Abstract
The study presented here investigates the number weighted particle size distributions of aerosols generated in the laboratory from lead oxide and lead sulfide dusts and sampled by Institute of Occupational Medicine (IOM) and closed face cassette (CFC) samplers as determined by scanning electron microscopy (SEM). The wall deposits and filter deposits from each sampler were characterized separately. A Mann-Whitney statistical analysis revealed that differences in the number weighted distributions of particles captured by the filter and the wall were not significant over the size range (up to 20 μm aerodynamic equivalent diameter) present in these laboratory-generated aerosols. Furthermore, for these samples it was not possible to distinguish an absolute difference between the IOM and CFC filter catches. By comparing direct measurements of aerodynamic equivalent diameter (AED) made by an Aerodynamic Particle Sizer (APS) to AEDs calculated from SEM images, empirical shape factors for lead oxide and lead sulfide were determined. To validate this approach APS and SEM measurements of the AED of 2 μm and 6 μm physical diameter monodisperse glass and polystyrene microspheres were made. Using the shape factors of spheres and the known densities of these materials, it was found that the SEM determinations of AED agreed with the APS results. To demonstrate the reliability of the redeposition method of sample preparation, lead sulfide and lead oxide aerosols were briefly sampled by IOM samplers such that sufficient particles were collected for SEM examination directly on the filter but not so many that particles were likely to touch or overlap. Half of each filter was analyzed in the SEM directly; the other half was ultrasonically removed and re-deposited for analysis by SEM. There were no statistically significant differences in their number weighted size distributions, demonstrating that the sample treatment process does not change the size distribution of these particular aerosols.
INTRODUCTION
Historically, the standard method to determine particles in air was collection of the aerosol in an impinger followed by counting and comparing the concentration to a standard in terms of numbers of particles per unit volume of air. However, this methodology assumed that all particles were contributing equally to health effects. There are two reasons why this may not be the case; firstly, the particles may not all contain the toxin of concern, and, secondly, the dose of a systemic poison is a function of the mass of toxin which is a function of the particle size. Improvements in chemical analysis allowed a more direct determination of dose, and chemical analysis worked well with collection of particles on filters which became popular in the 1960s and 1970s. At that time, the filter catch was considered to be a reasonable representation of the particles that could enter a worker's breathing zone and be inhaled. The possibility of particles of relevance that might not be collected on the filter was not considered. Eventually the filter was incorporated into a plastic holder, known as the “closed-face cassette” (CFC) that kept it free from accidental damage. This CFC has been a standard in U.S. workplace air sampling methods ever since, and it has some advantages of cost and disposability. In recent years it has become clear that not all particles in the worker's breathing zone are collected on the filter. Some may not reach the filter while others may bounce off. Some may enter the sampler but fail to reach the filter because they are deposited on the internal surfaces of the sampler by gravitational settling, inertial impaction, or electrostatic attraction. Particles that fail to enter the sampler are considered as aspiration losses, while particles that enter the sampler, but fail to deposit on the filter are considered transport losses. In all cases, particle size is likely a factor. The Occupational Safety and Health Administration (OSHA) has confirmed an opinion that all particles entering the CFC are considered the sample, whether they collect on the filter or elsewhere within the sampler (i.e., the transport losses). Advice on how to assess the non-filter portion of the sampler is available (CitationStones et al. 2004), and all OSHA samples for the analysis of metal particulate are treated this way. On the other hand, methods from the National institute for Occupational Safety and Health (NIOSH) do not include a step to incorporate the transport losses (although some discussion of the issue takes place in the preamble pages to the Methods manual). NIOSH methods are much more widely followed than are OSHA methods, and laboratories performing analysis according to NIOSH methods do not generally incorporate the transport losses. Clients requesting analyses of samples by NIOSH methods may not be aware that their results may not be comparable to those from identical samples taken and analyzed according to OSHA procedures.
Several agencies have proposed that an aerosol sample should be selected from the range of airborne particle size in much the same fraction as that aspirated by a worker when breathing (e.g., ISO 7708). The specific size fraction proposed for many chemicals that act as systemic poisons, such as lead, is that fraction that would enter the nose or mouth under typical conditions of ambient wind speed and breathing rates, which has been termed the “inhalable” fraction. A sampler to have the same aspiration performance as the inhalable fraction has been developed at the Institute of Occupational Medicine in the UK and hence is known as the IOM sampler. It was developed on the basis that all particles entering the sampler would be included as the sample. Early in the development tests it was shown that not all particles entering the plane of the orifice were deposited on the filter, but instead a portion could be found attached to the inner walls of the sampler (CitationMark and Vincent 1986). Where total sample mass was the metric of interest, the particles deposited on the walls could easily be included through weighing the entire filter holder. In later studies when it was preferred to analyze the sample by chemical methods the wall deposits were simply rinsed into the flask containing the filter. No procedure for doing this has ever been standardized or validated, or included in any UK method. In fact, it is actually not possible to include the IOM wall deposits in some UK methods, where X-ray fluorescence analysis is performed on the filter deposit only. A typical response to these issues, seen on both sides of the Atlantic, has been to suggest that the transport losses are either negligible in comparison to the filter catch, or that they form a constant proportion of the filter mass, so that they can safely be ignored. As more evidence is published it has become apparent that neither case generally applies.
