Abstract
Numerous sources of liquid aerosols are to be found in industrial environments. Such aerosols may, for instance, be cutting fluids, pesticides, etc., that are harmful or even toxic to humans. To control and reduce worker exposure to potentially toxic aerosols, these latter are usually filtered through fibrous filters. When non-saturated air traverses a clogged filter, however, the drops deposited on the fibers may evaporate. Consequently, workers are exposed to greater amounts of more concentrated vapors than the initial state of the filtered aerosol. Furthermore, exposure readings are distorted by an artifact that may be significant. This study offers an experimental approach to long-term monitoring of the evaporation of a semi-volatile n-hexadecane liquid aerosol deposited on filters of varying efficiency. Results were modeled using two semi-empirical models for identifying the basic parameters of liquid aerosol evaporation on fibers. For the first time ever it has been demonstrated that the Fick's first law, as previously suggested by models proposed in the literature, does not control evaporation kinetic.
INTRODUCTION
Systems for collecting aerosols on fibrous media are used both to quantify aerosol exposure levels and to eliminate them from the environment. These systems force the aerosol through a filter to collect its particulate phase. The deposited particles, which are subjected to air movement, are liable to evaporate partially or totally. In the occupational health and safety field, this leads to an underestimation of aerosol exposure levels, and to a gradual release of the vapors by the clogged filters into the atmosphere in the workplace and/or into workers' respiratory tracts.
Although collection mechanisms during the filtration of aerosols by fibrous filters are becoming more widespread, the related evaporation phenomena have not been studied in depth. The need for greater knowledge of evaporation or re-entrainment relating to the filtration of semi-volatile liquid aerosols such as oil mist or crop protection products led to this study.
Earlier studies on this subject focused on environmental issues, with loss through evaporation during air sampling of solid particles such as ammonium nitrate (NH4NO3) (CitationZhang et al. 1987b; CitationCheng et al. 1998; CitationFuruuchi et al. 2001) and polycyclic aromatic hydrocarbons (PAHs) (Lane, 1999). More recently, other studies have focused on the re-entrainment in vapor form of cutting oils deposited on fibrous filters, when treating metalworking oil mist (CitationCooper et al. 1998; CitationRiss et al. 1999) or during control sampling of ambient concentrations of aerosols (CitationVolckens et al. 1999; CitationSimpson et al. 2000; CitationVolckens et al. 2002; CitationPark et al. 2003; CitationSimpson et al. 2003; CitationVerma et al. 2006; CitationLim et al. 2008).
A number of experimental and modeling studies of the behavior of free aerosols have been conducted (CitationZhang et al. 1987a; CitationLehtinen et al. 1998; CitationLongest et al. 2005; CitationHopkins et al. 2006; CitationDebry et al. 2007; CitationLim et al. 2008). The complexity of the phenomena involved in the evaporation of collected aerosols, however, in particular those relating to the interactions between drops and fibers, accounts for the lack of experimental and theoretical studies in this field. The stakes, however, are high in terms of workplace prevention, both with respect to air control through sampling and to air purification. In 1999, the Institut National de Recherche et de Sécurité (INRS) estimated that 215,000 people were directly exposed to cutting oils (insoluble or soluble) in France (CitationLafontaine et al. 2002). Improving existing theoretical models to predict the loss through evaporation of a collected semi-volatile liquid aerosol requires more knowledge of the physical phenomena involved. The aim of this study was to produce experimental data on the evaporation of a collected model liquid aerosol, allowing us to identify the evaporation kinetic. For this purpose, we used a monodispersed pure n-hexadecane aerosol as a model, which is representative of the semi-volatile compounds included in the formulation of straight metalworking oils. According to the theory, the most important parameters, which can significantly influence evaporation, are the aerosol particle size and the structure of the fibrous filters. Consequently, we tested three different model aerosol particle sizes and five filters of varying efficiency.
Finally, the results are represented by two semi-empirical models containing governing evaporation parameters which are discussed to identify the evaporation process.
STATE OF THE ART
Few models representing the volatilization of collected aerosols are available. Among them are those of CitationFuruuchi et al. (2001) and CitationRaynor et al. (1999), who have developed theoretical models based on diffusion mass transfer theory. The total flow φ tot (t) (g.s–1) of vapors downstream of a clogged filter is calculated using the individual flow φ (x,t) of each particle. φ (x,t) is a function of the position of the particle within the thickness of filter x and of time t. φ (x,t) is estimated by the evaporation flow of a spherical particle suspended in still air (Fick's first law), corrected by a factor f representing the interaction between particle and fiber:
φ tot (t) is calculated using iterative methods that interested readers will find in the aforementioned articles. The experimental results of Furuuchi et al. plotted , show that evaporation occurs in two stages. First of all, evaporation occurs at constant rate when the mass of aerosol is sufficient to saturate air flow with vapor. φ tot (t) is proportional to the air flow Q (m3.s–1) traversing the filter and can be simplified according to Equation (Equation2) to obtain the same result as the iterative method used by the authors (dashed curve noted “Saturation” in ).
