Abstract
We report a positive matrix factorization (PMF) analysis of organic particulate material (PM) emissions of aircraft engine exhaust that includes data from five different aircraft engines and two different fuels (petroleum jet fuel and a Fischer-Tropsch fuel) collected over three field missions. PMF of aerosol mass spectrometer (AMS) data was used to identify six distinct factors: two lubrication oil factors, two aliphatic factors, an aromatic factor, and a siloxane factor. Of these, the lubrication oil factors and the siloxane factor were noncombustion sources. The siloxane factor was attributed to silicone tubing used in the sampling system deployed in one of the three missions included in this study, but not the other two. The two lubrication oil factors correlate with the two different lubrication oils used by the aircraft engines evaluated in this study (Mobil II and Air BP) as well as minor differences presumably due to variation in the blend stocks, temperature history, and analytical factors. Overall, the sum of the aliphatic and aromatic factors decreased with increasing power, as expected based on known trends in VOC emissions. The aliphatic #1 factor correlated with soot emissions, especially at power conditions where EIm-soot was greater than 30 mg kg?1. The aliphatic factor #2 mass spectrum shared some similarities with ambient aerosol organic PM present during the tests and correlated most strongly with dilution levels, two observations that suggest that aliphatic #2 contains components found in ambient aerosol. The aromatic factor correlated with benzene emissions, especially at low power conditions were EIm-benzene was greater than 0.03 mg kg?1. Our results improve the current understanding of aircraft PM composition.
Copyright 2014 American Association for Aerosol Research
INTRODUCTION
As submicron particles, gas turbine engine particle material (PM) emissions may potentially impact human health (Stettler et al. Citation2011; Levy et al. Citation2012), local air quality (Dodson et al. Citation2009; Arunachalam et al. Citation2011; Hsu et al. Citation2011, Citation2012; Zhu et al. Citation2011), and global climate (Lee et al. Citation2010; Dallara et al. Citation2011; Dorbian et al. Citation2011; Jacobson et al. Citation2011; Unger Citation2011). Since aircraft use is predicted to increase in the coming decades (Lee et al. Citation2009) and since post-treatment of aircraft engine exhaust is not feasible, better understanding of the combustion emissions is required so that the potential impacts can be properly accounted for in atmospheric models and to improve aircraft combustor designs.
TABLE 1 Conditions, engine characteristics, and fuel composition data for APEX-3 and AAFEX aircraft emissions tests
Motivated by these environmental concerns, researchers have performed studies to advance the current state of knowledge of gas turbine engine NOX (Herndon et al. Citation2004; Wormhoudt et al. Citation2007; Wood et al. Citation2008; Timko et al. Citation2010a; Lee et al. Citation2011; Santoni et al. Citation2011), volatile organic compounds (Spicer et al. Citation1994; Herndon et al. Citation2006, 2009; Knighton et al. Citation2007; Yelvington et al. Citation2007; Timko et al. Citation2010c; Beyersdorf et al. Citation2012), and PM emissions (Herndon et al. Citation2005b, 2008;
Lobo et al. Citation2007, 2011; Hagen et al. Citation2009; Onasch et al. Citation2009; Bulzan et al. Citation2010; Timko et al. Citation2010b). Partly because the knowledge base was limited and partly due to the potential impacts, particular emphasis has been placed on characterizing turbine engine PM emissions. Recent studies confirm that gas turbine PM is composed of nonvolatile soot particles and volatile materials, themselves consisting primarily of sulfate and organic components (Onasch et al. Citation2009; Timko et al. Citation2010b, Citation2013). Here, the term “volatile” refers to its vapor state at the elevated temperatures of the engine exit plane (>500 K). By mass, volatile PM can dominate PM emissions during low power operation, during operation at low ambient temperature (<275 K), and/or for combustion of high sulfur fuels (Timko et al. Citation2010b, Citation2013). Depending on conditions and engine technology, the volatile PM can coat existing soot particles, enter a nucleation/growth mode, or exist as a separate size mode (Timko et al. Citation2013). In most cases, the PM mass is contained in size modes with characteristic physical diameters less than 150 nm, and in all cases particles smaller than 300 nm represent the majority of PM mass.
Several recent studies provide information on the composition of aircraft volatile PM. Onasch et al. (Citation2009) reported that the aircraft volatile PM is a complex mixture of sulfate and organic compounds. Timko et al. (Citation2010b) reported that—under idle conditions—organic material could constitute more than 90% of the mass of the nucleation/growth mode material. The components present in the organic PM have been incompletely characterized. Timko et al. (Citation2010b) identified a lubrication oil component in the volatile PM and Yu et al. (Citation2010, Citation2012) found that the lubrication oil component is a common feature in the commercial aircraft fleet. Aside from the lubrication oil components, the remaining organic PM composition matrix remains un-resolved.
In this work, we use positive matrix factorization (PMF) to analyze aerosol mass spectrometer (AMS) composition data from two major aircraft engine measurement campaigns—Aircraft Particles Experiment (APEX-3) and the first and second Alternative Aviation Fuels Experiments (AAFEX-1 and AAFEX-2). The three field studies provide an opportunity to evaluate composition differences that depend on engine technology (using APEX-3 data) and fuel composition (AAFEX data). The objectives of this work were to: (1) quantify the lubrication oil contribution and (2) identify and quantify additional relative contributions present in the unresolved complex mixture.
METHODS
Field Studies
All data are from the APEX-3, AAFEX-1, and AAFEX-2 field studies (Bulzan et al. Citation2010). lists the relevant experimental details. The three-field studies complement one another and provide an opportunity to capture PM composition differences that depend on engine technology and fuel composition. A primary focus of APEX-3 was the comparison of the trace gas and PM emissions performance of a range of different commercial aircraft engines running on “standard” petroleum jet fuels. A primary focus of the two AAFEX studies was the comparison of trace gas and PM emissions performance for a single aircraft engine type running on a petroleum jet fuel; synthetic jet fuels derived from the Fischer-Tropsch (FT) process; and 50/50 volume blends of the FT fuels and the petroleum jet fuel. The primary difference between the FT fuels and the petroleum jet fuels is that the FT fuels have nearly zero sulfur (<10 ppmw) and aromatic contents (<1 vol%), whereas the petroleum jet fuels contain >100 ppmw of sulfur and >10 vol% aromatics. provides composition details for the different fuel types.
lists the engines evaluated during the APEX-3 and AAFEX field campaigns, and provides their maximum thrust ratings. As shown in , the APEX-3 engines cover a wide range in rated thrusts, from the CJ6108A which is a low-thrust turbo-jet engine designed for an executive jet, to the PW4158, a high-thrust engine used on commercial airliners. With the exceptions of the CJ6108A and CFM56–2C1 engines (both of which are maintained by NASA), all of the test engines were part of the commercial carrier or cargo fleets. Timko et al. (Citation2010a) provide more detail on the APEX-3 aircraft engines and fuels; their trace gas (Timko et al. Citation2010a; Beyersdorf et al. Citation2012) and PM emissions (Timko et al. Citation2010b) performance have been reported previously. Bulzan et al. (Citation2010) and Wey et al. (Citation2007) provide more detail on the engine emissions performance of the DC-8 aircraft and its CFM56–2C1 engines used during the AAFEX campaigns.
