Abstract
Mineral dust is the second largest atmospheric emission by mass and one of the least understood sources. The shape of the particles depends on their composition and has implications for particle optical properties and reactive surface area. Mineral dust particles are often approximated as spheroids to model their optical properties. In this study, scanning electron microscopy (SEM) is used to measure the aspect ratios of calcite, quartz, NX-illite, kaolinite (KGa-1b and KGa-2), and montmorillonite (STx-1b and SWy-2). In addition to traditional SEM images of the top of the particles, the SEM substrates are oriented approximately normal to the electron beam in order to image the side of the particles. In this manner, aspect ratios for the top and side orientation of the particles are determined. Calcite particles have an aspect ratio of approximately 1.3 in both orientations, while quartz particles have an aspect ratio of 1.38 in the top orientation and 1.64 in the side orientation. The clay minerals studied all exhibited plate-like structures with aspect ratios of 1.35 to 1.44 for the top orientation and 4.80 to 9.14 for the side orientation. These values are used to estimate the specific surface areas (SSAs) of the minerals, which are compared to Brunauer-Emmett-Teller (BET) surface area measurements. Through this study, we present a simple method for determining the aspect ratios of aerosolized samples, rather than relying on literature values of model systems. As a result, this technique should provide a better method for determining the optical properties of mineral dust particles.
Copyright 2014 American Association for Aerosol Research
INTRODUCTION
Aerosol particles affect the radiative balance of the Earth through their interactions with light and clouds as well as their influence on atmospheric composition (Forster et al. Citation2007). How particles scatter and absorb light is determined by their shape, composition, and size. Mineral dust aerosol particles also act as nuclei for liquid water and ice clouds (Broadley et al. Citation2012; Pinti et al. Citation2012; Atkinson et al. Citation2013). In addition, heterogeneous chemistry on mineral dust aerosol particles can alter atmospheric composition (McNaughton et al. Citation2009; Fischer et al. Citation2010).
Mineral dust aerosol particles are the second largest emission by mass into the atmosphere, amounting to 1000 to 3000 Tg per year (Dentener et al. Citation1996; Ginoux et al. Citation2001; Forster et al. Citation2007; Ginoux et al. Citation2012). A major source of mineral dust comes from dust storms in arid regions that entrain large amounts of particulates in the atmosphere, which can subsequently be transported long distances from the source region (Cahill Citation2003; Zhao et al. Citation2003; Fairlie et al. Citation2007). Up to 50% of mineral dust emissions originate from anthropogenic sources such as agriculture (Forster et al. Citation2007; Ginoux et al. Citation2012).
Aluminosilicate clay minerals are one of the most common types of mineral dust, accounting for 50–64 wt.% of Saharan dust (McNaughton et al. Citation2009) and up to 85% of Asian dust (Liu Citation1985). Mineral dust is one of the most common ice nuclei, and aluminosilicate clay minerals are one of the most ice-active types of mineral dust (Broadley et al. Citation2012; Pinti et al. Citation2012). Ice nuclei from field samples have been studied in great detail using scanning electron microscopy (SEM) and scanning transmission X-ray microscopy (STXM; Wang et al. Citation2012; Hiranuma et al. Citation2013). Among mineral dust components, clay minerals have the largest aspect ratios, with disk-like shapes and measured aspect ratios of approximately 4 to 500 (Nadeu Citation1985; Inoue and Kitagawa Citation1994). These shapes cause aluminosilicate clay minerals to have different optical properties than particles that have aspect ratios closer to unity. In addition, these particles have a large surface area to volume ratio because of their large aspect ratio. Because they have a larger surface area to volume ratio than other types of particles, they are more active toward ice nucleation and heterogeneous chemistry per unit volume.
Clay minerals are composed of negatively charged aluminosilicate layers composed of alternating layers of tetrahedrally coordinated silicon and octahedrally coordinated aluminum, resulting in alternating tetrahedral and octahedral layers. Al3+ can be substituted into the tetrahedral layers and divalent cations (e.g., Mg2+) can be substituted into the octahedral layers, which results in the net negative charge. Monovalent cations (e.g., Na+ and K+) and calcium tend to sit in the interlayer sites, which holds the aluminosilicate layers together through electrostatic interactions (Moore and Reynolds Citation1997). The number of tetrahedral to octahedral layers, for example, 1:1 or 2:1, defines the particle type (Martin et al. Citation1991). For example, kaolinite is a 1:1 clay, while montmorillonite and illite are 2:1 clays that vary in the number and composition of interlayer ions (Grim Citation1968).
Two other major components of mineral dust are quartz and calcite. Quartz is the largest component of China Loess, at greater than 50 wt.% (Liu Citation1985). Calcium carbonate is a highly reactive component of mineral dust and a major fraction of Asian dust, contributing up to 12 wt.% (Liu Citation1985; McNaughton et al. Citation2009). A study of Asian dust collected over the Eastern North Pacific estimated that 5–30% of calcium carbonate was converted to CaSO4 or Ca(NO3)2 during transport (McNaughton et al. Citation2009).