The CFC sampler was not designed to sample a physiologically relevant size fraction, but because of its wide application it has been studied to see if it does sample the inhalable size range of particles. However, while in laboratory and field comparisons of the IOM and CFC the internal wall deposits in the IOM have always been included, equivalent wall deposits in the CFC rarely have been included. Where they have been included they have been shown to be of a similar magnitude to those found in the IOM, and sometimes even exceeding the deposit on the filter (CitationDemange et al. 1990, Citation2002; CitationHarper and Demange 2007). One argument that could be made for not including the CFC wall deposits is the possibility that the size of the wall deposit particles may be larger than those on the filter, and perhaps above the limit of inhalability. For example, it has been suggested that the filter-only catch of the IOM better represents the inhalable fraction of the aerosol in slowly moving air (CitationKenny et al. 1997; CitationWitschger et al. 2004) although CitationKenny et al. (1999) showed a higher collection efficiency in their work. However, the convention for sampling in slowly moving air should first be agreed upon (CitationLidén and Harper 2006), and it is not within the purview of the present study to address this issue. The overall objective of this work is to prepare and demonstrate a method to compare particle size distributions of samples taken with CFC and IOM samplers at a variety of industrial sites, such that separate characterization of wall and filter deposits can be made, and further that sub-populations of particles with lead or other elements can be identified and characterized in these samplers.
Thus, the primary goal of these experiments is to determine if the measured wall and filter deposits of two widely used samplers, the CFC and IOM samplers, agree with respect to particle size for the particular cases of lead oxide and lead sulfide dusts in a limited range of particle size as studied here. A scanning electron microscope (SEM) method was devised to allow a sub-population of an aerosol sample to be identified and characterized as to composition and size, information that is not available from chemical analysis of the entire sample or from any other technique.
A second goal of these experiments is to compare the performance of the IOM and CFC samplers in sampling typical lead-containing aerosols, although those studied here include particles larger than respirable size but do not include the full range of inhalable size particles.
A third goal of these experiments is to compare the size distribution of particles as determined by two distinct methods (SEM and Aerodynamic Particle Sizer (APS)) to explore the conditions under which they can be meaningfully compared. The method based on SEM starts with a physical size measurement, i.e., of the projected area of a particle, and uses assumed values of shape factors and bulk density to calculate aerodynamic equivalent diameter. The APS makes a direct measurement of aerodynamic behavior and from it calculates a diameter. Using an assumed volumetric shape factor and a known particle density, a dynamic shape factor for lead oxide and lead sulfide aerosols can be determined by fitting the SEM results to the APS measurements. To demonstrate the method monodisperse glass microspheres and polystyrene beads (i.e., particles of known shape factors and densities) were investigated in the same way, and their known sizes compared to the APS and SEM measurements.
A fourth goal of these experiments is to demonstrate that the ultrasonic method of removing particles from a filter followed by redeposition does not alter the size distribution of the parent particle population, at least for the lead oxide and lead sulfide powders studied here. If the parent population contained a large fraction of agglomerates, then it might be suspected that the ultrasonic process might de-agglomerate them and bias any subsequent size distribution analysis towards smaller sizes. This question has been addressed for some samples, but it was thought necessary to repeat the study for the particular materials studied here (CitationCasuccio 2004; CitationCasuccio 1983; and CitationKim and Hopke 1988).
METHODOLOGY
Lead-Containing Particles
Two different lead-containing particles were employed in this study, lead (II) oxide (99.9%, 200 mesh, 9.53 g/cm3, Alfa Aesar, A Johnson Matthey Company, Ward Hill, MA, USA) and lead (II) sulfide (99.9%, 200 mesh, 7.73 g/cm3, Alfa Aesar).