Both APEX-3 and AAFEX used an experimental protocol that has been described previously (Wey et al. Citation2007). Here, we summarize only the most significant details. For all tests, the aircraft were parked and chocked and the aircraft engines were operated at a range of power conditions, ranging from 4% full rated thrust (to simulate a “low” idle) to 100% full rated thrust (to simulate take-off). Emissions were extracted using stainless steel probes anchored at fixed locations equal to 15, 30, 43, and 50 m downstream of the engine. Samples were also extracted at the engine exit nozzle, but previous research found that engine exit nozzle samples provided scant information on volatile PM (Timko et al. Citation2010b, Citation2013) and these samples are excluded from further analysis. Exhaust gas samples were delivered to the PM characterization suite via stainless steel tubes (approximately 20 m distant), housed in the Aerodyne Mobile Laboratory (AML) (Kolb et al. Citation2004; Herndon et al. Citation2005a). Particle losses were measured on site during each of the tests using NaCl test particles. In summary, typical 50% cut diameters measured during APEX-1 (Wey et al. Citation2007) were on the order of 50 nm. We present data un-corrected for particle line loss with the understanding that the absolute mass loadings will be under-represented. Since our focus is on identifying the contributions to organic PM, the uncertainty regarding absolute mass loadings will not compromise any of our primary conclusions.
To account for variations in sample dilution, all data are reported as emission indices (EI), that is, mass of emissions species emitted per mass of fuel burned. Units for PM species are mg kg−1 and for trace gas species the units are g kg−1. As is common practice (Canagaratna et al. Citation2004; Herndon et al. Citation2005b; Timko et al. Citation2010a), above ambient levels of CO2 served as an exhaust tracer, so that the EI for emission “X” can be defined as:
The Aerosol Mass Spectrometer
The particle composition data we present here were acquired using one of two types of AMS: the compact time-of-flight aerosol mass spectrometer (C-ToF-AMS, Aerodyne Research Inc.) or a high-resolution time-of-flight aerosol mass spectrometer (HR-ToF-AMS, Aerodyne Research Inc.). The C-ToF-AMS m/Δm resolution is approximately 500–1,000, sufficient for unit mass resolution, whereas the SP-AMS m/Δm is greater than <1,500, permitting mass defect differentiation between fragments with the same unit mass but with different amounts of oxygen (e.g., C2H5 vs. CHO). The C-ToF-AMS was used for the majority of our aircraft PM measurements and accordingly analysis of C-ToF-AMS data is the focus of this article. We performed HR-ToF-AMS measurements for a subset of the engines/fuels evaluated in our studies (specifically the CFM56–2C1 engine tested during AAFEX-2).
The general operation of the AMS (Jayne et al. Citation2000; Drewnick et al. Citation2005; Canagaratna et al. Citation2007) and AMS particle focusing (Liu et al. Citation1995; Liu et al. Citation2007) have been described previously in the literature and used for a wide range of emissions sources (Canagaratna et al. Citation2004; Herndon et al. Citation2005c; Lack et al. Citation2009; Massoli et al. Citation2012). Onasch et al. (Citation2009), Timko et al. (Citation2010b, Citation2011, Citation2013), and Yu et al. (Citation2010) have described the use of the AMS specifically to characterize aircraft exhaust PM. Briefly, the AMS converts aerosols into a particle beam that is focused onto a tungsten vaporizer (typically held at 600°C), vaporized by electron impact ionization (70 eV), and analyzed using a time-of-flight mass spectrometer (Tofwerk). Consistent with common practice, we use the Allan et al. (Citation2004) fragmentation algorithm to separate the organic component of the exhaust gas matrix from other components (primarily nitrate and sulfate). The AMS has several known sources of uncertainty, as reviewed previously (Jayne et al. Citation2000; Canagaratna et al. Citation2007; Timko et al. Citation2010b). Since aircraft PM is dominated by particles <100 nm (Lobo et al. Citation2007) which the AMS cannot analyze quantitatively, the discussion of data here will focus on the qualitative aspects of composition and relative contributions—rather than absolute mass loadings.
Supporting Measurements
We performed both trace gas and PM measurements to help interpret the AMS composition measurements which are the focus of this article. Specifically, we used the multiangle absorption photometer (MAAP) to measure total black carbon soot emissions (Petzold et al. Citation2005; Slowik et al. Citation2007) and the proton-transfer reaction mass spectrometer to measure select VOC emissions, specifically benzene (Lindinger et al. Citation1998; Knighton et al. Citation2007; Blake et al. Citation2009). Both of these instruments have been described previously.
PMF Model
PMF is a multivariate factor analysis technique developed by Paatero's group (Paatero and Tapper Citation1993; Paatero Citation1997). In recent years, PMF has been used to successfully analyze AMS mass spectra of complex organic aerosol (Lanz et al. Citation2007;
Ulbrich et al. Citation2009). The use of PMF in AMS applications has been reviewed by Zhang et al. (Citation2011). AMS spectra are complicated by the fact that the spectra consist of overlapping ion fragmentation patterns from all of the individual nonrefractory species present in the aerosol. PMF is used to express the AMS data as a sum of constant factor profiles (AMS mass spectra) with varying contributions over the time period of the dataset (time series). Correlations of the factor mass spectra and time series with source mass spectra and external tracers can then be used to connect individual factors with specific sources and/or processes.
The PMF analysis for this study was performed according to methods outlined by Zhang et al. (2011) and Ulbrich et al. (Citation2009). One specific departure from previous use of PMF to analyze AMS data was the elimination of two masses dominated by gas phase compounds in aircraft exhaust: m/z 18 (primarily H2O) and 44 (primarily CO2). In ambient samples, both of these signals arise from both H2O/CO2 vapor and a PM component that is likely associated with organic acids present in atmospheric aerosols. For freshly emitted aircraft exhaust, detailed gas phase analysis indicates negligible organic acid content (Knighton et al. Citation2007; Spicer et al. Citation2009). For fresh exhaust samples, CO2 levels are much higher (>1,000 ppm) and vary by as much as a factor of 2, making differentiation between exhaust CO2 and exhaust gas PM acid groups highly uncertain. Thus, rather than attempting to extract meaningful information from m/z 18 and 44, we instead opt to eliminate them from consideration. Analysis of photochemical aerosol generation potential of aircraft exhaust—which is not the aim of this article–would require a more sophisticated analysis of m/z 18 and 44.
While PMF does not need a priori assumptions of either mass spectral or time profiles of the factors (Lanz et al. Citation2007; Ulbrich et al. Citation2009), the appropriate number of factors needed for the final solution must be determined by the user based on the “goodness of fit” and the interpretability of the factors. In this analysis, we tested up to seven factors. For four factors or less, the model was only able to describe 98% of the variability in the data. The five factor solution described approximately 99% of the data variability, but we did not observe a factor for siloxane—a component that we strongly suspected should be present in the APEX-3 data set due to the use of silicone tubing in the APEX-3 sampling system. The six factor solution described approximately 99% of the data variability, and correctly identified the siloxane factor. The seven factor solution did not improve the goodness of fit and caused splitting of factors into categories with no obvious physical interpretation. For this reason, we accepted the six factor solution.
RESULTS AND DISCUSSION
We obtained four primary results from our study: (1) mass spectra factors obtained by application of the PMF model to the AMS data, (2) high resolution mass spectra supporting the physical attribution of the PMF factors, (3) mass loadings for the various PMF factors for different engines, engine operating conditions, and fuels, and (4) correlation of specific factors with other tracers of incomplete combustion, specifically soot and gas-phase benzene.
Factor Mass Spectra
provides the mass spectra of the organic PMF factors obtained from the six-factor solution. The factor mass spectra are initially identified by name according to their unique spectral features and the connection between individual factors and source influences is explored by comparisons with source mass spectra (this section) and external tracers (see section. 3.4). In this section, we focus on the physical interpretations of these factors.
FIG. 1 Mass spectra for the factors identified using PMF for the combined APEX-3 and AAFEX organic PM emissions dataset. (Color figure available online.)
