Abstract
This study introduces a spark discharge system (SDS) as a way to simulate welding fumes. The SDS was developed using welding rods as electrodes with an optional coagulation chamber. The size, morphology, composition, and concentration of the fume produced and the concentration of ozone (O3) and nitrogen oxides (NOX) were characterized. The number median diameter (NMD) and total number concentration (TNC) of fresh fume particles were ranged 10–23 nm and 3.1×107 − 6×107 particles/cm3, respectively. For fresh fume particles, the total mass concentration (TMC) measured gravimetrically ranged 85–760 μg/m3. The size distribution was stable over a period of 12 h. The NMD and TNC of aged fume particles were ranged 81–154 nm and 1.5×106−2.7×106 particles/cm3, respectively. The composition of the aged fume particles was dominated by Fe and O with an estimated stoichiometry between that of Fe2O3 and Fe3O4. Concentrations of O3 and NOX were ranged 0.07–2.2 ppm and 1–20 ppm, respectively. These results indicate that the SDS is capable of producing stable fumes over a long-period that are similar to actual welding fumes. This system may be useful in toxicological studies and evaluation of instrumentation.
Copyright 2014 American Association for Aerosol Research
1. INTRODUCTION
Welding produces “welding fume” which is mixture of metal oxide particles (Jenkins and Eagar Citation2005) and gases including ozone (O3) and nitrogen oxides (NOX) (Antonini Citation2003). Welding vaporizes metals found in the object being welded and materials used in the welding process (e.g., welding sticks used in shielded metal arc welding, SMAW). These metals react with ambient air, condense, and form metal oxide particles primarily of respirable size (Antonini Citation2003). In welding, O3 is produced in a photochemical reaction induced by ultraviolet light with ambient oxygen gas during the welding process (Liu et al. Citation2007), and NOX are formed by direct oxidation of ambient nitrogen (N2) at high temperatures produced by the arc or flame (Antonini Citation2003).
Welding fume particles range in size from 0.005 to 20 μm, although less than 10–30% of the fume mass is larger than 1 μm (Jenkins and Eagar Citation2005). When inhaled, sub-micrometer particles can deposit throughout the respiratory system. As a result of their small size and unique properties in vivo, the toxicological profiles of sub-micrometer particles, including nanoparticles, may differ considerably from those of larger particles composed of the same materials (Oberdörster et al. Citation1992). It has been established that adverse pulmonary health effects are primarily dependent on the size, solubility and chemical makeup of the particle (Kim et al. Citation2011a; Oprya el al. Citation2012). The welding fume particles that deposit in the respiratory tract can dissolve and/or translocate as nanoparticles from the lung, reach the bloodstream, and pass to other organs (Antonini et al., Citation2009; Antonini et al. Citation2010). The materials that are welded often contain alloying elements, such as manganese (Mn) common to all steel, chromium (Cr) in stainless steel, and cadmium (Cd) in plating and brazing materials. These metals contribute to the toxicity of welding fume and can cause acute and chronic adverse health effects (Antonini Citation2003).
O3 and NOX are potent oxidizing agents that can also contribute to adverse health effects. Prolonged exposure to low levels of O3 (as low as 0.08 ppm) can initiate pulmonary inflammatory reactions in normal humans (Devlin et al. Citation1991), potentiate allergic airway diseases (Holz et al. Citation2002). Repeated exposures to low levels (i.e., 0.2 ppm) of O3 led to persistent airway inflammation in healthy subjects (Jörres et al. Citation2000). Exposure to low level O3 (i.e., 0.06 ppm for 6.6 h) induced a significant decrease in lung function (i.e., forced expiratory volume in one second, FEV1) with inflammatory changes in the airways of healthy young adults (Kim et al. Citation2011b). Repeated low level (i.e., 2 ppm) exposure to nitrogen dioxide (NO2) induced pro-allergic responses (i.e., upregulation of IL-5, IL-10, IL-13, and ICAM-1 expression) in the bronchial epithelium of healthy human airways (Pathmanathan et al. Citation2003). Brief exposure to environmentally relevant concentration (i.e., 0.27 ppm) of NO2 can enhance the airway inflammatory reaction to allergen without any decrement in lung function in asthmatics (Barck et al. Citation2005). Prolonged exposure to low doses (i.e., 0.5 ppm) of nitric oxide (NO) induced interstitial lung damage in rats (Mercer et al. Citation1995).