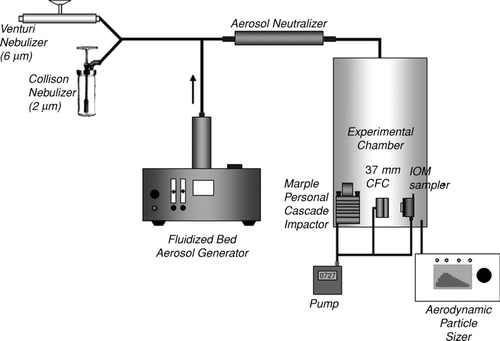
The experimental facility utilized in this study is shown in . Lead-containing particles were generated by a fluidized bed aerosol generator (Model 3400, TSI Inc., Shoreview, MN, USA). Lead-containing particles were directed through a Kr-85 electrostatic charge equilibrator (Model 3012, TSI Inc.) to the aluminum test chamber (28 cm in diameter and 68.5 cm in length) which was used in a previous study (CitationFeather and Chen, 2003). The size distribution of airborne particles was also monitored using the APS. Measurements with the APS at several locations near the bottom of the sampler chambers demonstrated that the particle size distribution was uniform by the time the particles moved from the entrance at the top of the chamber to the sample points near the bottom. After the three-hour sampling period was complete, the filter from each sampler was removed and placed in a borosilicate glass test tube with 5 ml of isopropyl alcohol. The particles were extracted from filters and suspended in the alcohol by a combined 2 min of vortex mixing (Fisher Scientific, Bohemia, NY, USA) and 10 min of ultrasonic agitation in an ultrasonic bath (Branson Ultrasonic Co., Danbury, CT, USA). The suspension was vacuum filtered through a 13 mm polycarbonate filter (0.2 μm pore size, Millipore, Billerica, MA, USA) using a side arm flask with filter holder (Millipore). After drying, the polycarbonate filter was placed on pin-type aluminum SEM specimen mount (Electron Microscopy Sciences, Hatfield, PA, USA) with carbon adhesive tabs (Electron Microscopy Sciences) for SEM analysis. It is well known that the polycarbonate filter is suited for SEM analysis due to the smooth surface texture that avoids problems with embedding filter fiber and with agglomeration of the particles (CitationCasuccio et al. 2004). Each SEM sample was coated with a thin layer of gold/palladium utilizing a sputter coater (SPI Module system, SPI Inc., West Chester, PA, USA). To prepare an SEM sample of the wall deposit of the lead-containing particles, the interior walls of the IOM sampler and the CFC were wiped with a polycarbonate filter wetted with isopropyl alcohol. The filter was then placed in a test tube with isopropyl alcohol and particles were extracted by the same method described above.
FIG. 1 Experimental setup for the collection of lead oxide and lead sulfide particles aerosolized by the fluidized bed and collection of glass and polymer spheres aerosolized by the Collison nebulizer and venturi feeder.
Because the APS measures the aerodynamic diameter at high velocity of air (outside of the Stokes regime), the APS over-sizes the particle diameter when the density of the particle is greater than unity (CitationWang and John 1987). Thus, the Stokes correction was applied to the APS data for the lead particles, which have a much higher density than the particles used in the factory calibration of the APS unit.
To address the fourth goal as to whether the ultrasonic method for removing particles from a filter might inadvertently change the parent population size distribution by de-agglomerating larger particles, a modified version of the experiment was performed.
Lead oxide was captured in an IOM sampler under the usual conditions except that the sampling period was shortened to three minutes. The filter was then removed and cut in half. One piece was coated with gold and palladium and analyzed by the SEM method without other treatment. The other half of the filter was treated by the usual method, i.e., particles were extracted from the filter into alcohol by ultrasonic agitation and the suspended particulate was re-deposited on a polycarbonate filter, coated with gold and palladium, and then analyzed by the SEM method. The short sampling time minimized the likelihood of particles overlapping on the filter, but was long enough to accumulate enough particles to make the SEM analysis feasible.
Standard Microsphere Particles
Two different standard microsphere particles were utilized in this study including 2 μm (physical diameter) borosilicate glass microsphere particles (Duke Scientific, 2.5 g/cm3, size standard deviation (Coefficient of Variation (C. V. (%)): 0.7μm (35%), Fremont, CA, USA) and 6 μm (physical diameter) fluorescent polymer microspheres (Duke Scientific, 1.05 g/cm3, 9% C. V.). The experimental setup for microsphere particles is shown in . A Collison nebulizer (BGI Inc., Waltham, MA, USA) was used for dispersing the 2 μm microspheres suspended in isopropyl alcohol and a venturi feeder (In-Tox, Albuquerque, NM, USA) was used for dispersing dry powder 6 μm microspheres. The 2 μm microspheres suspended in isopropyl alcohol were nebulized by 20 p.s.i.g. (approximately 12 L/min) HEPA filtered air. The venturi feeder was driven by 35 p.s.i.g. HEPA filtered air to aerosolize the powder of 6 μm glass spheres. The aerosolized microsphere particles entered the experimental chamber (25.4 cm × 27.9 cm × 30.5 cm) through a Kr-85 electrostatic charge equilibrator (Model 3012, TSI Inc.). The size distribution of the particles was measured with an APS (Model 3321, TSI Inc.) and microsphere particles were collected with 37 mm CFC (Polystyrene, SKC Inc., Eighty Four, PA, USA) or IOM (SKC Inc.) samplers during 15 min periods. The IOM and CFC samplers were loaded with a 25 mm (0.2 μm pore size, polycarbonate filter, Millipore) or 37 mm (0.2 μm pore size, polycarbonate filter, Millipore) filter, respectively. The Collison nebulizer operated continuously and the dust chamber was sampled concurrently with aerosol generation. However, the venturi feeder was operated for only a few seconds to fill the dust chamber with suspended particles. The high flow rate through the venturi feeder generates pressure transients in the chamber which would affect the flow rate through any sampler operating during that time. So for these experiments, all the sampling tubes into the chamber were pinched shut before the venturi was operated. After the flow through the nebulizer ended and the pressure in the chamber stabilized, the sample tubes were opened and the sampling pumps activated. This assured that these samples were collected under the nominal steady-state conditions. After sampling, a portion of each filter (described below) with sample deposit was cut and placed on a pin type aluminum SEM specimen mount with carbon adhesive tabs. Each sample was coated with a thin layer of gold/palladium utilizing a sputter coater for the scanning electron microscopy analysis.