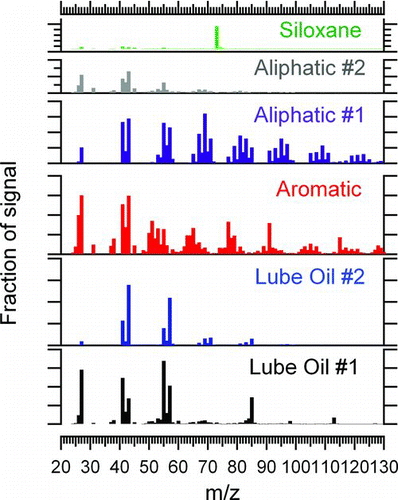
One component of the organic PM exhaust we clearly identify as lubrication oil, a known component of aircraft engine PM emissions (Timko et al. Citation2010b; Yu et al. Citation2010, Citation2012). Yu et al. (Citation2010) studied lubrication oil PM under well defined laboratory conditions and concluded that the mass spectra of pure synthetic lubrication oils could be differentiated from fuel-related hydrocarbons by strong signals at m/z 85 and 113 combined with weak signals for common ions derived from saturated and unsaturated hydrocarbons (i.e., delta series of 0 and +2 for unsaturated and saturated hydrocarbons, respectively; McLafferty and Tureček Citation1993). Timko et al. (Citation2010b) and Yu et al. (Citation2010) attributed the strong m/z 85 and 113 signals to synthetic esters (specifically the C6H9O and C8H13O fragments) that account for >90% of aircraft synthetic lubrication oil composition. The lubricating oil organic PM component is described by the sum of the two factors of the 6-factor PMF solution factors in this study; this result is robust, being obtained for six-factor PMF analyses on the total data set as well as for individual PMF analysis of the AAFEX and APEX-3 studies.
Lubrication oils are primarily available from two manufacturers (Mobil and BP), and the blend stock differs between the two manufacturers and even slightly between batches
from the same manufacturer. Accordingly, aerosol mass spectra for the two lubrication oils may be distinct from one another; however, the aerosol mass spectra have also been shown to be sensitive to the analytical conditions (specifically vaporizer temperature; Yu et al. Citation2010). In this study, the lubrication oil signatures of engines which are known to use Air BP lubrication oils (RB211–535E4-B) are dominated (10:1) by lube oil #1 factor, whereas engines which use Mobil II (CFM56, PW4158) are closer to a 1:1 mixture of lube oil #1 and lube oil #2 factors. The PMF results appear to support the contention by Yu et al. (Citation2010) that the two major lubricating oil types can be differentiated by using aerosol mass spectrometry.
Two factors are identified as likely products of incomplete combustion, one of them being labeled “aromatic” and the other being “aliphatic #1”. The aromatic factor attribution is made based on the occurrence of elevated signals associated with aromatic fragments: m/z 77 (benzene), 91 (toluene), 105 (xylene), 115 (indene), and 128 (naphthalene). Since even the heaviest of these fragments is too volatile to exist in the PM phase at equilibrium, we conclude that we are observing fragments of larger molecules that have sufficiently low volatility to partition into the PM phase. Aliphatic #1 is attributed based on the elevated signals at masses 41/43, 55/57, 69/71, 83/85, etc. Each of these masses corresponds to the alkane (e.g., mass 43 is C3H7 the primary fragment resulting from ionization of propane) and the corresponding hydrocarbon with a single double bond equivalent (e.g., mass 41 is C3H5 the primary fragment resulting from ionization of propene). Aliphatic #1 extends to masses greater than 120, suggesting that the parent ion family contains compounds consisting of at least nine carbon atoms. Jet fuel consists primarily of aliphatic hydrocarbons in the C8 to C14 range, and un-burned jet fuel may comprise a portion of the aliphatic #1 signal.
A second factor has some features in common with aliphatic #1, and we term this “aliphatic #2”. Like aliphatic #1, aliphatic #2 contains a series of clusters of masses separated by 14 amu, indicative of CH2 units. Aliphatic #2 is dominated by masses smaller than 60 amu, specifically masses 27 (C2H3), 41 (C3H5 and C2H3O), 43 (C3H7 and/or C2H5O), and 55 (C4H9 and C3H5O). Aliphatic factor #2 shares some features in common with aerosol mass spectra taken of ambient air taken at the airport just before and just after engine tests (data not shown), specifically the importance of ions at 27, 41, 43, and 55. Quantitatively, aliphatic #1, aliphatic #2, and ambient are differentiated based on their fractional contributions due to masses less than 60 amu; masses less than 60 amu contribute 5–10% of the total mass in ambient, less than 5% in aliphatic #2, and more than 30% in aliphatic #1. Based on this similarity, aliphatic #2 may have a contribution from the ambient aerosol.
In addition to the aliphatic, lubrication oil, and aromatic factors, we identified a factor with clear siloxane characteristics. Specifically, the siloxane factor was dominated by only a handful of masses: 73, 147, and 207. Timko et al. (Citation2009) previously ascribed these masses to siloxane contaminants associated with the use of silicone tubing. Silicone tubing was used for sampling exhaust in the APEX-3 campaign, so the identification of a siloxane factor was expected in the APEX-3 data. Between performing the APEX-3 and AAFEX measurements, we learned that silicone tubing could contribute a siloxane artifact to aircraft PM emissions, hence we removed silicone tubing entirely from the AAFEX sampling system. As anticipated, the siloxane artifact was not identified in the AAFEX data set, an observation that we take a strong corroborating evidence of the physical interpretation of this factor.
Comparison with High-Resolution Reference Mass Spectra
As discussed above, patterns in signature ions can be used to differentiate unit mass resolution factor mass spectra from each other. High-resolution mass spectra can be used to more explicitly provide information about the chemical composition of these ions. Here, we use high-resolution mass spectra of the organic PM emitted by the CFM56–2C1 engine while it was running JP-8 petroleum jet fuel () as a reference to investigate the assignments of the key signature ions observed in AMS spectra of aircraft organic PM. By “high resolution” we mean that m/Δm is greater than 1,500, sufficient to resolve C, H, O mass defects (i.e., hydrocarbons can be differentiated from oxygenated organics). By fitting the raw data to curves centered at known masses, we were able to apportion important masses to specific CxHy and CxHyOz fragments.
summarizes key results obtained during AAFEX-2 for the CFM56–2C1 engine burning JP-8 and listing the data by the corresponding unit masses, and organizing it using the PMF factors identified in . contains pie chart summaries of the data shown in , with the area of each pie chart being proportional to the fractional contribution of that category of fragment (lubrication oil, aromatic, and mixed) and the area of each slice of the pie charts being proportional to the contribution of hydrocarbons, singly oxygenated hydrocarbons, and doubly oxygenated hydrocarbons. separates lubrication oil and aromatics into two pie charts and provides a third chart termed “mixed fragments” which has contributions primarily from both aliphatic factors and both lubrication oil factors. As anticipated in our previous discussion, both the lube oil and aromatic components are easily identified by their fragment ions as they are dominated by expected fragments. Specifically, aromatic fragments accounted for at least 90% of all the aromatic fragments identified in , for example, 77 (benzene), 91 (toluene), 105 (xylenes), 115 (indene), and 128 (naphthalene). The C4H13O+ accounted for 10% of m/z 77, the most that any nonaromatic mass contributed to a mass attributed to the aromatic factor. Similarly, 85 and 113, the key masses that differentiate lubrication oil, were dominated by the C5H9O and C7H13O fragments associated with the synthetic esters used in lubrication oil blends.