The ability to control welding fume concentrations is critical in toxicological studies. Only few welding fume inhalation exposure systems have been developed because of the numerous different types of welding processes employed in the workplace and the difficulties with generating a fume with stable output over extended periods of time. Hicks et al. (Citation1984) used experienced welders to generate fumes from various welding types for the head-only exposure and the intratracheal instillation. Difficulties with this system included the need to hire experienced welders to operate the system and disruptions in output because the base metal and welding materials required replacement every few minutes. Others have developed robotic welders to generate fumes. Oh et al. (Citation2012) used a rotating stainless disc as a base metal and a welding rod restrained in the welding rod holder support to simulate SMAW fume, known informally as stick welding. Similarly, Zimmer et al. (2002) used a welding fume generation system comprised of a rotating cylinder as the base material. Antonini et al. (2006; 2007) developed a robotic welding fume generator to simulate gas metal arc welding (GMAW). The welding fume generation system used an automated, programmable six-axis robotic arm, a water-cooled arc welding torch, a wire feeder that supplied the wire to the torch, and an automatic welding torch cleaner to keep the welding nozzle free of debris and spatter. This system was coupled with an inhalation exposure system. Physical and chemical characterizations of the generated fumes were performed. Although quite representative of real welding exposures, a limitation of these robotic systems is their large size, high cost, and complex operation that introduce difficulties in performing laboratory experiments.
On the other hand, spark systems represent a simple way to reproducibly generate a metal oxide aerosol of controllable size and concentration (Roth et al. Citation2004). A spark is discharged across the electrode gap when a high voltage is supplied to metal electrodes, thereby vaporizing material from the electrodes and producing primary particles in the low nanometer size range via nucleation/condensation of the vapor. At the same time, these particles are oxidized by air ions and radicals produced by spark discharge. Primary particles collide with one another, due to a relative motion between them and adhere to form chain-like agglomerates (Meuller et al. Citation2012). This spark process is similar to SMAW. Many researchers have discussed and described the application of spark discharge in conductive nanoparticle (metals and carbon) generation for toxicology study (Roth et al. Citation2004; Kapp et al. Citation2004; Takenaka et al. Citation2006; Messing et al. Citation2013). However, none of these studies sought to simulate welding fume.
The focus of this study was twofold. First, we introduce a spark discharge system (SDS) as a way to simulate welding fume in a compact system that is inexpensive and easy to control. The SDS was developed using welding rods as electrodes with an optional chamber that can then be used to coagulate the particles. Second, the physicochemical characteristics of particles and gases produced by the SDS were characterized. In particular, for particles, the size, morphology, aggregation state, and composition of the welding fumes produced under an air environment were determined using a variety of analytical methods. This novel application of SDS can be used to simulate welding fume from SMAW for toxicological studies and instrument testing.
2. METHODS
2.1. Spark Discharge System
The SDS is shown in . Compressed air was passed through a trap to remove oil contaminants, a diffusion dryer to remove moisture, and a high efficiency particulate air (HEPA) filter to remove particles. The dry, clean air, controlled by a needle valve and monitored with a mass flowmeter (4146, TSI Inc., USA), was delivered at 5 L/min to the spark discharge chamber (volume 10.6 cm3). In the chamber, a spark discharge was formed between two identical electrodes. The electrodes were coaxial welding rods (Hard Surfacing Stick Electrodes Overlay, Hobart, USA) with a core rod (diameter 3.2 mm = 1/8 in.) and a coating layer (diameter 6.4 mm = 1/4 in). Elemental and compositional specifications of these electrodes are provided in . The electrical circuit included a resistance of 0.5 MΩ (two 1 MΩ resistors arranged in parallel), a capacitance of 1 nF, and an applied voltage of 5 kV.
TABLE 1 Elemental and compositional specification of test electrodes (Hobart Citation2008)
FIG. 1. Experimental setup. Sampling ports #1 and #2 are located before and after the coagulation chamber, respectively.
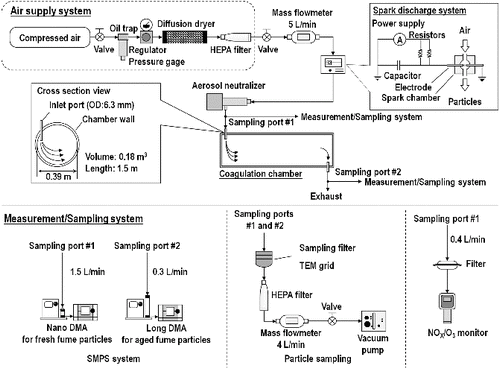
The aerosol produced by the spark was passed through an aerosol neutralizer (3087, TSI Inc., USA) to neutralize the charge on the aerosol to a Boltzmann distribution. Optionally, particles were delivered to a coagulation chamber, consisting of a circular steel cylinder with a volume of 0.18 m3 (inner diameter of 0.39 m and length of 1.5 m). A coagulation time of 36 min was approximated from the volume of the coagulation chamber (0.180 m3) divided by airflow rate (5 L/min).