FIG. 1 Evaporation of collected ammonium nitrate on a TEOM filter for different masses. Experimental points taken from Furuuchi et al. 2001 and compared to the maximum theoretical evaporation rate noted “saturation.”
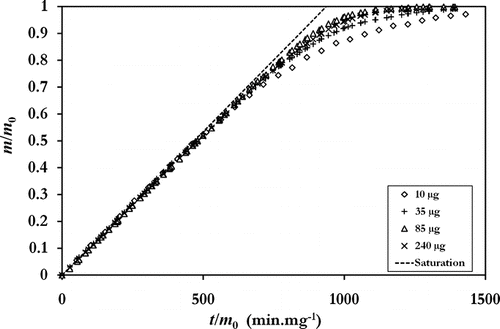
As particles are collected in a fibrous filter inducing a pressure drop, there is an increase in gas flow between upstream and downstream of the filter, which can increase φ tot (t). For a filter with the highest pressure drop value as measured by Furuuchi (8200 Pa), the increase is expected to be of circa 8% of the upstream flow if we consider the total thickness of the filter and the conditions of filtration used. For high-efficiency filters such as TEOM filters, however, it is accepted that almost 90% of the particles are collected in the first ten percent of the thickness of the filter. Thus, the flow variation, which is produced in the major collection area of the filter, is about 0.8% of the upstream flow. Consequently, in this case, we can neglect the effect of the pressure drop on evaporation kinetic. It is for this reason that the saturation curve plotted , which does not take into account the pressure drop effect, closely fits the first stage of the experimental evaporations obtained by Furuuchi.
The theoretical evaporation from the evaporated flow of individual particles (Equation [1]) appears to occur only belatedly in a second stage, when the mass of remaining aerosol no longer saturates airflow. The transition between these two evaporation stages would appear to be governed by the initial mass of the collected aerosol. In all the cases studied by the authors, the transition appears for a remaining mass lower than 30% of the initial deposited mass.
The correction factor f used by Furuuchi corrects for the loss of evaporation rate of collected solid particles by comparison to free ones, due to the contact between particles and fibers. This factor is hard to estimate, however, due to particle shape variations and other complex interactions between particles and fibers. Our study conditions were quite different as we were faced with liquid particles. Due to the high degree of liquid deformation, the correction factor f, applied to liquid particles, is expected to be different and superior than unity if we consider only the increase in surface area of deposited droplets exposed to air flow, by comparison to free ones. However, the estimation of f is problematic due to the amount of different interactions possible between droplets and fibers (chemical interactions, diameter and nature of fibers, deposited droplets geometry, etc.).
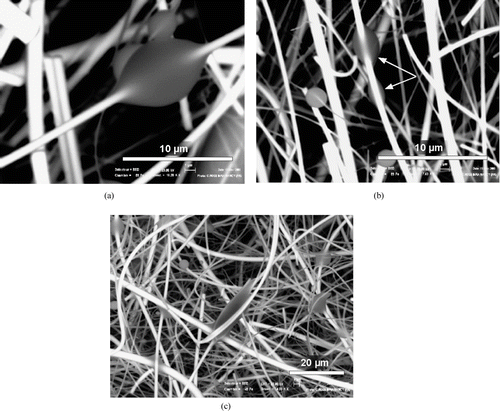
To illustrate the geometry variation of deposited drops collected on fibers, we observed poly-dispersed drops of pure n-hexadecane (C16) deposited on quartz fibrous filter with a scanning electron microscope (SEM) used at low vacuum and equipped with a Peltier cooling stage. With this device, the clogged filter can be frozen to minus 40°C and the frozen deposited droplets can be observed without evaporation, even under low vacuum conditions. The results show that the drops may develop highly variable geometries when they are in contact with fibers of the same type (). Drop geometry varies from a perfectly axisymetric shape () to total deformation through simultaneous contact with several fibers (), as a function of various parameters: number of points of contact between the drop and the fibers, volume of the drop, diameter of the fiber, liquid/fiber, and liquid/vapor surface tensions (CitationMcHale et al. 1997; CitationMcHale et al. 2002), the manner of deposition onto the fiber (interception, impaction), possible coalescence, etc. The total area of the gas/liquid interface developed by the drops, which is one of the keys to the problem, is thus unknown.
FIG. 2 Drops of n-hexadecane deposited on the quartz fibers of a W filter, observed by SEM. (a) axisymetric ondoloid drop in contact with only one fiber, (b) Clamshell shaped drop out of alignment with the bearing fiber, c) Drop deformed by simultaneous contact with several fibers.