Scanning Electron Microscopy (SEM) Analysis and Estimation of Aerodynamic Diameter
The aerodynamic particle size distribution of each filter sample was determined by a combination of scanning electron microscopy under computer control (SEM, JSM-6400, JEOL Ltd, Tokyo, Japan) and energy dispersive x-ray analysis (EDX, PGT Inc., Princeton, NJ, USA). A sequence of fields at random locations on the face of the stub were chosen and a backscattered electron (BSE) image of each field acquired in turn and transferred to the PGT IMIX version 9.0 software. After acquisition the image was converted from a gray scale to a two-color version, and the IMIX Feature Analysis software identified the location and size of all distinct features in the image and numbered them. Then the IMIX software directed the SEM system to irradiate each identified feature in sequential order with its electron beam fixed at the center of the feature for 5 s and an x-ray spectrum simultaneously recorded. The imaging and analyses were done at 20 kV accelerating potential and approximately 1.5 nA beam current.
A projected area diameter of the particle (i.e., the diameter of a circle with the same area as that of the particle image) was calculated and stored for each feature by the IMIX software. This diameter, together with assumed values for a shape factor, was entered into a spreadsheet calculation of an estimate of the particle's equivalent volume diameter, i.e., the diameter of a sphere of the same volume. From this assumed spherical volume an aerodynamic diameter is calculated from the particle's specific gravity and an assumed dynamic shape factor (CitationHinds 1999). (In these experiments the specific gravity is known a priori, but in application to field samples it will be estimated for each individual particle from the relative intensity of the x-ray emission lines for each element in its spectrum.)
The following equations were used for this calculation:
A sufficient number of selected fields were imaged and interrogated such that at least 1000 particles were randomly selected and analyzed by the SEM. Only lead-containing particles were selected from the leaded aerosol studies to determine the size distribution. (There was a small fraction of contaminant dust particles captured that were not from the lead containing sample.) The threshold for the differentiation between a lead-containing particle and otherwise was set from the proportion of lead (L-alpha line) x-ray intensity, i.e., if 50% or more of the total x-ray intensity originated from the lead L-alpha line, the particle was classified as lead. Carbon counts were ignored because of their ubiquitous appearance from the polycarbonate filter substrate (CitationCasuccio et al. 2004). Because the lead compounds studied here were reagent grade chemicals, the vast majority of particles observed were lead-containing and the results were insensitive to assumptions about the lead x-ray emission intensity. (In subsequent work with field samples, the question of thresholds to identify lead-containing particles will be treated differently.)
In order to compare the size distributions from the APS and SEM data, the number concentration data from the SEM were grouped into the same 52 size intervals that corresponded to the default size intervals used by the APS. Particles smaller than 0.5 μm were excluded from our result because a previous study has shown that the APS does not usefully size particles of this range. This is because the APS distinguishes particle size by differences in particle velocity when they are injected into a high velocity air stream. All particles below the 0.5 micron AED are rapidly accelerated to the airstream velocity and hence have the same velocity (CitationStein et al. 2003).
To compare distributions obtained by APS and SEM analyses each distribution was first normalized by dividing the incremental number concentration of each bin by the total particle concentration. The SEM distribution depends on assumed values of shape factors, but by assuming a spherical volume shape factor, the dynamic shape factor could be adjusted to generate agreement between the number weighted distributions of the APS measurements and the SEM calculations.
Comparison of the relative numbers of particles deposited on the wall and filter of a given sampler were based upon the average number of particles observed per field of view of the SEM on two stubs, one formulated from the filter and one from the wall wipe. The average number of particles per field was weighted by the total number of fields on the stub (which is different for 1500 × and 2000 × magnifications), and by the fraction of the original 5 milliliter suspension of particles deposited.