Current uncertainties in the radiative impacts of mineral dust aerosol include size distributions, particle shape, and composition-resolved emission concentrations (Johnson et al. Citation2009, Citation2012; Kok Citation2011). Both satellite and ground-based lidar measurements use algorithms that include particle shape to accurately derive aerosol mass loadings. Traditionally, shapes of particles in models are estimated by spheroids, where the axes of the spheroid are determined by the particle aspect ratios (ratio of the width to the length or height of the particle). By using a distribution of particle aspect ratios, the radiative properties of particles have been more effectively modeled in aerosol retrieval algorithms (Haapanala et al. Citation2012). Many studies have found that incorporating the shape distribution with a large range of aspect ratios has improved models, but lack a physical reason for their choice of aspect ratios (Kahnert Citation2004; Nousiainen et al. Citation2006; Merikallio et al. Citation2011). Hudson et al. (Citation2008) found that incorporating the shape of clay mineral particles (e.g., disc/needle) improves the agreement between models and laboratory results. Recently, Lindqvist et al. (Citation2014) used SEM to characterize the shape and composition of supermicron mineral dust aerosol particles. They found a large difference between the scattering properties of spheres, spheriods, and particles with the measured shapes and compositions (Lindqvist et al. Citation2014).
For the minerals of interest in this study, two aspect ratios are defined in reference to the substrate on which they are placed for this measurement. One aspect ratio is the ratio of the length to the width of the top of the particle (top-down orientation), and one is measured as the ratio of the length to the height of the side of the particle (side-on orientation). The axes are chosen such that the aspect ratio is greater than unity. For the larger of the two aspect ratios, it has been shown that calcite has an aspect ratio near 1, quartz has a slightly higher aspect ratio near 1.7–1.9 (Siegesmund et al. Citation2002), and clay minerals have aspect ratios from 3 to approximately 500 (Nadeau Citation1985; Inoue et al. 1994). Previous studies have determined the high aspect ratios of clay minerals using transmission electron microscopy (TEM) and atomic force microscopy (AFM). To measure the aspect ratio using TEM, particles are coated (generally with Pt) at a known angle, approximately 10 degrees from normal. Due to the angle of the coating process, the height of the particle shields part of the substrate from being coated. The height of the particles can be calculated based on the width of the uncoated region. Using the two dimensional projection of the particles visible in the TEM image and the height of the particle, the aspect ratios of the particle in the top-down and side-on orientations can be determined (Robertson et al. Citation1954; Jepson and Rowse Citation1975; Nadeau Citation1985, Citation1987; Inoue and Kitagawa Citation1994; Becket et al. Citation1997; Tumolva et al. Citation2012). This method assumes that the particle has no surface irregularities that may affect the observed height of the particle and that the particles do not move during the coating process. This technique has been used extensively to determine the volume of mineral dust particles. Aspect ratios for clay minerals have been as high as 1.68 for montmorillonite, 1.31 for kaolinite, and 5.33 for illite for the top-down orientation and as high as 448, 18, and 120, respectively, for the side-on orientation (Nadeau Citation1985). AFM has also been used to measure the dimensions of clay minerals without the use of a coating (Lindgreen et al. Citation1991; Schleicher et al. Citation1993; Bickmore et al. Citation2002; Tumolva et al. Citation2012). In addition to microscopy methods used to study the dimensions of the particles, surface area measurements through gas adsorption studies (described below) can be used with microscopy methods to find the dimensions of the particles (Hofmann et al. Citation1961; Schuttlefield et al. Citation2007). Because of the need for a coating for the TEM measurement of the side aspect ratio and the nature of the AFM technique, these methods are slow. As a result, they are not used routinely to study field and laboratory samples. Instead, in studies where aspect ratios are needed for analysis, literature values are used, which may not be representative of the exact sample of interest. The development of techniques to easily measure aspect ratios for samples would help our analysis of field and laboratory samples because each sample of interest could be measured rather than depending on literature values for representative compounds. Where additional information is needed for complex particles, more time-consuming techniques such as electron tomography and focused ion beam SEM imaging can be used to obtain three-dimensional shape and composition information of particles (van Poppel et al. Citation2005; Adachi et al. Citation2007; Adler et al. Citation2013; Conny Citation2013).
Particle shape is also important for the measurement of specific surface area (SSA), which impacts aerosol reactivity and ice nucleation activity (Brantley and Mellot Citation2000; Hoose and Möhler Citation2012). There have been multiple strategies used to obtain particle surface area, including scanning probe microscopy and gas adsorption. AFM has been used to study minerals, but it is time consuming to analyze a sufficient number of particles with this technique and it can miss some of the interior detail (Bickmore et al. Citation2002; Metz et al. Citation2005). The major method used for minerals is the measurement of Brunauer-Emmett-Teller (BET) surface area, where the adsorption of a nonreactive gas to the surface of the particles is used to determine the surface area (Brunauer et al. Citation1938; Madsen Citation1977; van Olphen and Fripiat Citation1979; Pruett and Webb Citation1993; Bereznitski et al. Citation1998; Schuttlefield et al. Citation2007; Steudel et al. Citation2009; Sanders et al. Citation2010; Broadley et al. Citation2012). BET measurements can show variability for particles depending on the gas used with up to a 37% difference shown between N2 and Kr adsorption (Brantley and Mellott Citation2000). For standard materials, differences from the same sample are observed from 0.19% to −12.77% for a high SSA material (Hackley and Stefaniak Citation2013). New methods for the measurement of geometric surface area may provide a lower bound for the surface area of minerals.
In our study, we demonstrate a method for calculating the dimensions of mineral dust particles in the submicron regime using SEM. Previously, SEM has generally been used to look at particles larger than a micron and for elemental identification with energy dispersive X-ray spectroscopy (Laird Citation2001; Krueger et al. Citation2003; Clayton and Pearce Citation2007; Chen et al. Citation2013). As described above, previous studies using TEM have required lengthy sample preparation techniques and additional assumptions to ascertain the height of particles. While AFM studies have been shown to give accurate detail of the particles, these methods are time consuming for the collection of information on hundreds of single particles. In contrast, high resolution SEM requires no sample preparation and large numbers of particles can be analyzed quickly. We demonstrate our method on a variety of commercially available minerals.