TABLE 2 High-resolution mass spectrometer molecular ion assignments
FIG. 2 Composition of the different categories of fragments identified in by fitting the high-resolution mass spectrometer data, as shown in . Here, “mixed fragments” refer to fragments with contributions from the lubrication oil, aliphatic, and aromatic factors. (Color figure available online.)
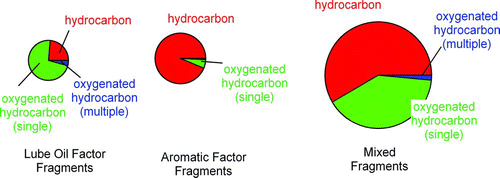
The high-resolution data do not provide unambiguous chemical composition differentiation (e.g., O/C ratio data) of the two aliphatic fragments from one another and from minor lubrication oil fragments. The compositions of the primary “hydrocarbon” masses (e.g., 41/43, 55/57, etc.) are listed in , showing that they are all mixtures of CxHy and CxHyOz compounds. The primary exception to this rule is mass 41, which is dominated by the hydrocarbon, C3H5 (97%) though both mass 57 (C4H9) and 69 (C5H9) are also 90% hydrocarbon. Overall, the masses selected in as representative of aliphatic #1 and #2 are on average 70% CxHy and 30% CxHyOz. Based solely on the overall contributions, the factors we term aliphatic #1 and #2 likely contain some oxygen. However, because the lubrication oil factor contains some masses that overlap with the aliphatics listed in that would carry some oxygen content of their own, the high resolution mass spectra of the mixed fragments cannot be used to separately quantify the oxygen content of aliphatic #1 and #2.
PM Mass Loadings
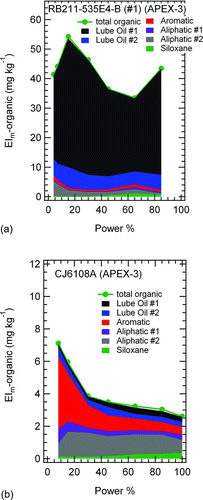
Having identified six PMF factors in the PM mass spectra and assigned to them physical meanings, we apportioned the organic PM mass loadings among the six factors. provides representative data from an RB211–535E4-B () and CJ6108A () engine. We provide data for these two engines because Timko et al. (Citation2010b) reported that the RB211 (low) and CJ6108A (high) engines span the range of VOC emissions for the engines in our dataset. Additionally, Timko et al. (Citation2010b) found a distinct size mode in the RB211 exhaust and tentatively apportioned it to lubrication oil based on similarities with the mass spectrum of pure lubrication oil. For these reasons, the RB211 and CJ6108A engines are good indicators for assessing how the PMF factors correlate with different sources. As expected, lubrication oil factors dominate the composition of the organic PM emissions of the RB211 (), whereas the aromatic and aliphatic factors emissions dominate the CJ6108A organic PM emissions (). Additionally, PMF identified the siloxane component, which contributed between 0.1 and 0.3 mg kg−1 to the overall budget, in the APEX-3 organic PM mass loadings (including the RB211 and CJ6108A). The “total” AMS measurement of organic PM is plotted in comparison to the sum of the individual factors in , showing that the PMF fitting routine captured >99% of the data variability. The observation of a dominant lubrication oil contribution to the RB211–535E4-B PM loading, a more pronounced aromatic/aliphatic contribution to the CJ6108A exhaust, and the overall goodness of fit all lend credibility to the chemical interpretation of the PMF factors.
FIG. 3 Organic PM mass loadings for the two representative aircraft engines: (a) RB211–535E4-B and (b) CJ6108A. Data are shown as “stacked plots” so that the total of the individual factors can be compared directly with the total organic PM loading measured by the AMS. (Color figure available online.)
summarizes the primary lubrication oil results. Of the various potential factors (engine operating power), engine manufacturer/model was found to exert the largest influence on lube oil EIs. For this reason, plots lubrication oil EIs by averaging data collected over all operating conditions for each engine tested. The data in show the lube oil EIm varies from about 0.5 mg kg−1 for the CJ6108A turbo-jet engine to approximately 40 mg kg−1 for the two RB211–535E4-B engines. The two CFM56 engines and the PW4158 engine fall within this range, much closer to the lower end. The differences in lubrication oil EIm can presumably be tied directly to differences in engine design, and differences in the efficiencies of the lubrication oil recovery systems likely play a role. Unfortunately for a scientific analysis, the lubrication oil recovery systems are engine manufacturer proprietary, making a definite conclusion impossible to reach. Additionally, differences in engine seal integrity and engine maintenance history—neither of which is known with certainty—may play a role in determining EIm. Our data make clear, however, that EIm lubrication oil varies with engine manufacturer and engine model.
FIG. 4 Lubrication oil EIs for the different engines, averaged over all available power conditions. (Color figure available online.)
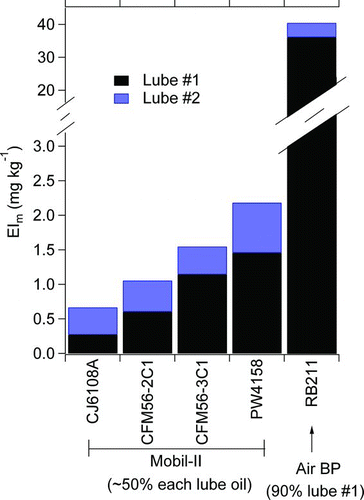
Aside from quantification data, also shows the relative contributions of lubrication oil factor #1 and #2 for each engine. The ratio of the two factors appears to depend on the type of lubrication oil used in a particular engine. RB211 engines operate with Air BP and exhibit a 10:1 ratio of lube #1 to lube #2, whereas all other engines (PW4158, CJ6108A, and CFM56–3C1) operated with Mobil-II and exhibit ratios near 1:1. In all cases, the ratio of the two lubrication oil factors was constant to within about 50% for a single engine operating over a range of powers from 4% idle to 100% takeoff. The fact that the ratio of lubrication oil factors and their absolute mass loadings are more dependent on engine and lubrication oil type used than on power conditions is consistent with the lubrication oil component being a noncombustion source of PM.
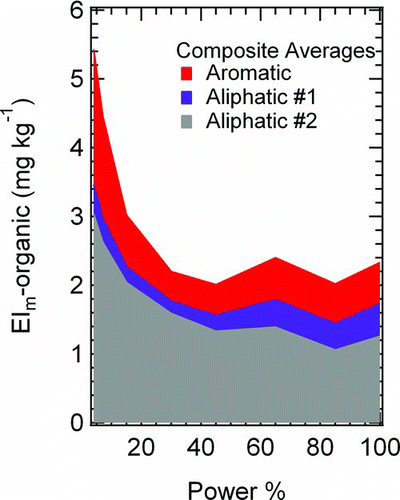
shows three factors (aliphatic #1 and #2 and aromatic), which are present for both the lowest VOC emitting engine (RB211–535E4-B) and the highest (CJ6108A) and which may have combustion related sources. Considering the entire engine dataset and eliminating data from combustion of FT fuels, the mass loadings attributed to these three factors vary over a reasonably narrow range, from approximately 6–8 mg kg−1 (at idle) to 1–2 mg kg−1 (at takeoff). The low variability in the combustion organic PM factors (e.g., aromatic, aliphatic #1, and aliphatic #2) justifies visualizing the data as a composite average, as shown in . The sum of the three organic PM factor EIs decrease with increasing engine power, as we would expect since the combustion-sourced organic PM should follow the known trends observed between VOC emissions and engine power (Knighton et al. Citation2007; Timko et al. Citation2010a;
Beyersdorf et al. Citation2012). Looking more closely at individual factors, the trends that we observed with respect to engine power are more complicated than would be predicted based strictly on global trends. Specifically, the inverse trend between organic PM emissions and engine power is dominated by aliphatic #2. The aromatic factor decreases slightly with increasing power up to 30% and then increases. Aliphatic #1 steadily increases with increasing power. The physical significance of each of these trends is discussed in more detail in Section 3.4.