2.2. Particle Size and Morphology
The aerosol produced by the SDS was characterized for the following conditions: without passing through the coagulation chamber (fresh fume) with loading currents of 0.25, 0.5, and 1 mA; and with passing through the coagulation chamber (aged fume) with loading currents of 1 and 3 mA. When the loading current increases, the generation rate and size of particles are also increased (Meuller et al. Citation2012). To identify operational conditions showing similar output to actual welding fumes, the loading currents were varied from 0.25 to 3 mA. The loading current was decreased from 1 to 0.25 mA to produce smaller sized particles for the fresh fume, and increased from 1 to 3 mA to produce lager sized particles for the aged fume. The spark and coagulation chambers were cleaned periodically with compressed dry particle-free air to eliminate the residual particles.
The size distributions of the generated particles were measured using a scanning mobility particle sizer (SMPS; 3936, TSI Inc., USA), consisting of a classifier controller (3080, TSI Inc., USA), a differential mobility analyzer (DMA), a condensation particle counter (CPC; 3776, TSI Inc., USA), and an aerosol neutralizer (3077; TSI Inc., USA). The SMPS was operated with a nano DMA (3085, TSI Inc., USA) to measure the fresh fume particles from 2.2 to 63.8 nm (mobility equivalent diameters) and a long DMA (3081, TSI Inc., USA) to measure the aged fume particles from 15.1 to 661.2 nm. The size distribution of each test atmosphere was measured five times. The size distributions of fresh fume particles were measured every 30 min for 12 h at loading currents of 0.25 and 0.5 mA to examine the long-term stability of generation.
To determine the total mass concentration (TMC), fresh and aged fume particles were sampled onto a polyvinyl chloride (PVC) membrane filter (5 μm, 37 mm, SKC Gulf Coast Inc., USA) at the sampling flow rate of 4 L/min for 12 h. A micro-balance (MT5, Mettler-Toledo, USA) was used to measure the weight of deposited particles. Gravimetric tests were conducted in triplicate.
To examine morphology, fresh and aged fume particles were sampled onto a transmission electron microscope (TEM) grid (200-mesh, Ni grid, Carbon layer, 01840N-F, Ted Pella Inc., USA). The TEM grid was placed on the PVC filter. Fresh fume particles (0.25 mA) and aged fume particles (3 mA) were sampled for 6 h and 5 min, respectively. A TEM (JEM-1230, JEOL Ltd., Japan) was used to evaluate the projected area (PA) diameter (dPA) and morphology of generated particles. The dPA is defined as the diameter of the circle having the same PA as the particle's two-dimensional silhouette. The PA was obtained using ImageJ software (version 1.47, NIH, USA) and dPA was calculated as follows:[1]
A total number of 100 and 70 agglomerates were counted for fresh and aged fume particles, respectively.
2.3. Chemical Characterization
Elemental analysis of aged fume particles was determined using energy dispersive X-ray (EDX) spectroscopy. To obtain EDX spectra, the fume particles deposited on the 200-mesh, carbon coated Ni grid were imaged by high resolution transmission electron microscopy (HRTEM) using a microscope (JEM-2100F, JEOL Ltd., Japan) in dark field mode. The EDX spectral peaks were compared against the standard peak positions of the elements given in . The elemental maps were obtained by rastering the electron beam over a specified region on the Ni grid. Mapping was conducted only for the aged fume particles because the loading of fresh fume particles collected on the grids was too low.
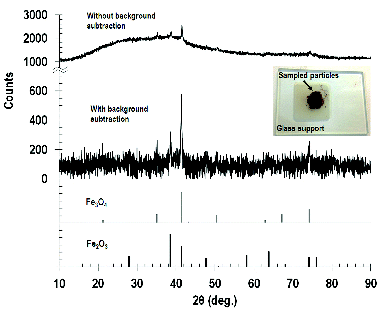
To further examine the phase of particles, aged fume particles with a loading current of 3 mA were collected onto a PVC filter for 36 h. Insufficient mass of particles were collected for lower loading currents. The solid phase of the particles were determined by X-ray diffraction (XRD; Miniflex II, Rigaku Corporation, Japan) using Co Kα radiation (λ = 179 pm) at a step size of 0.02°. Cobalt is used as the source of X-rays to minimize the background resulting from iron fluorescence. The XRD patterns obtained were matched against a database containing standard patterns using the JADE software (version 8, Materials Data, Inc., USA) to identify the phases present in these particles.