EXPERIMENTAL METHODS
Four types of clay minerals used were from the Source Clays Repository of the Clay Mineral Society: low-defect kaolinite, Washington County Georgia, USA (KGa-1b); high-defect kaolinite, Warren County Georgia, USA (KGa-2); montmorillonite, Gonzales County TX, USA (STx-1b); and Na-rich montmorillonite, Crook County WY, USA (SWy-2). The remaining minerals were: NX-illite (Arginotec, NX Nanopowder, B+M Notenkämper, Munich, Germany), calcium carbonate (>99.95%, Macron Chemicals), and silicon dioxide (quartz; >99%, Sigma Aldrich). Because the calcium carbonate and silicon dioxide were obtained from chemical companies, their physical and chemical properties may differ from geological sources. NX-Illite is a mixture of minerals that has been suggested to have a similar composition as mineral dust that undergoes long-range atmospheric transport (Broadley et al. Citation2012). In this study, we have investigated aerosolized samples, which may have a different composition than the bulk samples.
Aerosol particles were generated by directing a stream of nitrogen at 1.5 lpm toward the mineral dust while agitating it. The entrained dust particles were directed into a cascade impactor (PIXE International Corp., Tallahassee, FL, USA) backed with a pump at 1.0 lpm. Particles were impacted near the edge of the silicon wafer chips (Virginia Semiconductor Inc., Fredericksburg, VA, USA) for SEM analysis. For quartz particles, the silicon substrate was coated with a thin layer of Formvar 15/95 (Electron Microscopy Science, Hatfield, PA, USA) to prevent damage to the substrate from the particle impaction.
A FEI NanoSEM 630 FESEM operated at 3 keV was used to image the particles. Under these conditions, a resolution of <2 nm can be obtained. Particles were imaged in top-down and side-on configurations. Top-down refers to the standard SEM configuration. To image side-on, the substrates were placed in a perpendicular orientation at an angle of 5° with respect to normal, and a tilt correction was used to remove distortion. The particles were imaged at an orientation that allowed for the greatest emission of secondary electrons toward the detector, which results in the best resolution. Top-down and side-on images were analyzed to measure the aspect ratios using ImageJ (National Institutes of Health, Bethesda, MD, USA). In the top-down orientation, the aspect ratio is calculated from the ratio of the length to the width of the particle. The length is defined as the longest distance across the center of the particle; the width is measured across the longest distance in the orthogonal direction. In the side-on orientation, the aspect ratio is calculated as the ratio of the width to the height of the particle. In this study, we have investigated minerals that can be described by these two aspect ratios. We note, however, that some types of minerals have shapes that are best described by three aspect ratios (e.g., rectangular prisms). For these types of particles, the aspect ratio in the top-down orientation will not be close to unity. In this case, two aspect ratios will be averaged together in the side-on orientation. Using the top-down orientation, however, the length and width of the particles is measured, and the height is measured in the side-on orientation. By combining the top-down length and width measurements and the side-on measurement of height, the three aspect ratios can be determined. Only particles with a dimension less than 1 μm were analyzed due to large quartz impurities present in some of the clay mineral samples (Clayton and Pearce Citation2007). Using the top view of the particles, 198 to 511 particles were analyzed for each sample. Due to the low depth of field with the SEM for the side view, 90 to 167 particles were analyzed for each sample.
The geometric SSA of the particles is defined as area per unit mass. To calculate the particle surface area, we have assumed three geometries for the particles: hexagonal prisms, rectangular prisms, and spheroids. Using the top-down SEM image, the length and width of each particle is measured. The aspect ratio for the side-on orientation is used to obtain the height of the particle. Using the geometry of interest, the ratio of the surface area to volume is calculated for each particle. The volumes are then normalized by the density of the particles to give units of m2/g. The densities in units of g/cm3 used for the calculations were: 2.71 for calcite, 2.65 for quartz, 2.8 for illite, 2.65 for kaolinite, and 2.5 for montmorillonite (Haynes Citation2013). The SSAs for each particle are summed and divided by the number of particles to give an average SSA.
RESULTS AND DISCUSSION
To demonstrate the use of SEM for the measurement of the aspect ratios of mineral particles, we use commercially available samples of calcite, quartz, illite, kaolinite, and montmorillonite, as described above. We report the values derived for these commonly used samples, but this technique can be extended to any sample of interest. In , we show histograms of the aspect ratios for each type of mineral in the top-down and side-on orientations with representative SEM images. shows a box-and-whisker plot of the aspect ratios of all particle types measured for both orientations. The central value shown is the mean, the box indicates the 25th and 75th percentile, and the whiskers mark the 5th and 95th percentiles. summarizes the mean aspect ratios obtained with standard deviations. All of the particles have aspect ratios of approximately unity when only the top-down orientation is measured. The major difference between particle dimensions arises from the side-on view of the particles. There is no dependence of the aspect ratio on the size of the particles for the submicron diameters reported in this study. In the supermicron regime, a size dependence is present for some samples, such as the montmorillonite SWy-2, due to mineral contaminants. While both kaolinite samples are >96% pure, the STx-1b contains 67% montmorillonite and up to 3% quartz. The SWy-2 bulk sample contains 75% montmorillonite, 8% quartz, and 16% feldspar while the <2 micron fraction contains 95% montmorillonite and 4% quartz (Chipera and Bish Citation2001). Plots of the aspect ratio as a function of particle size for submicron and supermicron particles are provided in the online supplemental information. We note that the described SEM technique can be extended without alteration to study supermicron particles.