FIG. 5 Composite organic PM mass loadings for all of the engines considered in this study. Data are shown as “stacked plots” so that the total of the individual factors can be compared directly with the total organic PM loading measured by the AMS. (Color figure available online.)
Combustion of FT fuels greatly reduces the aromatic and aliphatic EIs to less than 1 mg kg−1 (data not shown), whereas combustion of the 50/50 blend results in aliphatic and aromatic EIs intermediate to JP-8 and FT. Unlike the lubrication oils, combustion of FT fuels and blended fuels may lead to a true reduction in the emissions of combustion related organic PM as combustion of these reduced aromatic fuels reduces emissions of soot and many VOCs (Bulzan et al. Citation2010). Using a sampling protocol that provides time for particles initially smaller than 80 nm to grow to sizes that are detected efficiently by the AMS, Timko et al. (Citation2013) quantified the reductions in organic PM emission observed with combustion of low aromatic fuels.
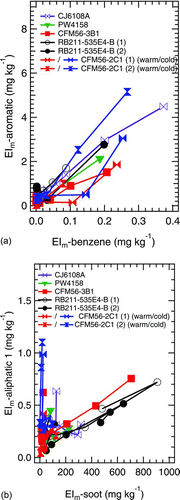
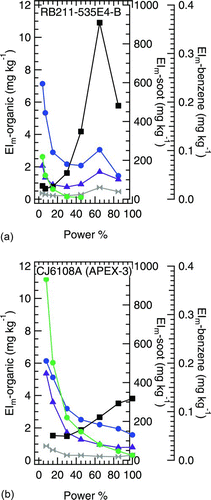
FIG. 6 Combustion-sourced organic PM EIs plotted as functions of power, alongside two other tracers of combustion inefficiency: benzene and black carbon soot. Data are shown for two aircraft engines: (a) RB211–535E4-B and (b) CJ6108A. ▪ Black carbon soot; • benzene (gas phase); ▴ aromatic; ▸◂ aliphatic #1; • aliphatic #2. (Color figure available online.)
Comparison with other Products of Incomplete Combustion
Having identified combustion sourced PM in the organic PM emissions, we sought to identify correlations with other known combustion emissions, measured at the same conditions as the organic PM factors. We chose gas-phase benzene (measured using PTR-MS) and black carbon soot (measured using the MAAP) for the purpose of identifying correlations. We select benzene as a representative hydrocarbon that has been shown to: (a) follow similar trends with respect to engine power as most other VOC emissions (Knighton et al. Citation2007; Beyersdorf et al. Citation2012) and (b) be sensitive to changes in fuel aromatic content (Timko et al. Citation2011). Specifically, benzene emissions take their highest values at engine idle and then rapidly fall to below detection limits (e.g., <0.02 mg kg−1 at 2,000 ppm CO2 dilution levels) as engine power increases above 15%. Black carbon soot makes the largest contribution to the overall PM mass budget and—in contrast to benzene—takes its lowest values at or near idle and increases with increasing engine power (Timko et al. Citation2010b). Because benzene and soot exhibit qualitatively different trends with respect to engine power, we speculate that they are formed due to two different types of engine inefficiencies—
one operative near idle, the other at higher engine powers. These
TABLE 3 Summary of identified components present in the organic PM emissions of aircraft engines
features make benzene and black carbon soot representative products of incomplete combustion.
plots the aliphatic and aromatic factors as functions of engine power for the two engines plotted previously in (i.e., RB211–535E4-B and CJ6108A). In addition to the organic PM data, contains benzene and black carbon soot EIs. Interestingly, the combustion-sourced organic PM factors follow trends similar to benzene at near-idle powers and up to about 30% engine power (EIm-benzene greater than about 0.03 mg kg−1). At higher powers, where EIm-soot was greater than about 30 mg kg−1, the combustion-sourced organic PM factors follow black carbon soot trends more closely. Particularly striking is that both aliphatic factors and the aromatic factor follow the maximum in black carbon soot observed at 65% engine thrust in . By comparison to the RB211–535E4-B, the CJ6108A emissions tend to follow trends similar to benzene more closely than soot, a likely consequence of the higher benzene to soot ratio for this engine and the reduced structure in the CJ6108A soot signature compared to the RB211–535E4-B. The clear conclusion from is that the combustion-sourced PM factors follow similar trends observed for other known combustion emissions.
takes the analysis shown in one step further by expanding the analysis to include all of the engines included in this study and by plotting individual PMF factors as functions of benzene () and soot () emissions. shows that gas-phase benzene and particle-bound aromatic are strongly correlated, especially at the low power conditions where EIm-benzene is greater than about 0.03 mg kg−1. shows that aliphatic #1 is strongly correlated with soot emissions for the high-power conditions where EIm-soot is greater than about 30 mg kg−1. As expected, the noncombustion organic PM factors (i.e., the two lubrication oils and siloxane) show no correlation with either soot or benzene (data not shown). Interestingly, aliphatic #2 does not correlate strongly with benzene or soot. Of all the correlations tested, aliphatic #2 loadings were found to correlate most strongly with calculated dilution levels, supporting our previous assertion that aliphatic #2 may in part be due to entrainment of ambient PM onto the aircraft PM emissions—either through gas-to-particle conversion (e.g., due to evaporation and recondensation of atmospheric PM in the aircraft engine) or via particle–particle microphysical processing. Uncertainty regarding aliphatic #2 notwithstanding, we take as clear evidence of the combustion origins of aromatic and aliphatic #1.
The correlation observed between aliphatic #1 and soot merits further discussion. Previously, Knighton et al. (Citation2007) reported that aircraft VOC emissions scale with one another, at least at near-idle conditions. Later, Beyersdorf et al. (Citation2012) showed that the near-idle scaling broke down with engine power settings greater than about 30%. Specifically, Beyersdorf et al. (Citation2012) showed that emissions of alkanes do not scale with other hydrocarbons and decrease more slowly with increasing engine power–the same conditions which favor soot production. Moreover, a recent FTIR study of the black carbon soot surface indicated that presence of alkane components in the absorption spectra (Cain et al. Citation2010). These two results seem to link aliphatic #1 with soot emissions, suggesting that aliphatic #1 and soot have similar combustion origins, that the soot surface itself contains aliphatic components that are removed during evaporation and ionization in the AMS and detected as aliphatic #1, or that soot provides a favorable surface for gas-to-particle conversion of aliphatic #1.
CONCLUSION
We used the PMF source receptor model to divide aircraft organic PM emissions measured at the APEX-3 and AAFEX campaigns into six factors: two lubrication oil factors, two combustion factors, one factor that seems to be associated with atmospheric PM, and a sampling line siloxane contaminant. summarizes these assignments, providing the most important masses associated with each one. We find that the two lubrication oil factors, each characterized by masses corresponding to the synthetic esters present in aircraft lubrication oils as verified by their mass defects, are required to describe the two major aircraft lubrication oils—Air BP and Mobil II. In addition to capturing differences between the two lubrication oil sources, variability in the ratio between the two lubrication oil factors likely describes differences in the blend stocks, lubrication oil temperature history, and sampling protocol. Siloxane was attributed to the use of silicone tubing in the sampling system; the siloxane factor was observed in the organic PM characterized at APEX-3 (where silicone tubing was included in the sampling system) but not at AAFEX (when the silicone tubing was carefully removed). As expected, none of the “non-combustion” factors showed strong dependence with respect to engine operating power.