In order to free ourselves from the assumptions made at the microscopic scale, we decided to analyze the evaporation of the collected aerosol in its globality, with respect to time. The goals of the study were firstly to observe the evaporation of a deposited model semi-volatile liquid aerosol, while monitoring the downstream vapor concentration over time. Secondly, we wanted to focus our attention on three major parameters which, in our opinion, can greatly influence the evaporation rate of the collected aerosol. These tested parameters were the particle size of the deposited aerosol, the initially deposited mass of aerosol on the filter and the nature of the filter. Finally, in order to analyze evaporation behavior according to the tested parameters and to extract as much information as possible from the experimental data, we needed to create two descriptive semi-empirical models. These models were only used as tools to calculate chosen variables and analyze them against the tested parameters. In this way, new data extracted from experimentations were produced, thus helping us gain a better understanding of the evaporation behavior of the semi-volatile liquid aerosol collected on fibrous filter for future theoretical modeling.
EQUIPMENT AND METHOD
The method comprised two distinct phases: filter clogging and evaporation of the collected aerosol, with the aim to entirely control the evaporation step.
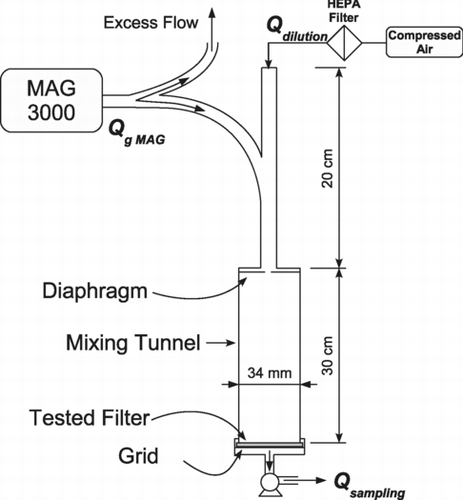
Filter clogging was achieved by the system schematized in . The Monodispersed Aerosol Generator (MAG 3000, Palas), produces a mono-dispersed aerosol of n-hexadecane (C16), through condensation of vapor on the NaCl nucleus, at a rate of 3.5 L.min− 1. According to the desired mass of deposited aerosol for each test, the mono-dispersed aerosol can be diluted with HEPA-filtered dry air. Homogenization was performed using a diaphragm with a 3 mm orifice diameter, followed by a 30 cm mixing tunnel. The characteristics of the mono-dispersed aerosols were measured with an optical spectrometer (Aerosol Spectrometer model 1.109, Grimm) at the filter collection point. The aerosol property values are given in .
TABLE 1 Size distribution of mono-dispersed aerosols of the C16 tested
The collected mass varies according to sampling time and dilution. The mass of the deposited aerosol M w was determined by weighing the filter before and after each clogging process (balance with a precision of ± 5 μ g). In all cases, filter clogging time was less than 1 min to limit evaporation of the first drops of collected aerosols during this phase. The sampling rate Q sampling was fixed at 1 L.min− 1 for all experiments.
Due to the short clogging time, there was no need to perform specific conditioning of filters. Filter mass measurement, before and after a one-minute exposure to clean air, showed no significant difference (circa 1 μ g).
After the clogging process, filters were placed in the system that evaporates the collected aerosol as shown in . Clean dry air (relative humidity < 1%) was injected at rate Q f , homogenized by two beds of steel wool and passed through the clogged filter being tested. Average filtration velocity was kept constant at v = 5 ± 0.15 cm.s–1. The working diameter of filter exposed to the clean air was d filtre = 3.4 cm, i.e., an area of A f ≈ 9.1 cm2. The temperature of the injected air (23 ± 1°C) was measured by miniature type-T thermocouples with a precision of ± 0.2°C. The pressure drop of the filters was continuously monitored during evaporation by means of a differential pressure measuring transducer (Keller PD-41X) accurate to within ± 0.6 Pa in the measurement range used.
FIG. 4 Diagram of the system for monitoring the evaporation of the liquid aerosols collected by the fibrous filter.