TABLE 1 Average aspect ratios and standard deviations for the minerals used in this study for both the top-down and side-on orientations
FIG. 1. (a) A representative scanning electron microscopy (SEM) image of the calcite particles in the top-down orientation. (b) A representative SEM image of the calcite particles in the side-on orientation, imaged at an angle of 5˚ from normal. (c) The distribution of calcite aspect ratios obtained from SEM images. The fraction of particles by number is plotted vs. aspect ratio. (d) SEM images of quartz particles in the top-down orientation and the (e) side-on orientation. (f) The distribution of quartz aspect ratios obtained from the SEM images. The tick marks label the bin to the left of the number, making the aspect ratio of the first bin 1.0.
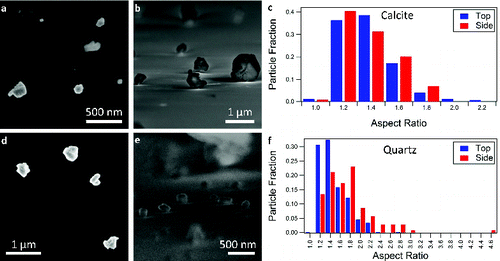
FIG. 2. SEM images of KGa-1b (kaolinite) in the (a) top-down and (b) side-on orientations. (c) The distribution of aspect ratios determined for KGa-1b in the two orientations. (d) SEM images of KGa-2 (kaolinite) in the top-down and (e) side-on orientations. (f) The distribution of aspect ratios for KGa-2. The tick marks in (c) and (f) denote the bin to the left of the number.
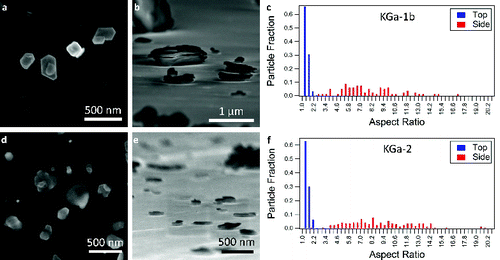
FIG. 3. SEM images of NX-illite in the (a) top-down and (b) side-on orientations. (c) The distribution of aspect ratios determined for NX-illite from the SEM images. (d) SEM images of STx-1b (montmorillonite) in the top-down and (e) side-on orientations. (f) The measured distribution of aspect ratios for STx-1b. (g) SEM images of SWy-2 in the top-down and (h) side-on orientations. (i) The distribution of aspect ratios for SWy-2. For (c), (f), and (i), the tick marks denote the bin to the left of the number.
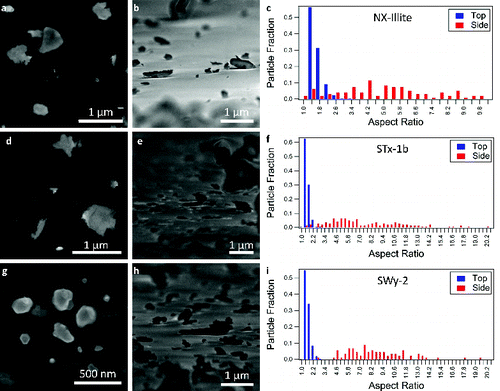
FIG. 4. Comparison of the aspect ratios for each species studied. For each type of mineral, the aspect ratios observed for the top-down and side-on orientations are shown. In the box and whiskers plots, the center line is the average of the data, the bottom of the box is the 25th percentile, the top of the box is the 75th percentile, and the whiskers correspond to the 5th and 95th percentiles.
The top-down and side-on orientations of calcite have similar aspect ratios of 1.29 ± 0.20 and 1.27 ± 0.20, respectively (a–c, ). The range of aspect ratios observed is shown in c, where the peaks that represent the measured aspect ratios for top-down and side-on orientations match closely. a and b show SEM images that illustrate that the particles have a near unity aspect ratio in both orientations. Note that surface roughness affects the aspect ratios of these particles, which accounts for the aspect ratios being slightly greater than one.
Quartz has a higher aspect ratio than calcite in the top-down orientation of 1.38 ± 0.28, and deviates more from calcite with its side-on aspect ratio of 1.64 ± 0.49 (d–f, ). These aspect ratios indicate that quartz is slightly elongated, while calcite is approximately spherical, in agreement with the literature (Siegesmund et al. Citation2002). f shows that the distribution of the aspect ratios for quartz in the side-on orientation peaks at a higher value than for calcite. From the top-down and side-on SEM images of quartz, the surface of these particles is smooth in contrast to the roughness of the calcite particles (d and e).
The aspect ratios of the clay minerals in the top-down orientation range from 1.35 to 1.44, in agreement with literature values of approximately 1.4 (; Robertson et al. Citation1954; Nadeau Citation1985; Becket et al. Citation1997). These values are larger than the aspect ratio of calcite in the top-down orientation at 1.29 ± 0.20, but are similar to that of quartz at 1.38 ± 0.28 (). This result indicates that when looking at the particles in the top-down orientation in SEM or TEM, the particles look approximately spherical or cubic (a and d, , d and g). The range of aspect ratio values for the top-down orientation is similar for all clay minerals used in this study (c and f, c, f, and i).
