Abstract
Photo-absorption by black carbon (BC) aerosol is believed to be enhanced by the internal mixing of BC with volatile compounds. We investigate this effect quantitatively with laboratory experiments. Graphite particles are used as a surrogate for BC, with coatings of oleic acid and glycerol. The photo-absorption of coated graphite with known shell and core diameters are directly measured by a photo-acoustic technique. The size distribution and coating thickness are quantified using a single-particle soot photometer (SP2). The absorption is amplified by ∼30% even with a small coating thickness (shell/core diameter ratio (D p/D c) = 1.2). The amplification reaches as high as 2 at D p/D c = 2. We calculate the amplification of the absorption using a shell/core model of Mie theory. The model generally reproduces the measured amplification well.
1. INTRODUCTION
Black carbon (BC) aerosol, a by-product of incomplete combustion, is a strong absorber of visible solar radiation. Although BC generally constitutes a small fraction of the aerosol mass, its photo-absorption plays an important role in controlling atmospheric radiation. It influences climate on regional and global scales through heating (CitationIPCC 2007). Furthermore, the heating of clouds by BC may evaporate cloud droplets, leading to a reduction of the cloud albedo and warming of the Earth surface (CitationHansen et al. 1997; CitationAckerman et al. 2000). BC aerosol also accelerates the melting of polar ice and changes snow albedo when deposited on ice and snow (CitationHansen and Nazarenko 2004). Accurate estimates of photo-absorption by BC are needed to improve the assessment of these effects.
BC is believed to be hydrophobic and mostly externally mixed with non-refractory compounds upon emission (CitationShiraiwa et al. 2007; CitationSchwarz et al. 2008a); with time, however, it becomes hydrophilic and more internally mixed through condensation and coagulation. In polluted urban air, BC becomes internally mixed on a time scale of ∼ 12 h with a coating of organics and sulfate (CitationMoteki et al. 2007; CitationShiraiwa et al. 2007). BC aerosol was observed to be thickly coated with a shell/core diameter of 1.5 in air transported from the Asian continent in a few days (CitationShiraiwa et al. 2008).
The absorption of BC can be significantly enhanced by internal mixing with other compounds according to Mie theory (CitationBond et al. 2006). Aircraft measurements indicated that coatings enhance light absorption in the ambient BC column by at least 30% in the free troposphere (CitationSchwarz et al. 2008b). Photo-absorption by BC was estimated to be enhanced by a factor of 1.5–1.6 by internal mixing in the Asian outflow (CitationShiraiwa et al. 2008). This absorption enhancement effects has been widely inferred in previous ambient measurements without clear laboratory results. However, they are still not well understood and remain a significant uncertainty in our understanding of the optical properties of BC aerosols.
Direct measurements of the amplification of BC by internal mixing are still limited. Previous studies focused on the optical properties of coated BC with ozonolysis products of α -pinene (CitationSchnaiter et al. 2005), water (CitationMikhailov et al. 2006), oleic acid (CitationSlowik et al. 2007), wax (CitationGangl et al. 2008), and sulfate (CitationZhang et al. 2008; CitationKhalizov et al. 2009). In most of these studies, the photo-absorption coefficients were measured by taking the difference between the extinction and scattering coefficients. Photo-absorption was shown to be amplified by BC coating. However, we still need to characterize the amplification of the photo-absorption of coated BC as a function of coating thickness along with comparison with Mie theory in detail.
In this study, we measure the amplification of the photo-absorption by BC as a function of shell/core diameter ratio (i.e., coating thickness). Graphite particles are used as a surrogate for BC. The physical properties (i.e., refractive index, density) of graphite are similar to those of ambient BC (CitationBond and Bergstrom 2006). For example, the density and incandescence temperature of graphite are 2.28 g cm–3 (CitationMichelsen 2003) and ∼ 3500 K (CitationMoteki and Kondo 2007), respectively, whereas they are 2.0 g cm–3 (CitationSlowik et al. 2007) and ∼ 4000 K (CitationSchwarz et al. 2006), respectively, for ambient BC. Accurate measurement of absorption is required to quantify the absorption enhancement. Filter-based methods suffer from multiple scattering caused by the filter and artificial changes to the mixing state of aerosol on the filter (CitationCappa et al. 2008; CitationLack et al. 2008; CitationKondo et al. 2009). Estimation of absorption by the difference between extinction and scattering leads to large uncertainties (CitationAndreae and Gelencser 2006). In this study, the absorption is measured directly using the photoacoustic technique (CitationArnott et al. 2003), which can provide non-destructive, airborne measurements. BC particles with known core diameters are coated with organic compounds using a tandem differential mobility analyzer (TDMA) system (CitationMoteki and Kondo 2007). The coating thickness and size distribution of coated particles are measured using a single-particle soot photometer (SP2). The amplification of absorption is measured as a function of the coating thickness and compared with model calculations based on Mie theory.
2. INSTRUMENTATION
2.1. Shape and Optical Properties of Graphite Particles
Graphite particles (Alfa Aesar, Inc., Ward Hill, MA, USA) used in this study were observed to be non-aggregates by transmittance electron microscopy (TEM) (, CitationMoteki and Kondo 2007), suggesting that their shapes changed very little by the condensation of organics (CitationMoteki and Kondo 2007). The mass-equivalent diameters (D c) of graphite particles with given mobility diameters were measured using a differential mobility analyzer (DMA)—aerosol particle mass analyzer (APM) system (CitationMcMurry et al. 2002). D c is calculated as D c = (6M/π ρ)1/3, where M is particle mass and ρ is a graphite particle density of 2.28 g cm–3 (CitationMichelsen 2003). D c with mobility diameters of 150, 200, 250, and 300 nm were measured to be 123, 185, 234, and 281 nm, respectively. The dynamic shape factors were calculated to be ∼ 1.2, indicating that they were non-spherical. Note that graphite particles sampled by SP2 in the BC coating experiments (Section 3) range from 100 to 430 nm and the evaluation of the graphite shape factor did not fully cover this range.