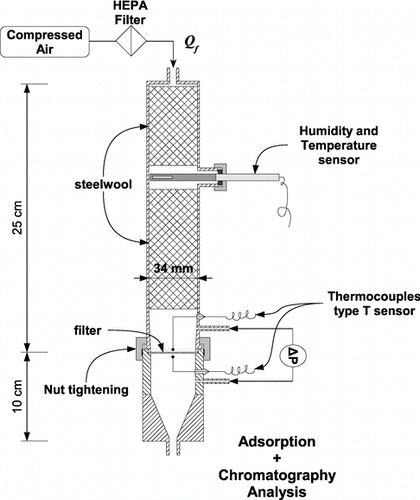
The vapors produced by the clogged filter were sequentially sampled at a rate of 1 L.min− 1 by adsorption in tubes containing activated carbon, until the entire initially collected aerosol mass had evaporated. Liquid desorption of the tubes by n-hexane doped with an internal standard n-heptadecane was then carried out. The desorption substance was then analyzed by gas chromatography with a flame ionization detector. The sampling times for each tube were adapted to the minimum mass quantifiable by GC, and were of at least 2 minutes (quantification limit = 0.02 μ g of adsorbed C16, resulting in a vapor concentration of 10 μ g.m− 3 for a 2 min sampling). Results were obtained by analyzing the samples between times t i − 1 and t i . The evaporated mass between times t i − 1 and t i is:
Therefore, the total evaporated mass at time t i is:
At the end of the evaporation phase, the total mass of aerosol quantified by the analytical technique was noted M ev,∞. This mass can be compared to the total mass initially deposited on the filter M w and determined by gravimetry. We defined the relative and absolute difference (respectively ε r and ε a ) between M ev,∞ and M w as:
The values of both differences are given in the online supplemental information for each test.
The characteristics of the tested filters are shown in .
TABLE 2 Characteristics of the tested filters
Semi-Empirical Model Development
The examination of an evaporation test of approximately 500 μ g of n-hexadecane aerosol collected on a W filter is presented in , where the change in concentration c of n-hexadecane vapor downstream of the clogged filter and the total evaporated mass M ev are traced over time. This result suggests that evaporation takes place into two distinct parts, as observed by CitationFuruuchi et al. (2001), which revolve around critical time t 1.
FIG. 5 Evaporation of a mono-dispersed n-hexadecane aerosol, DMM = 1 μ m, Filter W, filtration velocity of 5 cm.s− 1 and a temperature of 23°C.
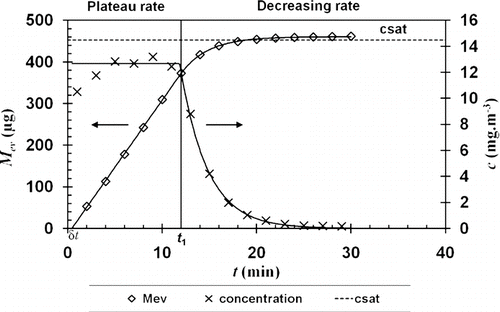
Initially, the concentration of C16 appeared to increase rapidly and level off at a maximum value of 13 mg.m–3, which is lower than the saturation concentration of approximately 14 mg.m–3. Total evaporated mass then progressed in a linear manner, showing constant maximum kinetics during this phase. At transition point t 1, vapor concentration fell sharply to a residual value that could not be quantified after 30 min. Total evaporated mass then leveled off at a peak value noted M ev,∞.
This two-stage evaporation behavior is comparable to all other results obtained during this study. The duration of each stage varied according to the mass initially deposited and, hence, the value of t 1, which can be null. Therefore, we can develop semi-empirical models that accurately describe the evaporation behavior of each test.
We chose to introduce physical parameters into models in order to obtain the best possible physical description and to extract them from experimental data (vapor concentration and deposited mass variations over time). We were then able to establish relationships between physical parameters, experimental conditions of each test, and time of filtration.
To do so, we can define the fraction of evaporated mass X 1 at time t 1 according to the following equation:
When t < t 1, changes in M ev (t) appear to be representable in linear form, which presupposes a plateau where the evaporation rate is at its maximum. Evaporation occurs at a “plateau rate.” The maximum theoretical evaporation rate R max equals:
When t > t 1, the evaporation rate decreases and appears to be modeled according to a first-order phenomenon in relation to the mass of aerosol present on the filter. Thus, evaporation then occurs at a “decreasing rate.”
The evaporation rate can be modeled using model A, which is defined by the following pair of equations:
This model ensures rate continuity at t = t 1, but not the continuity of its derivative. On average, we observed a relatively rapid transition from plateau rate at the start of evaporation. This rate setting is taken into account by a shift δ t of the origin with δ t < < t 1. The causes of this shift cannot be explained clearly. We can suppose temperature equilibrium between the inlet air flow and the clogged filter, even though the clogging and evaporation stages were conducted at the same room temperature. Reorganization of the collected drops can occur at the beginning of the filtration and can also explain this shift.