TABLE 2 Comparison of measured average aspect ratios to literature values for the clay minerals. The NX-illite is compared to measurements of illite
The aspect ratios measured for the side-on orientation of the clay mineral dust are significantly different than the other minerals. The two kaolinite samples studied (KGa-1b and KGa-2) have similar aspect ratios in both top-down and side-on orientations and similar particle morphologies (). The distribution of aspect ratios is wide, but similar for the two types of kaolinite (c and f). The particles have regular polygonal structures with sharp, defined edges and flat surfaces (a, b, d, and e). Literature values for the aspect ratio of kaolinite measured with TEM report median values of approximately 10 with a minimum of 2.8 and a maximum of 17.67 (; Robertson et al. Citation1954; Jepson and Rowse Citation1975; Nadeau Citation1987; Becket et al. Citation1997). The kaolinite aspect ratios obtained in this study have values near the median of the literature results.
Of the clay minerals, the lowest aspect ratio is measured for the NX-illite sample at 4.80 ± 2.23 (). The distribution of aspect ratios of the NX-illite particles is wide when compared to calcite or quartz, but smaller than the spread for the aspect ratios of kaolinite and montmorillonite (c). NX-Illite samples vary in composition, but are greater than 60% illite with additional contributions from illite-smectite mixed layers, feldspar, kaolinite, quartz, and carbonates (Broadley et al. Citation2012). As a result, the SEM images show different types of particles, rather than just illite (a and b). The measured aspect ratio is an average over this diverse composition, which could result in a lower aspect ratio compared to the other clay minerals in this study. TEM studies have reported aspect ratios for illite from 2.10 to 120 (; Nadeau Citation1985; Inoue and Kitagawa Citation1994; Becket et al. Citation1997). From the SEM images, the particles are irregular and feathery in morphology (a and b).
The montmorillonite particles (STx-1b and SWy-2) have the largest range of aspect ratios among the clay minerals (f and i) even though they have similar average aspect ratios to kaolinite (). They also have the largest variation in particle morphology. STx-1b particles have a wispy/flakey appearance with rough edges (d and e). SWy-2 has structures with flat regular features, but there are also particles similar in morphology to STx-1b and NX-illite. The SWy-2 structures were closer in morphology to the kaolinite samples than the STx-1b, but did not have the regular hexagonal structure of kaolinite (g and h). In our experimental measurements, the kaolinite and montmorillonite particles have similar mean aspect ratios in both the top-down and side-on orientations, but the montmorillonite particles have a much greater range of measured values (). This range of measurements is reflected in the larger standard deviation of the montmorillonite aspect ratios in comparison to kaolinite (). Previous studies of montmorillonite have had a much larger range of aspect ratios than kaolinite from 3.10 to 448 (; Nadeau Citation1985; Becket et al. Citation1997).
SSA was calculated from the SEM images of our aerosolized samples to compare with previously published BET and AFM measurements of bulk samples. As stated previously, the composition of the aerosolized, submicron samples may differ from the bulk composition. The particles studied have a variety of shapes, and as a result, we have calculated the SSA for each type of particle studied assuming that the particles are hexagonal prisms, rectangular prisms, and spheroids. Because BET measurements use a bulk sample for analysis, the polydispersity of the samples are included in the analysis as described in the Experimental Methods. This distribution is then used to calculate the bulk geometric SSA of the submicron fraction. The SSA of calcite and quartz were calculated and are expected to be close to BET measurements because of their smooth surfaces. When our measurements are compared to the BET measurements for the same species, there is good agreement for the kaolinite species between the SEM data, BET measurements, and AFM measurements (). We would expect good agreement in this case because of the smooth shape of the particles. The SSA of the SWy-2 montmorillonite sample was overestimated, which can be accounted for by the large difference between bulk composition of SWy-2 and the <2 μm fraction. While the bulk sample contains up to 8% quartz and 16% feldspar, the <2 μm fraction contains up to 4% quartz (Chipera and Bish Citation2001). Quartz has a smaller SSA than montmorillonite, which will especially effect the calculation of SSA in the supermicron regime. As a result, the bulk SSA reported in the literature will be smaller than our experimental estimate of the submicron fraction. The SSA of NX-illite is underestimated compared with measurements for illite because NX-illite is a mixture of minerals rather than pure illite (Broadley et al. Citation2012). The SSA of STx-1b is also significantly underestimated because these species are not regular polygons. Instead, they have a wispy/flakey structure that results in additional surface area that is accounted for in the BET measurement, but not in the SEM measurement. While BET measurements with N2 do not penetrate the interlayer spacing of montmorillonite (Grim Citation1968), they measure surface heterogeneities and pores, and therefore may result in larger values than our calculations. Water in interstitial spacings is removed by heating prior to BET measurements and depleted by the vacuum conditions in the SEM. As a result, both BET and SEM measurements are unlikely to be affected differently by the amount of interstitial water. In summary, our calculations based on the SEM images show that there is good agreement between the SSA measurements found with SEM and BET when the particles are smooth in shape, and that we obtain an underestimate for more complex particle morphologies.
TABLE 3 Specific surface area (SSA) measurements using SEM, BET, and AFM. The SSA calculations from the SEM measurements are performed assuming the particles are hexagonal prisms, rectangular prisms, and spheroids. NX-Illite is compared to BET measurements of illite
CONCLUSIONS
SEM provides a method of characterizing the aspect ratios of particles in the top-down and side-on orientations with little sample preparation. To demonstrate this technique, we have used common, commercially available components of mineral dust, including calcite, quartz, NX-illite, kaolinite (KGa-1b, KGa-2), and montmorillonite (STx-1b, SWy-2). In the top-down orientation (i. e., the usual SEM orientation), all mineral dust components studied have an aspect ratio of approximately unity. In the side-on orientation, the aspect ratio of calcite is approximately unity, and the aspect ratio of quartz is slightly greater than for the top-down orientation. The clay minerals that we investigated have significantly larger aspect ratios in the side-on orientation than calcite and quartz. NX-Illite has the smallest aspect ratio of the clay minerals and montmorillonite has the largest range of observed values. These aspect ratio measurements were coupled with the size distribution to estimate the geometric SSA of the particles. The results are similar to BET measurements for smooth kaolinite particles, while they underestimate the surface area determined with BET for nonsmooth NX-illite and STx-1b montmorillonite. The SEM method introduced in this article will allow a fast, easy measurement of the aspect ratios of mineral dust samples of interest. As a result, it has the potential to improve the calculation of the optical properties of nonspherical mineral dust particles for climate modeling and remote sensing.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the publisher's website.