The refractive index (m) of graphite was reported to be m graphite = 2.65–1.39i at a wavelength (λ) of 533 nm (CitationStagg and Charalampopoulos, 1993). The real and imaginary parts of the refractive index given by CitationBond and Bergstrom (2006) are in the range of 2.0–3.3 and 0.7–2.1, respectively.
For the interpretation of the measurements, the absorption cross section (σabs) of graphite was calculated by Mie theory assuming that the particles were dense and spherical. To evaluate the uncertainties due to this assumption, graphite particles were also assumed to not be dense but mixed with air voids in the particle mobility diameter (CitationBond et al. 2006). The volume fraction of air was calculated to be 20–45% for diameters of 150–300 nm. The refractive index of air-included graphite (m graphite-air) was computed using the Maxwell-Garnett theory of volume mixing model (CitationBohren and Huffman 1983), assuming an m air of 1.0–0.0i, resulting in an m graphite-air of 2.0–0.9i. To estimate the maximum uncertainty, a volume fraction of air of 45% was assumed. This value is in the range of values reported by CitationBond and Bergstrom (2006). In calculating σabs, we used both m graphite and m graphite-air to give a measure of the uncertainties of m and the non-sphericity.
2.2. PASS
The absorption coefficients (b abs) of graphite particles were measured using a Photo-Acoustic Soot Spectrometer Single-Wavelength (PASS-1) instrument (Droplet Measurement Technologies, Boulder, CO). In the PASS, sample air is irradiated by Nd:YAG laser light (λ = 532 ± 0.05 nm) modulated at about 1500 Hz, the acoustic resonance frequency of the cell. Light absorption by the sampled air causes periodic heating of the air to generate an acoustic wave in the resonator. The amplitude of the acoustic wave is detected by a microphone. By using a calibrated microphone and the simple linear relationship between acoustic signal and the light-absorption source, absorption coefficients can be obtained. The relative humidity of the air at the PASS was around 30% during the experiments. There was no significant RH change during measurements. More detailed descriptions of PASS have been given elsewhere (CitationArnott et al. 2000, Citation2003).
The PASS used in this study was calibrated using gaseous nitrogen dioxide (NO2) and ozone (O3) as light-absorbing substances (CitationArnott et al. 2000). NO2 was produced by titration of NO with O3. The NO2 concentration was determined from the mixing ratio of NO in N2 gas (5.04 ppm) and the fraction of NO oxidized using a commercial NO monitor (Thermoelectron, Model 42iTL). The overall accuracy of the measured b abs for NO2 was 24%, considering the uncertainties of the laser wavelength, NO2 absorption cross section, and the concentration of the NO2 gas. In addition, the PASS was also calibrated using O3. The variability of the absorption cross section of O3 is much smaller than NO2 in the 532 nm wavelength region. O3 was produced with Hg lamps and its concentration was measured with a UV-photometer (Dylec Model 1193) that had been calibrated with a NIST standard (CitationKondo et al. 2008). By comparing ozone concentrations at the inlet and outlet of the resonance cell in the PASS instrument, the ozone loss in the cell was estimated to be 3 ± 0.5%. We have considered this ozone loss in the calibration data analysis. The overall accuracy of the b abs measured using O3 was estimated to be 7%. The calibrated sensitivities using NO2 and O3 agreed to within 1%.
The photoacoustic response of the PASS to light-absorbing particles was measured using uncoated graphite particles. Mono-disperse graphite particles with D c of 185, 234, and 281 nm were generated using a DMA-APM system. The size transmission width of this system was ∼ 10%. It should be noted that multiply charged particles that had passed through the DMA were rejected by the APM and did not influence the measurement. The number concentration of mono-disperse graphite particles was monitored with a condensation particle counter (CPC).
shows the observed b abs versus number concentration of graphite particles, showing a good linear relationship (r 2 > 0.91 in all cases). The absorption cross section of graphite per particle, σabs,mea, was derived from the slope of the regression line. summarizes the obtained values. The uncertainty of the measured σabs,mea was estimated to be about 8%, considering the uncertainties of the number concentration (∼ 3%) and b abs (∼ 7%). The mass absorption cross section (C abs) was also derived assuming a graphite density of 2.28 g cm–3.
TABLE 1 Measured and calculated absorption cross sections (σabs) and mass absorption cross sections (C abs) of graphite particles. The units of σabs are 10–12 m2, C abs are m2 g–1
FIG. 1 Absorption coefficient (b abs) of uncoated graphite particles with D c of 185, 234, and 281 nm. Model calculations by Mie theory are also shown.