Equation (Equation8) is then integrated as follows:
The average vapor concentration ![]() i
and the evaporated mass Δ M
ev,i
determined for each sample contains an error composed of the sampling and the analytical methods. Consequently, at the end of the experiment, there was an error on the final evaporated mass M
ev,∞ equal to the sum of errors of all samples. For all tests, the relative difference ε
r
between M
w
and M
ev,∞ was less than 15%. Therefore, the quality of the monitoring method is good in comparison to the gravimetric method.
i
and the evaporated mass Δ M
ev,i
determined for each sample contains an error composed of the sampling and the analytical methods. Consequently, at the end of the experiment, there was an error on the final evaporated mass M
ev,∞ equal to the sum of errors of all samples. For all tests, the relative difference ε
r
between M
w
and M
ev,∞ was less than 15%. Therefore, the quality of the monitoring method is good in comparison to the gravimetric method.
The four parameters δ t, R max, t 1, and M ev,∞ are optimized from relations (9) and (10) according to the least-squares method. M ev,∞ is provided as an initial approximation through experimentation, such that M ev,∞ = M w .
When M w is insufficient and/or gas flow rate Q f is too high to achieve the R max kinetic value, evaporation is then too slow to saturate the gas flow rate and only the decreasing rate exists. The plateau model then fails because no point R max (a reference value) is reached. The kinetic is thus constantly dominated by interface transfer and changes constantly during evaporation. It would appear reasonable to retain the first-order phenomenon such as the one developed in the decreasing rate of model A, while retaining a start delay δ t′. The equation of evaporation speed according to model B then becomes:
Time constant τ accounts for evaporation kinetics and corresponds to the inverse of a first-order kinetic constant. Integration of Equation (Equation11) gives:
This dynamic model is governed by 3 parameters: M ev,∞, δ t′, and τ which are optimized using the same process as model A.
The choice of either model relies solely on the experimental results, with or without observation of a plateau rate at the onset of evaporation.
All tests and their modeling parameters of semi-empirical models are provided in the online supplemental information.
RESULTS: EXPLOITATION OF THE SEMI-EMPIRICAL MODELS
The temperatures upstream and downstream of the filters varied during evaporation by the same order of magnitude as the measuring errors. No variation in pressure drop was observed during evaporation, whatever the weight of the collected aerosol. From that, one can reasonably infer that overall flow varies little during the evaporation process.
The maximum experimental evaporation rates R max,exp of all the tests presenting a plateau rate, standardized by the maximum theoretical rate R max as a function of the weight of initially collected aerosol, were compared in . For all the filters except W, there was a good correlation between experimental plateau rate and the rate estimated by the relationship (7), namely R max,exp≅ R max, whatever the value of M ev,∞. The values of standardized rates greater than 1, are due to a mathematical artifact arising from the optimization of the semi-empirical model. Evaporation tests with collected mass greater than 600 μ g on filters A, B, D, and T were not conducted because the maximum theoretical rate was obtained for the lower mass values tested. It was therefore assumed that the maximum rate would be obtained at higher mass too.
FIG. 6 Standardized maximum rate as a function of the mass of collected C16, for all the tests. Group “W” includes results from W filters and group “other” includes all results obtained with filters A, B, D, and T.
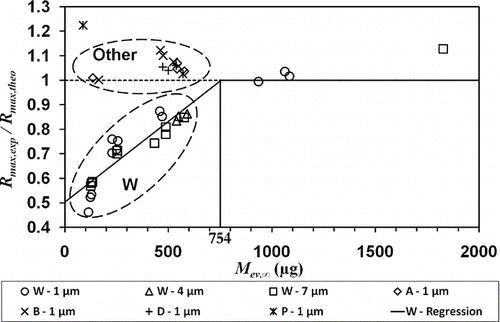
Concerning W filters, R max,exp clearly changes as a function of load, up to a critical mass of approximately 750 μ g, where R max is reached, regardless of size distribution. This different behavior of W filters is not explained clearly at the present time. The difference in structures of tested filters, which can cause different flow repartition in filters, is the strongest hypothesis that we can propose.
At the transition point t 1 between the plateau rate and the decreasing rate, the mass of remaining aerosol M a (t 1) is defined by M a (t 1) = M ev,∞(1 − X 1). The error on M a (t 1) means that the results of tests with loads ≥ 1 mg could not be used, as the absolute error ε a = |M ev,∞− M w | is greater than M a (t 1). Only tests with an absolute error ε a ≤ 40 μ g were used and traced in as a function of initial load M ev,∞.
FIG. 7 Mass of remaining aerosol at transition point t 1 as a function of initial load M ev,∞. M ev,∞ ≥ 88 μ g and ε a ≤ 40 μ g.