PMF divided the three remaining factors into two with aliphatic characteristics and one with aromatic components. High-resolution mass spectrometry clearly showed that the signature masses of the aromatic factor (e.g., 77, 91, 105, 115, and 128) were associated primarily with aromatic fragments (e.g., benzene, toluene, xylenes, indene, and naphthalene, respectively). One of the two aliphatic factors (termed “aliphatic #1”) clearly had characteristics associated with long-chain alkanes, specifically the importance of clusters of masses separated by 14 mass units corresponding to successive loss of CH2 groups during ionization. The second aliphatic factor (termed “aliphatic #2”) was dominated by fragments with masses less than 60, suggesting either a highly branched or oxygenated source. High-resolution mass spectrometry indicated that the aliphatic factors consists of ∼60% CxHy and ∼40% CxHyOz compounds.
The two aliphatic factors and the aromatic factor varied strongly with engine power, but less from engine to engine. Their sum decreased with increasing engine power, paralleling observations made for aircraft engine VOC emissions. More specifically, the aromatic factor was correlated with benzene emissions, especially at low power conditions when EIm-benzene <0.03 mg kg−1. Aliphatic #1 was correlated with soot emissions, especially for high power conditions when EIm-soot > 30 mg kg−1. Association of aliphatic #1 with soot may be consistent with observations that alkane emissions are diminished less strongly than other VOCs with increasing engine power. Moreover, the correlation between aliphatic #1 and soot suggests that aliphatic #1 is associated with the soot surface. We found no correlations between known combustion tracers (including soot and benzene) and the non-combustion contributions to PM mass (i.e., the two lubrication oil factors and siloxane). Interestingly, we found that aliphatic #2 correlated more strongly with calculated dilution levels than with any known combustion tracers. Based on this observation, we surmise that aliphatic #2 may be related with atmospheric aerosol that becomes associated with aircraft engine PM during combustion or postcombustion processing. Our results improve our understanding of the composition of aircraft engine exhaust PM and may have implications for potential human health, climate, and environmental effects.
Acknowledgments
The authors thank NASA for leading the APEX-3 and AAFEX measurement activities and for supporting our participation in AAFEX-1 (NRA # NNC07CB57C). FAA (via Missouri University of Science and Technology) supported our participation in AAFEX-2. NASA DAOF and Cleveland Hopkins Airport were gracious hosts for the field measurement campaigns. Discussions with Kathy Tacina, Chris Heath, Bruce Anderson, and Andreas Beyersdorf helped guide our analysis and discussion of our results. Scott Herndon's efforts made sure that Aerodyne's involvement in the APEX-3 and AAFEX campaigns were successful. Berk Knighton generously shared his PTR-MS benzene measurement data. Ezra Wood (APEX-3 and AAFEX) and Jon Franklin (AAFEX-2) were valuable team members who contributed to preparation, execution, and analysis efforts. John Jayne developed AMS operating procedures used in this study and advised the team during the data analysis effort.
REFERENCES
- Allan , J. D. , Delia , A. E. , Coe , H. , Bower , K. N. , Alfarra , M. R. Jimenez , J. L. 2004 . A Generalised Method for the Extraction of Chemically Resolved Mass Spectra from Aerodyne Aerosol Mass Spectrometer Data . J. Aerosol Sci. , 35 : 909 – 922 .
- Arunachalam , S. , Wang , B. , Davis , N. , Baek , B. H. and Levy , J. I. 2011 . Effect of Chemistry-Transport Model Scale and Resolution on Population Exposure to PM(2.5) from Aircraft Emissions During Landing and Takeoff . Atmos. Environ. , 45 : 3294 – 3300 .
- Beyersdorf , A. J. , Thornhill , K. L. , Winstead , E. L. , Ziemba , L. D. , Blake , D. R. Timko , M. T. 2012 . Power-Dependent Speciation of Volatile Organic Compounds in Aircraft Exhaust . Atmos. Environ. , 61 : 275 – 282 .
- Blake , R. S. , Monks , P. S. and Ellis , A. M. 2009 . Proton-Transfer Reaction Mass Spectrometry . Chem. Rev. , 109 : 861 – 896 .
- Bulzan , D. , Anderson , B. , Wey , C. , Howard , R. , Winstead , E. Beyersdorf , A. 2010 . Gaseous and Particulate Emissions Results of the NASA Alternative Aviation Fuel Experiment (AAFEX) 1195 – 1207 . Proceedings of the ASME Turbo Expo 2010, Vol. 2, Parts A and B
- Cain , J. P. , Gassman , P. L. , Wang , H. and Laskin , A. 2010 . Micro-FTIR Study of Soot Chemical Composition-Evidence of Aliphatic Hydrocarbons on Nascent Soot Surfaces . Phys. Chem. Chem.l Phys. , 12 : 5206 – 5218 .
- Canagaratna , M. R. , Jayne , J. T. , Ghertner , D. A. , Herndon , S. , Shi , Q. Jimenez , J. L. 2004 . Chase Studies of Particulate Emissions From In-Use New York City vehicles . Aerosol Sci. Technol. , 38 : 555 – 573 .
- Canagaratna , M. R. , Jayne , J. T. , Jimenez , J. L. , Allan , J. D. , Alfarra , M. R. Zhang , Q. 2007 . Chemical and Microphysical Characterization of Ambient Aerosols with the Aerodyne Aerosol Mass Spectrometer . Mass Spectrom. Rev. , 26 : 185 – 222 .
- Dallara , E. S. , Kroo , I. M. and Waitz , I. A. 2011 . Metric for Comparing Lifetime Average Climate Impact of Aircraft . AIAA J. , 49 : 1600 – 1613 .
- Dodson , R. E. , Houseman , E. A. , Morin , B. and Levy , J. I. 2009 . An Analysis of Continuous Black Carbon Concentrations in Proximity to an Airport and Major Roadways . Atmos. Environ. , 43 : 3764 – 3773 .
- Dorbian , C. S. , Wolfe , P. J. and Waitz , I. A. 2011 . Estimating the Climate and Air Quality Benefits of Aviation Fuel and Emissions Reductions . Atmos. Environ. , 45 : 2750 – 2759 .
- Drewnick , F. , Hings , S. S. , DeCarlo , P. , Jayne , J. T. , Gonin , M. Fuhrer , K. 2005 . A New Time-of-Flight Aerosol Mass Spectrometer (TOF-AMS) - Instrument Description and First Field Deployment . Aerosol Sci. Technol. , 39 : 637 – 658 .
- Hagen , D. E. , Lobo , P. , Whitefield , P. D. , Trueblood , M. B. , Alofs , D. J. and Schmid , O. 2009 . Performance Evaluation of a Fast Mobility-Based Particle Spectrometer for Aircraft Exhaust . J. Propul. Power , 25 : 628 – 634 .
- Herndon , S. C. , Jayne , J. T. , Lobo , P. , Onasch , T. B. , Fleming , G. Hagen , D. E. 2008 . Commercial Aircraft Engine Emissions Characterization of In-Use Aircraft at Hartsfield-Jackson Atlanta International Airport . Environ. Sci. Technol. , 42 : 1877 – 1883 .
- Herndon , S. C. , Jayne , J. T. , Zahniser , M. S. , Worsnop , D. R. , Knighton , B. Alwine , E. 2005a . Characterization of Urban Pollutant Emission Fluxes and Ambient Concentration Distributions Using a Mobile Laboratory with Rapid Response Instrumentation . Faraday Discuss. , 130 : 327 – 339 .