Comparison of total numbers of lead containing particles sampled as determined by SEM and APS is problematical. The total number of lead sulfide particles as determined by SEM was always smaller by 30 to 40% than the number as determined by APS, possibly because of incomplete recovery of particles from the filters. Other sources of uncertainty could be counting by the APS of contaminants and undercounting by the SEM of very small particles which are below the brightness threshold in binary imaging. Because of these uncertainties, it is not useful to compare absolute numbers of particles as determined by SEM and APS.
Statistical Analysis
Analyses of size distribution data were conducted to test for differences between distributions of the wall and filter catches of lead oxide and lead sulfide from both the CFC and IOM samplers, and to test for differences between the distributions of the IOM and CFC filter catches. Calculations were done using SAS 9.1 (SAS Institute, Cary, NC). Inferential statistics were then calculated, using theoretical and empirical distributions. Theoretical distributions were tested, first by fit testing the filter data to a probability distribution and then using the Anderson-Darling test to test both the wall deposit and filter deposit distributions to the probability distribution of best fit. Also, empirical distributions for wall and filter were tested using the Mann-Whitney U-test. The exponential distribution fit the filter data the best; however, both the wall and filter data rejected the hypothesis that the data came from the exponential distribution (tests also showed that both the wall and the filter failed to fit normal, log-normal, and gamma distributions). As the data failed to fit the theoretical distributions, empirical distributions for wall and filter were tested using the Mann-Whitney U-test.
RESULTS AND DISCUSSION
Lead-Containing Particles
shows the normalized number weighted size distribution data of lead oxide aerosol deposited on IOM filters and walls as determined by SEM. shows the data for measurements of the lead sulfide aerosol. Figures and show the results of analogous measurements of lead sulfide and lead oxide aerosols from CFC sampler walls and filters. The abscissa axis in all these figures is aerodynamic equivalent diameter as calculated from the SEM projected area diameter and the bulk density of the lead compound according to Equation (Equation2), using the volumetric shape factor of a sphere (0.52) and a dynamic shape factor chosen to match the SEM calculated distribution to the distribution as measured by APS.
FIG. 2 Normalized average number-weighted distribution of lead oxide particles obtained by IOM sampler filter (n = 3), and wall deposit (n = 3) analyzed by scanning electron microscope. The error bars are one standard deviation.
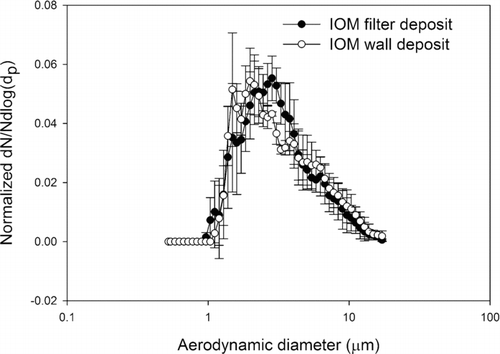
FIG. 3 Normalized average number-weighted distribution of lead sulfide particles obtained by IOM sampler filter (n = 3), and wall deposit (n = 3) analyzed by scanning electron microscope. The error bars are one standard deviation.
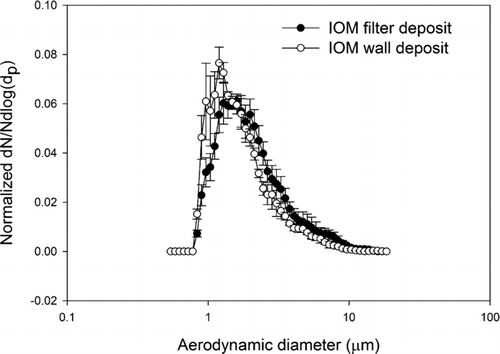
FIG. 4 Normalized average number-weighted distribution of lead oxide particles obtained by CFC sampler filter (n = 3), and wall deposit (n = 3) analyzed by scanning electron microscope. The error bars are one standard deviation.
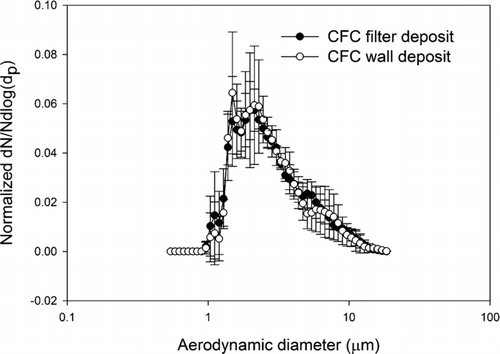
FIG. 5 Normalized average number-weighted distribution of lead sulfide particles obtained by CFC sampler filter (n = 3), and wall deposit (n = 3) analyzed by scanning electron microscope. The error bars are one standard deviation.