2.4. O3 and NOX Generation
A gas monitor (PortaSensII, ATI, USA) was used to monitor O3 and NOX concentrations when the SDS was operating. The sensitivity of the instrument was 0.01 ppm for O3 and 1 ppm for NOX. Gas concentrations were measured in triplicate for each test condition.
3. RESULTS AND DISCUSSION
3.1. Particle Size and Morphology
The size distribution of the fresh and aged fume particles obtained by SMPS is shown in with summary details provided in . Images showing the morphology and size of the generated welding fume particles are shown in . These data demonstrate that higher current supplied to the welding rod produces higher number concentrations and larger nanoparticles.
TABLE 2 Sample current and particle characteristics (size, total number concentration, and total mass concentration) for generated welding fume particles
FIG. 2. Size distributions of fresh (a) and aged (b) fume particles and continuous generation data (c) of fresh fume particles at 0.25 and 0.5 mA. TNC: Total number concentration; NMD: number median diameter; σg: geometric standard deviation.
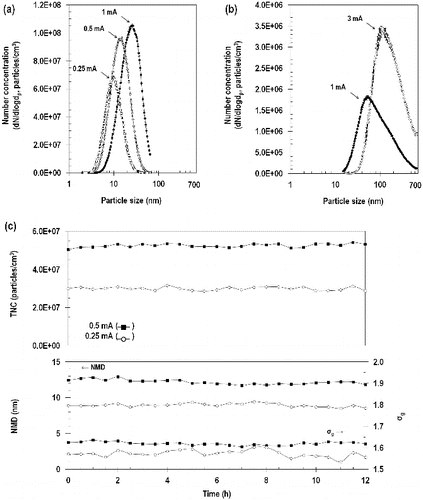
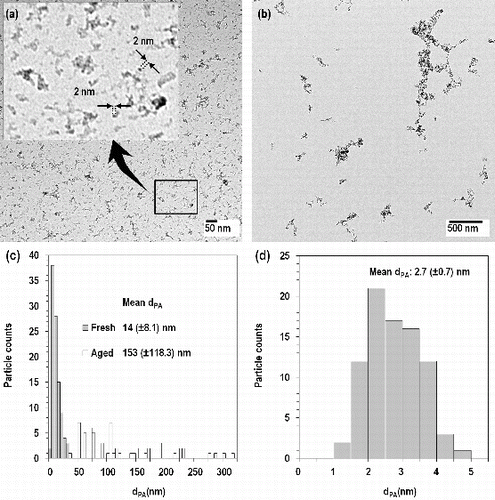
FIG. 3. TEM images of fresh (a) and aged (b) fume particles, projected area diameters (dPA) of fresh and aged fume particles (c) and primary single particles (d).
3.1.1. Fresh Fume Particles
For fresh fume particles, the number median diameter (NMD) increased from 10 nm at 0.25 mA to 23 nm at 1 mA. Similarly, the total number concentration (TNC) increased from 3.1×107 particles/cm3 at 0.25 mA to 6×107 particles/cm3 at 1 mA. Size distributions of fresh fume particles were log-normally distributed with a geometric standard deviation (σg) ranging from 1.52 to 1.64. As shown in , the concentration and size distribution of the fresh fume particles were extremely stable over 12 h. The coefficient of variation of the 30-min readings over the 12-h period were: 2.9% TNC, 2.7% NMD, and 1.0% σg at a loading current of 0.25 mA; and 1.7% TNC, 2.7% NMD, and 0.5% σg at a loading current of 0.5 mA.
As shown in , fresh fume particles appeared as clusters and chain-like aggregates formed from much smaller primary particles sized 2.7 (±0.7) nm. A histogram of the primary particle diameter determined from analysis of TEM images is shown in . However, these primary particles appeared as imperfect spheres. Roth et al. (Citation2004) explained that the primary particles in the spark discharge process form by nucleation and grow further either by collision with product molecules (condensation) or particles (coagulation). The colliding particles maintain their identity or fuse together into a single particle. This fusion can be complete or incomplete. In the case of iron oxide particles, incomplete fusion (partial sintering) occurred, and thus iron oxide particles appear as non-spherical particles. From TEM images, the mean dPA of the fresh fume particles at 0.25 mA was 14 (±8.1) nm, which is consistent although somewhat larger than NMD (10 nm) from the SMPS (). However, the standard deviation of dPA was ±8.1 nm and the difference of NMD and mean dPA was within the range of error.