- Herndon , S. C. , Onasch , T. B. , Frank , B. P. , Marr , L. C. , Jayne , J. T. Canagaratna , M. R. 2005b . Particulate Emissions from In-Use Commercial Aircraft . Aerosol Sci. Technol. , 39 : 799 – 809 .
- Herndon , S. C. , Rogers , T. , Dunlea , E. J. , Jayne , J. T. , Miake-Lye , R. and Knighton , B. 2006 . Hydrocarbon Emissions from In-Use Commercial Aircraft During Airport Operations . Environ. Sci. Technol. , 40 : 4406 – 4413 .
- Herndon , S. C. , Shorter , J. H. , Zahniser , M. S. , Nelson , D. D. , Jayne , J. Brown , R. C. 2004 . NO and NO2 Emission Ratios Measured from In-Use Commercial Aircraft During Taxi and Takeoff . Environ. Sci. Technol. , 38 : 6078 – 6084 .
- Herndon , S. C. , Shorter , J. H. , Zahniser , M. S. , Wormhoudt , J. , Nelson , D. D. Demerjian , K. L. 2005c . Real-Time Measurements of SO2, H2CO, and CH4 Emissions from In-Use Curbside Passenger Buses in New York City using a Chase Vehicle . Environ. Sci. Technol. , 39 : 7984 – 7990 .
- Herndon , S. C. , Wood , E. C. , Northway , M. J. , Miake-Lye , R. , Thornhill , L. Beyersdorf , A. 2009 . Aircraft Hydrocarbon Emissions at Oakland International Airport . Environ. Sci. Technol. , 43 : 1730 – 1736 .
- Hsu , H. H. , Adamkiewicz , G. , Houseman , A. , Vallarino , J. , Melly , S. Wayson , R. 2011 . Spatiotemporal Patterns of Ultrafine Particle Counts and Fine Particle Mass in Neighborhoods Surrounding an Airport . Epidemiology , 22 : S208 – S208 .
- Hsu , H. H. , Adamkiewicz , G. , Houseman , E. A. , Vallarino , J. , Melly , S. J. Wayson , R. L. 2012 . The Relationship Between Aviation Activities and Ultrafine Particulate Matter Concentrations Near a Mid-Sized Airport . Atmos. Environ. , 50 : 328 – 337 .
- Jacobson , M. Z. , Wilkerson , J. T. , Naiman , A. D. and Lele , S. K. 2011 . The Effects of Aircraft on Climate and Pollution. Part I: Numerical Methods for Treating the Subgrid Evolution of Discrete Size- and Composition-Resolved Contrails from all Commercial Flights Worldwide . J. Comput. Phys. , 230 : 5115 – 5132 .
- Jayne , J. T. , Leard , D. C. , Zhang , X. F. , Davidovits , P. , Smith , K. A. Kolb , C. E. 2000 . Development of an Aerosol Mass Spectrometer for Size and Composition Analysis of Submicron Particles . Aerosol Sci. Technol. , 33 : 49 – 70 .
- Knighton , W. B. , Rogers , T. M. , Anderson , B. E. , Herndon , S. C. , Yelvington , P. E. and Miake-Lye , R. C. 2007 . Quantification of Aircraft Engine Hydrocarbon Emissions using Proton Transfer Reaction Mass Spectrometry . J. Propul. Power , 23 : 949 – 958 .
- Kolb , C. E. , Herndon , S. C. , McManus , B. , Shorter , J. H. , Zahniser , M. S. Nelson , D. D. 2004 . Mobile Laboratory with Rapid Response Instruments for Real-Time Measurements of Urban and Regional Trace Gas and Particulate Distributions and Emission Source Characteristics . Environ. Sci. Technol. , 38 : 5694 – 5703 .
- Lack , D. A. , Corbett , J. J. , Onasch , T. , Lerner , B. , Massoli , P. Quinn , P. K. 2009 . Particulate Emissions from Commercial Shipping: Chemical, Physical, and Optical Properties . J. Geophys. Res. –Atmos. , 114:D00F04
- Lanz , V. A. , Alfarra , M. R. , Baltensperger , U. , Buchmann , B. , Hueglin , C. and Prevot , A. S. H. 2007 . Source Apportionment of Submicron Organic Aerosols at an Urban Site by Factor Analytical Modelling of Aerosol Mass Spectra . Atmos. Chem. Phys. , 7 : 1503 – 1522 .
- Lee , B. H. , Wood , E. C. , Zahniser , M. S. , McManus , J. B. , Nelson , D. D. Herndon , S. C. 2011 . Simultaneous Measurements of Atmospheric HONO and NO(2) via Absorption Spectroscopy using Tunable Mid-Infrared Continuous-Wave Quantum Cascade Lasers . Appl. Phys. B-Lasers and Opt. , 102 : 417 – 423 .
- Lee , D. S. , Fahey , D. W. , Forster , P. M. , Newton , P. J. , Wit , R. C. N. Lim , L. L. 2009 . Aviation and Global Climate Change in the 21st Century . Atmos. Environ. , 43 : 3520 – 3537 .
- Lee , D. S. , Pitari , G. , Grewe , V. , Gierens , K. , Penner , J. E. Petzold , A. 2010 . Transport Impacts on Atmosphere and Climate: Aviation . Atmos. Environ. , 44 : 4678 – 4734 .
- Levy , J. I. , Woody , M. , Baek , B. H. , Shankar , U. and Arunachalam , S. 2012 . Current and Future Particulate-Matter-Related Mortality Risks in the United States from Aviation Emissions During Landing and Takeoff . Risk Anal. , 32 : 237 – 249 .
- Lindinger , W. , Hansel , A. and Jordan , A. 1998 . Proton-Transfer-Reaction Mass Spectrometry (PTR-MS): On-Line Monitoring of Volatile Organic Compounds at pptv Levels . Chem. Soc. Rev. , 27 : 347 – 354 .
- Liu , P. , Ziemann , P. J. , Kittelson , D. B. and McMurry , P. H. 1995 . Generating Penetrating Particle Beams of Controlled Dimensions and Divergence. 2. Experimental Evaluation of Particle Motion in Aerodynamic Lenses and Nozzle Expansions . Aerosol Sci. Technol. , 22 : 314 – 324 .
- Liu , P. S. K. , Deng , R. , Smith , K. A. , Williams , L. R. , Jayne , J. T. Canagaratna , M. R. 2007 . Transmission Efficiency of an Aerodynamic Focusing Lens System: Comparison of Model Calculations and Laboratory Measurements for the Aerodyne Aerosol Mass Spectrometer . Aerosol Sci. Technol. , 41 : 721 – 733 .
- Lobo , P. , Hagen , D. E. and Whitefield , P. D. 2011 . Comparison of PM Emissions from a Commercial Jet Engine Burning Conventional, Biomass, and Fischer-Tropsch Fuels . Environ. Sci. Technol. , 45 : 10744 – 10749 .
- Lobo , P. , Hagen , D. E. , Whitefield , P. D. and Alofs , D. J. 2007 . Physical Characterization of Aerosol Emissions from a Commercial Gas Turbine Engine . J. Propul. Power , 23 : 919 – 929 .
- Massoli , P. , Fortner , E. C. , Canagaratna , M. R. , Williams , L. R. , Zhang , Q. Sun , Y. 2012 . Pollution Gradients and Chemical Characterization of Particulate Matter from Vehicular Traffic near Major Roadways: Results from the 2009 Queens College Air Quality Study in NYC . Aerosol Sci. Technol. , 46 : 1201 – 1218 .