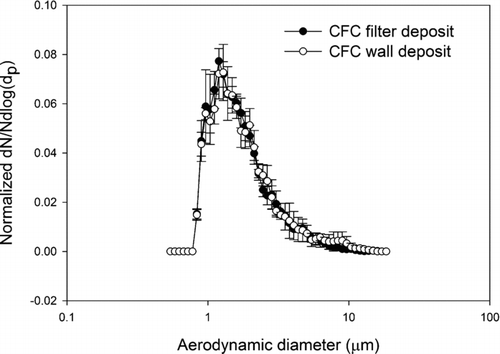
Comparing the wall and filter deposit size distributions, visual inspection of the data indicates that the number-weighted size distributions, wall and filter, from a given aerosol are very similar. And in fact the Mann-Whitney U-test analyses for both the CFC and IOM samplers’ wall and filter data failed to reject the hypothesis that they come from different distributions (p > 0.05). In other words, for the lead oxide and lead sulfide aerosols studied here a measurement of particle size distribution from the filter deposit alone represents the parent size distribution as well as a measurement that includes the wall deposit. This is true for both the CFC and IOM samplers. Comparing the results from the IOM and CFC samplers, it was found that their filter deposits are not significantly different, both for lead sulfide and lead oxide. These results suggest that for aerosols such as these the CFC and IOM samplers perform identically when the metric is particle number. In the comparisons of IOM and CFC filter and wall results by SEM, the conclusions are independent of any assumptions about shape factor. However, in comparing the SEM results to the APS results some assumption must be made about the relationship of projected area diameter and aerodynamic equivalent diameter. By incorporating a dynamic shape factor correction according to the method of CitationHinds (1999), the SEM calculation can be fit to the APS measurements. This approach was verified in the spherical particle experiments, where both particles were known to be spherical and the particle density was also known. This result is illustrated in where direct measures by the APS of the AEDs of spherical particles of known physical size and shape are compared to AEDs derived from SEM measurements and bulk particle density. The 2 μm and 6 μm physical diameter particles have, by Equation (Equation2), AEDs of 3.2 μm, and 6.1 μm, respectively. These data demonstrate that with suitable shape factors and knowledge of particle density, the SEM method of determining particle size distribution can be valid for number-weighted particle size distributions for the 2 μm and 6 μm examples. The ratio of number of particles deposited on the wall and the filter are between 0.1% and 8% in both the IOM and CFC samplers. The average ratio for IOM and CFC sampler were 1 and 2%, respectively. These are relatively small numbers when compared with the ratios found in a field sampling study in a lead ore processing mill, where the average wall deposits were 19 and 17% for IOM and CFC samplers, respectively (CitationHarper et al. 2006). Because the data from current study were collected in a calm air chamber that does not provide turbulent air flow around the samplers, there is a smaller possibility of wall deposits.
FIG. 6 Normalized average number-weighted distribution of 2 (a) and 6 (b) μm (physical diameter) glass and polymer spherical particles by APS and by scanning electron microscope.
The SEM derived values of AED fit the APS measurements with a dynamic shape factor of 2.8 for lead oxide and 1.5 for lead sulfide. The value for lead oxide is extreme when compared with other materials. This may indicate that the individual particle density is less than the bulk density or that the volumetric shape factor differs from that of sphere. Fitting to a single parameter instead of treating the particle density, volumetric shape factor, and dynamic shape factor separately may also be the reason that fitting only the dynamic shape factor yields an unusually large value. In accordance with Cheng et al. (1990) the APS underestimates the size of a non-spherical particle and this might make the dynamic shape factor large in this study. In addition, for the calculation of aerodynamic size of the lead-containing particles, the spherical particle's volume shape factor (α v = 0.52) in Equation (Equation1) was used, but for non-spherical particles the volume shape factor will always be less than this. Assuming the limiting value of 0.52 may be one of the reasons that the calculated dynamic shape factor was large. In any case, the SEM image data combined with an estimate of particle density and shape factor can be fit reasonably well to the number-weighted aerodynamic size distribution.
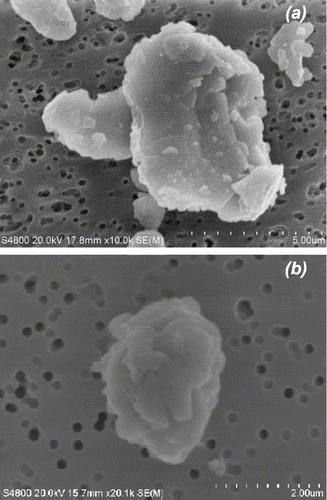
The fact that the two lead compounds might have very different dynamic shape factors is not unexpected. shows secondary electron images of a typical lead oxide and lead sulfide particle obtained at high magnification by a Field Emission Scanning Electron Microscopy (FESEM, Model S-4800–2, Hitachi High Technologies America Inc., Pleasanton, CA, USA). The shape of the lead oxide is flatter than that of the lead sulfide which gives lead oxide a greater dynamic shape factor. It also causes it to have a smaller volume shape factor. Together these factors could make lead oxide particles and sulfide particles of the same physical size demonstrate different aerodynamic behavior. CitationO'Brien et al. (1986) compared SEM derived physical size and APS measures of aerodynamic size distributions for an iron-containing aerosol. They noted that differences in measurements of size distribution of as much as a factor of two between APS and SEM. They attributed the difference to differences between the bulk density and the effective single particle density of the material they were studying. However, for the materials presented here, there is no a priori reason to assume that the individual particle density differs from the bulk material density.