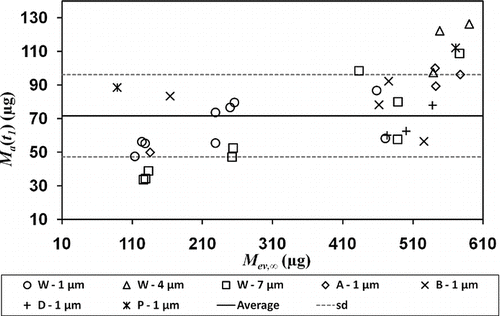
M a (t 1) appeared constant overall, whatever the type of filter and droplet size distribution tested. The average transition mass was equal to 70 ± 25 μ g. This effect can be explained by a standardization of the drop size distribution when these drops are collected on fibers. According to the ability of liquids to spray over the surface of fibers, the formation of liquid films on fibers is possible (CitationBrochard-Wyart 1986). Film thickness, however, is estimated to be less than 10 nm for n-hexadecane deposed on a W filter (CitationQuéré et al. 1999) and cannot provide the necessary flow for evaporation from its surface due to Plateau-Rayleigh instabilities. Therefore, the standardization of drop size distribution is due to unidentified interactions between drops and fibers.
During the plateau rate stage, we demonstrated that the collected mass is sufficient to produce high vapor levels, close to saturation vapor concentration. Consequently, evaporation rate is not governed by the maximum evaporation rate calculated by the diffusion theory. Thus, the application of this theory could not be experimentally verified.
Conversely, at the beginning of the decreasing rate stage, evaporation kinetic started to become dominated by interface transfer. Hence, the evaporation process can be identified experimentally and should, according to the literature, be able to be modeled by Fick's first law if all drops are independent of each other (very low collected/remaining mass). Indirectly, the identification of drop–fiber interactions can be expected if the experimental evaporation rate diverges from this theory.
If one applies Equation (Equation1) to a set of N i spheres with the same diameter, by introducing the mass of aerosol present on filter M a (t), one obtains Equation (Equation13).
Coagulation of drops collected on fibers can put this equation in default by causing size poly-dispersion of the deposited droplets, according to the initial filter load. The more highly clogged the filter, the more the coagulation occurs. Therefore, to justify the use of Equation (Equation13) with a mono-dispersed size distribution of collected drops, we observed, under an optical microscope, two W filters clogged with high mass of n-hexadecane (1 mg) mono-dispersed at DMM equal to 1 and 7 μ m ().
FIG. 8 Optical microscopic observations of W filters clogged with 1 mg n-hexadecane. (a) DMM = 1 μ m, (b) DMM = 7 μ m. Magnification ×200.
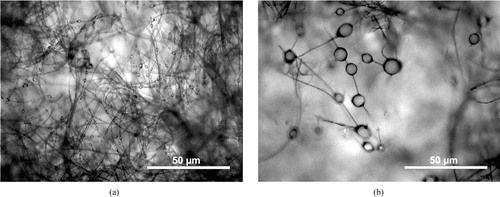
We can note that, even at high deposited mass, collected droplets diameters are homogenous and are of the same size as the initially generated free droplets. Thus, the coalescence probability is low for this range of deposited mass on W filters and is assumed to be quasi nonexistent for lower mass. Consequently, Equation (Equation13) can be applied with the assumption of mono-dispersion of the collected droplets for low collected aerosol mass.
One could expect the evaporation rate to behave according to the remaining mass M a (t) to the one-third power. This behavior, however, was not observed experimentally. represents evaporation rate R(t) standardized by maximum theoretical rate R max as a function of the mass of remaining deposited aerosol M a (t), which was equal to M a (t) = M ev,∞− M ev (t), for three tests on W filters and two different initial charges of aerosol. Calculations from Equation (Equation13) are also traced on this figure and were obtained with experimental values of c sat and M a (t 0) = M ev,∞. According to the theory, the diffusion coefficient of n-hexadecane used for calculations is equal to 4.4 10–6 m2.s–1 for these experimentations. The value of the correction coefficient f was optimized to fit the highest value of the relative rate obtained by the experimentations.
FIG. 9 Standardized evaporation rate as a function of residual mass at decreasing rate, for three tests (1, 2, and 3). W filter and DMM = 1 μ m. (a) M ev,∞≈ 30 μg. (b) M ev,∞≈ 90 μ g.
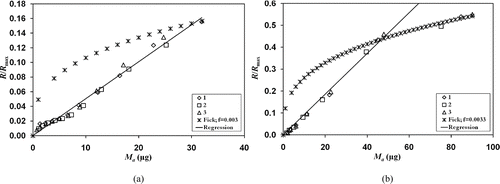
The experimental evaporation kinetics are clearly linear as a function of M a (t), for tests with initial collected mass M ev,∞≈ 30 μg. When M a ≤ 7 μg approximately, the experimental error on M a was lower than 10%, rapidly increasing as M a decreased, thus accounting for the observed deviations. It was still possible to observe the linear behavior of the evaporation kinetics for charges of C16 in the order of M ev,∞≈ 90 μ g. The evaporation kinetics decreased from the first order when M a was less than circa 40 μ g. These observations also hold true for all sizes distribution and types of filter tested. For greater initial loads, the accumulation of measurements over a long period decreased observation precision at the end of evaporation and the first-order kinetics could not be determined with precision.