As shown in , the TMC measured by SMPS did not agree well with that measured gravimetrically. The TMC measured by SMPS was 282 μg/m3 at 0.25 mA and 7200 μg/m3 at 1 mA, whereas the TMC measured gravimetrically was 85 μg/m3 at 0.25 mA and 760 μg/m3 at 1 mA. The TMC from SMPS was calculated from the measured number concentration assuming a particle density of 7870 kg/m3 (bulk iron density) and spherical shape. The particles were observed to be highly nonspherical. Thus, the assumption of sphericity is problematical and the actual particle density is likely substantially lower than that of bulk iron.
These results show that the SDS when operated without the coagulation chamber can be used to generate fresh fume particles which consist primarily of nanoparticles in the range of 3 to 20 nm over a long time period. A major advantage of this method is that freshly produced nanoparticles can be immediately delivered to animals or cells without intermediary steps, such as nebulized suspension of powders. Generally, nanoparticles can be generated by dispersion of solutions or suspensions by nebulizers or electrospray, but the impurities of the dispersed liquid will contaminate the particles (Roth et al. Citation2004). Although nanoparticle production using vaporization of material in a tube furnace with subsequent homogenous nucleation, condensation, and coagulation offers similar benefits, the tube furnace system is considerably more complex and costly than the SDS used here. Additionally, impurities that evaporate from crucibles and tube materials are incorporated in the particles generated with a tube furnace system (Roth et al. Citation1994). Another advantage of SDS is that size and generation rate of fresh fume particles can be readily controlled by a loading current.
3.1.2. Aged Fume Particles
As expected when the fresh fume was passed through the coagulation chamber at a loading current of 1.0 mA, the NMD increased from 23 to 81 nm, and the TNC decreased from 6.0×107 to 1.5×106 particles/cm3 (; ). When the current was increased to 3 mA, the NMD increased to 154 nm and the TNC increased to 2.7×106 particles/cm3. Size distributions of aged fume particles were log-normally distributed but the σg was approximately 2, which was substantially larger than that for the fresh fume particles.
The size distribution of the aged fume particles observed at 3 mA is similar to that of a field study analyzing welding fume particles (Zimmer Citation2002; Stephenson et al. Citation2003). Using TEM, Zimmer (Citation2002) identified the particles formed during welding to range in size from 50 to 300 nm. Stephenson et al. (Citation2003) reported that welding produced an approximately lognormal particle mode with a 120-nm count median and a σg of 2.07.
Similar to fresh fume particles (), aged fume particles also appear as clusters and chain-like aggregates formed from much smaller primary particles (). The shape of aged fume particles is similar to one of welding fume sampled by Stephenson et al. (Citation2003) and Antonini et al. (Citation2006). The mean dPA of the aged particles was 153 (±118.3) nm which agrees well with the NMD of 154 nm in . These aged fume particles can be used to evaluate instruments for welding fumes. Conventional methods to produce test aerosol have used salt, oil, and polystyrene sphere particles. Their shapes are cubes or spheres which are different from those of welding fumes. The SDS can be an alternative method to evaluate the instruments which measure the welding fumes.
As shown in , the TMC measured gravimetrically (730 μg/m3 at 1 mA and 2700 μg/m3 at 3 mA) were substantially lower than those from the SMPS (4.5×104 μg/m3 at 1 mA and 2.4×105 μg/m3 at 3 mA) (). The difference of TMC from SMPS and the gravimetric method for aged fume particles was substantially greater than that for fresh fume particles. A possible reason is the shape of the aged fume that the difference of particle density and assumed density was increased. The shape of aged fume particles was totally different with sphere and more complex than one of fresh fume particles as shown in . We may expect that the density of aged fume particle is smaller than 150 kg/m3 from the result of gravimetric method.
The maximum TMC of 2700 μg/m3 is comparable to 8-h average concentrations measured in actual factories (3000 to 11300 μg/m3, Balkhyour and Goknil, Citation2010). However, in toxicology studies, the required test concentration is often higher than 2700 μg/m3. In traditional methods, welding fumes have been generated at concentrations near or above the recommended welding fume threshold exposure limit value-time weighted average (TLV-TWA) of 5000 μg/m3 for 8 h/day (ACGIH Citation2001). Antonini et al. (Citation2006, Citation2007) have proposed welding fume concentrations of 1000–40,000 μg/m3 and 15,000–40,000 μg/m3, respectively. These higher values of concentration can be easily realized by increasing the power input with increasing the loading current or the applied voltage (Meuller et al. Citation2012).