FIG. 7 Secondary electron image of airborne lead oxide (a) and lead sulfide (b) obtained by the field emission scanning electron microscope.
There are limitations to generalizing the results of these experiments. The lead oxide and lead sulfide aerosols were generated by a fluidized bed generator from materials that were 200 mesh and smaller. Under the operating conditions used here very few large particles were transported from the fluidized bed generator into the sample chamber. If gravitational settling of very large particles (greater than 20 μm) onto the sampler walls were an issue, then these experiments would not reveal it because the aerosols used did not include very large particles. On the other hand, diffusion of smaller particles to the sampler walls or any other mechanism that preferentially deposited smaller particles on the walls would be apparent in these experiments.
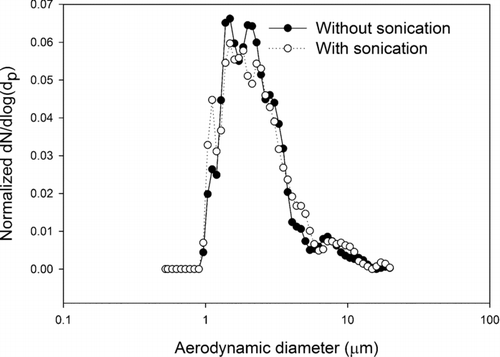
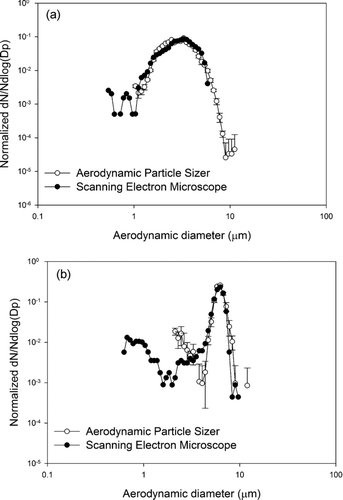
The extraction method of using the sonication and vortexing method as described in methodology section does not change the size distribution of the particle population on the filter. Two different number-weighted distributions of lead oxide particles for this experimental result are shown in . Two potential mechanisms could be at play, either the method could separate agglomerates that existed in the parent aerosol population, or the method might remove particles from the filter in a size dependent manner. To test the method, comparisons of particle size distributions from a single IOM filter, one half observed directly and one half sonicated and redeposited were performed. The exposure times were very brief, so that the particle loading was dense enough to make SEM images with a reasonable number of particles visible at a high magnification, but not so dense that there was a significant chance for overlap of particles. The measured size distributions are indistinguishable by visual inspection and by the Mann-Whitney U-test. This indicates that either there were no agglomerates in the original population or that the sonication process does not de-agglomerate any that existed. This does not resolve the general question as to whether or not sonication de-agglomerates. In the planned future studies of field samples it will be necessary to perform analogous sampling and measurements to determine if the sonication process changes the measurement of size distribution.
CONCLUSION
The size distributions of lead oxide and lead sulfide aerosols in the size range from 0.5 μm to 14 μm were investigated by SEM in order to determine if any difference between wall and filter deposits occurs when they are sampled by either IOM or CFC samplers. It was found that the number distributions of aerodynamic diameters were, for a given compound, not significantly different between wall and filter deposits.
The size distributions of filter deposits were compared between samplers, and it was found that for these particular lead compound samples, the two samplers performed similarly.
The aerodynamic equivalent diameter distributions of these aerosols as determined by an APS agree with the distributions calculated from the SEM images using bulk densities, spherical volumetric shape factors, and dynamic shape factors of 2.8 for lead oxide and 1.5 for lead sulfide. To further validate the SEM approach to calculating AEDs, an SEM determination of aerodynamic equivalent diameters of mono-disperse standard spheres in the 2 μm and 6 μm size range was performed and found to agree with measurements made by the APS and with values calculated from the nominal physical size and density using shape factors appropriate for spherical particles.
The method of ultrasonic and vortex removal of aerosol from sampler filter media into alcoholic suspension followed by redeposition onto filters suitable for microscopic analysis was demonstrated to not modify particle size distributions for the two lead compounds studied here.
This article not subject to United States copyright law.
Many thanks go to Paul Baron (NIOSH/DART) and William Lindsley (NIOSH/HELD) for reviewing this manuscript prior to journal submission.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
REFERENCES
- Casuccio , G. S. , Schlaegel , S. F. , Lersch , L. T. , Huffman , G. P. , Chen , T. and Shah , N. 2004 . Measurement of Fine Particulate Matter Using Electron Microscopy Techniques . Fuel Processing Technol. , 85 : 763 – 779 .