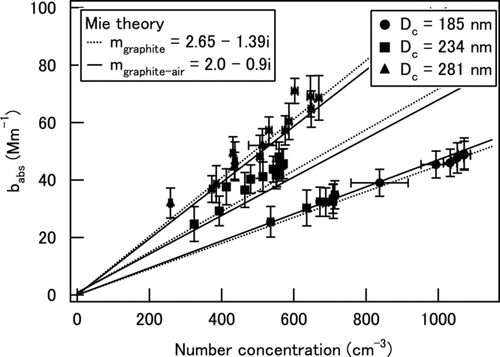
The σabs and C abs were calculated using a homogeneous sphere model of Mie theory (CitationBohren and Huffman 1983). The calculated σabs and C abs values using both m graphite and m graphite-air are shown in . The two model-calculated σabs,cal and C abs,cal agreed to within 5%. The σabs,cal (C abs,cal) agreed with σabs,mea (C abs,cal) to within 10%, which is similar to the uncertainty of the measurements. These results show the consistency of the b abs values measured by PASS and calculated by Mie theory for bare graphite particles.
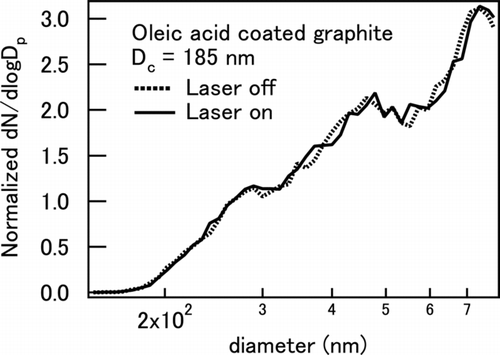
If mass transfer from particles to gas occurs, the b abs of particles measured with the PASS may be underestimated significantly because of the latent heat (CitationRaspet et al. 2003). Therefore, the absorption measurement of coated BC particles by PASS might be influenced by evaporation of volatile components of the coated BC (CitationArnott et al. 2003). If coating materials (i.e., glycerol and oleic acid) evaporate from BC by its heating through energy absorption of the laser beam, it will lead to a reduction of the photo-acoustic signal and cause an additional uncertainty in the photo-absorption measurement. In order to assess this effect, the size distributions of coated graphite particles were measured downstream of the PASS using a DMA–CPC system. The laser power of the PASS was turned on and off to measure the difference in the size distribution. shows an example of the number size distribution of oleic acid–coated graphite (D c = 185 nm). The number size distribution was normalized by total number concentration as (dN/dlogD p)/N. No significant changes were detected by the absorption of the laser beam. These comparisons were made with various D c (100–400 nm) and coating thicknesses (0–400 nm), showing no detectable changes in the size distribution. These results show that coated oleic acid and glycerol did not evaporate significantly by irradiation of the BC particles by the laser beam.
2.3. SP2
The number and mass size distributions and mixing state of graphite particles were measured using an SP2 based on the laser-induced incandescence (LII) technique. The basic measurement principle and schematic diagrams of the SP2 have been described previously (CitationGao et al. 2007; CitationMoteki and Kondo 2007), and the detailed calibration of the SP2 was discussed previously (CitationShiraiwa et al. 2008).
In brief, graphite particles are introduced to the Nd:YAG laser (λ = 1064 nm). They elastically scatter and absorb laser light. They are heated by absorption to their vaporization temperature and incandesce (producing the LII signal). The incandescent light is detected by photo-multiplier tubes, which are calibrated using graphite particles. The intensity of the LII signal is a function of BC mass (M). The calibration of the LII detector was made using uncoated BC that was mass-selected by the APM. The mass-equivalent diameter of the BC cores (D c) was calculated by assuming a density (ρ) of the graphite particles of 2.28 g cm–3 (CitationMichelsen 2003). The scatter of the distribution of the LII signal for mono-disperse graphite particles leads to an uncertainty of D c of 10%. The detection range of D c was 100–430 nm, with a detection efficiency of almost 100%. Particles larger than 430 nm were detected but not sized because the LII detector was saturated.
The scattering signal is detected by a two-element avalanche photodiode (CitationGao et al. 2007), which is calibrated using polystyrene latex (PSL) particles. The scattering signal is a function of the scattering cross section of the particles. The conversion of scattering cross section to shell diameter (D p) was made using the shell/core model of Mie theory, with an uncertainty of about 15% in the size range of 200–750 nm. This uncertainty was mainly caused by uncertainties in the refractive index of graphite and coating materials (CitationShiraiwa et al. 2008).
The mixing state, or shell/core diameter ratio (D p/D c), was calculated by dividing the shell diameter (D p) by the mass-equivalent diameter (D c). Considering the propagation of uncertainties of D c and D p, the uncertainty of R is 18%. Coating thickness is characterized as (D p – D c)/2.