2014_Veghte_aspect_ratio_AST_SI.zip
Download Zip (261.5 KB)ACKNOWLEDGMENTS
We thank S. K. Sihvonen for useful discussions. We acknowledge the Materials Characterization Lab run by the Penn State Materials Research Institute for use of the FEI NanoSEM 630 FESEM.
FUNDING
This study was supported by startup funding from the Pennsylvania State University.
REFERENCES
- Adachi, K., Chung, S. H., Friedrich, H., and Buseck, P. R. (2007). Fractal Parameters of Individual Soot Particles Determined using Electron Tomography: Implications for Optical Properties. J. Geophys. Res., 112:D14202, doi:10.1029/2006JD008296.
- Adler, G., Koop, T., Haspel, C., Taraniuk, I., Moise, T., Koren, I., et al. (2013). Formation of Highly Porous Aerosol Particles by Atmospheric Freeze-Drying In Ice Clouds. Proc. Nat. Acad. Sci. USA, 110:20414–20419.
- Atkinson, J. D., Murray, B. J., Woodhouse, M. T., Whale, T. F., Baustian, K. J., Carslaw, K. S., et al. (2013). The Importance of Feldspar for Ice Nucleation by Mineral Dust in Mixed-Phase Clouds. Nature, 498:355–358.
- Becket, R., Murphy, D., Tadjiki, S., Chittleborough, D., and Giddings, J. (1997). Determination of Thickness, Aspect Ratio and Size Distributions for Platey Particles using Sedimentation Field-Flow Fractionation and Electron Microscopy. Colloid Surface A: Physicochem. Eng. Aspects, 120:17–26.
- Bereznitski, Y., Jaroniec, M., and Maurice, P. (1998). Adsorption Characterization of Two Clay Minerals Society Standard Kaolinites. J. Colloid Interface Sci., 205:528–530.
- Bickmore, B., Nagy, K., Sandlin, P., and Crater, T. (2002). Quantifying Surface Area of Clays by Atomic Force Microscopy. Am. Mineral., 87:780–783.
- Brantley, S. L., and Mellott, N. P. (2000). Surface Area and Porosity of Primary Silicate Mineral. Am. Mineral., 85:1767–1783.
- Broadley, S. L., Murray, B. J., Herbert, R. J., Atkinson, J. D., Dobbie, S., Malkin, T. L., et al. (2012). Immersion Mode Heterogeneous Ice Nucleation by an Illite Rich Powder Representative of Atmospheric Mineral Dust. Atmos. Chem. Phys., 12:287–307.
- Brunauer, S., Emmett. P. H., and Teller, E. (1938). Adsorption of gases in multimolecular layers. J. Am. Chem. Soc., 60(2):309–319.
- Cahill, C. F. (2003). Asian Aerosol Transport to Alaska During ACE-Asia. J. Geophys. Res., 108(D23):8664, doi: 10.1029/2002JD003271.
- Chen, H., Grassian, V. H., Laxmikant, S. V., and Laskin, A. (2013). Chemical Imaging Analysis of Environmental Particles using the Focused Ion Beam/Scanning Electron Microscopy Technique: Microanalysis Insights into Atmospheric Chemistry of Fly Ash. Analyst, 138:451–460.
- Chipera, S. J., and Bish, D. L. (2001). Baseline Studies of the Clay Minerals Society Source Clays: Powder x-Ray Diffraction Analyses. Clay Clay Miner., 49(5):398–409.
- Clayton, T., and Pearce, R. B. (2007). Rapid Chemical Analysis of the <2 μm Clay Fraction using an SEM/EDS Technique. Clay Miner., 42:549–562.
- Conny, J. M. (2013). Internal Composition of Atmospheric Dust Particles from Focused Ion-Beam Scanning Electron Microscopy. Environ. Sci. Technol., 47:8575–8581.
- Dentener, F., Carmichael, G. R., Zhang, Y., Lelieveld, J., and Crutzen, P. J. (1996). Role of Mineral Aerosol as a Reactive Surface in the Global Troposphere. J. Geophys. Res., 101(D17):22869–22889, doi: 10.1029/96JD01818.
- Dentener, F., Kinne, S., Bond, T., Boucher, O., Cofala, J., Generoso, S., et al. (2006). Emissions of Primary Aerosol and Precursor Gases in the Years 2000 and 1750, Prescribed Data-Sets for AeroCom. Atmos. Chem. Phys., 6:2703–2763.
- Fairlie, T. D., Jacob, D., and Park, R. J. (2007). The Impact of Transpacific Transport of Mineral Dust in the United States. Atmos. Environ., 41:1251–126.
- Fischer, E.V., Jaffe, D. A., Marley, N. A., Gaffney J. S., and Marchany-Rivera, A. (2010). Optical Properties of Aged Asian Aerosols Observed Over the U.S. Pacific Northwest. J. Geophys. Res., 115:D20209, doi: 10.1029/2010JD013943.