3.2. Chemical Characteristics
The EDX elemental analysis of collected particles generated under coagulated conditions at 3 mA is shown in . The peak at approximately 7.5 keV corresponds to the Ni grid used to support the sample and is not from the aged fume particles. The composition of the aged fume particles was similar to that of test electrodes and dominated by Fe and O. The fractions of Fe and O were 0.08 and 0.15, respectively. From this result, a projected stoichiometry of iron oxide is between Fe2O3 and Fe3O4.
FIG. 4. EDX spectrum (a) and elemental maps (b) of aged fume particles. These maps are for Fe, O, Si, Cr and Ni. For EDX spectrum, the square region in the top left image of was analyzed. Particles were sampled on the Ni grid.
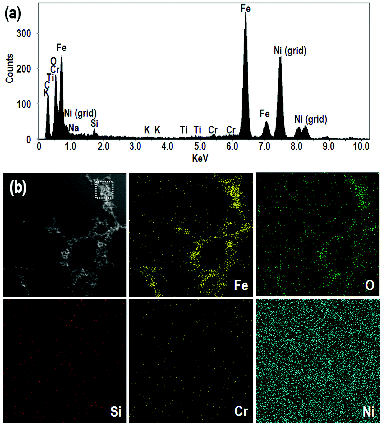
The first image in is for aged fume particles at 3 mA. The image of these particles is combined with EDX maps. These maps correspond to Fe, O, Si, Cr, and Ni, respectively. The dots in these images indicate the positions of each element in the first image. For example, Fe and O are concentrated in the regions corresponding to the particles in the first image, which shows that the particles contain Fe and O in agreement with spectrum shown in .
The powder XRD pattern () indicates that there are crystalline as well as amorphous materials present in the sample. In particular, the XRD data indicates that the crystalline fraction of spark-generated particles consists mainly of Fe2O3 (hematite) and Fe3O4 (magnetite), consistent with the EDX analysis. Sampled particles were reddish brown in color as shown in the inset of and have similar appearance to the laboratory synthesized 2 nm α-Fe2O3 nanoparticles. The broad band observed in the XRD patterns without any background subtraction indicates the presence of amorphous regions as well in these samples. In addition, majority of the background noise in the XRD patterns result from the fluorescence of iron and some from the X-rays scattered from air and the sample holder.
3.3. O3 and NOX Generation
The concentrations of O3 and NOX produced when the SDS was operating are provided in . A higher loading current resulted in higher concentrations of O3 and NOX. O3 and NOX emission was proportional to the loading current. The O3 and NOX concentrations increased from 0.07 and 1 ppm at 0.25 mA to 2.22 and 20 ppm at 3 mA.
TABLE 3 O3 and NOX concentrations measured for spark discharge system (SDS) under different operating conditions
Although other researchers have described the application of spark discharge for toxicology study (Kapp et al. Citation2004; Roth et al. Citation2004; Takenaka et al. Citation2006; Messing et al. Citation2013), they have not focused on gases since they used spark discharge with inert gas such as argon or N2. Our results of using spark discharge in an air environment show that O3 and NOX are produced, like that typical of SMAW in an occupational setting. However, we are unable to control the O3 and NOX emissions. In some toxicological studies, particles and gases need to be separated. In future work, we will investigate the use of inert gas, such as N2, for the spark environment with mixing of oxygen after particles are formed to minimize O3 and NOX production.
These gas concentrations are similar to those observed during welding on worksites. Steel (Citation1968) measured concentration of O3 in the range of 0.1 to 0.6 ppm in 40 shipyards where three different welding processes were used. Liu et al. (Citation2007) observed that O3 concentrations were highest (∼0.20 ppm) for SMAW and gradually decreased to 0.06 ppm 10 min after the welding was completed. NO2 levels in the welding area can be as high as 7 ppm during flux-cored arc welding (Antonini Citation2003). Cole et al. (Citation2007) conducted GMAW and gas tungsten arc welding processes for various combinations of base and filler alloys to quantify gas emissions such as O3, NO, and NO2. O3 concentration was ranged from 0.37 to 4.70 ppm. NO and NO2 concentrations were 0.52–16.6 ppm and 0.24–7.85 ppm, respectively.
4. CONCLUSION
This study introduces a method of particle generation using spark discharge to simulate welding fume. The SDS was developed using welding rods as electrodes with an optional chamber that can be used to coagulate the particles. Operating the spark under an air environment, we characterized the size, morphology, concentration, and composition of the welding fume particles and concentrations of O3 and NOX. These results indicate that the generated fume is comparable to welding fume particles in workplace settings. Our SDS may be useful in toxicological studies and instrument testing. The SDS as described here is limited to SMAW. A further limitation of this study is that only one welding rod was tested. Future studies will focus on particle generation in inert gas environments to eliminate these gases using various types of welding rod used in SMAW.