3. EXPERIMENTAL SETUP
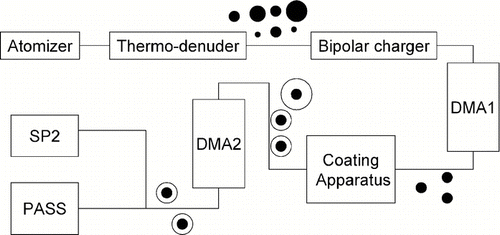
Photo-absorption of uncoated and coated graphite particles at given BC diameters and coating thickness were investigated using the experimental setup depicted in . The system consists of an atomizer, a tandem differential mobility analyzer (TDMA) with a vapor condensing system (coating apparatus) (CitationMoteki and Kondo 2007), PASS, and SP2. Particles generated by the atomizer were heated to 400°C to remove non-refractory compounds by the thermo-denuder. The coating apparatus was bypassed for producing uncoated graphite particles. DMAs were used to select the size of both the core and shell mobility diameters, and the APM was not used in this experiment. The coating thickness was changed by controlling the temperature of the oil bath of the coating apparatus. The sheath/sample flow ratio of the DMA was maintained at 8 for core mobility diameter selection. It was maintained at 3 for shell mobility diameter selection in order to maintain the high number concentrations of coated graphite particles necessary for good signal-to-noise in the PASS. Considering the transfer function of the DMA, the transmission widths of the core and shell mobility diameters were about ± 15% and ± 30%, respectively. The overall uncertainty of the shell/core mobility diameter ratio was estimated to be 34% by considering the propagation of uncertainties. Coated graphite particles with D c of 185, 234, and 281 nm and coating thicknesses of 0–300 nm were produced. The D p/D c ratios of particles were in the range of 1–2.5, which covers the ratios observed in the tropical atmosphere (CitationSchwarz et al. 2008b) and East Asia (CitationShiraiwa et al. 2008). The absorption coefficients (b abs) of coated graphite particles were measured by PASS. Core and shell diameters (D c and D p) were determined by SP2 and these values were used in the analysis of absorption amplification. The terminology used in this experiment is summarized in .
TABLE 2 Terminology of effective diameters used in this study
FIG. 3 Schematic of the laboratory experiment for measuring absorption of organics-coated graphite particles. The system uses differential mobility analyzers (DMA1 and DMA2), a single particle soot photometer (SP2), and a photo-acoustic absorption spectrometer (PASS).
Glycerol and oleic acid were used as coating materials. The refractive indices of coatings of glycerol and oleic acid at λ = 589 nm have been reported to be 1.47 – 0i (CitationThe Chemical Society of Japan 1993) and 1.46 – 0i (CitationJapan Oil Chemist's Society 2001), respectively. The refractive index of organics at λ = 550 nm was reported to be 1.45 – 0i (Krekov 1993), suggesting that the optical properties of glycerol and oleic acid are similar to that of organics in general.
4. EXPERIMENTAL RESULTS
4.1. Size Distribution and Mixing State
Graphite particles that passed through DMA1 () were not purely mono-disperse but poly-disperse to some extent due to multiple charging. Therefore the size distribution of the graphite particles after passing through DMA2 was also not purely mono-disperse, as measured by the SP2 downstream of DMA2 (). shows the number size distribution of graphite particles coated by oleic acid measured by the SP2 for graphite particles initially selected for a mobility core diameter of 200 nm by DMA1. The size distributions are shown for different shell mobility diameters of 200, 300, 400, and 500 nm selected by DMA2. Again, the size distributions were normalized by the total graphite number concentrations. The maximum D c measured by SP2 was 430 nm, and particles larger than 430 nm were detected but not sized, as discussed in Section 2.3. The diameters of these particles were assumed to be 430 nm in the presented size distributions and further analysis.
FIG. 4 (a) Distribution of graphite mass-equivalent core diameter (D c) and (b) distribution of shell/core diameter ratio (D p/D c) of oleic acid-coated graphite particles with D c of 185 nm and the shell mobility diameters of 200, 300, 400, and 500 nm selected by DMA2.
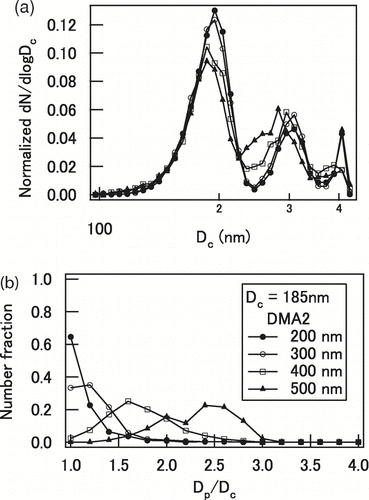
The core mobility diameter of 200 nm corresponds to a D c of 185 nm as discussed in Section 2.1. The peak at D c = 185 nm corresponds to singly charged particles and the peaks at D c = 280 and 400 nm correspond to doubly and triply charged particles, respectively. The normalized size distribution of D c depended little on the shell mobility diameter selection by DMA2. The average number ratios of singly, doubly, and triply charged particles (D c = 185 nm) were 68–74, 21–25, and 5–7%, respectively. The number fractions of the singly charged particles were larger than 85% for the graphite particles with 250 and 300 nm core mobility diameters selected by DMA1. The sampling of multiply charged particles influences the absorption measurements, which is further discussed in Section 4.2.
depicts the number fraction of the particles versus D p/D c of singly charged particles (D c = 185(± 30) nm) obtained by SP2. For uncoated graphite particles, the fraction of particles peaked at D p/D c = 1, as expected. With the increase in the shell mobility diameter selection by the DMA2 from 300 to 500 nm, the median of D p/D c measured by SP2 shifted from 1.2 to 2.4. The shell/core mobility diameter ratio of particles can be obtained by TDMA. The difference between shell/core mobility diameter ratio and D p/D c measured by SP2 was ∼ 15%, which is within the range of the uncertainties of the TDMA (∼ 34%) and SP2 (∼ 18%). Note that D p/D c ratios measured by SP2 are used for further analysis, because the distribution of D p/D c can be taken into account in the analysis. The number fraction of the particles versus D p/D c of multiply charged particles (D c = 280 and 400 nm) was also investigated. Their D p/D c ratios also increased with the increase in the shell mobility diameters selected by DMA2. The difference in the median D p/D c between singly and multiply charged particles was ∼ 6 (± 11)%.