- Forster, P., Ramaswamy, V., Artaxo, P., Berntsen, T., Betts, R., Fahey, D. W., et al. (2007). Changes in Atmospheric Constituents and in Radiative Forcing, in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. Averyt, M. Tignor, H. Miller, eds., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Ginoux, P., Chin, M., Tegen, I., Prospero, J.M., Holben, B., Dubovik, O., et al. (2001). Sources and Distributions of Dust Aerosol Simulated with the GOCART Model. J. Geophys. Res., 106(D17):20255–20273, doi: 10.1029/2000JD000053.
- Ginoux, P., Prospero, J. M., Gill, T. E., Hsu, N. C., and Zhao, M. (2012). Global-Scale Attribution of Anthropogenic and Natural Dust Sources and Their Emission Rates Based upon MODIS Deep Blue Aerosol Products. Rev. Geophys., 50:RG3005. doi:10.1029/2012RG000388.
- Grim, R. E. (1968). Clay Mineralology, 2nd ed. McGraw-Hill, New York.
- Haapanala, P., Raisanen, P., Kahnert, M., and Nousiainen, T. (2012). Sensitivity of the Shortwave Radiative Effect of Dust on Particle Shape: Comparison of Spheres and Spheroids. J. Geophys. Res., 117:D08201, doi:10.1029/2011JD017216.
- Hackley, V. A., and Stefaniak, A. B. (2013). “Real-World” Precision, bias, and Between-Laboratory Variation for Surface Area Measurement of a Titianium Dioxide Nanomaterial in Powder Form. J. Nanopart. Res., 15:1742.
- Haynes, W. M., Ed. (2013). CRC Handbook of Chemistry and Physics, 94th ed. CRC Press: Boca Raton, FL.
- Hiranuma, N., Brooks, S. D., Moffet, R. C., Glen, A., Laskin, A., Gilles, M. K., et al. (2013). Chemical Characterization of Individual Particles and Residuals of Clud Drolets and Ice Crystals Collected on Board Research Aircraft in the ISDAC 2008 Study. J. Geophys. Res. Atmos., 118:6564–6579, doi: 10.1002/jgrd.50484.
- Hofmann, V., Boehm, H. P., and Gromes, W. (1961). Die Abmessungen der Kristalle der Tonminerale. Zeitschrift Fur Anorganische Und Allgemeine Chemie, 308:143–154.
- Hoose, C., and Möhler, O. (2012). Heterogeneous Ice Nucleation on Atmospheric Aerosols: A Review of Results From Laboratory Experiments. Atmo. Chem. Phys., 12:9817–9854.
- Hudson, P. K., Gibson, E. R., Young, M. A., Kleiber, P. D., and Grassian, V. H. (2008). Coupled Infrared Extinction and Size Distribution Measurements for Several Clay Components of Mineral Dust Aerosol. J. Geophys. Res., 113:D01201, doi:10.1029/2007JD008791.
- Inoue, A., and Kitagawa, R. (1994). Morphological Characteristics of Illitic Clay Minerals from a Hydrothermal System. Am. Mineral., 79:700–711.
- Jepson, W., and Rowse, J. (1975). The Composition of kaolinite – An Electron Microscope Microprobe Study. Clay Clay Mineral., 23:310–317.
- Johnson, B., Christopher, S., Haywood, J., Osborne, S., McFarlane, S., Hsu, C., et al. (2009). Measurements of Aerosol Properties from Aircraft, Satellite and Ground-Based Remote Sensing: A Case-Study from the Dust and Biomass-Burning Experiment (DABEX). Q. J. R. Meteorol. Soc., 135:922–934.
- Johnson, M., Meskhidze, N., and Kiliyanpilakkil, P. (2012). A Global Comparison of GEOS-Chem-Predicted and Remotely-Sensed Mineral Dust Aerosol Optical Depth and Extinction Profiles. J. Adv. Model. Earth Sys., 4:M07001, doi:10.1029/2011MS000109.
- Kahnert, F. M. (2004). Reproducing the Optical Properties of Fine Desert Dust Aerosols using Ensembles of Simple Model Particles. J. Quant. Spectrosc. Radiat. Transfer, 85:231–249.
- Kok, J. F. (2011). A Scaling Theory for the Size Distribution of Emitted Dust Aerosols Suggests Climate Models Underestimate the Size of the Global Dust Cycle. Proc. Natl. Acad. Sci., 108(3):1016–1021.
- Krueger, B. J., Grassian, V. H., Iedema, M. J., Cowin, J. P., and Laskin, A. (2003). Probing Heterogeneous Chemistry of Individual Atmospheric Particles using Scanning Electron Microscopy and Energy-Dispersive x-Ray Analysis. Anal. Chem., 75:5170–5179.
- Laird, D. (2001). Nature of Clay-Humic Complexes in an Agricultural Soil: II. Scanning Electron Microscopy Analysis. Soil Sci. Soc. Am. J., 65:1419–1425.
- Lindgreen, H., Garnaes, J., Hansen, P., Besenbacher, F., Laegsgaard, E., Stensgaard, I., et al. (1991). Ulatrafine Particles of North Sea Illite/Smectite Clay Minerals Investigated by STM and AFM. Am. Mineral., 76: 1218–1222.
- Lindqvist, H., Jokinen, O., Kandler, K., Scheuvens, D., and Nousiainen, T. (2014). Single Scattering by Realistic, Inhomogeneous Mineral Dust Particles with Stereogrammetric Shapes. Atmos. Chem. Phys., 14:143–157.