FUNDING
This work was supported by a pilot research grant from the Environmental Health Sciences Research Center at the University of Iowa (NIH P30 ES005605). Financial support was also provided by National Institute for Occupational Safety and Health (1 R01 OH010238).
REFERENCES
- ACGIH. (2001). Welding Fumes, not Otherwise Specified, 7th edn. Documentation of the Threshold Limit Values for Chemical Substances, Vol. 3. American Conference of Governmental Industrial Hygienists, ACGIH, Cincinnati, OH, pp. 1726–1727.
- Antonini, J. (2003). Health Effects of Welding. Crit. Rev. Toxicol., 33(1):61–103.
- Antonini, J. M., Afshari, A. A., Stone, S., Chen, B., Schwegler-Berry, D., Fletcher, W. G., et al., (2006). Design, Construction, and Characterization of a Novel Robotic Welding Fume Generator and Inhalation Exposure System for Laboratory Animals. J. Occup. Envrion. Hyg., 3(4):194–203.
- Antonini, J. M., Stone, S., Roberts, J. R., Chen, B., Schwegler-Berry, D., Afshari, A. A., et al. (2007). Effect of Short-Term Stainless Steel Welding Fume Inhalation Exposure on Lung Inflammation, Injury, and Defense Responses in Rats. Toxicol. Appl. Pharm., 223(3):234–245.
- Antonini, J. M., Sriram, K., Benkovic, S. A., Roberts, J. R., Stone, S., Chen, B. T., et al. (2009). Mild Steel Welding Fume Causes Manganese Accumulation and Subtle Neuroinflammatory Changes but not Overt Neuronal Damage in Discrete Brain Regions of Rats After Short-term Inhalation Exposure. Neurotoxicology, 30(6):915–925.
- Antonini, J. M., Roberts, J. R., Chapman, R. S., Soukup, J. M., Ghio, A. J., and Sriram, K. (2010). Pulmonary toxicity and extrapulmonary tissue distribution of metals after repeated exposure to different welding fumes. Inhal. Toxicol., 22(10):805–816.
- Balkhyour, M. A., and Goknil, M. K. (2010). Total fume and metal concentrations during welding in selected factories in Jeddah, Saudi Arabia. Int. J. Environ. Res. Publ. Health, 7(7), 2978–2987.
- Barck, C., Lundahl, J., Halldén, G., and Bylin, G. (2005). Brief Exposures to NO2 Augment the Allergic Inflammation in Asthmatics. Environ. Res., 97(1):58–66.
- Cole, H., Epstein, S., and Peace, J. (2007). Particulate and Gaseous Emissions When Welding Aluminum Alloys. J. Occup. Environ. Hyg., 4(9):678–687.
- Devlin, R. B., McDonnell, W. F., Mann, R., Becker, S., House, D. E., Schreinemachers, D., et al. (1991). Exposure of Humans to Ambient Levels of Ozone for 6.6 Hours Causes Cellular and Biochemical Changes in the Lung. Am. J. Resp. Cell Mol., 4(1):72–81.
- Hicks, R., Lam, H. F., Al-Shamma, K. J., and Hewitt, P. J. (1984). Pneumoconiotic Effects of Welding-Fume Particles from Mild and Stainless Steel Deposited in the Lung of the Rat. Arch. Toxicol., 55(1):1–10.
- Hobart. (2008). Material Safety Data Sheet #230 by Selectrode Industries, Inc.
- Holz, O., Mucke, M., Paasch, K., Bohme, S., Timm, P., Richter, K., et al. (2002). Repeated Ozone Exposures Enhance Bronchial Allergen Responses in Subjects with Rhinitis or Asthma. Clin. Exp. Allergy, 32(5):681–689.
- Jenkins, N. T., and Eagar, T. W. (2005). Chemical Analysis of Welding Fume Particles. Weld. J., 84(6):87s–93s.
- Jörres, R. A., Holz, O., Zachgo, W., Timm, P., Koschyk, S., Muller, B., et al. (2000). The Effect of Repeated Ozone Exposures on Inflammatory Markers in Bronchoalveolar Lavage Fluid and Mucosal Biopsies. Am. J. Resp. Crit. Care., 161(6):1855–1861.
- Kapp, N., Kreyling, W., Schulz, H., Im Hof, V., Gehr, P., Semmler, M., et al. (2004). Electron Energy Loss Spectroscopy for Analysis of Inhaled Ultrafine Particles in Rat Lungs. Microsc. Res. Techiq., 63(5):298–305.