4.2. Enhanced Absorption by Coatings
The mass absorption cross section C abs was derived by dividing b abs by the mass concentrations of graphite particles. For example, C abs of uncoated graphite particles was 5.1 (± 0.9) m2 g–1 at D c = 185 nm. C abs increased with D p/D c, becoming ∼ 10 (± 1.9) m2 g–1 at D p/D c = 2.
The absorption measurements were influenced by multiply charged particles, and the uncertainty in C abs was estimated as follows. As a first step, we calculated the mass absorption cross section of singly (D c = 185 nm), doubly (D c = 280 nm), and triply (D c = 400 nm) charged uncoated graphite particles using m graphite and m graphite-air. They were 6.1 ± 0.1, 3.7 ± 0.1, and 2.3 ± 0.1 m2 g–1, respectively, each of which being an average value between m graphite and m graphite-air. As D c is based on BC mass measurement, we can calculate the relative mass of individual multiply charged particles with respect to singly charged particles. Also considering the number fraction of multiply charged particles, the fraction of the observed absorption due to singly charged particles was calculated to be 55%, which shows the significant contribution (45%) of multiply charged particles to the observed absorption. The theoretical C abs was defined as the ratio of theoretical absorption to graphite mass for the observed size distribution. It was calculated to be 4.3 m2 g–1, which is lower by 19% than the observed C abs of 5.1 (± 0.9) m2 g–1. In the same way, for D c = 234 and 281 nm the fraction of the observed absorption due to singly charged particles was calculated to be 71 and 78%, respectively. The differences between theoretical and observed C abs were 10 and 18%, respectively. We assume that 10–19% differences give a measure of the uncertainty of C abs.
For coated graphite particles, it is difficult to estimate uncertainties of C abs in the same way, because absorption amplification by coatings should be assumed to calculate the theoretical C abs of coated graphite particles, leading to a circular argument. Here we assume the uncertainties for coated graphite particles are the same as bare graphite particles. The estimated uncertainties are summarized in .
TABLE 3 Uncertainties of calculated and observed absorption coefficient (b abs), mass absorption cross section (C abs), and absorption amplification factor (γ)
The absorption amplification factor (γ) was calculated by the ratios of C abs (D c) for coated and uncoated graphite particles. The derived γ is plotted in for D c = (a) 185 nm, (b) 234 nm, and (c) 281 nm as functions of D p/D c ratio and coating thickness ((D p–D c)/2). The error of γ was estimated to be ∼ 25%, caused by the propagation of the error of C abs. The bottom axis is the median D p/D c ratio of singly charged particles. The horizontal bars of the median D p/D c represent the uncertainty caused by the distribution of D p/D c, as discussed in Section 4.1.
FIG. 5 Observed absorption amplification factor (γ) for organics-coated graphite particles with D c of (a) 185, (b) 234, and (c) 281 nm. The bottom axis shows the median of the shell/core diameter ratio (D p/D c) and the top axis shows the coating thickness (nm). Shell/core model calculations are also shown for m graphite and m graphite-air.
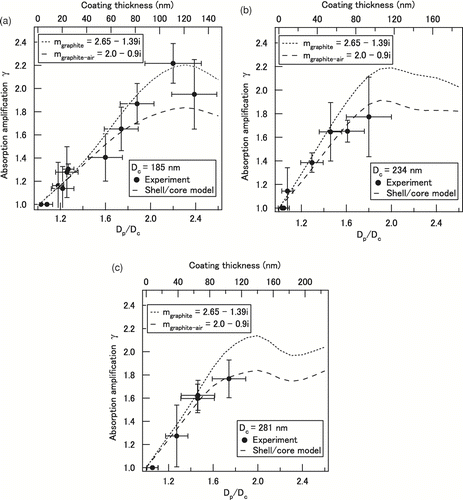
The absorption increased by about 30% even with a thin coating of D p/D c≈ 1.2. Much larger enhancements were observed at larger D p/D c ratios. The maximum γ was about 2.2 when D p/D c reached about 2.2. This is a clear demonstration of the effect of amplification of photo-absorption by the coating of BC particles. The maximum γ of 2.0 was also observed by soot coated with secondary organic aerosol (CitationSchnaiter et al. 2005).
The photo-absorption increased monotonically with the increase in the D p/D c ratios for graphite particles. However, BC particles in the atmosphere have fractal structures in general. The collapse of aggregates due to the capillary force of coatings can lead to reduction in the absorption, because some spherules that make up aggregates become screened by other spherules in compact forms (CitationSchnaiter et al. 2005; CitationBond et al. 2006). Indeed no significant amplification was observed for flame-generated soot aggregates coated with 10–50-nm thick oleic acid (CitationSlowik et al. 2007). Therefore, for a more realistic assessment of photo-absorption by ambient BC, this factor has to be taken into account at small D p/D c ratios.
4.3. Comparison with Mie Theory
We now compare the model calculations based on Mie theory with the laboratory measurements. The C abs and γ were calculated using the shell/core model of Mie theory (CitationBohren and Huffman 1983), which was reported to be a good proxy for internally mixed BC with arbitrary eccentricity (CitationSchnaiter et al. 2005).
shows the calculated γ using the shell/core model with m graphite-air and m graphite for D c = 185, 234, and 281 nm. The calculated γ for m graphite-air is ∼ 15% smaller than that for m graphite in both models. At D c = 185 nm, the model agreed with the observed γ reasonably well. At D c = 234 and 281 nm, the shell/core model reproduced well the observed γ. Overall, the γ calculated by the shell/core model agreed with that observed within the uncertainties of the observed γ (y-axis) and the median of D p/D c (x-axis).