- McLafferty , F. W. and Tureček , F. 1993 . Interpretation of Mass Spectra, , 4th ed. , 226 – 232 . Sausalito, CA : University Science Books .
- Onasch , T. B. , Jayne , J. T. , Herndon , S. , Worsnop , D. R. , Miake-Lye , R. C. Mortimer , I. P. 2009 . Chemical Properties of Aircraft Engine Particulate Exhaust Emissions . J. Propul. Power , 25 : 1121 – 1137 .
- Paatero , P. 1997 . Least Squares Formulation of Robust Non-Negative Factor Analysis . Chemometr. Intell. Lab. Syst. , 37 : 23 – 35 .
- Paatero , P. and Tapper , U. 1993 . Analysis of Different Modes of Factor-Analysis as Least-Squares Fit Problems . Chemometr. Intell. Lab. Syst. , 18 : 183 – 194 .
- Petzold , A. , Schloesser , H. , Sheridan , P. J. , Arnott , W. P. , Ogren , J. A. and Virkkula , A. 2005 . Evaluation of Multiangle Absorption Photometry for Measuring Aerosol Light Absorption . Aerosol Sci. Technol. , 39 : 40 – 51 .
- Santoni , G. W. , Lee , B. H. , Wood , E. C. , Herndon , S. C. , Miake-Lye , R. C. Wofsy , S. C. 2011 . Aircraft Emissions of Methane and Nitrous Oxide during the Alternative Aviation Fuel Experiment . Environ. Sci. Technol. , 45 : 7075 – 7082 .
- Slowik , J. G. , Cross , E. S. , Han , J. H. , Davidovits , P. , Onasch , T. B. Jayne , J. T. 2007 . An Inter-Comparison of Instruments Measuring Black Carbon Content of Soot Particles . Aerosol Sci. Technol. , 41 : 295 – 314 .
- Spicer , C. W. , Holdren , M. W. , Cowen , K. A. , Joseph , D. W. , Satola , J. Goodwin , B. 2009 . Rapid Measurement of Emissions from Military Aircraft Turbine Engines by Downstream Extractive Sampling of Aircraft on the Ground: Results for C-130 and F-15 Aircraft . Atmos. Environ. , 43 : 2612 – 2622 .
- Spicer , C. W. , Holdren , M. W. , Riggin , R. M. and Lyon , T. F. 1994 . Chemical-Composition and Photochemical Reactivity of Exhaust from Aircraft Turbine-Engines . Ann. Geophys. –Atmos. Hydr. Space Sci. , 12 : 944 – 955 .
- Stettler , M. E. J. , Eastham , S. and Barrett , S. R. H. 2011 . Air Quality and Public Health Impacts of UK Airports. Part I: Emissions . Atmos. Environ. , 45 : 5415 – 5424 .
- Timko , M. T. , Fortner , E. , Franklin , J. , Yu , Z. , Wong , H. W. Onasch , T. B. 2013 . Atmospheric Measurements of the Physical Evolution of Aircraft Exhaust Plumes . Environ. Sci. Technol. , 47 : 3513 – 3520 .
- Timko , M. T. , Herndon , S. C. , Blanco , E. D. , Wood , E. C. , Yu , Z. H. Miake-Lye , R. C. 2011 . Combustion Products of Petroleum Jet Fuel, a Fischer-Tropsch Synthetic Fuel, and a Biomass Fatty Acid Methyl Ester Fuel for a Gas Turbine Engine . Combust. Sci. Technol. , 183 : 1039 – 1068 .
- Timko , M. T. , Herndon , S. C. , Wood , E. C. , Onasch , T. B. , Northway , M. J. Jayne , J. T. 2010a . Gas Turbine Enginer Emissions - Part I: Volatile Organic Compounds and Nitrogen Oxides . J. Eng. Gas Turb. Power-Trans. ASME , 132 : 061504
- Timko , M. T. , Onasch , T. B. , Northway , M. J. , Jayne , J. T. , Canagaratna , M. R. Herndon , S. C. 2010b . Gas Turbine Engine Emissions - Part II: Chemical Properties of Particulate Matter . J. Eng. Gas Turb. Power –Trans. ASME , 132 : 061505
- Timko , M. T. , Yu , Z. , Onasch , T. B. , Wong , H. W. , Miake-Lye , R. C. Beyersdorf , A. J. 2010c . Particulate Emissions of Gas Turbine Engine Combustion of a Fischer-Tropsch Synthetic Fuel . Energy & Fuels , 24 : 5883 – 5896 .
- Timko , M. T. , Yu , Z. H. , Kroll , J. , Jayne , J. T. , Worsnop , D. R. Miake-Lye , R. C. 2009 . Sampling Artifacts from Conductive Silicone Tubing . Aerosol Sci. Technol. , 43 : 855 – 865 .
- Ulbrich , I. M. , Canagaratna , M. R. , Zhang , Q. , Worsnop , D. R. and Jimenez , J. L. 2009 . Interpretation of Organic Components from Positive Matrix Factorization of Aerosol Mass Spectrometric Data . Atmos. Chem. Phys. , 9 : 2891 – 2918 .
- Unger , N. 2011 . Global Climate Impact of Civil Aviation for Standard and Desulfurized Jet Fuel . Geophys. Res. Lett. , 38 : L20803
- Wey , C. C. , Anderson , B. E. , Wey , C. , Miake-Lye , R. C. , Whitefield , P. and Howard , R. 2007 . Overview on the Aircraft Particle Emissions Experiment . J. Propul. Power , 23 : 898 – 905 .
- Wood , E. C. , Herndon , S. C. , Timko , M. T. , Yelvington , P. E. and Miake-Lye , R. C. 2008 . Speciation and Chemical Evolution of Nitrogen Oxides in Aircraft Exhaust Near Airports . Environ. Sci. Technol. , 42 : 1884 – 1891 .
- Wormhoudt , J. , Herndon , S. C. , Yelvington , P. E. , Miake-Lye , R. C. and Wey , C. 2007 . Nitrogen Oxide (NO/NO2/HONO) Emissions Measurements in Aircraft Exhausts . J. Propul. Power , 23 : 906 – 911 .
- Yelvington , P. E. , Herndon , S. C. , Wormhoudt , J. C. , Jayne , J. T. , Miake-Lye , R. C. Knighton , W. B. 2007 . Chemical Speciation of Hydrocarbon Emissions from a Commercial Aircraft Engine . J. Propul. Power , 23 : 912 – 918 .
- Yu , Z. H. , Herndon , S. C. , Ziemba , L. D. , Timko , M. T. , Liscinsky , D. S. Anderson , B. E. 2012 . Identification of Lubrication Oil in the Particulate Matter Emissions from Engine Exhaust of In-Service Commercial Aircraft . Environ. Sci. Technol. , 46 : 9630 – 9637 .
- Yu , Z. H. , Liscinsky , D. S. , Winstead , E. L. , True , B. S. , Timko , M. T. Bhargava , A. 2010 . Characterization of Lubrication Oil Emissions from Aircraft Engines . Environ. Sci. Technol. , 44 : 9530 – 9534 .
- Zhang , Q. , Jimenez , J. L. , Canagaratna , M. R. , Ulbrich , I. M. , Ng , N. L. Worsnop , D. R. 2011 . Understanding Atmospheric Organic Aerosols via Factor Analysis of Aerosol Mass Spectrometry: A Review . Anal. Bioanal. Chem. , 401 : 3045 – 3067 .
- Zhu , Y. , Fanning , E. , Yu , R. C. , Zhang , Q. and Froines , J. R. 2011 . Aircraft Emissions and Local Air Quality Impacts from Takeoff Activities at a Large International Airport . Atmos. Environ. , 45 : 6526 – 6533 .