. Fluid Mech. , 177 : 133 – 166 .
- Li , W. 1993 . The Hygroscopicity of Indoor Aerosol Particles , New York : Clarkson University .
- Li , W. and Hopke , P. K. 1993 . Initial Size Distributions and Hygroscopicity of Indoor Combustion Aerosol Particles . Aerosol Sci. Technol. , 19 : 305 – 316 .
- Longest , P. W. and Kleinstreuer , C. 2005 . Computational Models for Simulating Multicomponent Aerosol Evaporation in the Upper Respiratory Airways . Aerosol Sci. Technol. , 39 : 124 – 138 .
- Longest , P. W. , Kleinstreuer , C. and Buchanan , J. R. 2004 . Efficient Computation of Micro–Particle Dynamics Including Wall Effects . Computers & Fluids , 33 ( 4 ) : 577 – 601 .
- Longest , P. W. and Oldham , M. J. 2006 . Mutual Enhancements of CFD Modeling and Experimental Data: A Case Study of One Micrometer Particle Deposition in a Branching Airway Model . Inhal. Toxicol. , 18 ( 10 ) : 761 – 772 .
- Longest , P. W. and Vinchurkar , S. 2007a . Effects of Mesh Style and Grid Convergence on Particle Deposition in Bifurcating Airway Models With Comparisons to Experimental Data . Medical Engineering and Physics , 29 ( 3 ) : 350 – 366 .
- Longest , P. W. and Vinchurkar , S. 2007b . Validating CFD Predictions of Respiratory Aerosol Deposition: Effects of Upstream Transition and Turbulence . J. Biomech. , 40 : 305 – 316 .
- Longest , P. W. , Vinchurkar , S. and Martonen , T. B. 2006 . Transport and Deposition of Respiratory Aerosols in Models of Childhood Asthma . J. Aerosol Sci. , 37 : 1234 – 1257 .
- Longest , P. W. and Xi , J. 2007 . Effectiveness of Direct Lagrangian Tracking Models for Simulating Nanoparticle Deposition in the Upper Airways . Aerosol Sci. Technol. , 41 : 380 – 397 .
- Lumb , A. B. 2000 . Nunn's Applied Respiratory Physiology , Oxford : Butterworth Heinemann .
- Ma , B. and Lutchen , K. R. 2006 . an Anatomically Based Hybrid Computational Model of the Human Lung and Its Application to Low Frequency Oscillatory Mechanics . Ann. Biomed. Engineer. , 34 ( 11 ) : 1691 – 1704 .
- Martonen , T. B. 1986 . “ Surrogate Experimental Models for Studying Particle Deposition in the Human Respiratory Tract: An Overview ” . In Aerosols , Edited by: Lee , S. D. Chesea, Michigan : Lewis Publishers .
- Martonen , T. B. 1992 . Deposition Patterns of Cigarette Smoke in Human Airways . Amer. Indust. Hygiene Assoc. J. , 53 ( 1 ) : 6 – 18 .
- Martonen , T. B. , Hoffmann , W. and Lowe , J. E. 1987 . Cigarette Smoke and Lung Cancer . Health Physics , 52 ( 2 ) : 213 – 217 .
- Matida , E. A. , Finlay , W. H. and Grgic , L. B. 2004 . Improved Numerical Simulation of Aerosol Deposition in an Idealized Mouth-Throat . J. Aerosol Sci. , 35 : 1 – 19 .
- Matida , E. A. , Nishino , K. and Torii , K. 2000 . Statistical Simulation of Particle Deposition on the Wall from Turbulent Dispersed Pipe Flow . Intl. J. Heat and Fluid Flow , 21 : 389 – 402 .
- Mcaughey , J. J. , Pritchard , J. N. , Black , A. , Hoare , G. C. and Knight , D. A. 1991 . Tar Retention and Regional Lung Deposition Male and Female Smokers Switching to Products With Lower Tar Yields , Oxfordshire, , U. K. : Didcot . AEA Technology Report AEAT–0159
- Mcfadden , E. R. , Pichurko , B. M. and Bowman , H. F. 1985 . Thermal Mapping of the Airways in Humans . J. Appl. Physiol. , 58 : 564 – 570 .
- Morsi , S. A. and Alexander , A. J. 1972 . An Investigation of Particle Trajectories in Two-Phase Flow Systems . J. Fluid Mech. , 55 ( 2 ) : 193 – 208 .
- Muller , W. , Hess , G. D. and Scherer , P. W. 1990 . A Model of Cigarette Smoke Particle Deposition . Am. Ind. Hyg. Assoc. J. , 51 : 245 – 256 .
- NCRP . 1997 . Deposition, Retention and Dosimetry of Inhaled Radioactive Substances , Bethesda : National Council on Radiation Protection and Measurements .