The b abs was also calculated by the shell/core model using the observed size distribution and mixing state of graphite particles. Particles larger than 430 nm were detected but not sized by SP2 and these particles (ca. 1–5% in number fraction) were assumed to be 430 nm for the present calculation, which would lead to an underestimation of the calculated b abs. For uncoated graphite particles, b abs calculated using m graphite-air and m graphite agreed to within ∼ 3%. The calculated b abs reproduced the observed b abs well (r 2 > 0.95), with slopes of 0.76 (m graphite) and 0.78 (m graphite-air) as shown in . For coated graphite, b abs values were calculated by the shell/core model of Mie theory. We fully took into account the co-existence of the singly and multiply charged particles for this calculation using the SP2 measurements. The uncertainties of D p/D c and dN/dlogD c measured by SP2 resulted in the 15% uncertainty of the calculated b abs.
FIG. 6 Comparison of the b abs (Mm–1) calculated using Mie theory and that observed by PASS for (a) uncoated and (b) coated graphite particles. For coated graphite, b abs was calculated assuming the shell/core model and no absorption enhancement. The dotted 1:1 line is a visual guide.
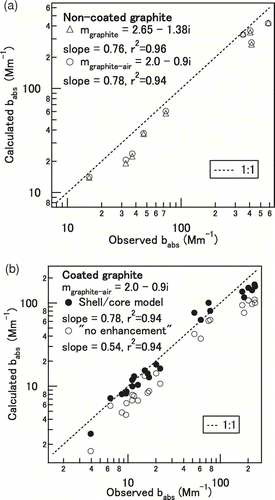
shows the correlation between the calculated b abs using m graphite-air and the observed b abs. In this figure, the calculations assuming D p/D c = 1 (“no enhancement”) are also shown for comparison. When the enhancement of absorption is not considered, the model significantly underestimates the observed b abs, with a slope of 0.54. On the other hand, the shell/core model reproduced well the observed b abs, with a slope of 0.78. The calculated b abs underestimated observed b abs by 22% on average. This difference is within the uncertainties of the observed b abs (∼ 7%) and calculated b abs (∼ 15%) as summarized in . It should be stressed here that the model reproduced observed values reasonably well only if the enhancement of absorption was taken into account. It has thus been shown that the shell/core model can be used to estimate the absorption properties of coated BC with reasonable accuracy.
5. SUMMARY AND CONCLUSION
The enhancement of photo-absorption of BC by coating with volatile organic compounds was measured in the laboratory. We used graphite particles as BC and oleic acid and glycerol as coating materials. The size distribution of BC and the shell/core diameter ratio (D p/D c) were quantified using an SP2. The absorption coefficients of airborne graphite particles were measured by a photo-acoustic absorption spectrometer (PASS) instrument, without significant evaporation of the coatings. Model calculations using Mie theory agreed well with the measured absorption coefficients for uncoated graphite particles.
The mass absorption cross section (C abs) increased with increasing coating thickness. It increased by about 30% at D p/D c = 1.2 and by a factor of about 2 at D p/D c∼ 2. This is a clear demonstration of the effect of amplification of photo-absorption by the coating of BC particles. The shell/core model calculations based on Mie theory (λ = 532 nm) well reproduced the measured amplification of photo-absorption by internal mixing, indicating that the shell/core model can be used to estimate the absorption properties of coated BC with reasonable accuracy.
BC particles in the atmosphere have fractal structures and have different compositions from graphite. For a more precise assessment of photo-absorption by ambient BC, this factor has to be taken into account.
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology—Japan (MEXT), the global environment research fund of the Japanese Ministry of the Environment (B-083), and the Japanese Science and Technology Agency (JST). The authors thank N. Takegawa, N. Moteki, and M. Kuwata for their support and stimulating discussions.
REFERENCES
- Ackerman , A. S. , Toon , O. B. , Stevens , D. E. , Heymsfield , A. J. , Ramanathan , V. and Welton , E. J. 2000 . Reduction of Tropical Cloudiness by Soot . Sci. , 288 ( 5468 ) : 1042 – 1047 .
- Andreae , M. O. and Gelencser , A. 2006 . Black Carbon or Brown Carbon? The Nature of Light-Absorbing Carbonaceous Aerosols . Atmos. Chem. Phys. , 6 : 3131 – 3148 .
- Arnott , W. P. , Moosmuller , H. , Sheridan , P. J. , Ogren , J. A. , Raspet , R. , Slaton , W. V. , Hand , J. L. , Kreidenweis , S. M. and Collett , J. L. 2003 . Photoacoustic and Filter-Based Ambient Aerosol Light Absorption Measurements: Instrument Comparisons and the Role of Relative Humidity . J. Geophys. Res.—Atmospheres , 108 ( D1 ) : 4034 DOI: 10.1029/2002jd002165
- Arnott , W. P. , Moosmuller , H. and Walker , J. W. 2000 . Nitrogen Dioxide and Kerosene–Flame Soot Calibration of Photoacoustic Instruments for Measurement of Light Absorption by Aerosols . Review of Scientific Instruments , 71 ( 12 ) : 4545 – 4552 .
- Bohren , C. F. and Huffman , D. R. 1983 . Absorption and Scattering of Light by Small Particles , New York : John Wiley and Sons .