Fick's first law, modified by Equation (Equation13), fails to correlate experimental evaporation behavior. Changes to theoretical evaporation rate according to the power one-third of the remaining mass is, therefore, physically unrealistic. Furthermore, the correction factors f used to fit the experimental results are very high. Experimental evaporation rates represent only 0.3% of the theoretical value. This strong discrepancy is due to high modifications of the evaporation process theoretically attempted. A strong modification in diffusion coefficient is not realistic as there is no change in physical properties of the gas passing through the filter (pressure, temperature, electrical charge). Modification of the saturated vapor concentration c sat is more highly probable with the effects of electrostatic interactions between liquids and fibers. A modification of this range, however, cannot explain the total difference between theoretical and experimental evaporation behavior.
Modelling the decreasing rate with semi-empirical models A and B according to first-order kinetics thus appears to be fully justified. This means that evaporation is not governed only by the diffusion process observed during the evaporation of drops in suspension in still air. Unfortunately, the evaporation process observed in these experimentations cannot be identified at the present time and more research, based on microscopic observations, is needed.
CONCLUSION
The evaporation of a semi-volatile mono-dispersed liquid aerosol of n-hexadecane deposited on various fibrous filters was modeled using a semi-empirical model based on two rates of evaporation, namely the plateau rate and the decreasing rate.
During the plateau rate, when the collected mass is the highest, experimental evaporation rate nearly reached the theoretical maximum evaporation rate simply defined by φ tot (t) = c sat Q. This equation is applicable to both solid and liquid particles collected on fibrous filters, as the evaporation rate is limited only by the air flow passing through the filter and not by interactions between particles and fibers (collected surface mass superior of 1 g.m–2). For lower loads, our results on liquid particles showed an influence of the structure of the filters and/or the nature of their fibers on the experimental maximal evaporation rate.
Independently of initial drop size distribution, collected mass and filter type, the mass of aerosol remaining on the filters during the transition between the two evaporation rates was identical, whatever the type of filter. Hence, fibrous filter complexity induces an averaging of the key parameters theoretically involved in evaporation kinetic.
The most important point of this study was the observation of evaporation kinetics for very low loads. Experimental observations disproved the use of the pure diffusion theory (Fick's laws), as recommended in models from the literature. Results showed that the evaporation rate of a few drops deposited on fibers and exposed to a pure air flow, is directly proportional to their mass rather than to their diameter, as propounded by the diffusion theory. The reasons for this behavior, however, are not clearly explained by the experimental results presented in this article.
Future work will pursue the identification of the evaporation process involved in the evaporation of one semi-volatile droplet deposed on a fiber. Thus, new knowledge will be obtained and used to develop new evaporation models of low-mass of semi-volatile liquid aerosols collected on fibrous filters.
uast_a_467946_sup_12979442.zip
Download Zip (38.8 KB)[Supplementary materials are available for this article. Go to the publisher's online edition of Aerosol Science and Technology to view the free supplementary files.]
REFERENCES
- Brochard-Wyart , F. 1986 . Instabilité Des Films Mouillant Des Fibres . Comptes rendus de l'Academie des Sci. , 303 ( 12 ) : 1077 – 1080 .
- Cheng , Y.-H. and Tsai , C.-J. 1998 . Evaporation Loss of Ammonium Nitrate Particles during Filter Sampling . J. Aerosol Sci. , 29 ( 1–2 ) : 243
- Cooper , S. J. and Leith , D. 1998 . Evaporation of Metalworking Fluid Mist in Laboratory and Industrial Mist Collectors . Amer. Indust. Hyg. Assoc. J. , 59 ( 1 ) : 45 – 51 .
- Davies , C. N. 1973 . Air Filtration , London/New York : Academic Press .
- Debry , E. , Fahey , K. , Sartelet , K. , Sportisse , B. and Tombette , M. 2007 . Technical Note: A New SIze REsolved Aerosol Model (SIREAM) . Atmos. Chem. Phys. , 7 ( 6 ) : 1537 – 1547 . J1—ACP
- Furuuchi , M. , Fissan , H. and Horodeck , J. 2001 . Evaporation Behavior of Volatile Particles on Fibrous Filter Flushed with Particle-Free Dry Air . Powder Technology , 118 ( 1–2 ) : 171 – 179 .
- Hopkins , R. J. and Reid , J. P. 2006 . A Comparative Study of the Mass and Heat Transfer Dynamics of Evaporating Ethanol/Water, Methanol/Water, and 1–Propanol/Water Aerosol Droplets . J. Phys. Chem. B. , 110 ( 7 ) : 3239 – 3249 .