- Kim, C. S., Alexis, N. E., Rappold, A. G., Kehrl, H., Hazucha, M. J., Lay, J. C., et al. (2011b). Lung Function and Inflammatory Responses in Healthy Young Adults Exposed to 0.06 ppm Ozone for 6.6 Hours. Am. J. Resp. Crit. Care., 183(9):1215–1221.
- Kim, J. S., Adamčaková-Dodd, A., O’Shaughnessy, P. T., Grassian, V. H., and Thorne, P. S. (2011a). Effects of Copper Nanoparticle Exposure on Host Defense in a Murine Pulmonary Infection Model. Part. Fibre. Toxicol., 8(1), article 29, 14 p.
- Liu, H. H., Wu, Y. C., and Chen, H. L. (2007). Production of Ozone and Reactive Oxygen Species After Welding. Arch. Environ. Con. Tox., 53(4):513–518.
- Mercer, R. R., Costa, D. L., and Crapo, J. D. (1995). Effects of Prolonged Exposure to Low Doses of Nitric Oxide or Nitrogen Dioxide on the Alveolar Septa of the Adult Rat Lung. Lab. Invest., 73(1):20–28.
- Messing, M. E., Svensson, C. R., Pagels, J., Meuller, B. O., Deppert, K., and Rissler, J. (2013) Gas-Borne Particles with Tunable and Highly Controlled Characteristics for Nanotoxicology Studies. Nanotoxicology, 7(6):1052–1063.
- Meuller, B. O., Messing, M. E., Engberg, D. L., Jansson, A. M., Johansson, L. I., Norlén, S. M., et al. (2012). Review of Spark Discharge Generators for Production of Nanoparticle Aerosols. Aerosol Sci. Technol., 46(11):1256–1270.
- Oberdörster, G., Ferin, J., Gelein, R., Soderholm, S. C., and Finkelstein, J. (1992). Role of the Alveolar Macrophage in Lung Injury: Studies with Ultrafine Particles. Environ. Health Persp., 97:193–199.
- Oh, J. H., Yang, M. J., Heo, J. D., Yang, Y. S., Park, H. J., Park, S. M., et al. (2012). Inflammatory Response in Rat Lungs with Recurrent Exposure to Welding Fumes a Tanscriptomic Approach. Toxicol. Ind. Health, 28(3):203–215.
- Oprya, M., Kiro, S., Worobiec, A., Horemans, B., Darchuk, L., Novakovic, V., et al. (2012). Size Distribution and Chemical Properties of Welding Fumes of Inhalable Particles. J. Aerosol Sci., 45:50–57.
- Pathmanathan, S., Krishna, M. T., Blomberg, A., Helleday, R., Kelly, F. J., Sandström, T., et al. (2003). Repeated Daily Exposure to 2 ppm Nitrogen Dioxide Upregulates the Expression of IL-5, IL-10, IL-13, and ICAM-1 in the Bronchial Epithelium of Healthy Human Airways. Occup. Environ. Med., 60(11):892–896.
- Roth, C., Ferron, G. A., Karg, E., Lentner, B., Schumann, G., Takenaka, S., et al. (2004). Generation of Ultrafine Particles by Spark Discharge. Aerosol Sci. Technol., 38(3):228–235.
- Roth, C., Scheuch, G., and Stahlhofen, W. (1994). Clearance Measurements with Radioactively Labelled Ultrafine Particles. Ann. Occup. Hyg., 38(inhaled particles VII):101–106.
- Steel, J. (1968). Respiratory Hazards in Shipbuilding and Shiprepairing. Ann. Occup. Hyg., 11(2):115–121.
- Stephenson, D., Seshadri, G., and Veranth, J. M. (2003). Workplace Exposure to Submicron Particle Mass and Number Concentrations from Manual Arc Welding of Carbon Steel. AIHA J., 64(4):516–521.
- Takenaka, S., Karg, E., Kreyling, W. G., Lentner, B., Möller, W., Behnke-Semmler, M., et al. (2006). Distribution Pattern of Inhaled Ultrafine Gold Particles in the Rat Lung. Inhal. Toxicol., 18(10):733–740.
- Zimmer, A. T. (2002). The Influence of Metallurgy on the Formation of Welding Aerosols. J. Environ. Monitor., 4(5):628–632.
- Zimmer, A. T., Baron, P. A., and Biswas, P. (2002). The Influence of Operating Parameters on Number-Weighted Aerosol Size Distribution Generated from a Gas Metal Arc Welding Process. J. Aerosol Sci., 33(3):519–531.