- Bond , T. C. and Bergstrom , R. W. 2006 . Light Absorption by Carbonaceous Particles: An Investigative Review . Aerosol Sci. Technol , 40 ( 1 ) : 27 – 67 . DOI: 10.1080/02786820500421521
- Bond , T. C. , Habib , G. and Bergstrom , R. W. 2006 . Limitations in the Enhancement of Visible Light Absorption due to Mixing State . J. Geophys. Res.—Atmospheres , 111 ( D20 ) : D20211 DOI: 10.1029/2006jd007315
- Cappa , C. D. , Lack , D. A. , Burkholder , J. B. and Ravishankara , A. R. 2008 . Bias in Filter-Based Aerosol Light Absorption Measurements Due to Organic Aerosol Loading: Evidence from Laboratory Measurements . Aerosol Sci. Technol. , 42 ( 12 ) : 1022 – 1032 . DOI: 10.1080/02786820802389285
- Gangl , M. , Kocifaj , M. , Videen , G. and Horvath , H. 2008 . Light Absorption by Coated Nano-Sized Carbonaceous Particles . Atmos. Environment , 42 ( 11 ) : 2571 – 2581 .
- Gao , R. S. , Schwarz , J. P. , Kelly , K. K. , Fahey , D. W. , Watts , L. A. , Thompson , T. L. , Spackman , J. R. , Slowik , J. G. , Cross , E. S. , Han , J. H. , Davidovits , P. , Onasch , T. B. and Worsnop , D. R. 2007 . A Novel Method for Estimating Light-Scattering Properties of Soot Aerosols Using a Modified Single-Particle Soot Photometer . Aerosol Sci. Technol. , 41 ( 2 ) : 125 – 135 . DOI: 10.1080/02786820601118398
- Hansen , J. and Nazarenko , L. 2004 . Soot Climate Forcing Via Snow and Ice Albedos . Proceedings of the National Academy of Sci. of the United States of America , 101 ( 2 ) : 423 – 428 . DOI: 10.1073/pnas.2237157100
- Hansen , J. , Sato , M. and Ruedy , R. 1997 . Radiative Forcing and Climate Response . J. Geophys. Res.—Atmospheres , 102 ( D6 ) : 6831 – 6864 .
- IPCC . 2007 . Climate Change 2007: The Physical Science Basis , Cambridge, , UK : Cambridge University Press . Contribution of Working Group 1 to the 4th Assessment Report of the IPCC
- Japan Oil Chemist's Society . 2001 . “ Lipid and Surfactants ” . In Handbook of Oil Chemistry, , 4th ed. , Tokyo, , Japan : Maruzen .
- Khalizov , A. F. , Xue , H. X. , Wang , L. , Zheng , J. and Zhang , R. Y. 2009 . Enhanced Light Absorption and Scattering by Carbon Soot Aerosol Internally Mixed with Sulfuric Acid . J. Phys. Chem. A , 113 ( 6 ) : 1066 – 1074 . DOI: 10.1021/jp807531n
- Kondo , Y. , Morino , Y. , Fukuda , M. , Kanaya , Y. , Miyazaki , Y. , Takegawa , N. , Tanimoto , H. , McKenzie , R. , Johnston , P. , Blake , D. R. , Murayama , T. and Koike , M. 2008 . Formation and Transport of Oxidized Reactive Nitrogen, Ozone, and Secondary Organic Aerosol in Tokyo . J. Geophys. Res.—Atmospheres , 113 ( D21 ) : D21310 DOI: 10.1029/2008jd010134
- Kondo , Y. , Sahu , L. , Kuwata , M. , Miyazaki , Y. , Takegawa , N. , Moteki , N. , Imaru , J. , Han , S. , Nakayama , T. , KimOanh , N. T. , Hu , M. , Kim , Y. J. and Kita , K. 2009 . Stabilization of the Mass Absorption Cross Section of Black Carbon for Filter-Based Absorption Photometry by the use of a Heated Inlet . Aerosol Sci. Technol. , 43 ( 8 ) : 741 – 756 .
- Lack , D. A. , Cappa , C. D. , Covert , D. S. , Baynard , T. , Massoli , P. , Sierau , B. , Bates , T. S. , Quinn , P. K. , Lovejoy , E. R. and Ravishankara , A. R. 2008 . Bias in Filter-Based Aerosol Light Absorption Measurements due to Organic Aerosol Loading: Evidence from Ambient Measurements . Aerosol Sci. Technol. , 42 ( 12 ) : 1033 – 1041 . DOI: 10.1080/02786820802389277
- McMurry , P. H. , Wang , X. , Park , K. and Ehara , K. 2002 . The Relationship Between Mass and Mobility for Atmospheric Particles: A New Technique for Measuring Particle Density . Aerosol Sci. Technol. , 36 ( 2 ) : 227 – 238 .