- Lafontaine , M. , Delsaut , P. and Morel , Y. 2002 . “ Risques Liés à L'utilisation Des Fluides De Coupe ” . In Cahiers de notes documentaires—Hygiène et sécurité du travail (ND 2164–186–02):37.
- Lane , D. A. , ed. 1999 . Gas and Particle Phase Measurements of Atmos. Organic Compounds , Newark, NJ : Gordon and Breach . Advances in Environmental, Industrial, and Process Control Technologies, Vol. 2
- Lehtinen , K. E. J. , Kulmala , M. , Vesala , T. and Jokiniemi , J. K. 1998 . Analytical Methods to Calculate Condensation Rates of a Multicomponent Droplet . J. Aerosol Sci. , 29 ( 9 ) : 1035 – 1044 .
- Lim , E. W. C. , Heng Koh , S. , Kuang Lim , L. , Hoon Ore , S. , Kiat Tay , B. , Ma , Y. and Wang , C.-H. 2008 . Experimental and Computational Studies of Liquid Aerosol Evaporation . J. Aerosol Sci. , 39 ( 7 ) : 618 – 634 .
- Longest , P. W. and Kleinstreuer , C. 2005 . Computational Models for Simulating Multicomponent Aerosol Evaporation in the Upper Respiratory Airways . Aerosol Sci. Technol. , 39 ( 2 ) : 124 – 138 .
- McHale , G. , Kab , N. A. , Newton , M. I. and Rowan , S. M. 1997 . Wetting of a High-Energy Fiber Surface . J. Coll. Inter. Sci. , 186 ( 2 ) : 453 – 461 .
- McHale , G. and Newton , M. I. 2002 . Global Geometry and the Equilibrium Shapes of Liquid Drops on Fibers . Colloids and Surfaces A: Physicochemical and Engineering Aspects , 206 ( 1–3 ) : 79 – 86 .
- Park , D. , Kim , S. and Yoon , C. 2003 . Loss of Straight Metalworking Fluid Samples from Evaporation during Sampling and Desiccation . AIHA Journal. , 64 ( 6 ) : 837 – 841 .
- Quéré , D. and Richard , D. 1999 . Viscous Drops Rolling on a Tilted Non–Wettable Solid . Europhysics Letters. , 48 ( 3 ) : 286 – 91 .
- Raynor , P. C. and Leith , D. 1999 . Evaporation of Accumulated Multicomponent Liquids from Fibrous Filters . Ann. Occupat. Hyg. , 43 ( 3 ) : 181 – 192 .
- Riss , B. , Ewald , W. and Wilhelm , H. 1999 . Quantification of Re-Evaporated Mass from Loaded-Mist Eliminators . J. Environ. Monitor. , 1 : 373 – 377 .
- Simpson , A. T. , Groves , J. A. , Unwin , J. and Piney , M. 2000 . Mineral Oil Metal Working Fluids (MWFs)—Development of Practical Criteria for Mist Sampling . Ann. Occup. Hyg. , 44 ( 3 ) : 165 – 172 .
- Simpson , A. T. , Stear , M. , Groves , J. A. , Piney , M. , Bradley , S. D. , Stagg , S. and Crook , B. 2003 . Occupational Exposure to Metalworking Fluid Mist and Sump Fluid Contaminants . Ann. Occup. Hyg. , 47 ( 1 ) : 17 – 30 .
- Verma , D. K. , Shaw , D. S. , Shaw , M. L. , Julian , J. A. , McCollin , S. A. and des Tombe , K. 2006 . An Evaluation of Analytical Methods, Air Sampling Techniques, and Airborne Occupational Exposure of Metalworking Fluids . J. Occupat. Environ. Hyg. , 3 ( 2 ) : 53 – 66 .
- Volckens , J. , Boundy , M. , Leith , D. and Hands , D. 1999 . Oil Mist Concentration: A Comparison of Sampling Methods. . American Industrial Hygiene Association Journal. , 60 ( 5 ) : 684 – 689 .
- Volckens , J. and Leith , D. 2002 . Filter and Electrostatic Samplers for Semivolatile Aerosols: Physical Artifacts . Environ. Sci. Technol. , 36 ( 21 ) : 4613 – 4617 .
- Zhang , S. H. and Davis , E. J. 1987a . Mass Transfer from a Single Micro–Droplet to a Gas Flowing at Low Reynolds Number . Chem. Engineer. Comm. , 50 ( 1–6 ) : 51 – 67 .
- Zhang , X. Q. and McMurry , P. H. 1987b . Theoretical Analysis of Evaporative Losses from Impactor and Filter Deposits . Atmos. Environ. , 21 ( 8 ) : 1779 – 1789 .