- Norman , V. 1977 . An Overview of the Vapor Phase, Semivolatile and Nonvolatile Components of Cigarette Smoke . Tobacco Sci. , 3 : 28 – 58 .
- Pedley , T. J. 1977 . Pulmonary Fluid Dynamics . Annual Review of Fluid Mechanics , 9 : 229 – 274 .
- Phalen , R. F. , Oldham , M. J. and Mannix , R. C. 1994 . Cigarette Smoke Deposition in the Tracheobronchial Tree: Evidence for Colligative Effects . Aerosol Sci. Technol. , 20 : 215 – 226 .
- Pritchard , J. N. and Black , A. 1984 . “ An Estimation of the Tar Particulate Material Depositing in the Respiratory Tracts of Healthy Male Middle– and Low-Tar Cigarette Smokers ” . In Aerosols , Edited by: Liu , Pue and Fissan . 989 – 992 . New York : Elsevier Science .
- Robinson , R. and Yu , C. P. 1998 . Theoretical Analysis of Hygroscopic Growth Rate of Mainstream and Sidestream Cigarette Smoke Particles in the Human Respiratory Tract . Aerosol Sci. Technol. , 28 : 21 – 32 .
- Robinson , R. J. and Yu , C. P. 1999 . Coagulation of Cigarette Smoke Particles . J. Aerosol Sci. , 30 ( 4 ) : 533 – 548 .
- Robinson , R. J. and Yu , C. P. 2001 . Deposition of Cigarette Smoke Particles in the Human Respiratory Tract . Aerosol Sci. Technol. , 34 : 202 – 215 .
- Sarangapani , R. and Wexler , A. S. 1996 . Growth and Neutralization of Sulfate Aerosols in Human Airways . J. Applied Physiol. , 81 ( 1 ) : 480 – 490 .
- Schlesinger , R. B. , Gurman , J. L. and Lippmann , M. 1982 . Particle Deposition Within Bronchial Airways: Comparisons Using Constant and Cyclic Inspiratory Flows . Ann. Occup. Hyg. , 26 : 47 – 64 .
- Stober , W. 1984 . Lung Dynamics and Uptake of Smoke Constituents by Nonsmokers—A Survey . Preventive Medicine , 13 ( 6 ) : 589 – 601 .
- US Department of Health and Human Services . 2004 . The Health Consequences of Smoking: A Report of the Surgeon General , U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordination Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health .
- US Department of Health and Human Services . 2006 . The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General—Executive Summary , U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordination Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health .
- Vargaftik , N. B. 1975 . Tables on Thermophysical Properties of Liquids and Gases , Washington, DC : Hemisphere .
- Wang , Y. and James , P. W. 1999 . On the Effect of Anisotropy on the Turbulent Dispersion and Deposition of Small Particles . Intl. J. Multiphase Flow , 22 : 551 – 558 .
- WHO . 2002 . Tobacco Smoke and Involuntary Smoking IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 83
- Wilcox , D. C. 1998 . Turbulence Modeling for CFD, , 2nd Ed. , California : DCW Industries, Inc. .
- Woodman , G. , Newman , S. P. , Pavia , D. and Clarke , S. W. 1984 . Temperature and Calibration Corrects to Puff Volume Measurements in Cigarette Smoke . Phys. Med. Biol. , 29 ( 11 ) : 1437 – 1440 .
- Wynder , E. L. and Graham , E. A. 1950 . Tobacco Smoking as a Possible Etiologic Factor in Bronchiogenic Carcinoma. A Study of Six Hundred and Eighty-Four Proved Cases . JAMA , 143 : 329 – 336 .
- Xi , J. and Longest , P. W. 2007 . Transport and Deposition of Micro-Aerosols in Realistic and Simplified Models of the Oral Airway . Ann. Biomed. Engineer. , 35 ( 4 ) : 560 – 581 .
- Xi , J. and Longest , P. W. 2008 . Effects of Oral Airway Geometry Characteristics on the Diffusional Deposition of Inhaled Nanoparticles . ASME J. Biomech. Engineer. , 130 : 011008
- Yang , C. P. , Callagher , R. P. , Weiss , N. S. , Band , P. R. , Thomas , D. B. and Russel , D. A. 1989 . Differences in Incidence Rates of Cancers of the Respiratory Tract by Anatomic Subside and Histological Type: An Etiologic Implication . J. National Cancer Institute , 81 ( 21 ) : 1828 – 1831 .
- Yeh , H. C. and Schum , G. M. 1980 . Models of Human Lung Airways and their Application to Inhaled Particle Deposition . Bull. Math. Biology , 42 : 461 – 480 .
- Zhang , Z. , Kleinstreuer , C. , Donohue , J. F. and Kim , C. S. 2005 . Comparison of Micro- and Nano-Size Particle Depositions in a Human Upper Airway Model . J. Aerosol Sci. , 36 ( 2 ) : 211 – 233 .