- Michelsen , H. A. 2003 . Understanding and Predicting the Temporal Response of Laser-Induced Incandescence from Carbonaceous Particles . J. Chemical Physics , 118 ( 15 ) : 7012 – 7045 . DOI: 10.1063/1.1559483
- Mikhailov , E. F. , Vlasenko , S. S. , Podgorny , I. A. and Ramanathan , V. 2006 . Optical Properties of Soot-Water Drop Agglomerates: An Experimental Study . J. Geophys. Res.—Atmospheres , 111 ( D7 ) : 16 DOI: 10.1029/2005jd006389
- Moteki , N. and Kondo , Y. 2007 . Effects of Mixing State on Black Carbon Measurements by Laser-Induced Incandescence . Aerosol Sci. Technol. , 41 ( 4 ) : 398 – 417 . DOI: 10.1080/02786820701199728
- Moteki , N. , Kondo , Y. , Miyazaki , Y. , Takegawa , N. , Komazaki , Y. , Kurata , G. , Shirai , T. , Blake , D. R. , Miyakawa , T. and Koike , M. 2007 . Evolution of Mixing State of Black Carbon Particles: Aircraft Measurements Over the Western Pacific in March 2004 . Geophys. Res. Letters , 34 ( 11 ) : L11803 DOI: 10.1029/2006gl028943
- Raspet , R. , Slaton , W. V. , Arnott , W. P. and Moosmuller , H. 2003 . Evaporation-Condensation Effects on Resonant Photoacoustics of Volatile Aerosols . J. Atmos. and Oceanic Technol. , 20 ( 5 ) : 685 – 695 .
- Schnaiter , M. , Linke , C. , Möhler , O. , Naumann , K. H. , Saathoff , H. , Wagner , R. , Schurath , U. and Wehner , B. 2005 . Absorption Amplification of Black Carbon Internally Mixed with Secondary Organic Aerosol . J. Geophys. Res.—Atmospheres , 110 ( D19 ) : D19204 DOI: 10.1029/2005jd006046
- Schwarz , J. P. , Gao , R. S. , Fahey , D. W. , Thomson , D. S. , Watts , L. A. , Wilson , J. C. , Reeves , J. M. , Darbeheshti , M. , Baumgardner , D. G. , Kok , G. L. , Chung , S. H. , Schulz , M. , Hendricks , J. , Lauer , A. , Karcher , B. , Slowik , J. G. , Rosenlof , K. H. , Thompson , T. L. , Langford , A. O. , Loewenstein , M. and Aikin , K. C. 2006 . Single-Particle Measurements of Midlatitude Black Carbon and Light-Scattering Aerosols from the Boundary Layer to the Lower Stratosphere . J. Geophys. Res.—Atmospheres , 111 ( D16 ) DOI: 10.1029/2006jd007076
- Schwarz , J. P. , Gao , R. S. , Spackman , J. R. , Watts , L. A. , Thomson , D. S. , Fahey , D. W. , Ryerson , T. B. , Peischl , J. , Holloway , J. S. , Trainer , M. , Frost , G. J. , Baynard , T. , Lack , D. A. , de Gouw , J. A. , Warneke , C. and Del Negro , L. A. 2008a . Measurement of the Mixing State, Mass, and Optical Size of Individual Black Carbon Particles in Urban and Biomass Burning Emissions . Geophys. Res. Lett. , 35 ( 13 ) : L13810 DOI: 10.1029/2008gl033968
- Schwarz , J. P. , Spackman , J. R. , Fahey , D. W. , Gao , R. S. , Lohmann , U. , Stier , P. , Watts , L. A. , Thomson , D. S. , Lack , D. A. , Pfister , L. , Mahoney , M. J. , Baumgardner , D. , Wilson , J. C. and Reeves , J. M. 2008b . Coatings and Their Enhancement of Black Carbon Light Absorption in the Tropical Atmosphere . J. Geophys. Res.—Atmospheres , 113 ( D3 ) : D03203 DOI: 10.1029/2007jd009042
- Shiraiwa , M. , Kondo , Y. , Moteki , N. , Takegawa , N. , Miyazaki , Y. and Blake , D. R. 2007 . Evolution of Mixing State of Black Carbon in Polluted Air from Tokyo . Geophys. Res. Lett. , 34 ( 16 ) : L16803 DOI: 10.1029/2007gl029819
- Shiraiwa , M. , Kondo , Y. , Moteki , N. , Takegawa , N. , Sahu , L. K. , Takami , A. , Hatakeyama , S. , Yonemura , S. and Blake , D. R. 2008 . Radiative Impact of Mixing State of Black Carbon Aerosol in Asian Outflow . J. Geophys. Res.—Atmospheres , 113 : D24210 DOI: 10.1029/2008jd010546
- Slowik , J. G. , Cross , E. S. , Han , J. H. , Davidovits , P. , Onasch , T. B. , Jayne , J. T. , Williams , L. R. , Canagaratna , M. R. , Worsnop , D. R. , Chakrabarty , R. K. , Moosmuller , H. , Arnott , W. P. , Schwarz , J. P. , Gao , R. S. , Fahey , D. W. , Kok , G. L. and Petzold , A. 2007 . An Inter-Comparison of Instruments Measuring Black Carbon Content of Soot Particles . Aerosol Sci. Technol. , 41 ( 3 ) : 295 – 314 . DOI: 10.1080/02786820701197078
- Stagg , B. J. and Charalampopoulos , T. T. 1993 . Refractive-Indexes of Pyrolitic-Graphite, Amorphous-Carbon, and Flame Soot in the Temperature-Range 25-degrees-C TO 600-degrees-C . Combustion and Flame , 94 ( 4 ) : 381 – 396 .
- The Chemical Society of Japan . 1993 . Handbook of Chemistry, , Basic 4th ed. , Tokyo, , Japan : Maruzen .
- Zhang , R. Y. , Khalizov , A. F. , Pagels , J. , Zhang , D. , Xue , H. X. and McMurry , P. H. 2008 . Variability in Morphology, Hygroscopicity, and Optical Properties of Soot Aerosols During Atmospheric Processing . Proceedings of the National Academy of Sci. of the United States of America , 105 ( 30 ) : 10291 – 10296 . DOI: 10.1073/pnas.0804860105