- Casuccio , G. S. 1983 . The Use of Scanning Electron Microscopy in Environmental Studies . J. Air Pollution Control Assoc. , 33 ( 10 ) : 937 – 948 .
- Cheng , Y. S. , Chen , B. T. and Yeh , H. C. 2009 . Behaviour of Isometric Nonspherical Aerosol Particles in the Aerodynamic Particle Sizer . J. Aerosol Sci. , 21 ( 5 ) : 701 – 710 .
- Kenny , L. C. Aitken , R. J. 1999 . The Sampling Efficiency of Personal Inhalable Aerosol Samplers in Low Air Movement Environments . J. Aerosol Science , 30 ( 5 ) : 627 – 638 .
- Kim , D. and Hopke , P. K. 1988 . Classification of Individual Particles Based on Computer–Controlled Scanning Electron-Microscopy Data . Aerosol Sci. Technol. , 9 ( 2 ) : 133 – 151 .
- Demange , M. , Gendre , J. C. , Herve-Bazin , B. , Carton , B. and Peltier , A. 1990 . Aerosol Evaluation Difficulties Due to Particle Deposition on Filter Holder Inner Walls . Ann. Occupat. Hyg. , 34 ( 4 ) : 399 – 403 .
- Demange , M. , Görner , P. , Elcabache , J. and Wrobel , R. 2002 . Field Comparison of 37–mm Closed–Face Cassettes and IOM Samplers . Appl. Occupat. Environ. Hyg. , 17 ( 3 ) : 200 – 208 .
- Feather , G. A. and Chen , B. T. 2003 . Design and Use of a Settling Chamber for Sampler Evaluation Under Calm-Air Conditions . Aerosol Sci. Technol. , 37 : 261 – 270 .
- Harper , M. and Demange , M. 2007 . Analytical Performance Criteria: Concerning Sampler Wall Deposits in the Chemical Analysis of Airborne Metals . J. Occupational and Environ. Hygiene , 4 : D81 – 86 .
- Harper , M. , Pacolay , B. , Hintz , P. and Andrew , M. 2006 . A Comparison of Portable XRF and ICP–OES Analysis for Lead on Air Filter Samples from a Lead Ore Concentrator Mill and a Lead–Acid Battery Recycler . J. Environ. Monitoring , 8 ( 3 ) : 384 – 392 .
- Hinds , W. C. 1999 . Aerosol Technology , New York : John Wiley & Sons Inc. .
- “ International Organization for Standardization: ISO 7708: Air Quality–Particle size fraction definitions for health-related sampling ” . Geneva, , Switzerland : ISO . http://www.iso.org (in series)
- Kenny , C. L. , Aitken , R. , Chalmers , C. , Fabries , J. F. , Gonzales-Fernandes , E. , Kromhout , H. , Liden , G. , Mark , D. , Riediger , G. and Prodi , V. 1997 . A Collaborative European Study of Personal Inhalable Aerosol Sampler Performance . Ann. Occupat. Hyg. , 41 : 135 – 153 .
- Lidén , G. and Harper , M. 2006 . The need for an international sampling convention for inhalable dust in calm air . J. Occupat. Environ. Hyg. , 3 ( 10 ) : D94 – D101 .
- Mark , D. and Vincent , J. H. 1986 . A New Personal Sampler for Airborne Total Dust in Workplaces . Ann. Occupat. Hyg. , 30 : 89 – 102 .
- O'Brien , D. , Baron , P. and Willeke , K. 1986 . Size and Concentration Measurement of an Industrial Aerosol . Amer. Indust. Hyg. Assoc. J. , 47 ( 7 ) : 168 – 392 .
- Stein , S. W. , Myrdal , P. B. , Gabrio , B. J. , Obereit , D. and Beck , T. J. 2003 . Evaluation of a New Aerodynamic Particle Sizer Spectrometer for Size Distribution Measurements of Solution Metered Dose Inhalers . J. Aerosol Med. , 16 ( 2 ) : 107 – 119 .
- Stones , F. , Edwards , S. , Crane , D. and Schultz , G. 2004 . “ The Deposition of Sample Analyte on Cassette Walls ” . Atlanta, GA : Poster Presentation, American Industrial Hygiene Conference & Exposition .
- Wang , H. C. and John , W. 1987 . Particle Density Correction for the Aerodynamic Particle Sizer . Aerosol Sci. Technol. , 6 : 191 – 198 .
- Witschger , O. , Grinshpun , S. A. , Fauvel , S. and Basso , G. 2004 . Performance of Personal Inhalable Aerosol Samplers in Very Slowly Moving Air When Facing the Aerosol Source . Ann. Occupat. Hyg. , 48 : 351 – 368 .