- Liu, T. (1985). Loess in China. China Ocean Press, Beijing.
- Madsen, F.T. (1977). Surface area measurements of clay minerals by glycerol sorption on a thermobalance. Thermochim. Acta, 21:89–93.
- Martin, R. T., Bailey, S. W., Eberl, D. D., Fanning, D. S., Guggenheim, S., Kodama, H., et al. (1991). Report of the Clay Minerals Society Nomenclature Committee: Revised Classification of Clay Materials. Clay Clay Mineral, 39(3):333–335.
- McNaughton, S., Clarke, A., Kapustin, V., Shinozuka, Y., Howell, S., Anderson, B., et al. (2009). Observations of Heterogeneous Reactions Between Asian Pollution and Mineral Dust Over Eastern North Pacific During INTEX-B. Chem. Phys., 2:8469–8539.
- Merikallio, S., Lindqvist, H., Nousiainen, T., and Kahner, M. (2011). Modelling Light Scattering by Mineral Dust using Spheroids: Assessment of Applicability. Atmos. Chem. Phys., 11:5347–5363.
- Metz, V., Raanan, H., Pieper, H., Bosback, D., and Ganor, J. (2005). Toward the Establishment of a Reliable Proxy of the Reactive Surface Area of Smectite. Geochimica et Cosmochimica Acta 69(10):2581–2591.
- Moore, D. M., and Reynolds, R. C., Jr. (1997). X-Ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed. Oxford University Press, New York.
- Nadeau, P. H. (1985). The Physical Dimensions of Fundamental Clay Particles. Clay Mineral, 20:499–514.
- Nadeau, P. H. (1987). Relationships Between the Mean Area, Volume and Thickness for Dispersed Particles of Kaolinites and Micaceous Clays and Their Application to Surface Area and Ion Exchange Properties. Clay Mineral, 22:351–356.
- Nousiainen, T., Kahnert, M., and Veihelmann, B. (2006). Light Scattering Modeling of Small Feldspar Aerosol Particles using Polyhedral Prisms and Spheroids. J. Quant. Spectrosc. Radiat. Transfer, 101:471–487, doi: 10.1016/j.jqsrt.2006.02.038.
- Pinti, V., Marcolli, C., Zobrist, B., Hoyle, C. R., and Peter, T. (2012). Ice Nucleation Efficiency of Clay Minerals in the Immersion Mode. Atmos. Chem. Phys., 12:5859–5878.
- Pruett, R. J., and Webb, H. L. (1993). Sampling and Analysis of KGa-1b Well-Crystallized Kaolin Source Clay. Clay Clay Mineral, 41:514–519.
- Robertson, R. H. S., Brindley, G. W., and Mackenzie, R. C. (1954). Mineralogy of Kaolin Clays from Pugu. Tanganyika, Am. Mineral., 39:118–138.
- Sanders, R. L., Washton, N. M., and Mueller, K. T. (2010). Measurement of the Reactive Surface Area of Clay Minerals using Solid-State NMR Studies of a Probe Molecule. J. Phys. Chem. C, 114:5491–5498.
- Schleicher, B., Jung, T., and Burtscher, H. (1993). Characterization of Ultrafine Aerosol Particles Adsorbed on Highly Oriented Pyrolytic Graphite by Scanning Tunneling and Atomic Force Microscopy. J. Colloid Interface Sci., 161:271–277.
- Schuttlefield, J. D., Cox, D., and Grassian, V. H.(2007). An Investigation of Water Uptake on Clays Minerals using ATR-FTIR Spectroscopy Coupled with Quartz Crystal Microbalance Measurements. J. Geophys. Res., 112:D21303 doi:10.1029/2007JD008973.
- Siegesmund, S., Weiss, T., and Vollbrecht, A. (2002). Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies, Geological Society, Vol. 205. Special Publications, London, pp. 137–147.
- Steudel, A., Batenburg, L. F., Fischer, H. R., Weidler, P. G., and Emmerich, K. (2009). Alteration of Non-Swelling Clay Minerals and Magadiite by Acid Activation. Appl. Clay Sci., 44:95–104.
- Tumolva, L., Park, J., and Park, K. (2012). Combination of Transmission Electron and Atomic Force Microscopy Techniques to Determine Volume Equivalent Diameter of Submicrometer Particles. Microsc. Res. Techniq., 17:505–512.
- van Olphen, H, and Fripiat, J.J. (1979). Data Handbook for Clay Materials and Other Nonmetallic Minerals. Pergamon, New York.
- van Poppel, L. H., Friedrich, H., Spinsby, J., Chung, S. H., Seinfeld, J. H., and Buseck, P. R. (2005). Electron Tomography of Nanoparticle Clusters: Implications for Atmospheric Lifetimes and Radiative Forcing of Soot. Geophys. Res. Lett., 32:L24811, doi:10.1029/2005GL024461.
- Wang, B., Laskin, A., Roedel, T., Gilles, M. K., Moffet, R. C., Tivanski, A. V., et al. (2012). Heterogeneous Ice Nucleation and Water Uptake by Field-Collected Atmospheric Particles Below 273 K. J. Geophys. Res., 117:D00V19, doi: 10.1029/2012JD017446.
- Zhao, T., Gong, S., Zhang, X., and McKendry, I. (2003). Modeled Size-Segregated Wet and Dry Deposition Budges of Soil Dust Aerosol During ACE-Asia 2001: Implications for Trans-Pacific Transport. Geophys. Res., 10:D23, doi: 10.1029/2002JD003363.
